Structural Characterization of Dendrobium officinale Polysaccharides and Their Regulation Effect on Intestinal Microbiota During In Vitro Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction and Purification of DOP
2.3. Molecular Weight Determination by GPC
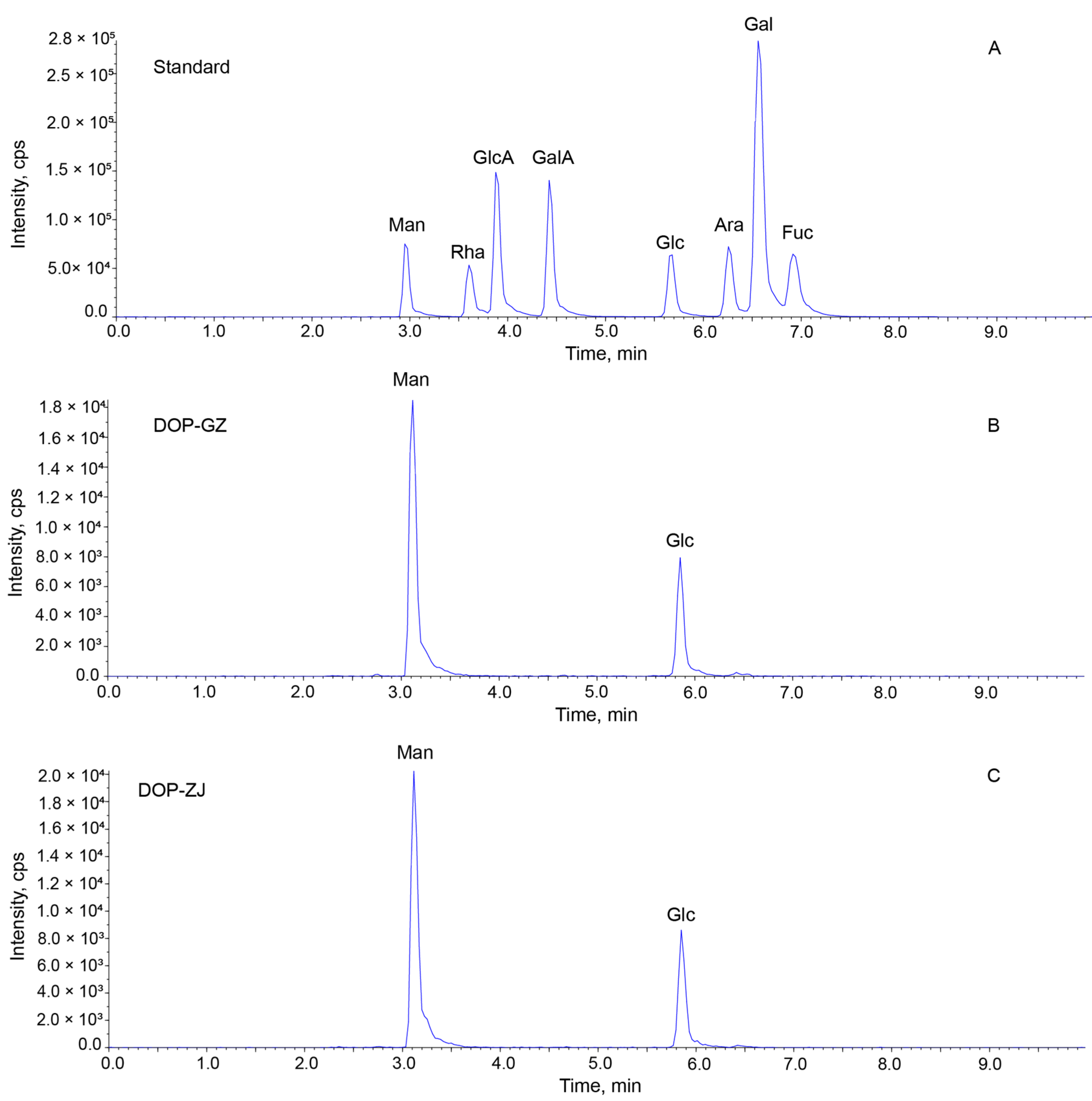
2.4. Monosaccharide Composition Analysis
