Medical Benefits and Polymer Applications of Grapes
Abstract
1. Introduction
2. Varieties
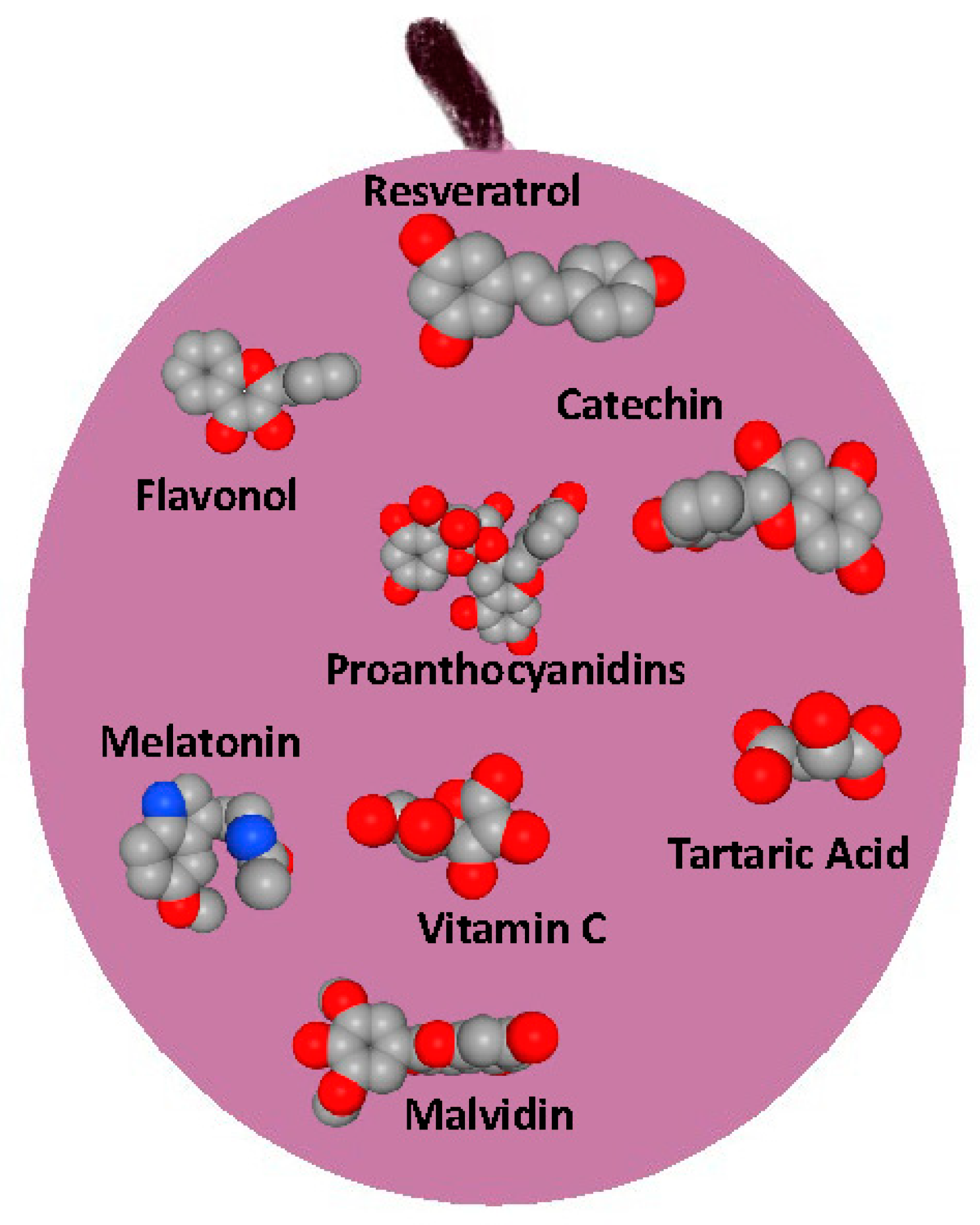
3. Bioactive Compounds
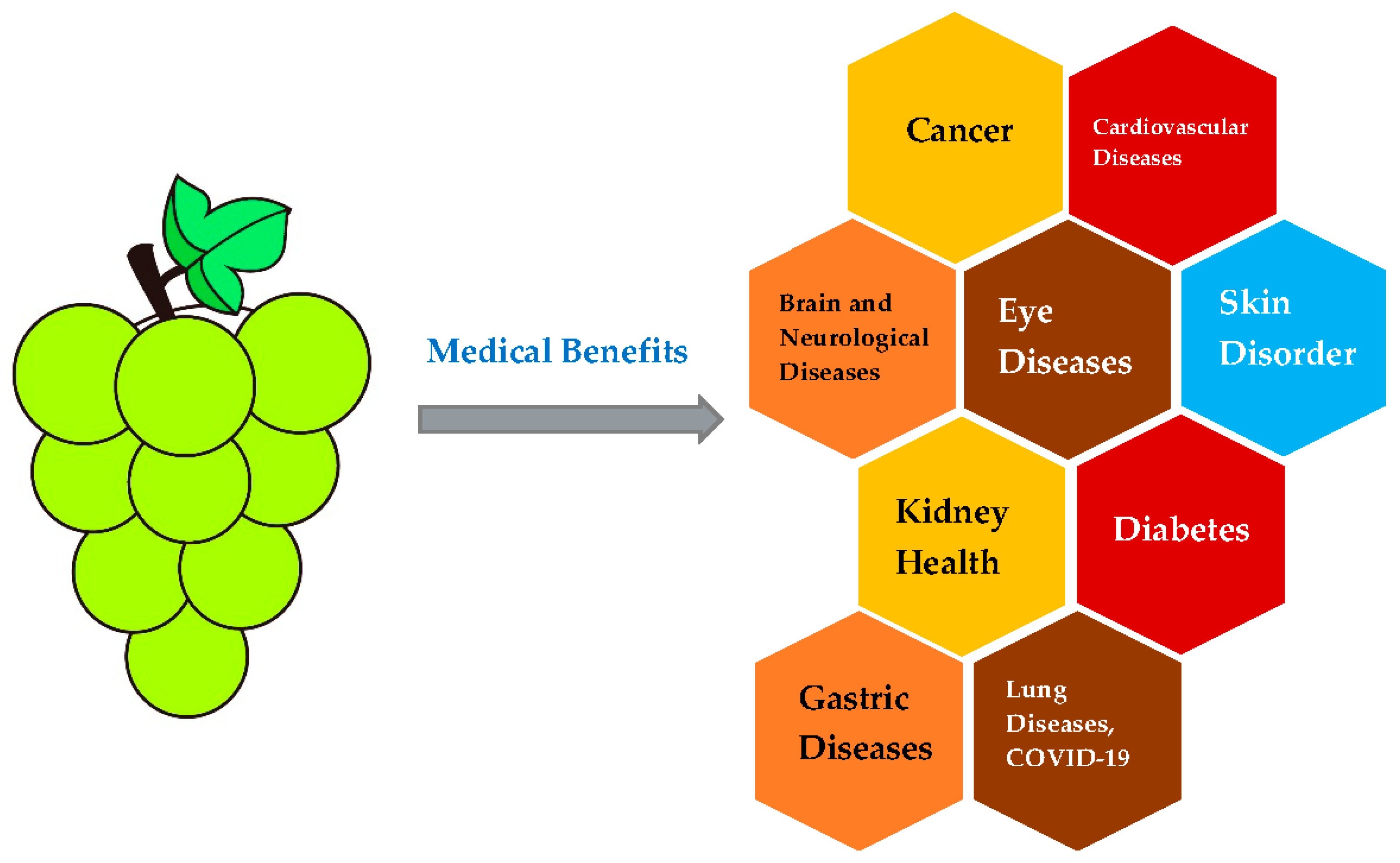
4. Medical Effects on Diseases
4.1. Cancer
4.2. Cardiovascular Disease
4.3. Brain and Neurological Disease
4.4. Eye Diseases
4.5. Skin Disorder and Wound Healing
4.6. Kidney Health
4.7. Diabetes
4.8. Gastric Diseases
4.9. Lung Diseases and COVID-19
5. Medical Polymer
5.1. Drug Delivery
5.2. Wound Dressing
5.3. Tissue Engineering
6. Discussion
6.1. Dietary Restrictions
6.2. Limitations and Future Research Perspectives
7. Conclusions
Funding
Conflicts of Interest
References
- Goor, A. The history of the grape-vine in the Holy Land. Econ. Bot. 1966, 20, 46–64. [Google Scholar] [CrossRef]
- Taskesenlioglu, M.Y.; Ercisli, S.; Kupe, M.; Ercisli, N. History of grape in Anatolia and historical sustainable grape production in Erzincan agroecological conditions in Turkey. Sustainability 2022, 14, 1496. [Google Scholar] [CrossRef]
- The New American Bible; Catholic Bible Press: Nashville, TN, USA, 1991.
- Alston, J.M.; Sambucci, O. Grapes in the world economy. In The Grape Genome; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–24. [Google Scholar]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Diaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020, 41, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. microRNA Pathological Mechanisms between Parkinson’s Disease, Alzheimer’s Disease, Glaucoma, and Macular Degeneration. Expert Rev. Mol. Med. 2023, 25, e24. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative stress and cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- López, M.; Martınez, F.; Del Valle, C.; Ferrit, M.; Luque, R. Study of phenolic compounds as natural antioxidants by a fluorescence method. Talanta 2003, 60, 609–616. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.-Y.; Zhao, C.-N.; Feng, X.-L.; Xu, X.-Y.; Cao, S.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, H.-B. Comparison of antioxidant activities of different grape varieties. Molecules 2018, 23, 2432. [Google Scholar] [CrossRef]
- Keskin, N.; Bilir Ekbic, H.; Kaya, O.; Keskin, S. Antioxidant activity and biochemical compounds of Vitis vinifera L. (cv.‘Katıkara’) and Vitis labrusca L. (cv.‘Isabella’) grown in Black Sea Coast of Turkey. Erwerbs-Obstbau 2021, 63, 115–122. [Google Scholar] [CrossRef]
- Wang, H. Biomaterials in medical applications. Polymers 2023, 15, 847. [Google Scholar] [CrossRef]
- Khan, N.; Fahad, S.; Naushad, M.; Faisal, S. Grape production critical review in the world. SSRN Electron. J. 2020, 3595842. [Google Scholar] [CrossRef]
- Possingham, J. Developments in the production of table grapes, wine and raisins in tropical regions of the world. In Proceedings of the International Symposium on Grape Production and Processing 785, Maharashtra, India, 6–11 February 2006; pp. 45–50. [Google Scholar]
- Péros, J.-P.; Launay, A.; Peyrière, A.; Berger, G.; Roux, C.; Lacombe, T.; Boursiquot, J.-M. Species relationships within the genus Vitis based on molecular and morphological data. PLoS ONE 2023, 18, e0283324. [Google Scholar] [CrossRef]
- Yamada, M.; Sato, A. Advances in table grape breeding in Japan. Breed. Sci. 2016, 66, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Rahemi, A.; Dodson Peterson, J.C.; Lund, K.T. Grape species. In Grape Rootstocks and Related Species; Springer: Berlin/Heidelberg, Germany, 2022; pp. 5–21. [Google Scholar]
