Transforming Agro-Waste Cutin into Sustainable Materials for Biomedical Innovations
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
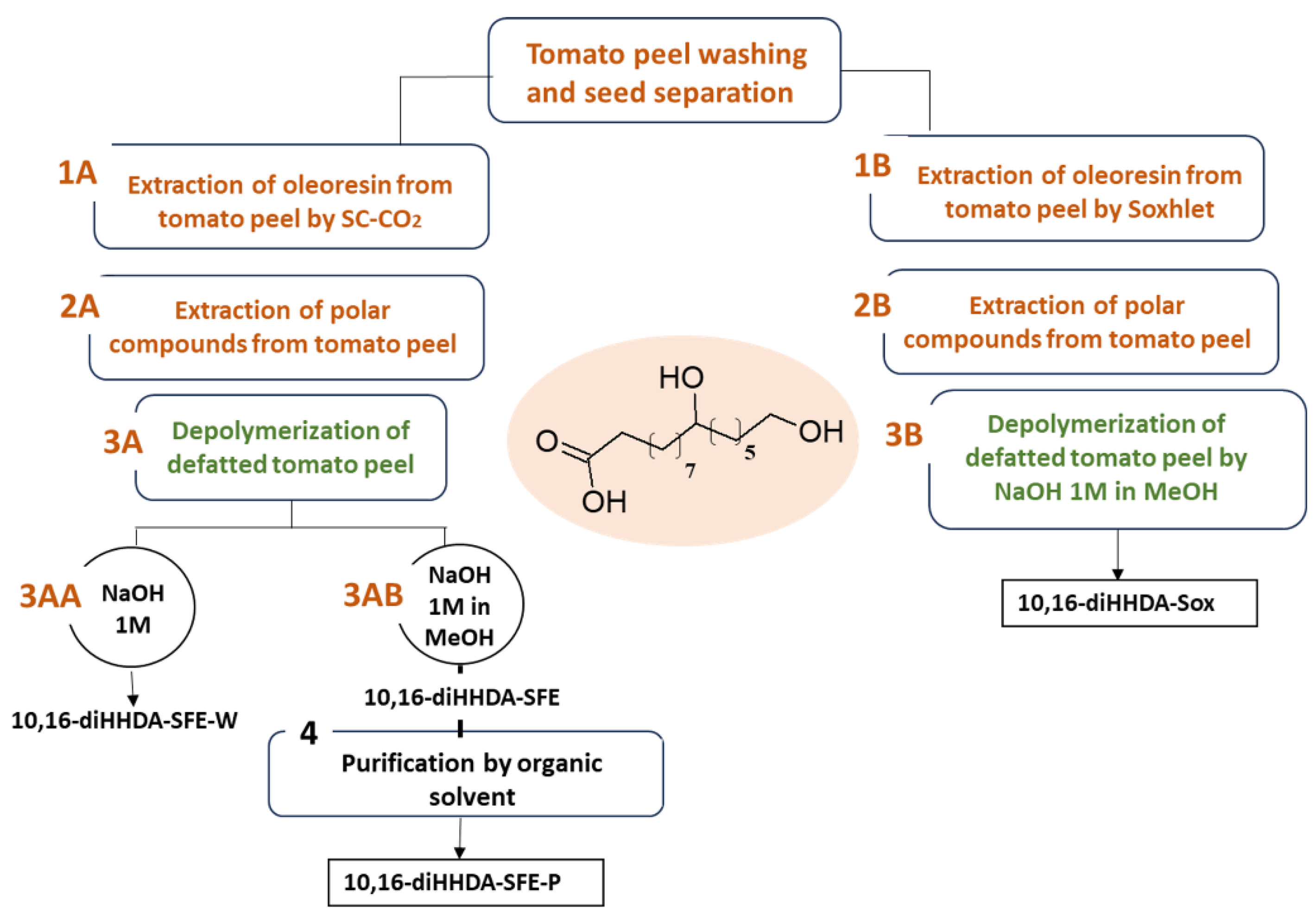
2.2. Monomers Isolation and Purification
2.3. Physico-Chemical Characterization
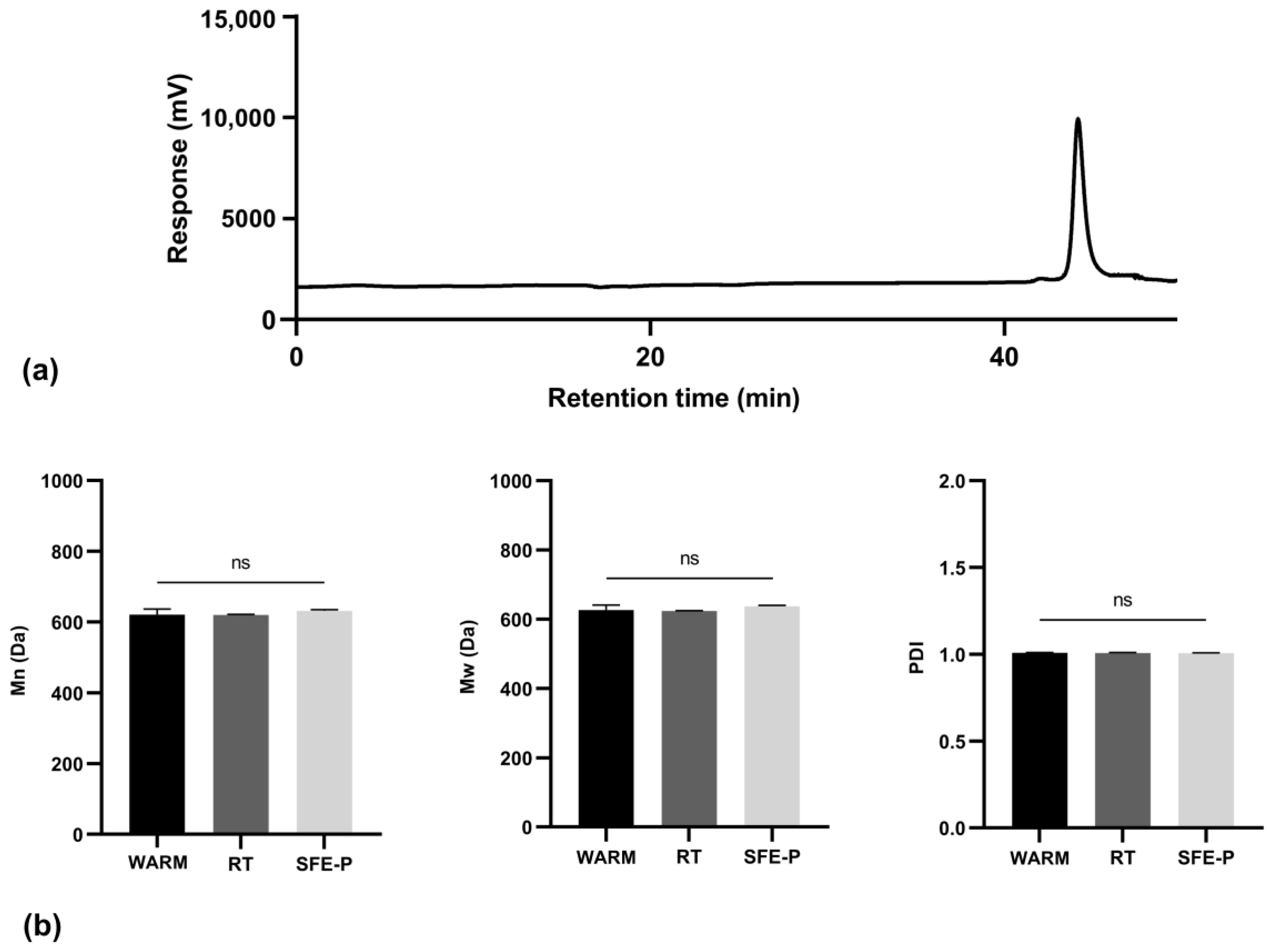
2.3.1. Molecular Weight Distribution of Monomers
2.3.2. Gas-Chromatography Analysis
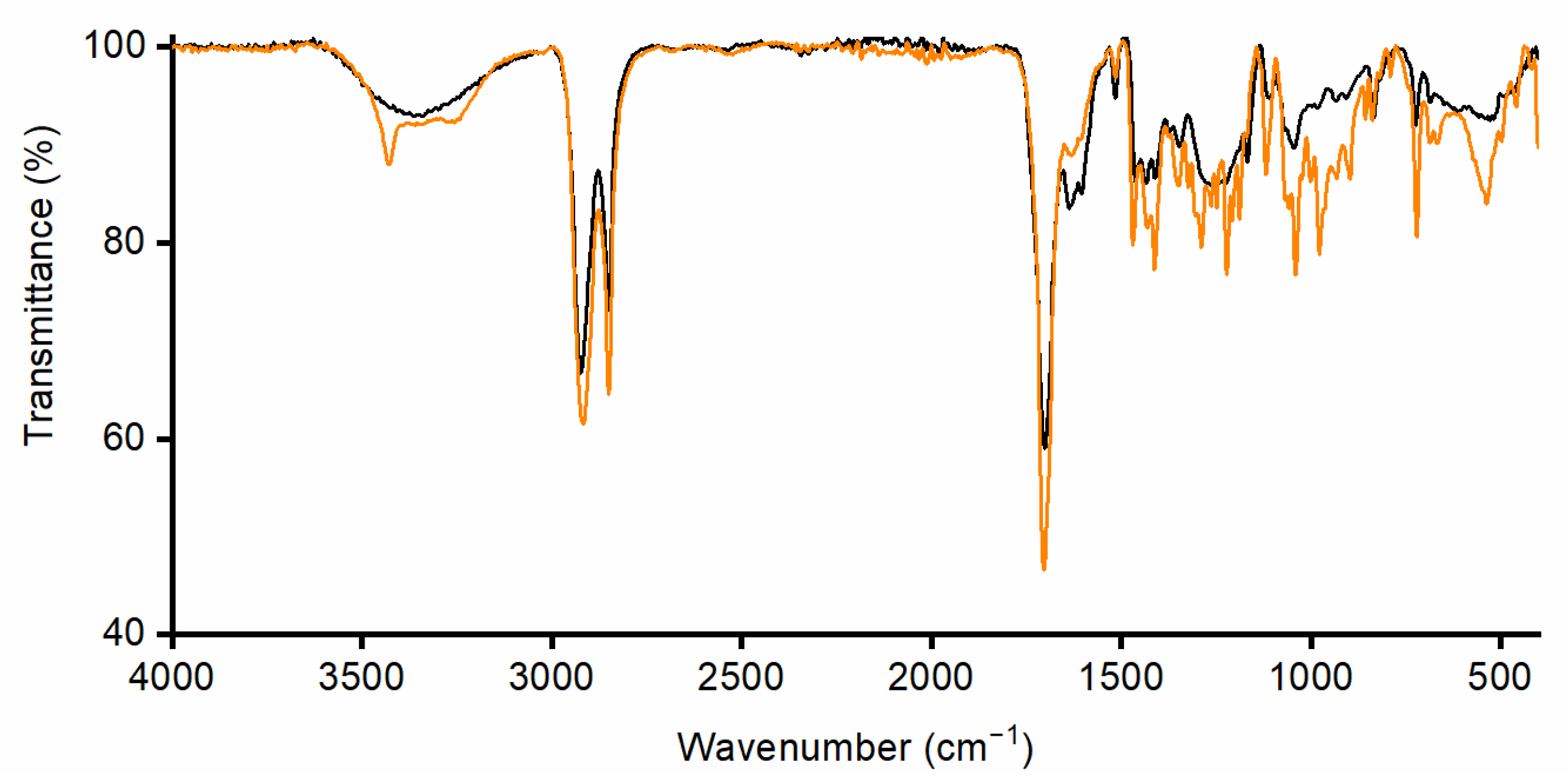
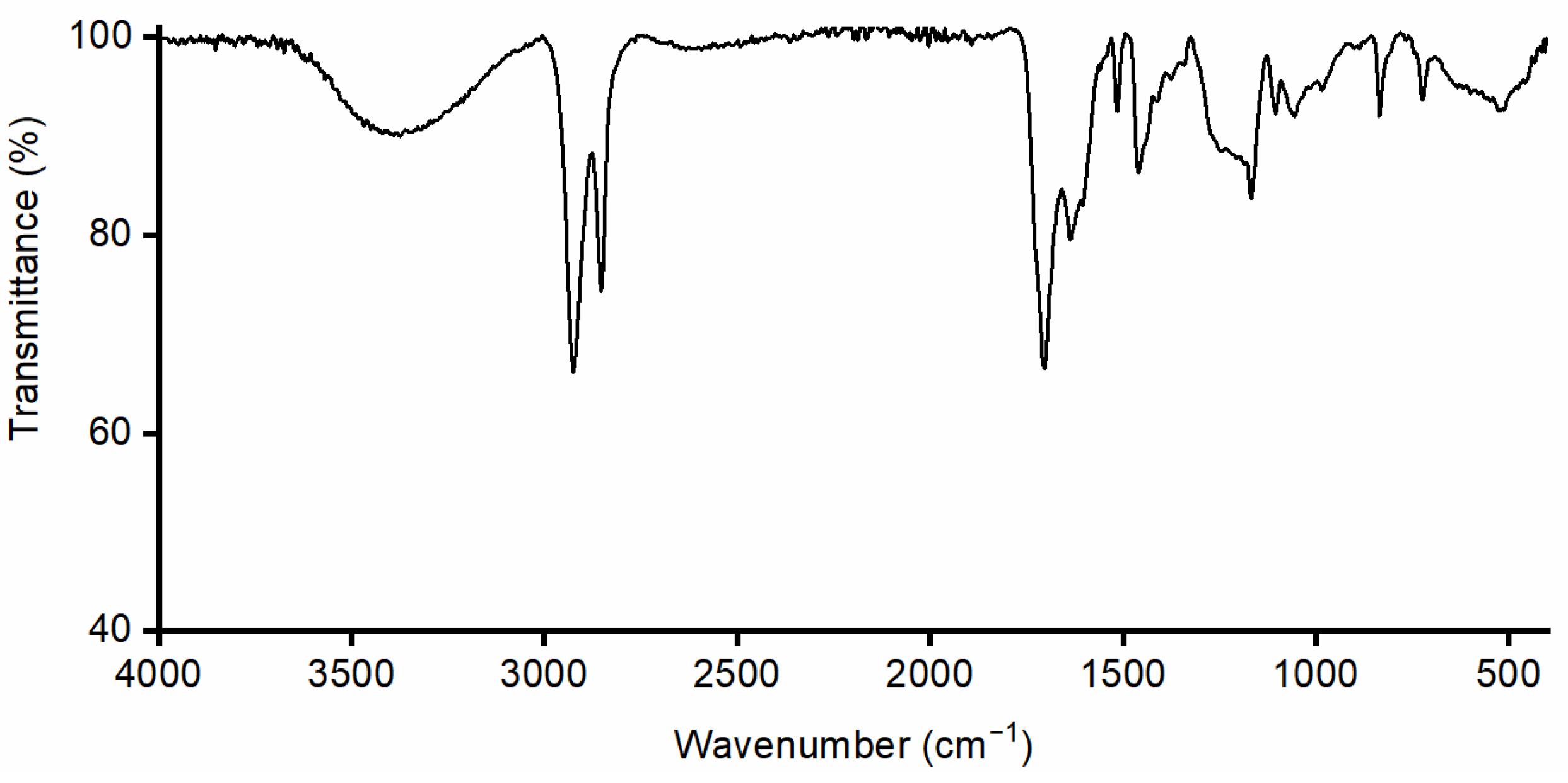
2.3.3. Spectroscopic Characterization of Monomers
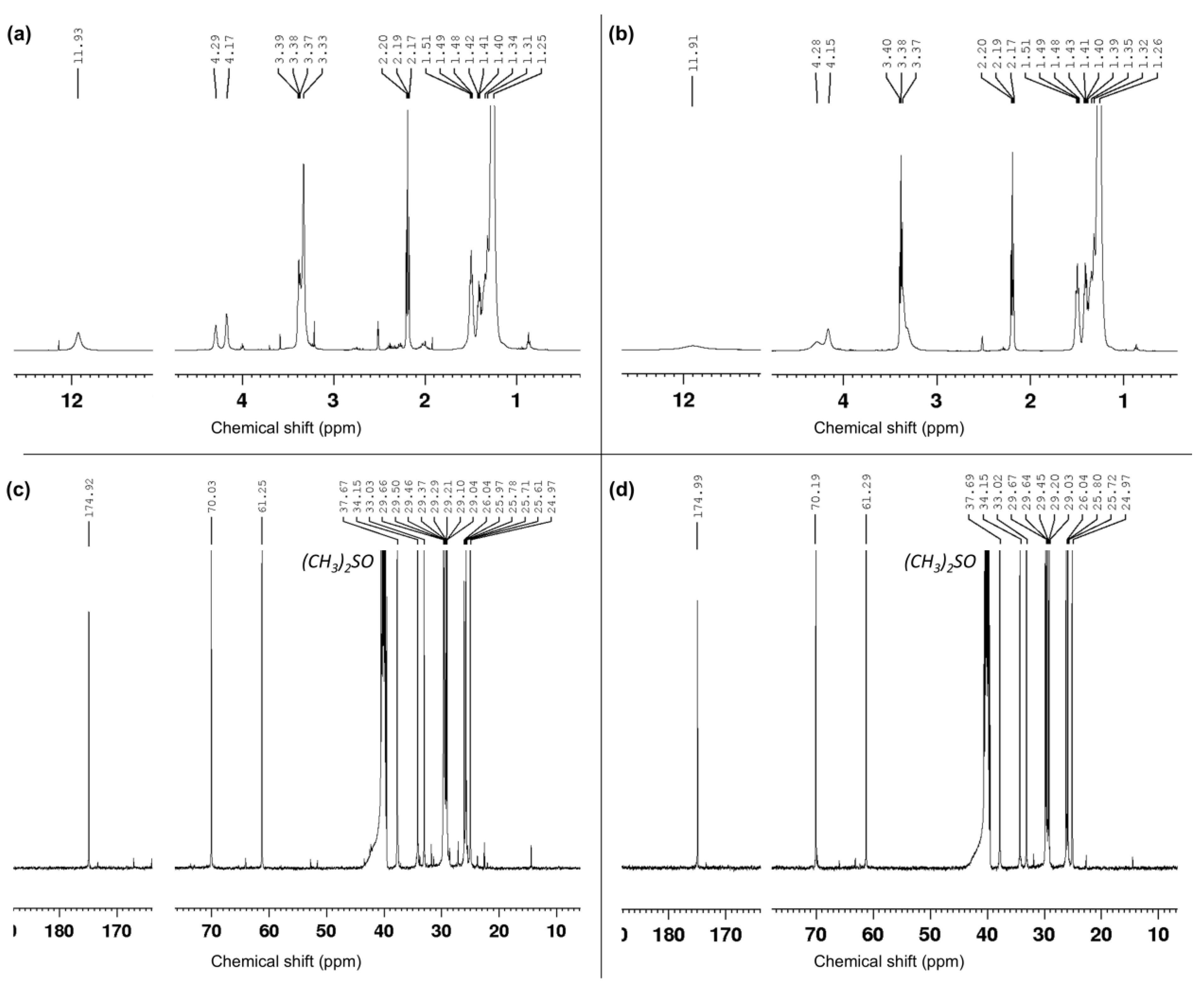
