Enhancement of Polypropylene Bonding Through Plasma–Ultrasonic Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
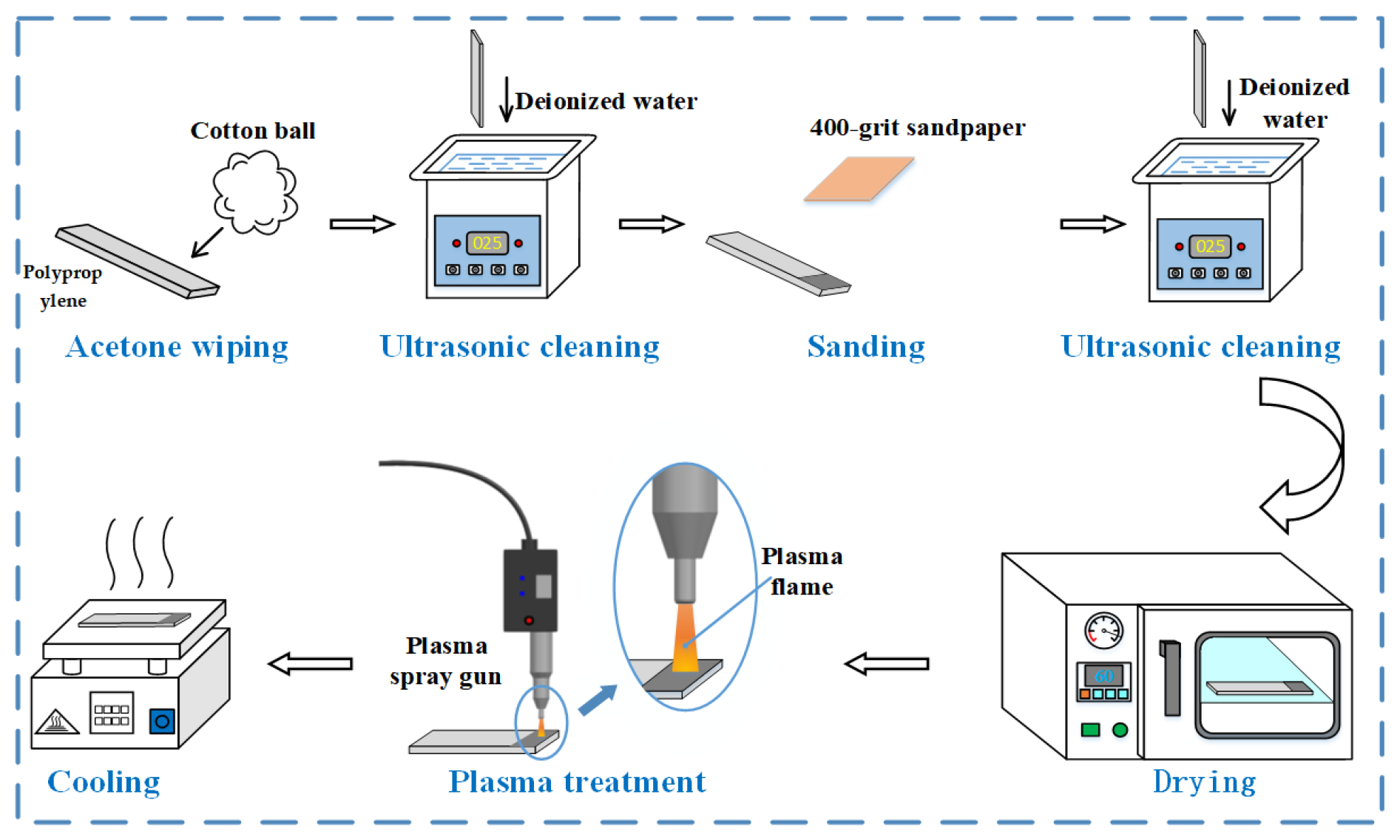
2.2. Surface Pre-Treatment Process
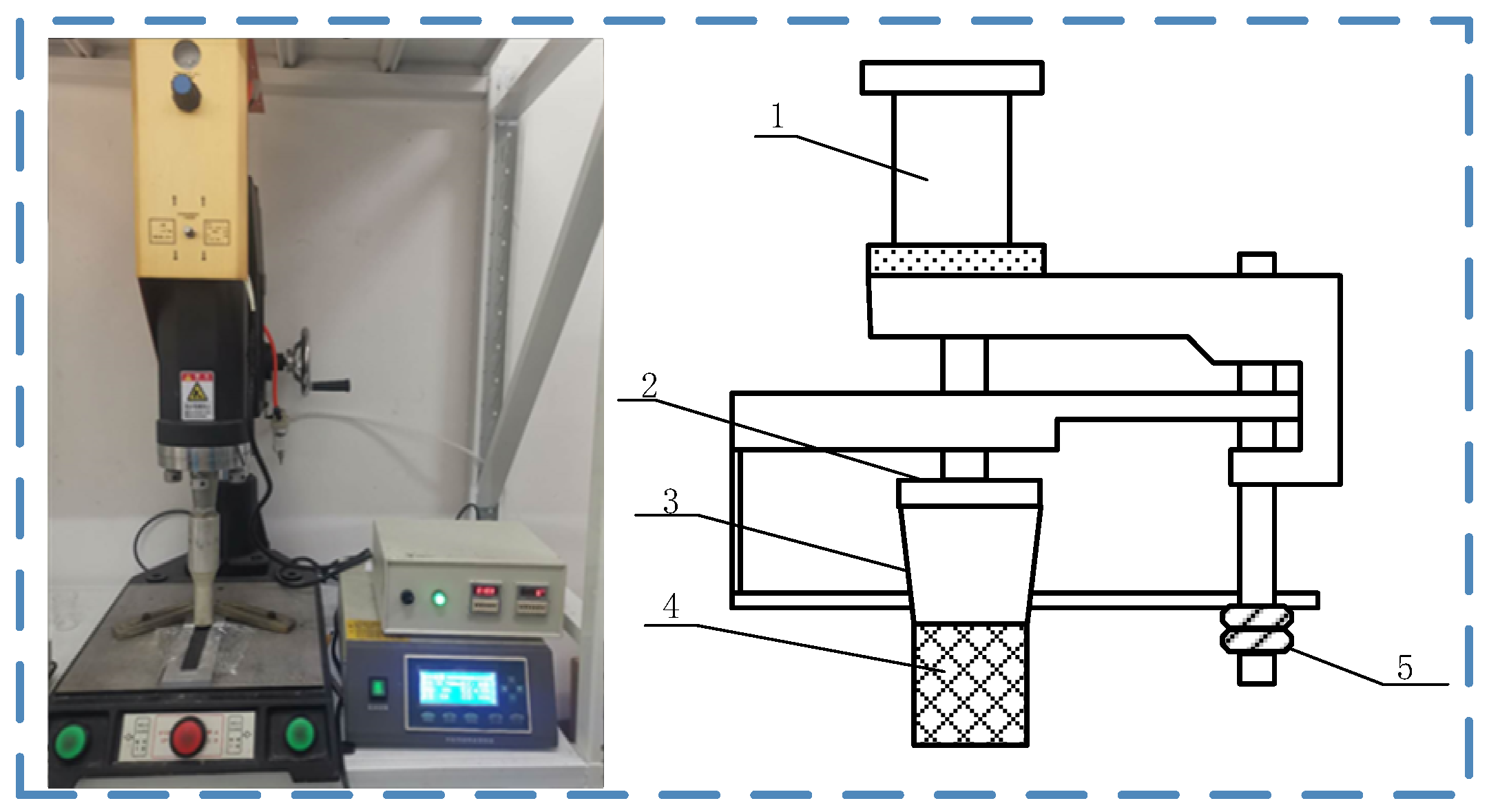
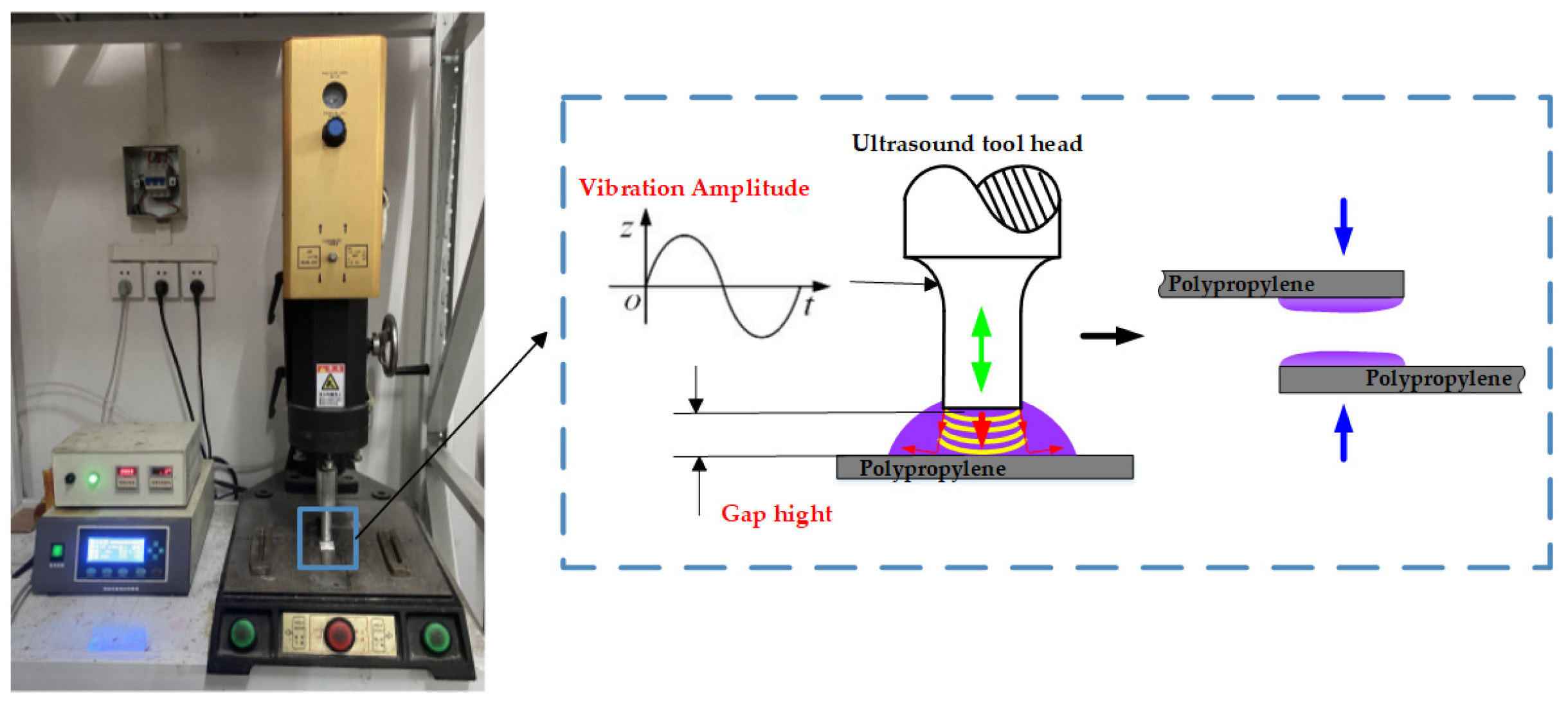
2.3. Ultrasonic Treatment Process
2.4. Characterization
2.4.1. Shear Strength Test
2.4.2. Contact Angle Measurement
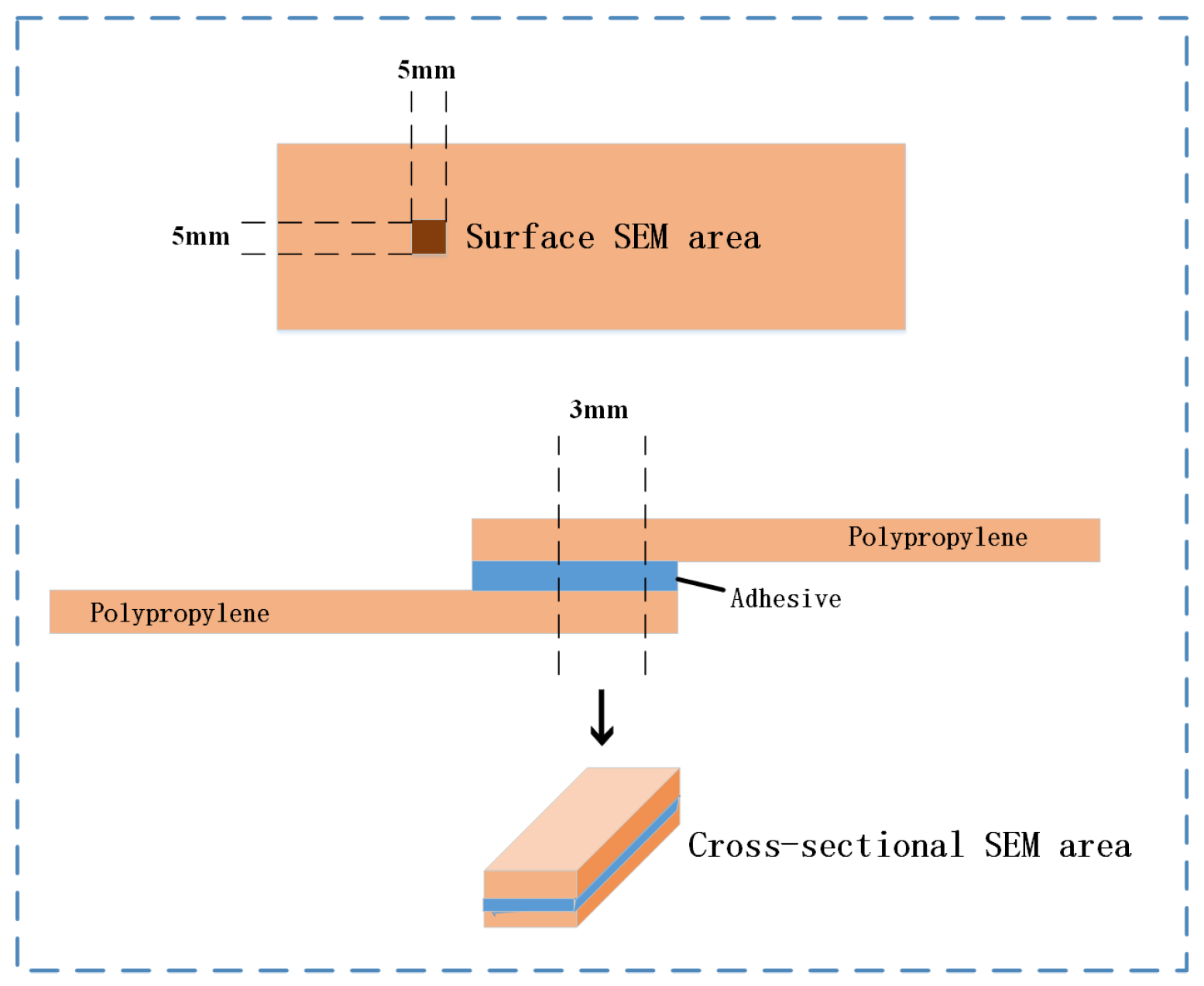
2.4.3. Surface Morphology and Chemical Composition Analysis
2.4.4. Surface Contact Angle and Chemical Functional Group
2.5. Molecular Dynamics Simulation
3. Results
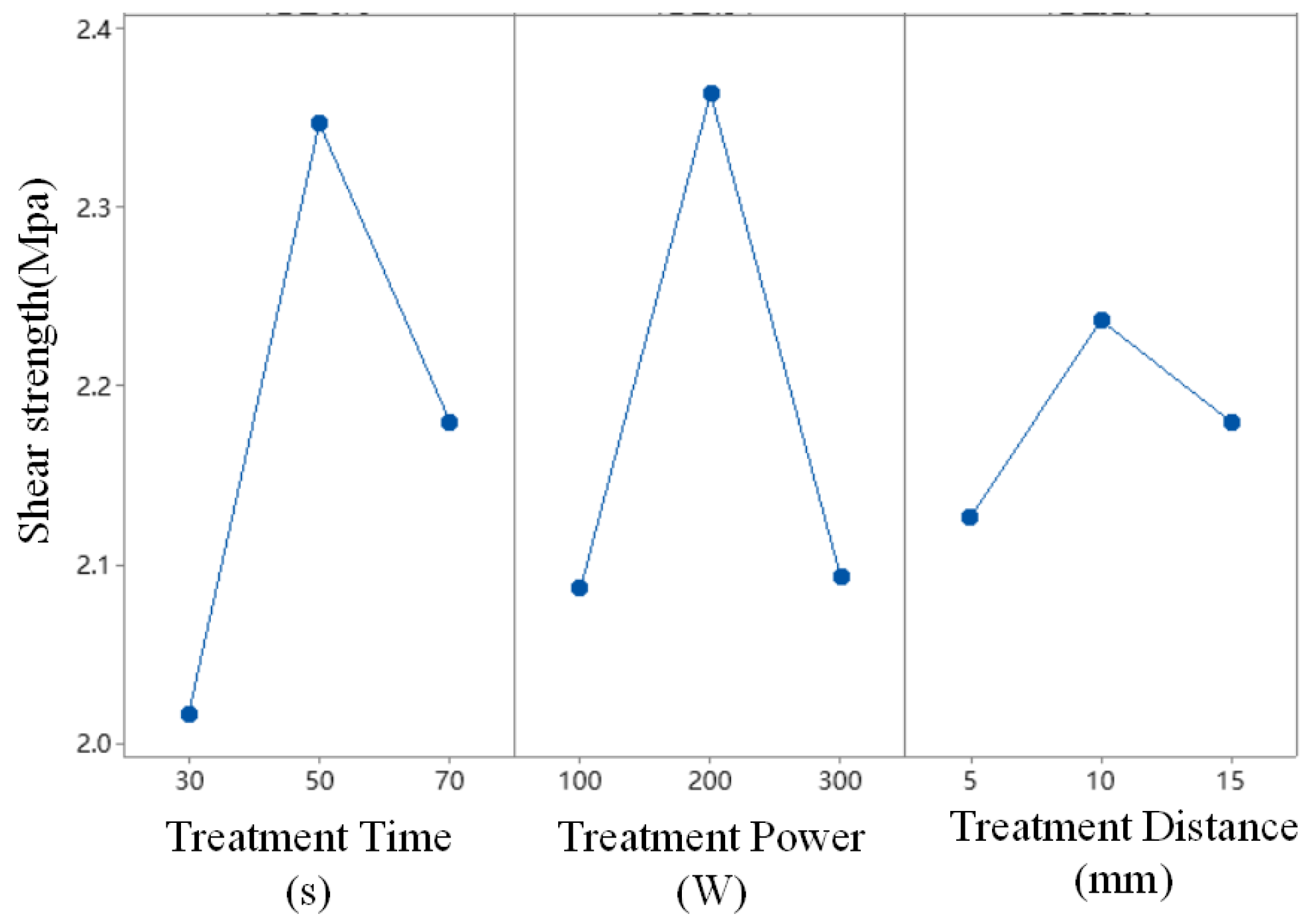
3.1. Optimization of Plasma Treatment Parameters
3.2. Ultrasonic Adhesive Application Optimization
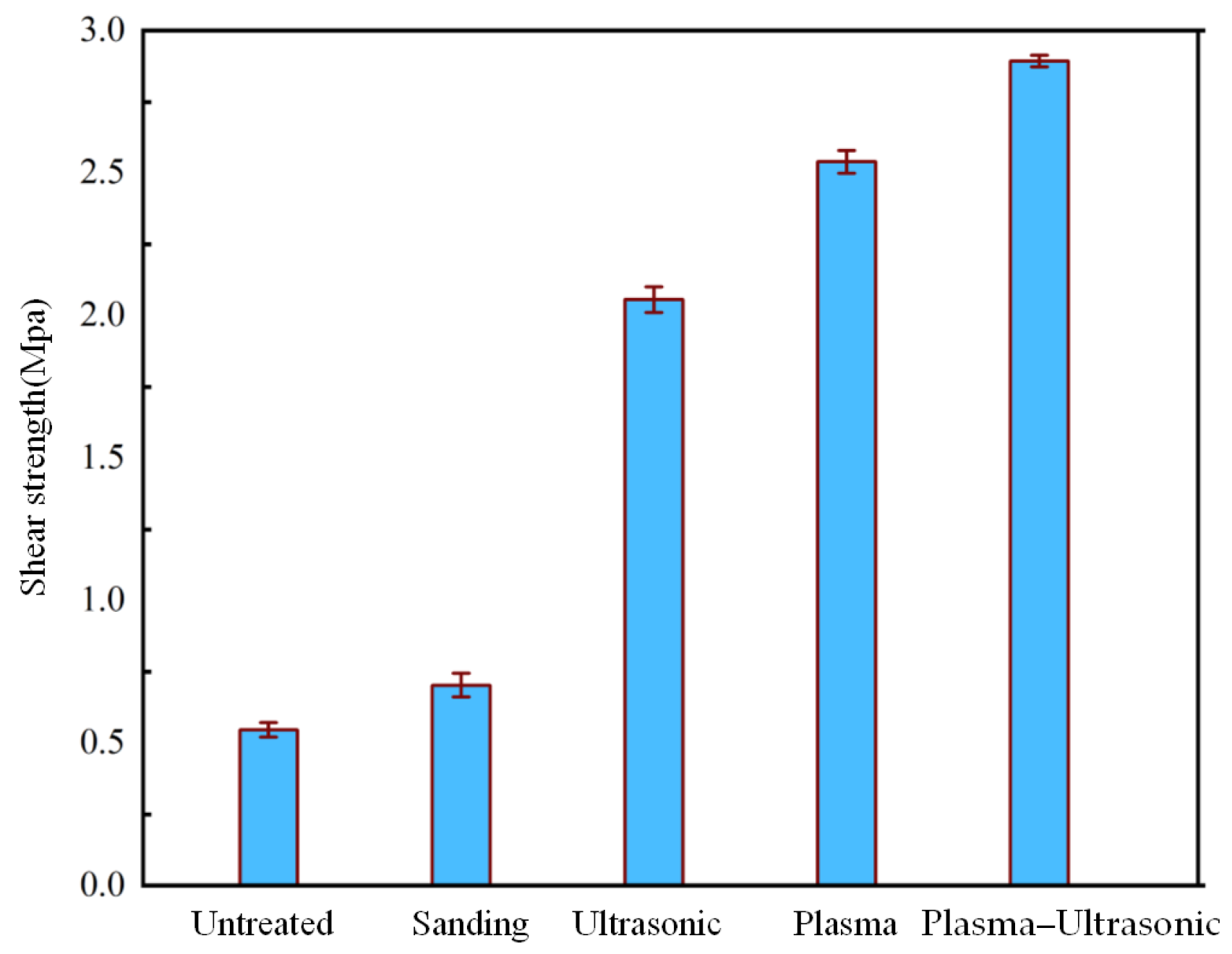
3.3. Shear Strength Testing Results
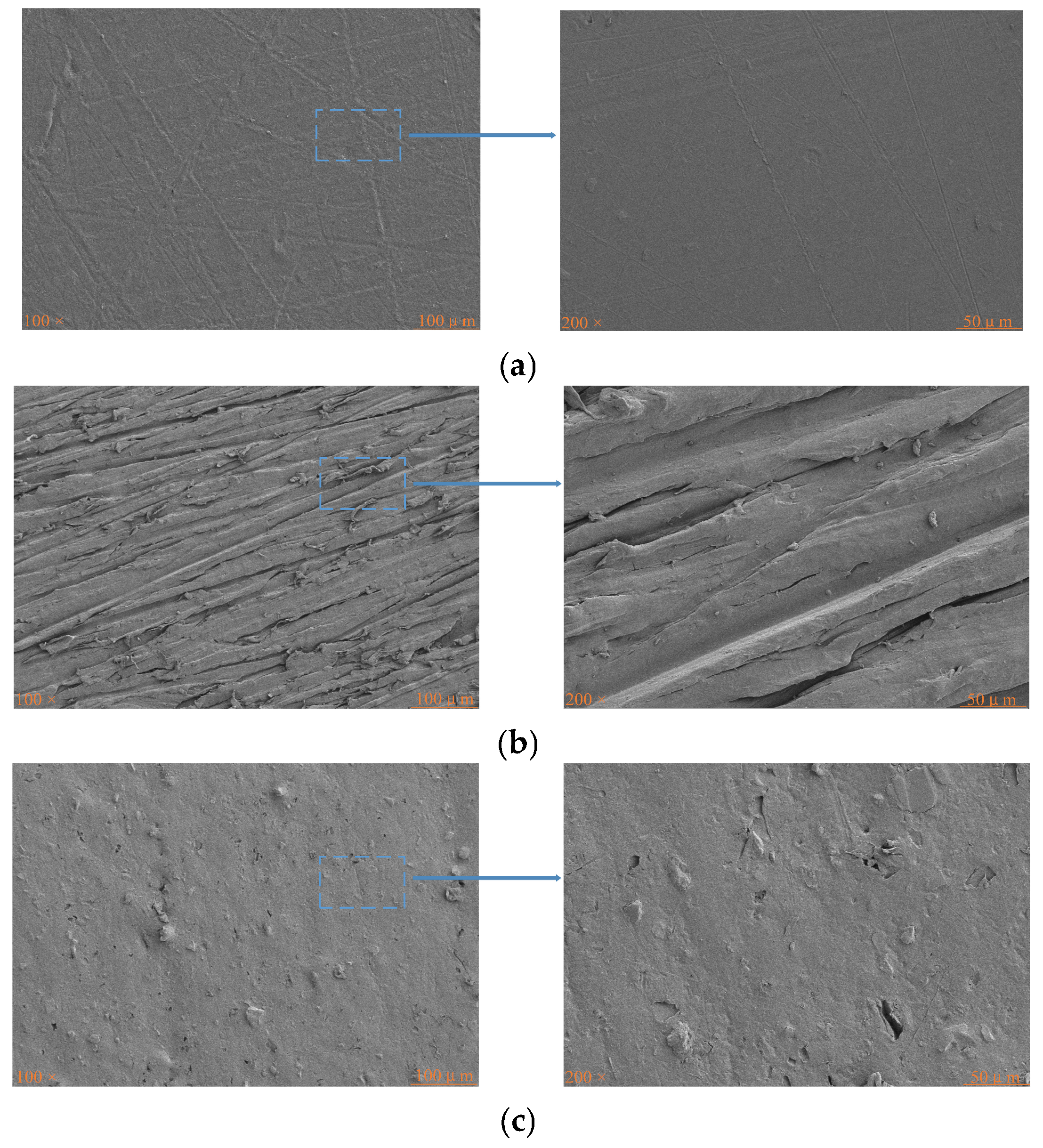
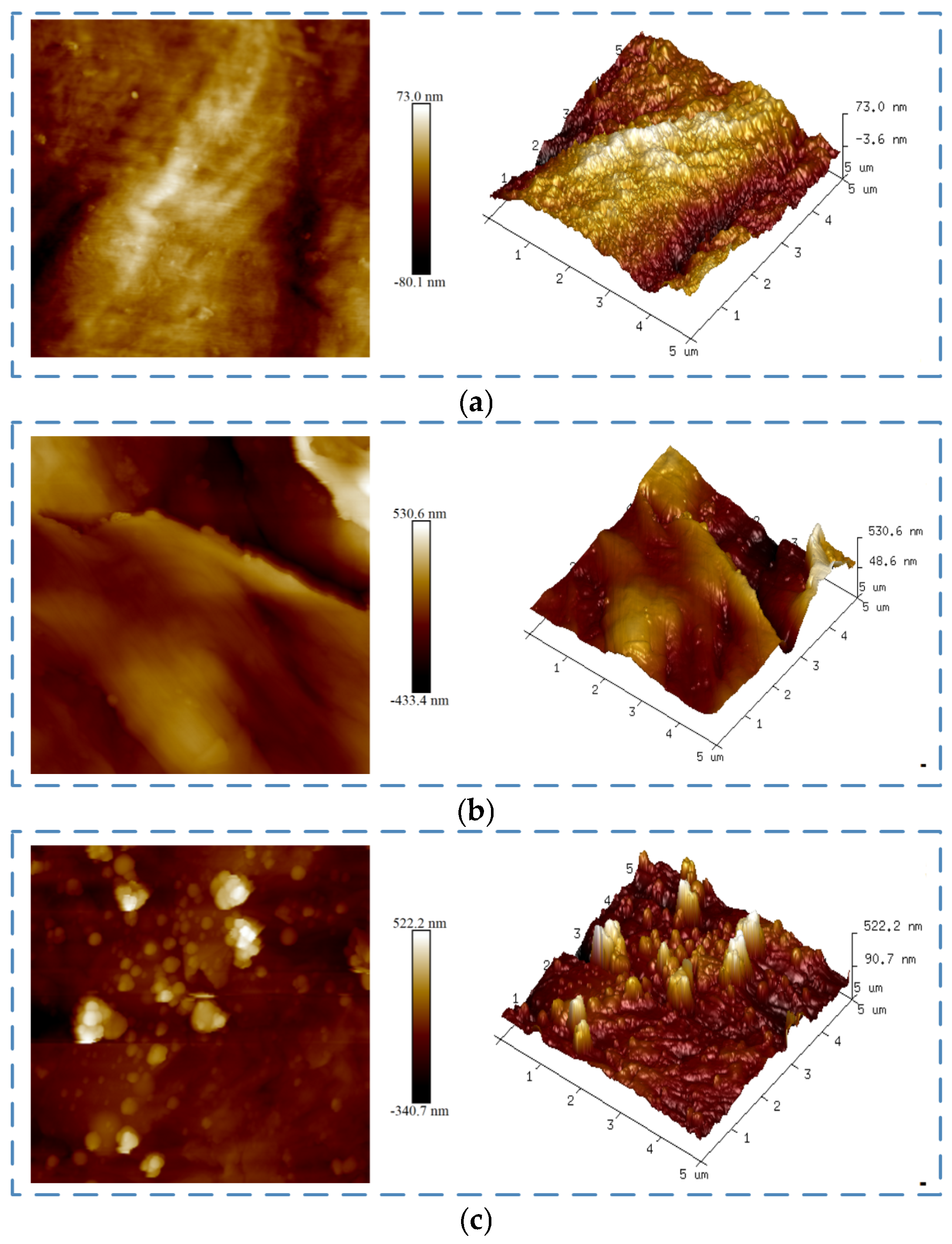
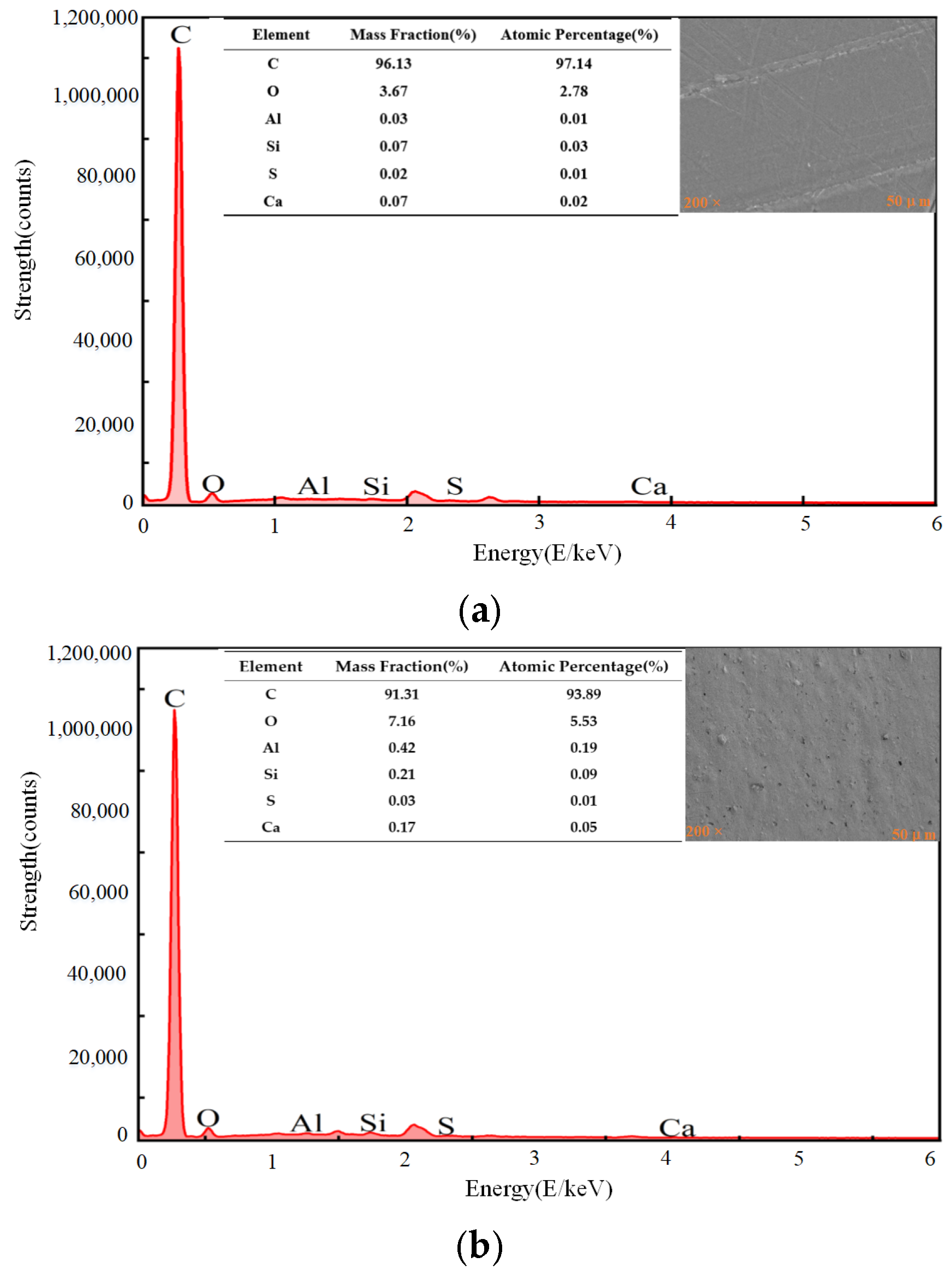
3.4. Surface Morphology and Chemical Composition Analysis
3.5. Surface Contact Angle and Chemical Functional Group
3.6. Molecular Dynamics Simulation Analysis
4. Conclusions
- (1)
- By the optimum process parameters of plasma–ultrasonic treatment, the plasma treatment process increased the shear strength of the PP specimens by 370.3% compared with untreated specimens, and the addition of ultrasonic treatment further increased the shear strength of the PP specimens by 10.6%. The coefficient of variation decreased from 0.53 in the untreated specimens to 0.32 for the plasma–ultrasonic ones, enhancing the stability of adhesion.