- Bonello, F.; Danieli, F.; Ragkousi, V.; Ferrandino, A.; Petrozziello, M.; Asproudi, A.; La Notte, P.; Pirolo, C.S.; Roseti, V. Aromatic Profiling of New Table Grape Varieties Using Gas Chromatography/Mass Spectrometry and Olfactometry. Plants 2024, 13, 1820. [Google Scholar] [CrossRef]
- Pérez, F.J.; Rubio, S. Relationship Between Bud Cold Hardiness and Budbreak in Two Vitis vinifera L Cultivars, Chardonnay and Thompson Seedless. J. Plant Growth Regul. 2022, 41, 840–847. [Google Scholar] [CrossRef]
- Shaw, A.B. The Niagara Peninsula viticultural area: A climatic analysis of Canada’s largest wine region. J. Wine Res. 2005, 16, 85–103. [Google Scholar] [CrossRef]
- Stewart, J.A.; Pajerowska-Mukhtar, K.M.; Bulger, A.; Kambiranda, D.; Nyochembeng, L.; Mentreddy, S.R. Muscadine, Resveratrol (RSV) synthesis, and the nutritional benefits to humans and plants. ACS Food Sci. Technol. 2023, 3, 3–14. [Google Scholar] [CrossRef]
- Conner, P.J.; Worthington, M.L. Muscadine grape breeding. Plant Breed. Rev. 2022, 46, 31–117. [Google Scholar]
- Li, S.-S.; Cheng, C.; Li, Z.; Chen, J.-Y.; Yan, B.; Han, B.-Z.; Reeves, M. Yeast species associated with wine grapes in China. Int. J. Food Microbiol. 2010, 138, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Buican, B.-C.; Colibaba, L.C.; Luchian, C.E.; Kallithraka, S.; Cotea, V.V. “Orange” Wine—The Resurgence of an Ancient Winemaking Technique: A Review. Agriculture 2023, 13, 1750. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; González, C.; Callejo, M.J.; Suárez-Lepe, J.A. Emerging preservation technologies in grapes for winemaking. Trends Food Sci. Technol. 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.J.; Wang, X.; Hawkins, T.; Painter, J.E. A Comprehensive review of raisins and raisin components and their relationship to human health. J. Nutr. Health 2017, 50, 203–216. [Google Scholar] [CrossRef]
- Jianying, F.; Bianyu, Y.; Xin, L.; Dong, T.; Weisong, M. Evaluation on risks of sustainable supply chain based on optimized BP neural networks in fresh grape industry. Comput. Electron. Agric. 2021, 183, 105988. [Google Scholar] [CrossRef]
- Yin, M.; Huo, L.; Li, N.; Zhu, H.; Zhu, Z.; Hu, J. Packaging performance evaluation and freshness intelligent prediction modeling in grape transportation. Food Control 2024, 165, 110684. [Google Scholar] [CrossRef]
- Toma, D.-I.; Manaila-Maximean, D.; Fierascu, I.; Baroi, A.M.; Matei, R.I.; Fistos, T.; Chican, I.E.; Fierascu, R.C. Applications of Natural Polymers in the Grapevine Industry: Plant Protection and Value-Added Utilization of Waste. Polymers 2024, 17, 18. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.-H. From fighting critters to saving lives: Polyphenols in plant defense and human health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From theory to practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Grieco, F. Functional properties of grape and wine polyphenols. Plant Foods Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Norton, E.L. Chemistry and reactivity of tannins in Vitis spp.: A review. Molecules 2020, 25, 2110. [Google Scholar] [CrossRef]
- Wang, H. Advantages of animal leather over alternatives and its medical applications. Eur. Polym. J. 2024, 214, 113153. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A review of sleep disorders and melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Iriti, M.; Rossoni, M.; Faoro, F. Melatonin content in grape: Myth or panacea? J. Sci. Food Agric. 2006, 86, 1432–1438. [Google Scholar] [CrossRef]