2.3.4. Nuclear Magnetic Resonance Spectroscopy
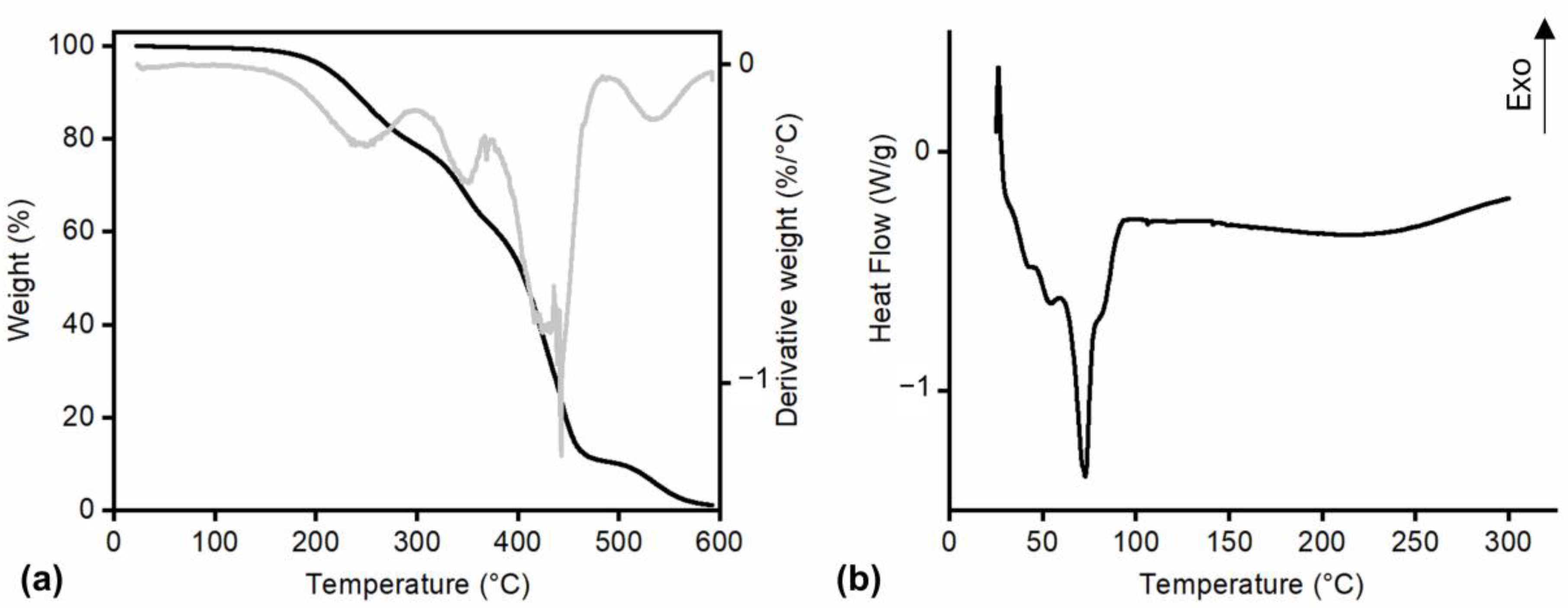
2.3.5. Thermal Characterization of Monomers
Thermogravimetric Analysis
Differential Scanning Calorimetry
2.4. Solvent Compatibility
Solubility of Cutin Monomer in Biomedical Solvents
2.5. Stability and Storage Conditions of the Monomers
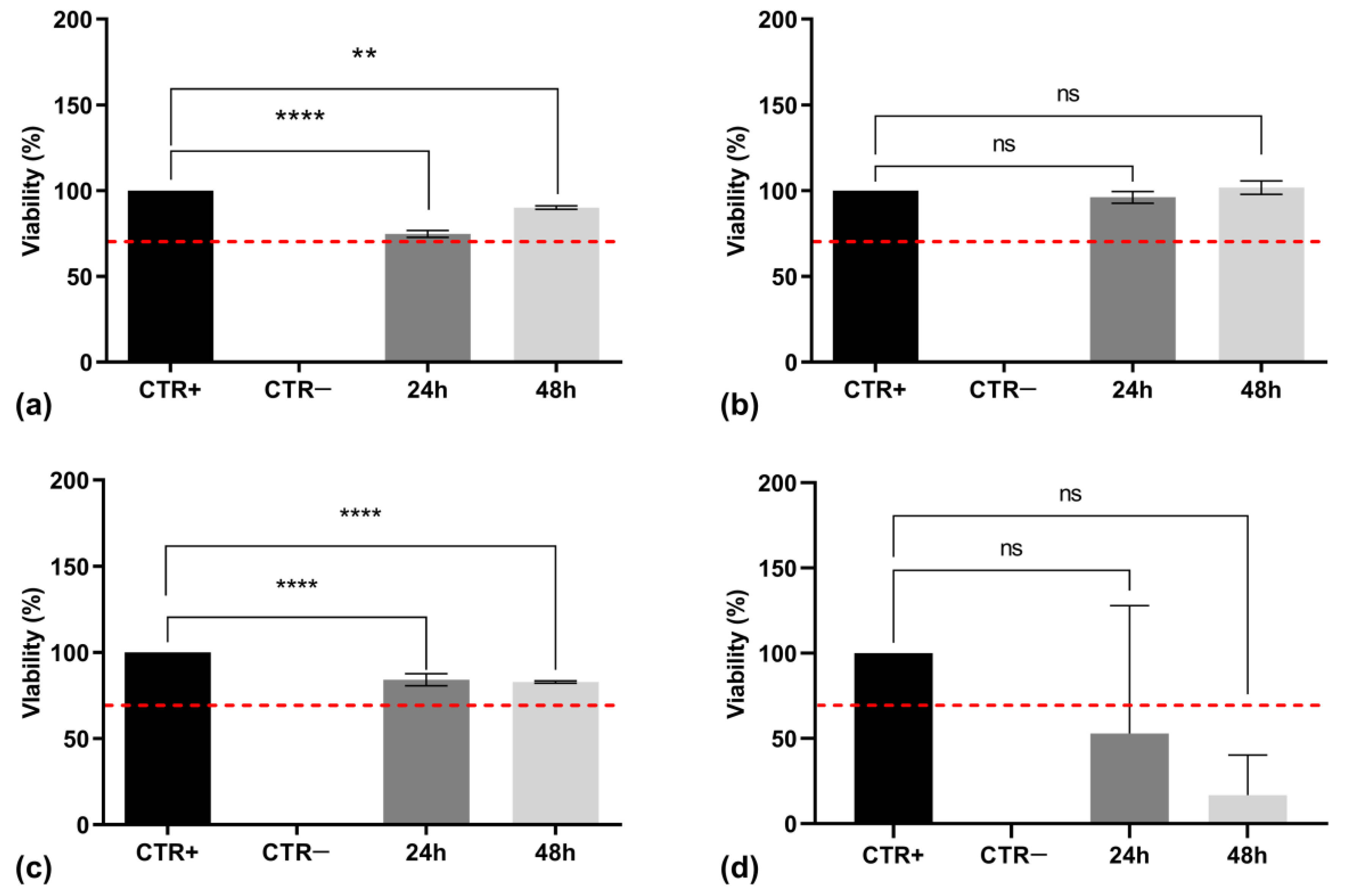
2.6. Cytotoxicity Assay
2.6.1. Cell Culture
2.6.2. MTT Assay
2.6.3. Morphological Characterization
2.6.4. Osmolarity
2.7. Statistical Analysis
3. Results and Discussion