- (2)
- The plasma–ultrasonic treatment makes full use of the surface roughness and wettability provided by plasma treatment, and under the ultrasonic action, the adhesive can better penetrate into the PP surface. By integrating plasma treatment with ultrasonic treatment, the bonding strength is further improved.
- (3)
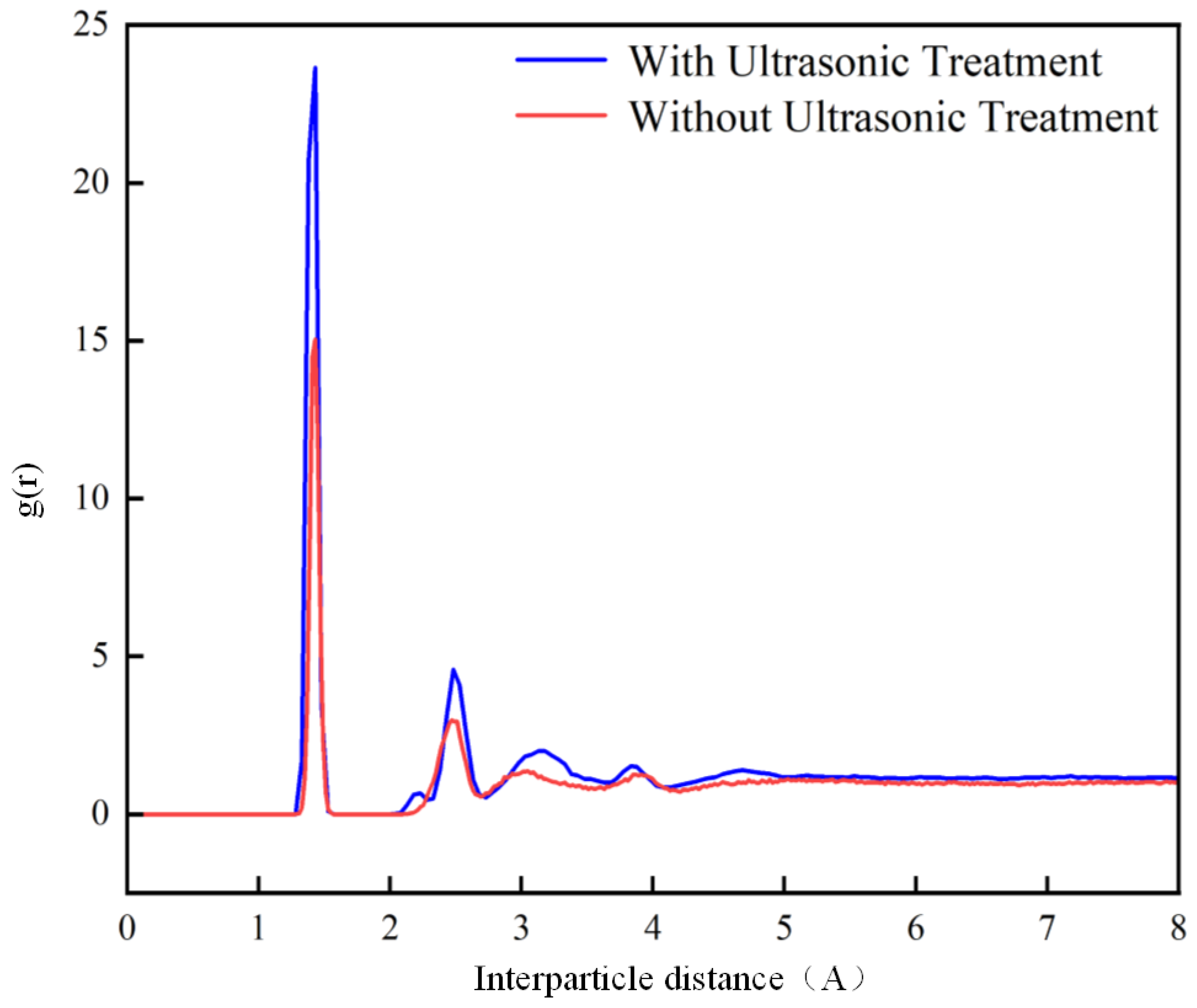
- Molecular dynamics results show that the plasma–ultrasonic process increases the binding energy of the adhesive system by 57%. Additionally, the plasma– ultrasonic process results in more bonds within the adhesive system, leading to a tighter interface bonding.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pious, C.V.; Thomas, S. Polymeric materials structure properties and applications. In Polymers; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 21–39. [Google Scholar]
- Watanabe, R.; Hagihara, H.; Sato, H. Structure-property relationships of polypropylene-based nanocomposites obtained by dis-persing mesoporous silica into hydroxyl-functionalized polypropylene. Part 1: Toughness, stiffness and transparency. Polym. J. 2018, 50, 1057–1065. [Google Scholar] [CrossRef]