- Iriti, M. Melatonin in grape, not just a myth, maybe a panacea. J. Pineal Res. 2009, 46, 353. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Keskin, N.; Kaya, O.; Ates, F.; Turan, M.; Gutiérrez-Gamboa, G. Drying grapes after the application of different dipping solutions: Effects on hormones, minerals, vitamins, and antioxidant enzymes in Gök Üzüm (Vitis vinifera L.) raisins. Plants 2022, 11, 529. [Google Scholar] [CrossRef]
- dos Santos Freitas, L.; Jacques, R.A.; Richter, M.F.; da Silva, A.L.; Caramão, E.B. Pressurized liquid extraction of vitamin E from Brazilian grape seed oil. J. Chromatogr. A 2008, 1200, 80–83. [Google Scholar] [CrossRef]
- Khan, S.H.; Iqbal, J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016, 44, 359–369. [Google Scholar] [CrossRef]
- Mato, I.; Suárez-Luque, S.; Huidobro, J.F. A review of the analytical methods to determine organic acids in grape juices and wines. Food Res. Int. 2005, 38, 1175–1188. [Google Scholar] [CrossRef]
- Kampa, M.; Nifli, A.-P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 79–113. [Google Scholar]
- Alshatwi, A.A. Catechin hydrate suppresses MCF-7 proliferation through TP53/Caspase-mediated apoptosis. J. Exp. Clin. Cancer Res. 2010, 29, 167. [Google Scholar] [CrossRef]
- Iannone, M.; Mare, R.; Paolino, D.; Gagliardi, A.; Froiio, F.; Cosco, D.; Fresta, M. Characterization and in vitro anticancer properties of chitosan-microencapsulated flavan-3-ols-rich grape seed extracts. Int. J. Biol. Macromol. 2017, 104, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Ferraz da Costa, D.C.; Pereira Rangel, L.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive compounds and metabolites from grapes and red wine in breast cancer chemoprevention and therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef]
- Bitting, R.L.; Tooze, J.A.; Isom, S.; Petty, W.J.; Grant, S.C.; Desnoyers, R.J.; Thomas, A.; Thomas, C.Y.; Alistar, A.T.; Golden, S.L. Phase I study of muscadine grape extract for patients with advanced cancer. Am. J. Clin. Oncol. 2021, 44, 239–246. [Google Scholar] [CrossRef]
- Paller, C.J.; Rudek, M.A.; Zhou, X.C.; Wagner, W.D.; Hudson, T.S.; Anders, N.; Hammers, H.J.; Dowling, D.; King, S.; Antonarakis, E.S. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate 2015, 75, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Rizvi, A. The pharmacological properties of red grape polyphenol resveratrol: Clinical trials and obstacles in drug development. Nutrients 2023, 15, 4486. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Vita, J.A. Grapes and cardiovascular disease. J. Nutr. 2009, 139, 1788S–1793S. [Google Scholar] [CrossRef]
- Rasines-Perea, Z.; Teissedre, P.-L. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef]
- Schön, C.; Allegrini, P.; Engelhart-Jentzsch, K.; Riva, A.; Petrangolini, G. Grape seed extract positively modulates blood pressure and perceived stress: A randomized, double-blind, placebo-controlled study in healthy volunteers. Nutrients 2021, 13, 654. [Google Scholar] [CrossRef]
- Renaud, S.d.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Almomen, S.M.; Guan, Q.; Liang, P.; Yang, K.; Sidiqi, A.M.; Levin, A.; Du, C. Daily intake of grape powder prevents the progression of kidney disease in obese type 2 diabetic ZSF1 rats. Nutrients 2017, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Rahman, H.; Behl, T.; Chowdhury, M.A.; Manirujjaman, M.; Bulbul, I.J.; Elshenaw, S.E.; Tit, D.M.; Bungau, S. Prospective role of polyphenolic compounds in the treatment of neurodegenerative diseases. CNS Neurol. Disord.-Drug Targets 2021, 20, 430–450. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Hoge, C.W.; McFarlane, A.C.; Vermetten, E.; Lanius, R.A.; Nievergelt, C.M.; Hobfoll, S.E.; Koenen, K.C.; Neylan, T.C.; Hyman, S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Primers 2015, 1, 15057. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.; Alkadhi, I.; Atrooz, F.; Patki, G.; Salim, S. Grape powder prevents cognitive, behavioral, and biochemical impairments in a rat model of posttraumatic stress disorder. Nutr. Res. 2015, 35, 65–75. [Google Scholar] [CrossRef]