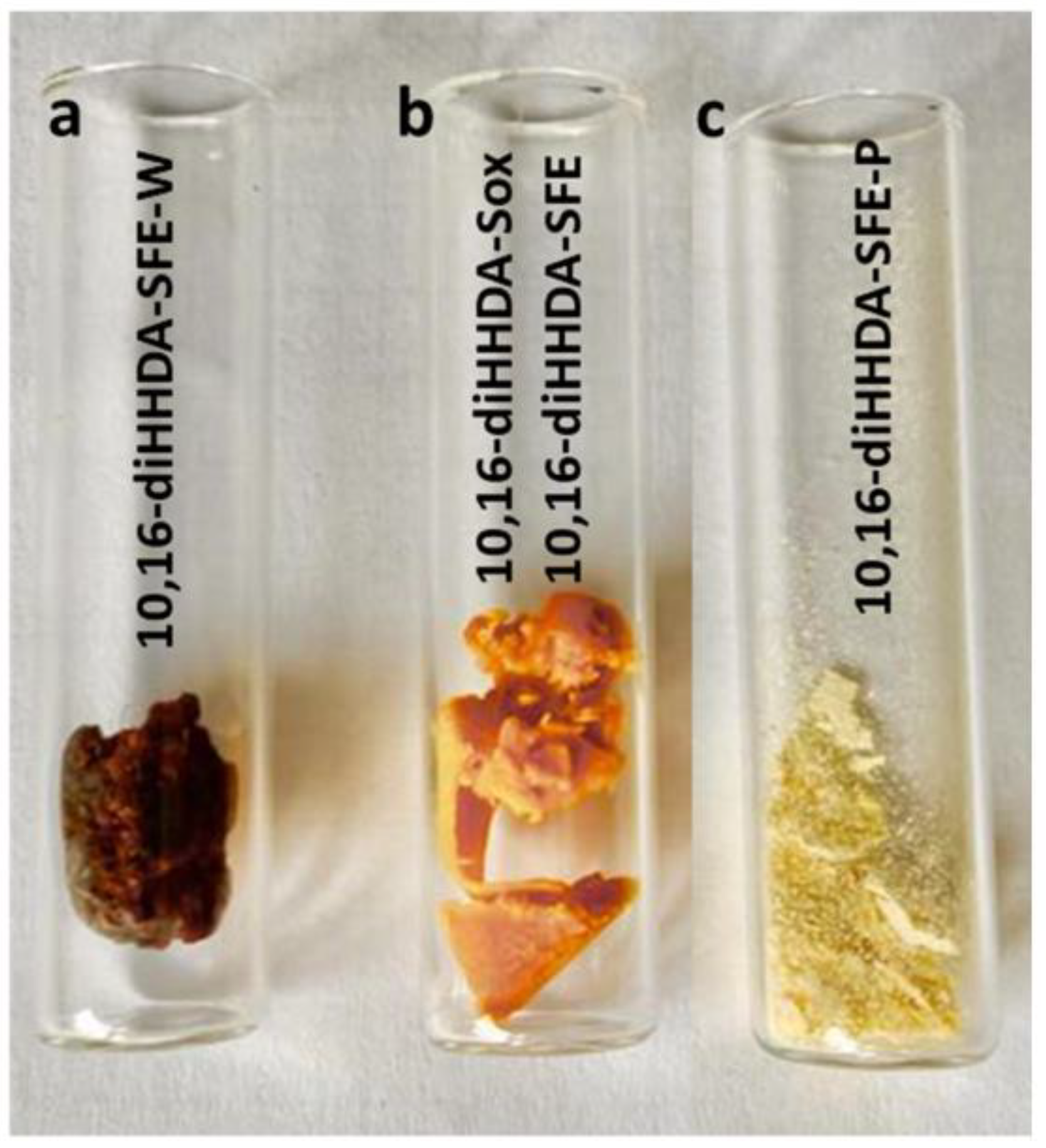
3.1. Monomers Isolation, Purification and Characterization
3.2. Physico-Chemical Characterization
3.2.1. Molecular Weight Distribution of Monomers
3.2.2. Thermal Characterization of Monomers
3.3. Solvent Compatibility
3.4. Solubility of Cutin in Biomedical Solvents
3.5. Stability and Storage Conditions of the Monomers
3.6. Cytotoxicity Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.M.; Lee, D. Effective Medical Waste Management for Sustainable Green Healthcare. Int. J. Environ. Res. Public Health 2022, 19, 14820. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Waste Management during the COVID-19 Pandemic: From Response to Recovery. 2020. Available online: https://www.unenvironment.org/resources/report/waste-management-during-covid-19-pandemic-response-recovery (accessed on 12 November 2024).
- World Health Organization. Healthcare Waste. Available online: https://www.who.int/en/news-room/fact-sheets/detail/health-care-waste (accessed on 12 November 2024).
- Aquino, A.C.T.; Goncalves, M.F.S.; Mol, M.P.G. Healthcare waste and circular economy principles: It is time to improve! Waste Manag. Res. 2024, 42, 857–859. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Heredia, A.; Dominguez, E.; Cingolani, R.; Bayer, I.S.; Athanassiou, A.; Benitez, J.J. Cutin from agro-waste as a raw material for the production of bioplastics. J. Exp. Bot. 2017, 68, 5401–5410. [Google Scholar] [CrossRef] [PubMed]
- Balla, E.D.; Klonos, P.A.; Kyritsis, A.; Bertoldo, M.; Guigo, N.; Bikiaris, D.N. Novel Biobased Copolymers Based on Poly(butylene succinate) and Cutin: In Situ Synthesis and Structure Properties Investigations. Polymers 2024, 16, 2270. [Google Scholar] [CrossRef]
- Manrich, A.; Moreira, F.K.; Otoni, C.G.; Lorevice, M.V.; Martins, M.A.; Mattoso, L.H. Hydrophobic edible films made up of tomato cutin and pectin. Carbohydr. Polym. 2017, 164, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Simoes, A.; Coelhoso, I.M.; Alves, V.D.; Brazinha, C. Recovery and Purification of Cutin from Tomato By-Products for Application in Hydrophobic Films. Membranes 2023, 13, 261. [Google Scholar] [CrossRef]
- Cifarelli, A.; Cigognini, I.M.; Bolzoni, L.; Montanari, A. Physical-Chemical Characteristics of Cutin Separated from Tomato Waste for the Preparation of Bio-Lacquers. Adv. Sci. Eng. 2019, 11, 33–45. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Agricultural Production Statistics 2000–2022. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/fba4ef43-422c-4d73-886e-3016ff47df52/content (accessed on 12 November 2024).
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 November 2024).
- World Processing Tomato Council. Available online: https://www.wptc.to (accessed on 12 November 2024).