- Agarwal, J.; Sahoo, S.; Mohanty, S.; Nayak, S.K. Progress of novel techniques for lightweight automobile applications through innovative eco-friendly composite materials: A review. Thermoplast. Compos. Mater. 2020, 33, 978–1013. [Google Scholar] [CrossRef]
- Belmonte, G.K.; Charles, G.; Strumia, M.C.; Weibel, D.E. Permanent hydrophilic modification ofpolypropylene and poly(vinyl alcohol) films by vacuum ultraviolet radiation. Appl. Surf. 2016, 382, 93–100. [Google Scholar] [CrossRef]
- Cognard, P. Handbook of Adhesives and Sealants, Volume 1—Basic Concepts and High Tech Bonding; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Chen, Y.; Xiang, W.; Zhang, Q.; Wang, H.; Hua, L. Improvement of Ni-CFRP interfacial properties using compound coupling agent treatment. Thin-Walled Struct. 2024, 195, 111334. [Google Scholar] [CrossRef]
- Miturska-Barańska, I.; Rudawska, A.; Doluk, E. The Influence of Sandblasting Process Parameters of Aerospace Aluminium Alloy Sheets on Adhesive Joints Strength. Materials 2021, 14, 6626. [Google Scholar] [CrossRef]
- Zhang, H.B.; Chen, Q. Recent progress of non-thermal plasma material surface treatment and functionalization. Acta Physica Sinica 2021, 70, 095203. [Google Scholar] [CrossRef]
- Williams, D.F.; Kellar, E.J.; Jesson, D.A.; Watts, J.F. Surface analysis of 316 stainless steel treated with cold atmospheric plasma. Appl. Surf. Sci. 2017, 403, 240–247. [Google Scholar] [CrossRef]