- Pereira, N.; Naufel, M.F.; Ribeiro, E.B.; Tufik, S.; Hachul, H. Influence of dietary sources of melatonin on sleep quality: A review. J. Food Sci. 2020, 85, 5–13. [Google Scholar] [CrossRef]
- Poza, J.J.; Pujol, M.; Ortega-Albás, J.J.; Romero, O. Melatonin in sleep disorders. Neurología 2022, 37, 575–585. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is melatonin the cornucopia of the 21st century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 441357. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Bi, W.; Ferruzzi, M.; Yemul, S.; Freire, D.; Mazzola, P.; Ho, L.; Dubner, L.; Pasinetti, G.M. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 2015, 89, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Singh, N.; Chaudhary, S.; Bellamkonda, V.; Kritikos, A.E.; Wise, A.S.; Rana, N.; McDonald, D.; Ayyagari, R. Retinal degeneration and Alzheimer’s disease: An evolving link. Int. J. Mol. Sci. 2020, 21, 7290. [Google Scholar] [CrossRef]
- Botto, C.; Rucli, M.; Tekinsoy, M.D.; Pulman, J.; Sahel, J.-A.; Dalkara, D. Early and late stage gene therapy interventions for inherited retinal degenerations. Prog. Retin. Eye Res. 2022, 86, 100975. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.B.; Hwang, J.-W.; Kim, Y.-S.; Kim, E.-K.; Park, P.-J. Ocular promoting activity of grape polyphenols—A review. Environ. Toxicol. Pharmacol. 2017, 50, 83–90. [Google Scholar] [CrossRef]
- Patel, A.K.; Davis, A.; Rodriguez, M.E.; Agron, S.; Hackam, A.S. Protective effects of a grape-supplemented diet in a mouse model of retinal degeneration. Nutrition 2016, 32, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The pathology associated with diabetic retinopathy. Vis. Res. 2017, 139, 7–14. [Google Scholar] [CrossRef]
- Calderon, G.; Juarez, O.; Hernandez, G.; Punzo, S.; De la Cruz, Z. Oxidative stress and diabetic retinopathy: Development and treatment. Eye 2017, 31, 1122–1130. [Google Scholar] [CrossRef]
- Schiano, E.; Vaccaro, S.; Scorcia, V.; Carnevali, A.; Borselli, M.; Chisari, D.; Guerra, F.; Iannuzzo, F.; Tenore, G.C.; Giannaccare, G. From Vineyard to Vision: Efficacy of Maltodextrinated Grape Pomace Extract (MaGPE) Nutraceutical Formulation in Patients with Diabetic Retinopathy. Nutrients 2024, 16, 2850. [Google Scholar] [CrossRef]
- Piazza, S.; Fumagalli, M.; Khalilpour, S.; Martinelli, G.; Magnavacca, A.; Dell’Agli, M.; Sangiovanni, E. A review of the potential benefits of plants producing berries in skin disorders. Antioxidants 2020, 9, 542. [Google Scholar] [CrossRef]
- Khanna, S.; Venojarvi, M.; Roy, S.; Sharma, N.; Trikha, P.; Bagchi, D.; Bagchi, M.; Sen, C.K. Dermal wound healing properties of redox-active grape seed proanthocyanidins. Free Radic. Biol. Med. 2002, 33, 1089–1096. [Google Scholar] [CrossRef]
- Soleymani, S.; Iranpanah, A.; Najafi, F.; Belwal, T.; Ramola, S.; Abbasabadi, Z.; Momtaz, S.; Farzaei, M.H. Implications of grape extract and its nanoformulated bioactive agent resveratrol against skin disorders. Arch. Dermatol. Res. 2019, 311, 577–588. [Google Scholar] [CrossRef]