- Saini, R.K.; Moon, S.H.; Keum, Y.S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, E.; Bianchi Oltolini, A.; Magni, M.; Beretta, G.; Cavallaro, M.; Suriano, R.; Turri, S. Crosslinked Polyesters as Fully Biobased Coatings with Cutin Monomer from Tomato Peel Wastes. Polymers 2024, 16, 682. [Google Scholar] [CrossRef]
- Simoes, R.; Miranda, I.; Pereira, H. Cutin extraction and composition determined under differing depolymerisation conditions in cork oak leaves. Phytochem. Anal. 2022, 33, 127–135. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waskiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.A.; Mohamed, A.; Abdulamir, A.S.; Abas, H.A. A Review on Supercritical Fluid Extraction as New Analytical Method. Am. J. Biochem. Biotechnol. 2008, 4, 345–353. [Google Scholar] [CrossRef]
- Ahmad, T.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gull, A. Supercritical Fluid Extraction: A Review. J. Biol. Chem. Chron. 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Nasti, R.; Beretta, G.; Trasatti, S.; Dorati, R.; Conti, B.; Genta, I. Process for the Preparation of a Cutin-Based Material and Biomedical Applications Thereof. WIPO Patent WO/2024/241267, 28 November 2024. [Google Scholar]
- Cifarelli, A.; Cigognini, I.; Bolzoni, L.; Montanari, A. Cutin isolated from tomato processing by-products: Extraction methods and characterization. In Proceedings of the 4th International Conference on Sustainable Solid Waste Management, Limassol, Cyprus, 23–25 June 2016; pp. 1–20. [Google Scholar]
- Ahmed, A.; Crawford, T.; Gould, S.; Ha, Y.S.; Hollrah, M.; Noor, E.A.F.; Dickman, M.B.; Dussault, P.H. Synthesis of (R)- and (S)-10,16-dihydroxyhexadecanoic acid: Cutin stereochemistry and fungal activation. Phytochemistry 2003, 63, 47–52. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 12 November 2024).
- Osman, S.; Gerard, H.; Fett, W.; Moreau, R.; Dudley, R. Method for the Production and Characterization of Tomato Cutin Oligomers. J. Agric. Food Chem. 1995, 43, 2134–2137. [Google Scholar] [CrossRef]
- Espana, L.; Heredia-Guerrero, J.A.; Segado, P.; Benitez, J.J.; Heredia, A.; Dominguez, A. Biomechanical properties of the tomato (Solanum lycopersicum) fruit cuticle during development are modulated by changes inthe relative amounts of its components. N. Phytol. 2014, 202, 790–802. [Google Scholar] [CrossRef]
- Del Baldo, M. When innovation rests on sustainability and food safety: Some experiences from Italian agri-food start-ups. Front. Sustain. 2022, 3, 889158. [Google Scholar] [CrossRef]
- Kranenburg, R.F.; Ramaker, H.-J.; Weesepoel, Y.; Arisz, P.W.F.; Keizers, P.H.J.; van Esch, A.; van Uxem, K.Z.; van den Berg, J.D.J.; Hulshof, J.W.; Bakels, S.; et al. The influence of water of crystallization in NIR-based MDMA⋅HCl detection. Forensic Chem. 2023, 32, 100464. [Google Scholar] [CrossRef]
- Dominguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles: An overview. N. Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef]
- Rubens, M.; Junkers, T. Comprehensive control over molecular weight distributions through automated polymerizations. Polym. Chem. 2019, 10, 6315–6323. [Google Scholar] [CrossRef]
- Arrighetti, L.; Ricci, L.; De Monte, C.; Aiello, F.; Massa, C.A.; Balzano, F.; Uccello Barretta, G.; Bronco, S. Innovative materials based on physical melt-blending of cutin from tomato waste and poly (lactic acid). Mater. Today Sustain. 2024, 27, 100852. [Google Scholar] [CrossRef]
- Benítez, J.J.; Heredia-Guerrero, J.A.; Guzmán-Puyol, S.; Barthel, M.J.; Domínguez, E.; Heredia, A. Polyhydroxyester Films Obtained by Non-Catalyzed Melt-Polycondensation of Natural Occurring Fatty Polyhydroxyacids. Front. Mater. 2015, 2, 59. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, 2009 ICH Topic Q3C (R4) Impurities: Guideline for Residual Solvents. Available online: https://www.tga.gov.au/sites/default/files/ich28395enrev4.pdf (accessed on 12 November 2024).
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecorini, G.; Tamburriello, M.; Tottoli, E.M.; Beretta, G.; Genta, I.; Conti, B.; Dorati, R.; Nasti, R. Transforming Agro-Waste Cutin into Sustainable Materials for Biomedical Innovations. Polymers 2025, 17, 742. https://doi.org/10.3390/polym17060742
Pecorini G, Tamburriello M, Tottoli EM, Beretta G, Genta I, Conti B, Dorati R, Nasti R. Transforming Agro-Waste Cutin into Sustainable Materials for Biomedical Innovations. Polymers. 2025; 17(6):742. https://doi.org/10.3390/polym17060742
Chicago/Turabian StylePecorini, Gianni, Martina Tamburriello, Erika Maria Tottoli, Giangiacomo Beretta, Ida Genta, Bice Conti, Rossella Dorati, and Rita Nasti. 2025. "Transforming Agro-Waste Cutin into Sustainable Materials for Biomedical Innovations" Polymers 17, no. 6: 742. https://doi.org/10.3390/polym17060742
APA StylePecorini, G., Tamburriello, M., Tottoli, E. M., Beretta, G., Genta, I., Conti, B., Dorati, R., & Nasti, R. (2025). Transforming Agro-Waste Cutin into Sustainable Materials for Biomedical Innovations. Polymers, 17(6), 742. https://doi.org/10.3390/polym17060742