- Wu, M.; Tong, X.; Wang, H. Effect of Ultrasonic Vibration on Adhesive Bonding of CFRP/Al Alloy Joints Grafted with Silane Coupling Agent. Polymers 2020, 12, 947. [Google Scholar] [CrossRef]
- Hieda, J.; Niinomi, M. Adhesive strength of medical polymer on anodic oxide nanostructures fabricated on biomedical β-type titanium alloy. Mater. Sci. Eng. 2014, 36, 244–251. [Google Scholar] [CrossRef]
- Mandolfino, C.; Lertora, E.; Gambaro, C.; Bruno, M. Improving adhesion performance of polyethylene surfaces by cold plasma treatment. Meccanica 2014, 49, 2299–2306. [Google Scholar] [CrossRef]
- Mandolfino, C. Polypropylene surface modification by low pressure plasma to increase adhesive bonding: Effect of process parameters. Surf. Coat. Technol. 2019, 366, 331–337. [Google Scholar] [CrossRef]
- Sanchis, M.R.; Blanes, V.; Blanes, M.; Garcia, D.; Balart, R. Surface modification of low density polyethylene (LDPE) film by low pressure O2 plasma treatment. Eur. Polym. J. 2006, 42, 1558–1568. [Google Scholar] [CrossRef]
- Hao, D.; Masahiro, S.; Atsushi, K.; Ryo, O. Theoretical prediction of the reaction probabilities between H, O, OH radicals and polypropylene surface. J. Phys. Chem. A 2024, 128, 1041–1048. [Google Scholar]
- Wang, H.; Chen, Z.Y.; Chen, Y.M. Mechanism study of bubble removal in narrow viscous fluid by using ultrasonic vibration. Jpn. J. Appl. Phys. 2019, 58, 115503. [Google Scholar] [CrossRef]