- Salem, Y.; Sunoqrot, S.; Rajha, H.N.; Abusulieh, S.; Afif, C.; Francis, H.; Touma, J.A.; Louka, N.; Maroun, R.G. Grape seed phenolic extracts encapsulation in polymeric nanoparticles: Characterization and in vitro evaluation against skin melanoma. J. Drug Deliv. Sci. Technol. 2024, 100, 106094. [Google Scholar] [CrossRef]
- Singh, C.K.; Mintie, C.A.; Ndiaye, M.A.; Chhabra, G.; Roy, S.; Sullivan, R.; Longley, B.J.; Schieke, S.M.; Ahmad, N. Protective effects of dietary grape against atopic dermatitis-like skin lesions in NC/NgaTndCrlj mice. Front. Immunol. 2023, 13, 1051472. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Zhu, J.; Du, C. Could grape-based food supplements prevent the development of chronic kidney disease? Crit. Rev. Food Sci. Nutr. 2020, 60, 3054–3062. [Google Scholar] [CrossRef] [PubMed]
- Turki, K.; Charradi, K.; Boukhalfa, H.; Belhaj, M.; Limam, F.; Aouani, E. Grape seed powder improves renal failure of chronic kidney disease patients. EXCLI J. 2016, 15, 424. [Google Scholar]
- Albrahim, T.; Robert, A. Renal protective effects of grape seed extract treatment against Eltroxin-induced hyperthyroidism, kidney damage, and oxidative stress in male mice. Environ. Sci. Pollut. Res. 2020, 27, 17963–17971. [Google Scholar] [CrossRef]
- Dave, A.; Park, E.-J.; Kofsky, P.; Dufresne, A.; Chakraborty, S.; Pezzuto, J.M. Long-Term Dietary Consumption of Grapes Affects Kidney Health in C57BL/6J Mice. Nutrients 2024, 16, 2309. [Google Scholar] [CrossRef]
- Wang, H. MicroRNA, diabetes mellitus and colorectal cancer. Biomedicines 2020, 8, 530. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef]
- Zunino, S.J. Type 2 diabetes and glycemic response to grapes or grape products. J. Nutr. 2009, 139, 1794S–1800S. [Google Scholar] [CrossRef]
- Moodi, V.; Abedi, S.; Esmaeilpour, M.; Asbaghi, O.; Izadi, F.; Shirinbakhshmasoleh, M.; Behrouzian, M.; Shahriari, A.; Ghaedi, E.; Miraghajani, M. The effect of grapes/grape products on glycemic response: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2021, 35, 5053–5067. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abdollahi, M.; Rahimi, R. Role of dietary polyphenols in the management of peptic ulcer. World J. Gastroenterol. WJG 2015, 21, 6499. [Google Scholar] [CrossRef]
- Saito, M.; Hosoyama, H.; Ariga, T.; Kataoka, S.; Yamaji, N. Antiulcer activity of grape seed extract and procyanidins. J. Agric. Food Chem. 1998, 46, 1460–1464. [Google Scholar] [CrossRef]
- Bagchi, M.; Milnes, M.; Williams, C.; Balmoori, J.; Ye, X.; Stohs, S.; Bagchi, D. Acute and chronic stress-induced oxidative gastrointestinal injury in rats, and the protective ability of a novel grape seed proanthocyanidin extract. Nutr. Res. 1999, 19, 1189–1199. [Google Scholar] [CrossRef]
- Silvan, J.M.; Gutierrez-Docio, A.; Guerrero-Hurtado, E.; Domingo-Serrano, L.; Blanco-Suarez, A.; Prodanov, M.; Alarcon-Cavero, T.; Martinez-Rodriguez, A.J. Pre-treatment with grape seed extract reduces inflammatory response and oxidative stress induced by Helicobacter pylori infection in human gastric epithelial cells. Antioxidants 2021, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.R.; Kim, J.N.; Kwon, M.J.; Lee, J.R.; Kim, S.C.; Lee, M.J.; Choi, W.-g.; Kim, B.J. Grape seed powder increases gastrointestinal motility. Int. J. Med. Sci. 2022, 19, 941. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, K.N.; Babu, S.C. Value chain management under COVID-19: Responses and lessons from grape production in India. J. Soc. Econ. Dev. 2021, 23, 468–490. [Google Scholar] [CrossRef]