- Du, L.Q.; Zhai, K.; Li, X.J. Ultrasonic vibration used for improving interfacial adhesion strength between metal substrate and high-aspect-ratio thick SU-8 photoresist mould. Ultrasonics 2020, 103, 106100. [Google Scholar] [CrossRef]
- Holtmannspotter, J.; Czarnecki, J.V.; Gudladt, H.J. The use of power ultrasound energy to support interface formation for structural adhesive bonding. Int. J. Adhes. Adhes. 2021, 30, 130–138. [Google Scholar] [CrossRef]
- Sundukov, S.K. Ultrasonic Technologies in Producing Adhesive Joints: A Review. Polym. Sci. 2023, 16, 868–874. [Google Scholar] [CrossRef]
- Benatar, A.; Gutowski, T.G. Ultrasonic welding of PEEK graphite APC-2 composites. Polym. Eng. Sci. 1989, 29, 1705–1721. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.T.; Ji, W. Improvement of interface anchoring by ultrasonic vibration for adhesively bonded CFRP/Al joints. J. Reinf. Plast. Compos. 2022, 41, 20–33. [Google Scholar] [CrossRef]
- Chen, W.X.; Yu, J.S.; Hu, W.; Chen, G.L. Partial hydrophilic modification of biaxially oriented polypropylene film by an atmospheric pressure plasma jet with the allylamine monomer. Appl. Surf. Sci. 2016, 387, 957–964. [Google Scholar] [CrossRef]
- Mandolfino, C.; Lertora, E.; Gambaro, C.; Pizzorni, M. Functionalization of Neutral Polypropylene by Using Low Pressure Plasma Treatment: Effects on Surface Characteristics and Adhesion Properties. Polymers 2019, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- And FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]



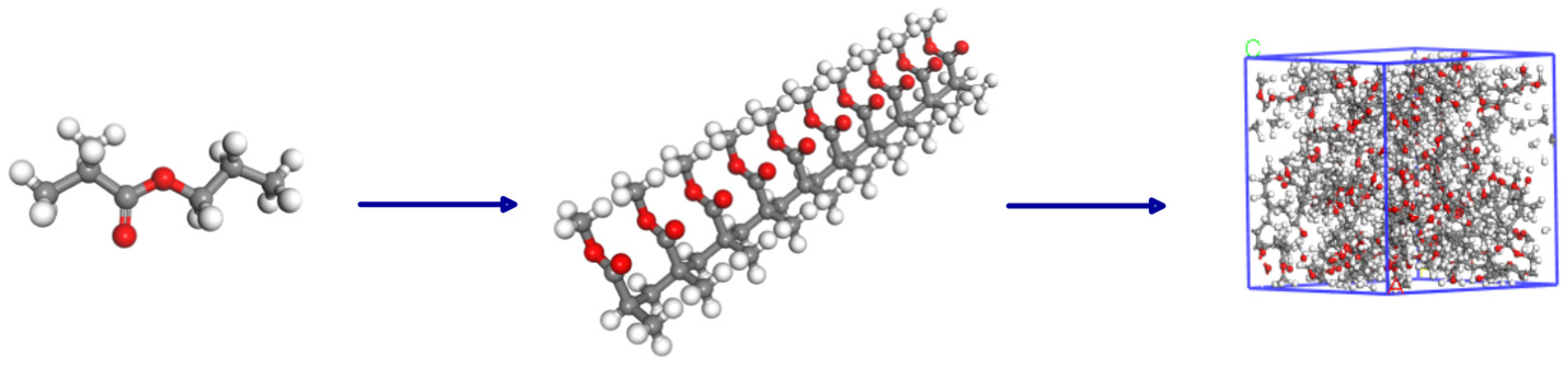
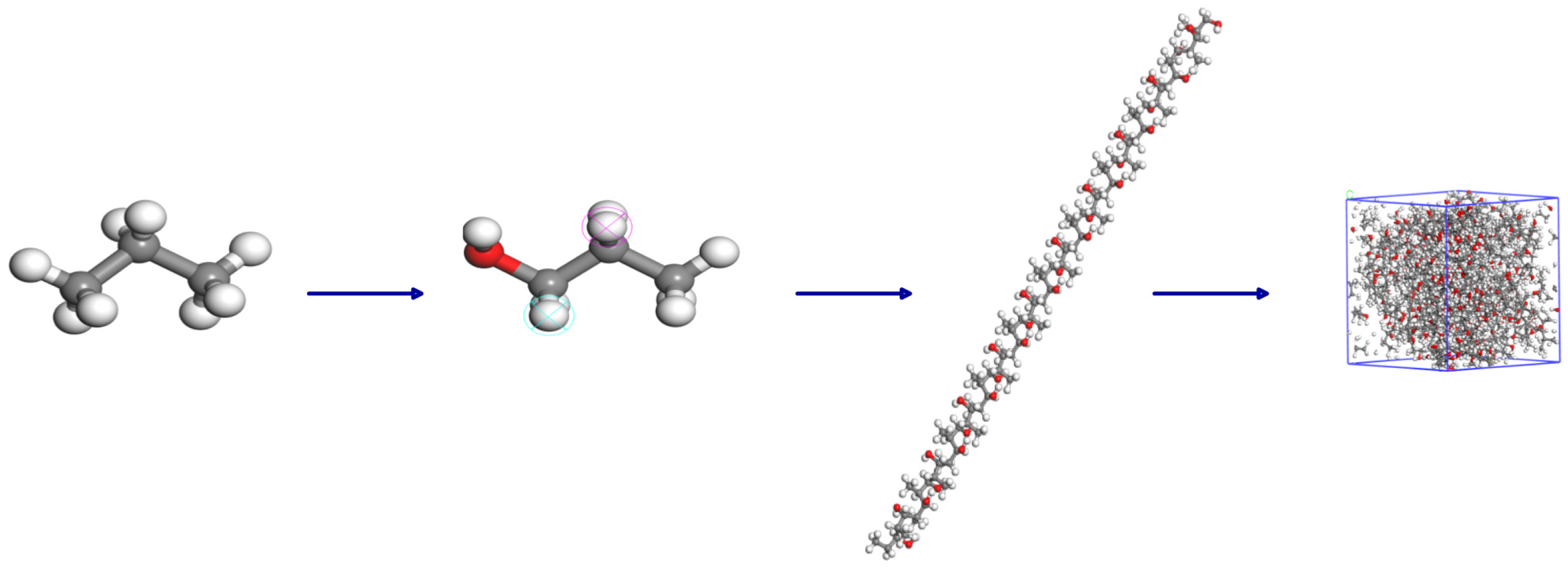
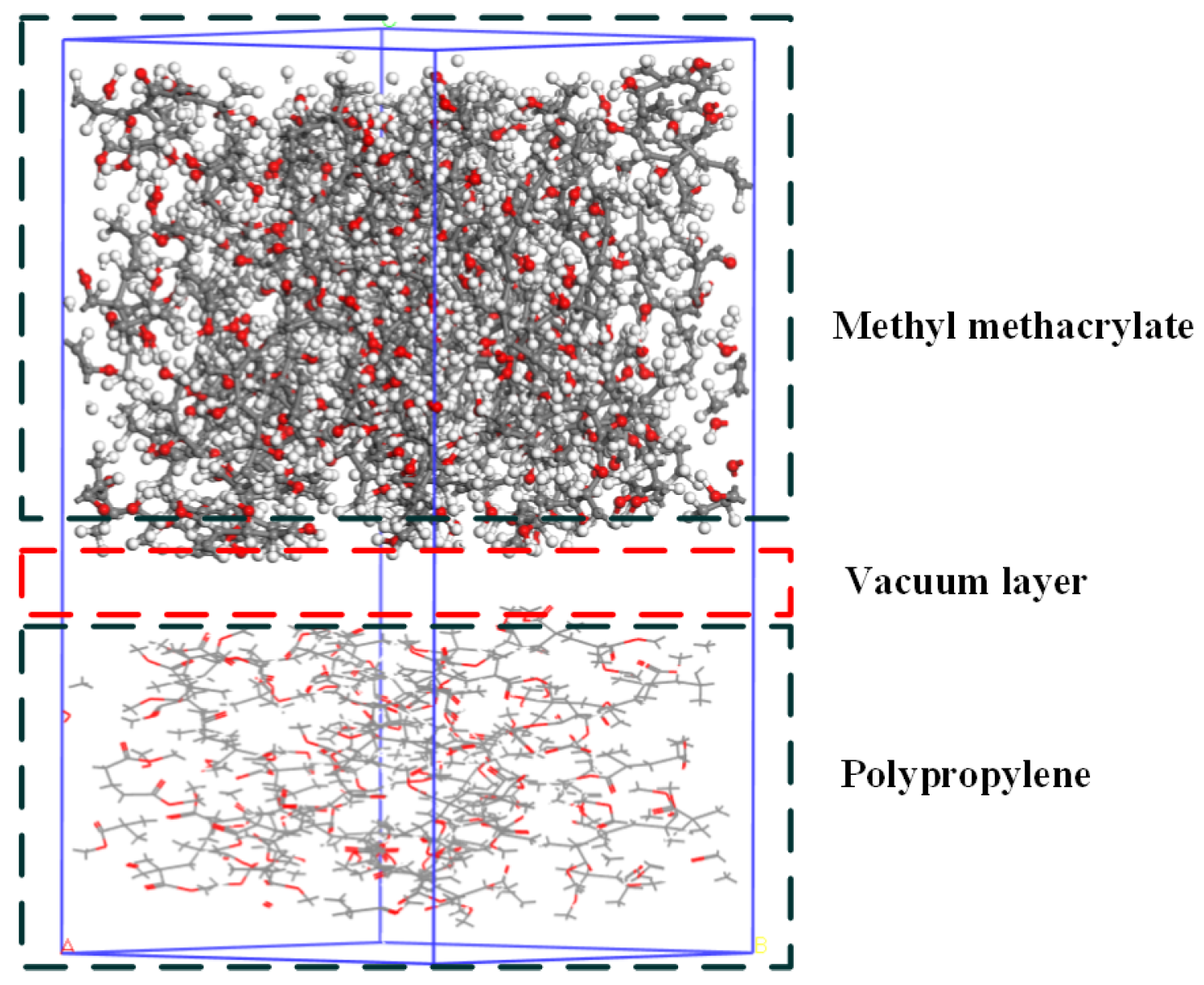





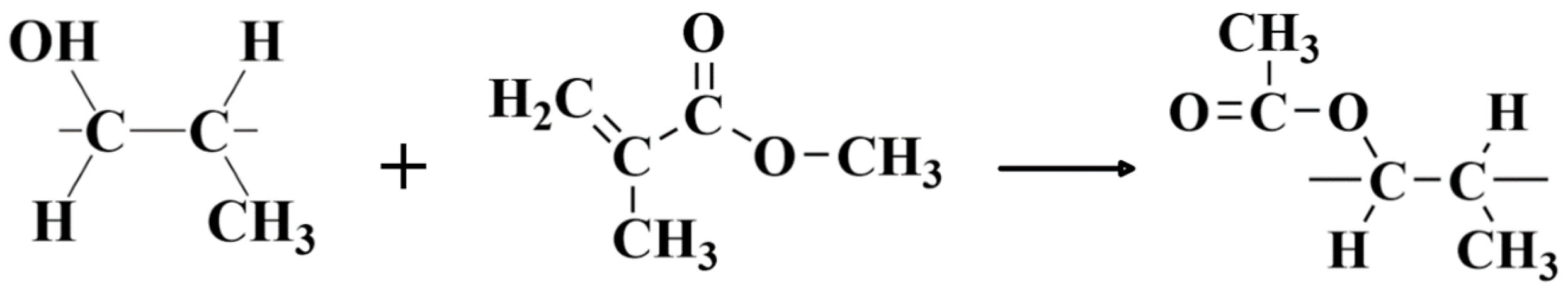
| Property | PP |
|---|---|
| Yield stress | 29 MPa |
| Elongation at break | 40% |
| Tensile modulus of elasticity | 527 MPa |
| Property | Adhesive |
|---|---|
| Tensile strength | 15.6 MPa |
| Elastic modulus | 413.7 MPa |
| Elongation at break | 45% |
| Levels | Factors | ||
|---|---|---|---|
| Treatment Power (W) | Treatment Time (s) | Treatment Distance (mm) | |
| 1 | 100 | 30 | 5 |
| 2 | 200 | 50 | 10 |
| 3 | 300 | 70 | 15 |
| Levels | Factors | ||
|---|---|---|---|
| Gap Height (mm) | Vibration Time (s) | Vibration Amplitude (µm) | |
| 1 | 0.5 | 5 | 16 |
| 2 | 1 | 10 | 20 |
| 3 | 1.5 | 15 | 24 |
| 4 | 2 | 20 | 28 |
| Scheme | Treatment Time (s) | Treatment Power (W) | Treatment Distance (mm) | Average Shear Strength (MPa) |
|---|---|---|---|---|
| 1 | 30 | 100 | 5 | 1.85 |
| 2 | 30 | 200 | 10 | 2.26 |
| 3 | 30 | 300 | 15 | 1.94 |
| 4 | 50 | 100 | 10 | 2.32 |
| 5 | 50 | 200 | 15 | 2.51 |
| 6 | 50 | 300 | 5 | 2.21 |
| 7 | 70 | 100 | 15 | 2.09 |
| 8 | 70 | 200 | 5 | 2.32 |
| 9 | 70 | 300 | 10 | 2.13 |
| Levels | Treatment Time | Treatment Power | Treatment Distance |
|---|---|---|---|
| 1 | 2.017 | 2.087 | 2.127 |
| 2 | 2.347 | 2.363 | 2.237 |
| 3 | 2.180 | 2.093 | 2.180 |