- Wang, H. COVID-19, anti-NMDA receptor encephalitis and microRNA. Front. Immunol. 2022, 13, 825103. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, H. Exploring Diversity of COVID-19 Based on Substitution Distance. Infect. Drug Resist. 2020, 13, 3887–3894. [Google Scholar] [CrossRef]
- Zannella, C.; Giugliano, R.; Chianese, A.; Buonocore, C.; Vitale, G.A.; Sanna, G.; Sarno, F.; Manzin, A.; Nebbioso, A.; Termolino, P. Antiviral activity of Vitis vinifera leaf extract against SARS-CoV-2 and HSV-1. Viruses 2021, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Kelleni, M.T. Resveratrol-zinc nanoparticles or pterostilbene-zinc: Potential COVID-19 mono and adjuvant therapy. Biomed. Pharmacother. 2021, 139, 111626. [Google Scholar] [CrossRef]
- Ghandaali, A.; Ardestani, M.M.; Hadi, S.; Nejatbakhsh, F.; Hadi, V.; Kazemi-Galougahi, M.H.; Mirghazanfari, S.M. The effects of almond porridge, grape extract, and pea syrup on fatigue severity and clinical symptoms of patients with COVID-19: A randomized controlled clinical trial. Clin. Cancer Investig. J. 2022, 11, 1–13. [Google Scholar]
- Chen, H.-F.; Wang, W.-J.; Chen, C.-Y.; Chang, W.-C.; Hsueh, P.-R.; Peng, S.-L.; Wu, C.-S.; Chen, Y.; Huang, H.-Y.; Shen, W.-J. The natural tannins oligomeric proanthocyanidins and punicalagin are potent inhibitors of infection by SARS-CoV-2. Elife 2023, 12, e84899. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Jain, K.K. An overview of drug delivery systems. In Drug Delivery Systems; Humana: New York, NY, USA, 2020; pp. 1–54. [Google Scholar]
- Locilento, D.A.; Mercante, L.A.; Andre, R.S.; Mattoso, L.H.; Luna, G.L.; Brassolatti, P.; Anibal, F.d.F.; Correa, D.S. Biocompatible and biodegradable electrospun nanofibrous membranes loaded with grape seed extract for wound dressing application. J. Nanomater. 2019, 2019, 2472964. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Scano, A.; Ebau, F.; Manca, M.; Cabras, V.; Marincola, F.C.; Manconi, M.; Pilloni, M.; Fadda, A.; Ennas, G. Novel drug delivery systems for natural extracts: The case study of Vitis Vinifera extract-SiO2 nanocomposites. Int. J. Pharm. 2018, 551, 84–96. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Vali, H.; Orsat, V. Value-adding to grape waste: Green synthesis of gold nanoparticles. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Sousa, M.; Massano, F.; Borges, A. Exploring grape pomace extracts for the formulation of new bioactive multifunctional chitosan/alginate-based hydrogels for wound healing applications. Food Biosci. 2024, 62, 105073. [Google Scholar] [CrossRef]
- Tahir, R.; Nazir, A.; Qadir, M.B.; Khaliq, Z.; Hareem, F.; Arshad, S.N.; Aslam, M. Fabrication and Physio-chemical characterization of biocompatible and antibacterial Vitis vinifera (grapes) loaded PVA nanomembranes for dermal applications. Mater. Today Commun. 2025, 42, 111178. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Q.; Chang, J.; Wu, C. Grape seed-inspired smart hydrogel scaffolds for melanoma therapy and wound healing. ACS Nano 2019, 13, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, M.; Jiang, H.; Fan, L.; Sun, B.; Yu, F.; Yang, X.; Lou, X.; He, C.; Wang, H. Green electrospun grape seed extract-loaded silk fibroin nanofibrous mats with excellent cytocompatibility and antioxidant effect. Colloids Surf. B Biointerfaces 2016, 139, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Khan, J.; Yadav, A. Applications of scaffolds in tissue engineering: Current utilization and future prospective. Curr. Gene Ther. 2024, 24, 94–109. [Google Scholar] [CrossRef]