| Delta | 0.330 | 0.277 | 0.110 |
| Rank | 1 | 2 | 3 |
| Scheme | Gap Height (mm) | Vibration Time (s) | Vibration Amplitude (µm) | Average Shear Strength (MPa) |
|---|---|---|---|---|
| 1 | 0.5 | 5 | 16 | 2.66 |
| 2 | 0.5 | 10 | 20 | 2.75 |
| 3 | 0.5 | 15 | 24 | 2.71 |
| 4 | 0.5 | 20 | 28 | 2.75 |
| 5 | 1 | 5 | 16 | 2.63 |
| 6 | 1 | 10 | 28 | 2.88 |
| 7 | 1 | 15 | 24 | 2.73 |
| 8 | 1 | 20 | 24 | 2.77 |
| 9 | 1.5 | 5 | 28 | 2.94 |
| 10 | 1.5 | 10 | 16 | 2.68 |
| 11 | 1.5 | 15 | 20 | 2.72 |
| 12 | 1.5 | 20 | 28 | 2.59 |
| 13 | 2 | 5 | 24 | 2.57 |
| 14 | 2 | 10 | 20 | 2.81 |
| 15 | 2 | 15 | 20 | 2.69 |
| 16 | 2 | 20 | 16 | 2.67 |
| Levels | Gap Height | Vibration Time | Vibration Amplitude |
|---|---|---|---|
| 1 | 2.718 | 2.700 | 2.732 |
| 2 | 2.752 | 2.780 | 2.665 |
| 3 | 2.732 | 2.712 | 2.808 |
| 4 | 2.685 | 2.695 | 2.683 |
| Delta | 0.067 | 0.085 | 0.143 |
| Rank | 3 | 2 | 1 |
| Group | Mean | 95% Confidence Interval |
|---|---|---|
| Untreated | 0.54 | 0.52–0.56 |
| Sanding | 0.69 | 0.65–0.73 |
| Ultrasonic | 2.05 | 1.99–2.11 |
| Plasma | 2.54 | 2.51–2.58 |
| Plasma–Ultrasonic | 2.81 | 2.79–2.83 |
| Treatment Method | Ra (nm) | Rq (nm) | Rmax (nm) |
|---|---|---|---|
| Untreated | 18.3 | 22.6 | 144 |
| Sanding Treatment | 170 | 130 | 993 |
| Plasma Treatment | 46.3 | 36.6 | 417 |
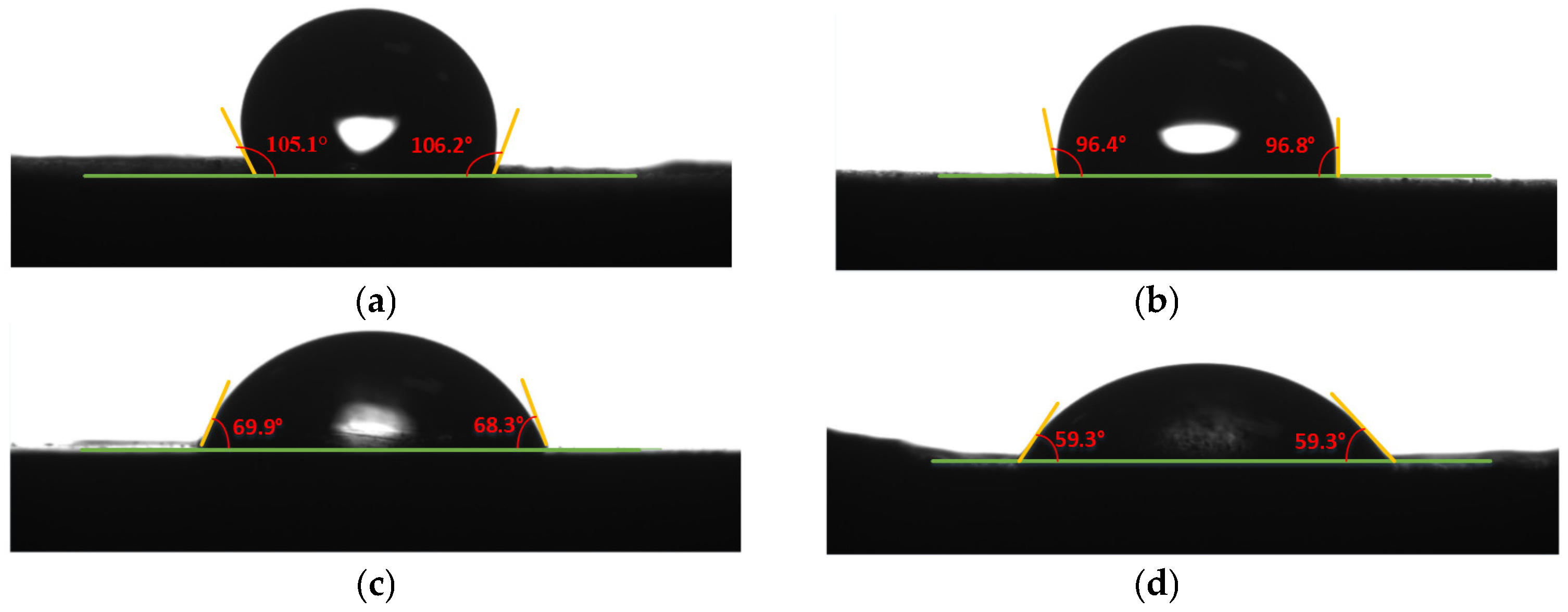
| Treatment Method | Left Contact Angle (°) | Right Contact Angle (°) | Average Contact Angle (°) |
|---|---|---|---|
| Untreated | 105.1 | 106.2 | 105.6 |
| Sanding Treatment | 96.4 | 96.8 | 96.7 |
| Plasma Treatment | 69.9 | 68.3 | 69.1 |
| Plasma–Ultrasonic Treatment | 59.3 | 59.3 | 59.3 |
| Treatment Method | PP Energy (kcal/mol) | Adhesive Energy (kcal/mol) | Total Energy (kcal/mol) | Interfacial Binding Energy (kcal/mol) |
|---|---|---|---|---|
| Untreated | −381.4 | 4959.9 | 4389 | 188.5 |
| Plasma Treatment | −462.1 | 4876.2 | 4145 | 269.1 |
| Plasma–Ultrasonic Treatment | −462.1 | 4979.1 | 4250 | 297.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yang, C.; He, L.; Yu, B.; Zhao, X.; Huang, Z. Enhancement of Polypropylene Bonding Through Plasma–Ultrasonic Treatment. Polymers 2025, 17, 726. https://doi.org/10.3390/polym17060726
Wang H, Yang C, He L, Yu B, Zhao X, Huang Z. Enhancement of Polypropylene Bonding Through Plasma–Ultrasonic Treatment. Polymers. 2025; 17(6):726. https://doi.org/10.3390/polym17060726
Chicago/Turabian StyleWang, Hui, Chuhao Yang, Limei He, Binbin Yu, Xiaobin Zhao, and Zongbin Huang. 2025. "Enhancement of Polypropylene Bonding Through Plasma–Ultrasonic Treatment" Polymers 17, no. 6: 726. https://doi.org/10.3390/polym17060726
APA StyleWang, H., Yang, C., He, L., Yu, B., Zhao, X., & Huang, Z. (2025). Enhancement of Polypropylene Bonding Through Plasma–Ultrasonic Treatment. Polymers, 17(6), 726. https://doi.org/10.3390/polym17060726