- Damaschin, R.P.; Lazar, M.M.; Ghiorghita, C.-A.; Aprotosoaie, A.C.; Volf, I.; Dinu, M.V. Stabilization of Picea abies spruce bark extracts within ice-templated porous dextran hydrogels. Polymers 2024, 16, 2834. [Google Scholar] [CrossRef]
- Vinayagamurthy, K.; Kumar, D.P.; Yalavarthi, K.; Ramudu, K.N.; Ravichandran, J.; Stephen, N.M.; Ponesakki, G. Biological Significance of Polyphenols as Functional Molecules in Biomaterial Preparations. Sci. Eng. Polyphen. Fundam. Ind. Scale Appl. 2024, 548–583. [Google Scholar]
- Mani, M.P.; Jaganathan, S.K. Fabrication and characterization of electrospun polyurethane blended with dietary grapes for skin tissue engineering. J. Ind. Text. 2020, 50, 655–674. [Google Scholar] [CrossRef]
- Yourdkhani, M.; Leme-Kraus, A.A.; Aydin, B.; Bedran-Russo, A.K.; White, S.R. Encapsulation of grape seed extract in polylactide microcapsules for sustained bioactivity and time-dependent release in dental material applications. Dent. Mater. 2017, 33, 630–636. [Google Scholar] [CrossRef]
- Bogdan, C.; Pop, A.; Iurian, S.M.; Benedec, D.; Moldovan, M.L. Research advances in the use of bioactive compounds from vitis vinifera by-products in oral care. Antioxidants 2020, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Mani, M.P.; Jaganathan, S.K. Engineering electrospun multicomponent polyurethane scaffolding platform comprising grapeseed oil and honey/propolis for bone tissue regeneration. PLoS ONE 2018, 13, e0205699. [Google Scholar] [CrossRef]
- Rao, K.M.; Kim, H.J.; Won, S.; Choi, S.M.; Han, S.S. Effect of grape seed extract on gelatin-based edible 3D-hydrogels for cultured meat application. Gels 2023, 9, 65. [Google Scholar] [CrossRef]
- Downer, S.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food is medicine: Actions to integrate food and nutrition into healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef]
- Duchêne, É.; Dumas, V.; Butterlin, G.; Jaegli, N.; Rustenholz, C.; Chauveau, A.; Bérard, A.; Le Paslier, M.C.; Gaillard, I.; Merdinoglu, D. Genetic variations of acidity in grape berries are controlled by the interplay between organic acids and potassium. Theor. Appl. Genet. 2020, 133, 993–1008. [Google Scholar] [CrossRef]
- Ayoub, N.; Badr, N.; Al-Ghamdi, S.S.; Alsanosi, S.; Alzahrani, A.R.; Abdel-Naim, A.B.; Nematallah, K.A.; Swilam, N. HPLC/MSn profiling and healing activity of a muco-adhesive formula of salvadora persica against acetic acid-induced oral ulcer in rats. Nutrients 2021, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Maret-Ouda, J.; Markar, S.R.; Lagergren, J. Gastroesophageal reflux disease: A review. JAMA 2020, 324, 2536–2547. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wang, H. The association between depression and gastroesophageal reflux based on phylogenetic analysis of miRNA biomarkers. Curr. Med. Chem. 2020, 27, 6536–6547. [Google Scholar] [CrossRef]
- Taraszewska, A. Risk factors for gastroesophageal reflux disease symptoms related to lifestyle and diet. Rocz. Państwowego Zakładu Hig. 2021, 72, 21–28. [Google Scholar] [CrossRef]
- Siener, R.; Seidler, A.; Voss, S.; Hesse, A. The oxalate content of fruit and vegetable juices, nectars and drinks. J. Food Compos. Anal. 2016, 45, 108–112. [Google Scholar] [CrossRef]
- Alelign, T.; Petros, B. Kidney stone disease: An update on current concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.; Kumar, P.; Reddy, T.; Wood, K.D.; Knight, J.; Assimos, D.G.; Holmes, R.P. Dietary oxalate and kidney stone formation. Am. J. Physiol.-Ren. Physiol. 2019, 316, F409–F413. [Google Scholar] [CrossRef] [PubMed]
- Price, G.; Patel, D.A. Drug Bioavailability; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Castro, M.L.; Ferreira, J.P.; Pintado, M.; Ramos, O.L.; Borges, S.; Baptista-Silva, S. Grape by-products in sustainable cosmetics: Nanoencapsulation and market trends. Appl. Sci. 2023, 13, 9168. [Google Scholar] [CrossRef]
- Costa, J.R.; Xavier, M.; Amado, I.R.; Gonçalves, C.; Castro, P.M.; Tonon, R.V.; Cabral, L.M.; Pastrana, L.; Pintado, M.E. Polymeric nanoparticles as oral delivery systems for a grape pomace extract towards the improvement of biological activities. Mater. Sci. Eng. C 2021, 119, 111551. [Google Scholar] [CrossRef]
- Micale, N.; Citarella, A.; Molonia, M.S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Hydrogels for the delivery of plant-derived (poly) phenols. Molecules 2020, 25, 3254. [Google Scholar] [CrossRef]
- Ivanov, Y.; Godjevargova, T. Antimicrobial polymer films with grape seed and skin extracts for food packaging. Microorganisms 2024, 12, 1378. [Google Scholar] [CrossRef]
- Qiu, Z.; Niu, W.; Wang, S.; Yu, F.; Yu, Y.; Fan, J.; Zheng, L.; Wang, Y.; Xiao, Z.; Xie, Y. Multifunctional composite film based on biodegradable grape skin and polyvinyl alcohol. Cellulose 2021, 28, 6467–6479. [Google Scholar] [CrossRef]
- Jamali, A.R.; Shaikh, A.A.; Chandio, A.D. Nano-based biodegradable food packaging of vitis-vinifera synthesized by PVA/ZnO nanocomposites. Phys. Chem. Res. 2023, 11, 449–458. [Google Scholar]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and synthetic polymers for biomedical and environmental applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Al-Shalawi, F.D.; Hanim, M.A.; Ariffin, M.; Kim, C.L.S.; Brabazon, D.; Calin, R.; Al-Osaimi, M.O. Biodegradable synthetic polymer in orthopaedic application: A review. Mater. Today Proc. 2023, 74, 540–546. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Hurlimann, J.; Thorbecke, G.; Hochwald, G. The liver as the site of C-reactive protein formation. J. Exp. Med. 1966, 123, 365–378. [Google Scholar] [CrossRef]
- Sarkhosh-Khorasani, S.; Hosseinzadeh, M. The effect of grape products containing polyphenols on C-reactive protein levels: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2021, 125, 1230–1245. [Google Scholar] [CrossRef]
- Cladis, D.P.; Weaver, C.M.; Ferruzzi, M.G. (Poly) phenol toxicity in vivo following oral administration: A targeted narrative review of (poly) phenols from green tea, grape, and anthocyanin-rich extracts. Phytother. Res. 2022, 36, 323–335. [Google Scholar] [CrossRef]

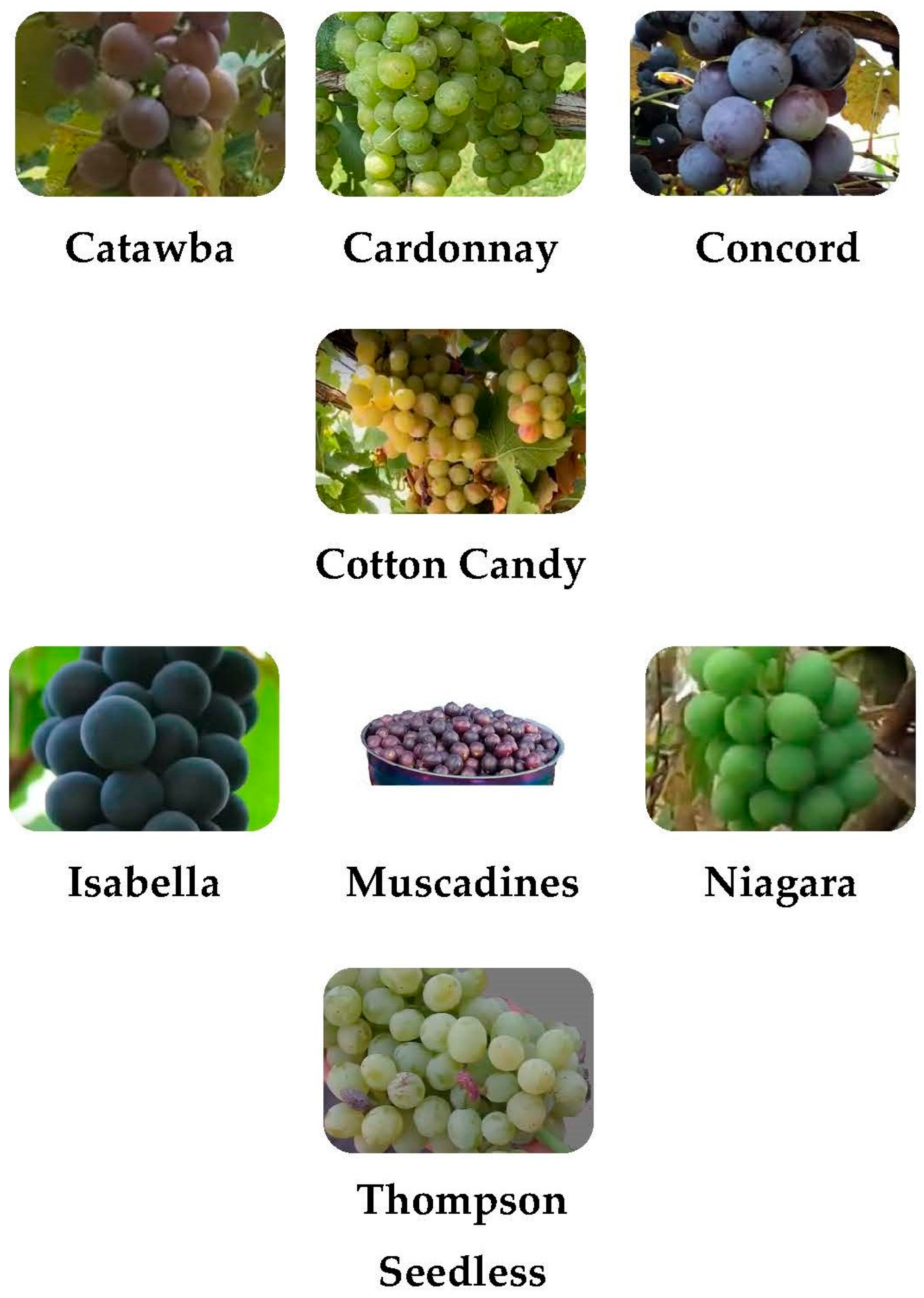


| Uses | Variety |
|---|---|
| Fresh Consumption | Thompson Seedless, Cotton Candy, Isabella, Concord, Niagara, Muscadine |
| Wine Production | Thompson Seedless, Chardonnay, Catawba, Isabella, Concord, Niagara, Muscadine |
| Raisin Production | Thompson Seedless |
| Juice, Jam, Jelly | Catawba, Isabella, Concord, Niagara, Muscadine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H. Medical Benefits and Polymer Applications of Grapes. Polymers 2025, 17, 750. https://doi.org/10.3390/polym17060750
Wang H. Medical Benefits and Polymer Applications of Grapes. Polymers. 2025; 17(6):750. https://doi.org/10.3390/polym17060750
Chicago/Turabian StyleWang, Hsiuying. 2025. "Medical Benefits and Polymer Applications of Grapes" Polymers 17, no. 6: 750. https://doi.org/10.3390/polym17060750
APA StyleWang, H. (2025). Medical Benefits and Polymer Applications of Grapes. Polymers, 17(6), 750. https://doi.org/10.3390/polym17060750