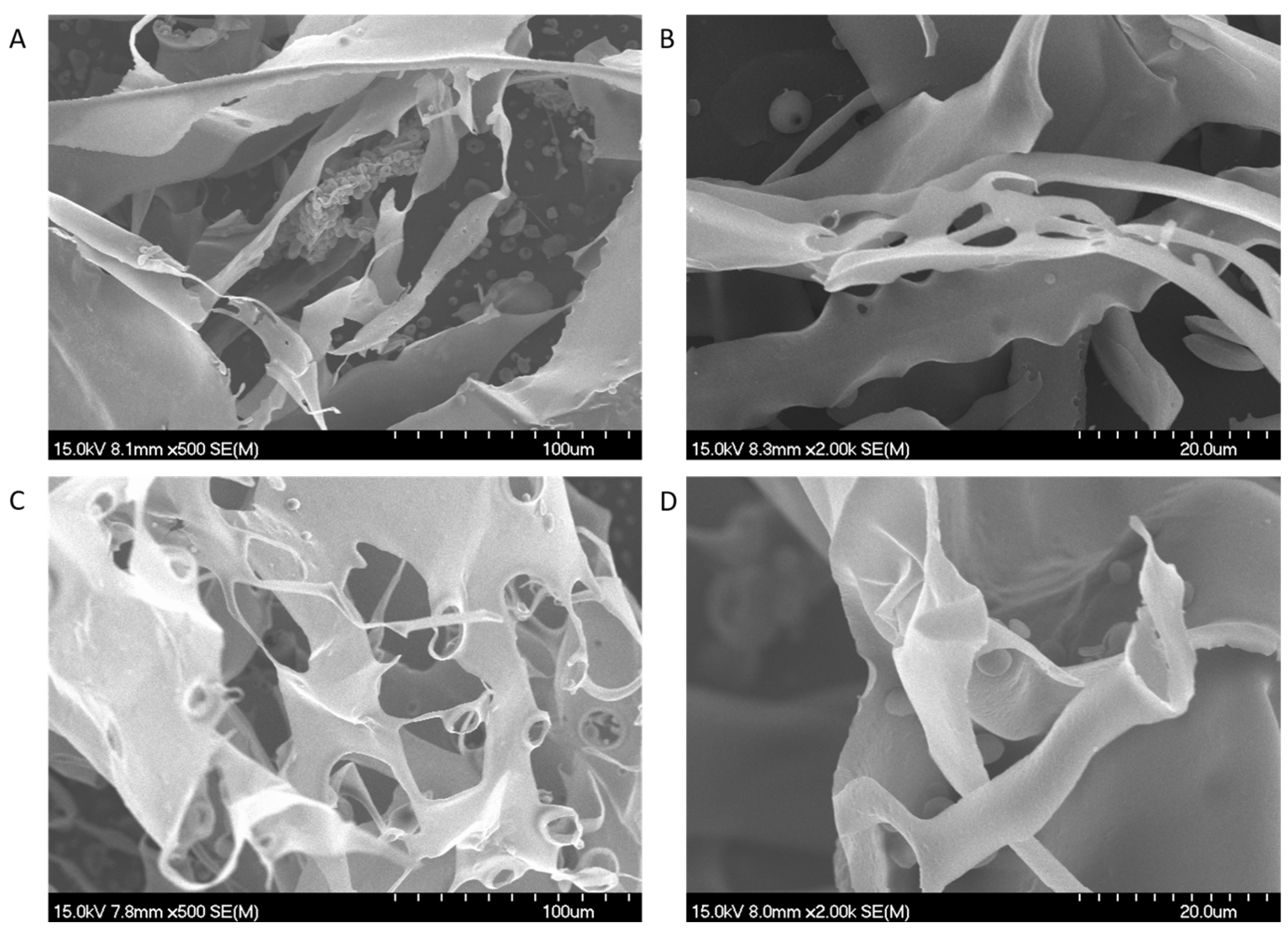
2.5. Scanning Electron Microscope (SEM) Analysis
2.6. Fourier Transform-Infrared (FT-IR) Analysis
2.7. Glycosidic Linkage Analysis by Methylation
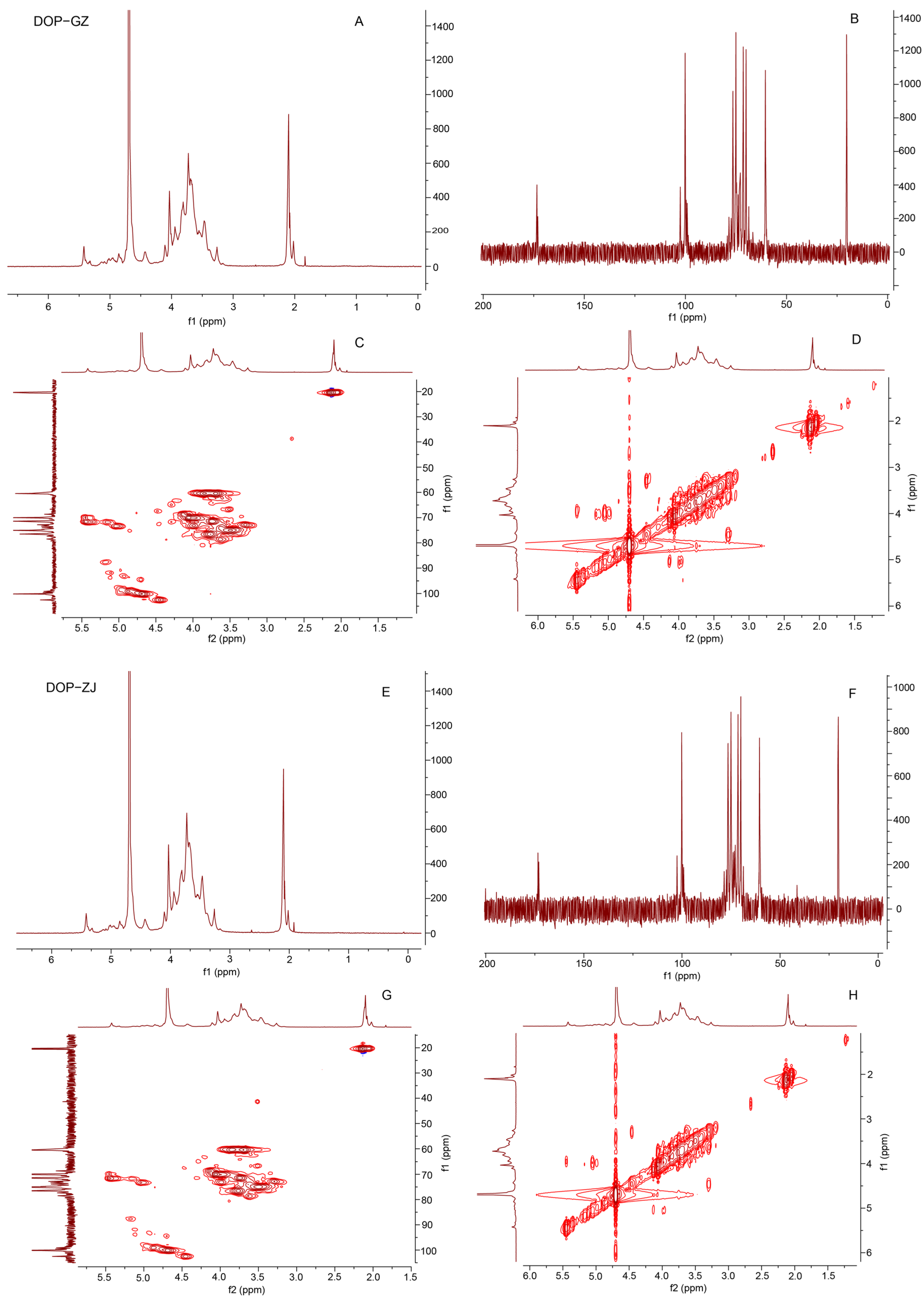
2.8. One-Dimensional and Two-Dimensional NMR Spectroscopy Analysis
2.9. α-Glucosidase Inhibition Assay
2.10. Gut Microbiota Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Purification of DOP
3.2. Molecular Weight Determination and Monosaccharide Composition Analysis
3.3. SEM Analysis
3.4. FT-IR Spectroscopy
3.5. Glycosidic Linkage by Methylation Analysis
3.6. NMR Analysis
3.7. Inhibitory Effects of DOPs on α-Glucosidase
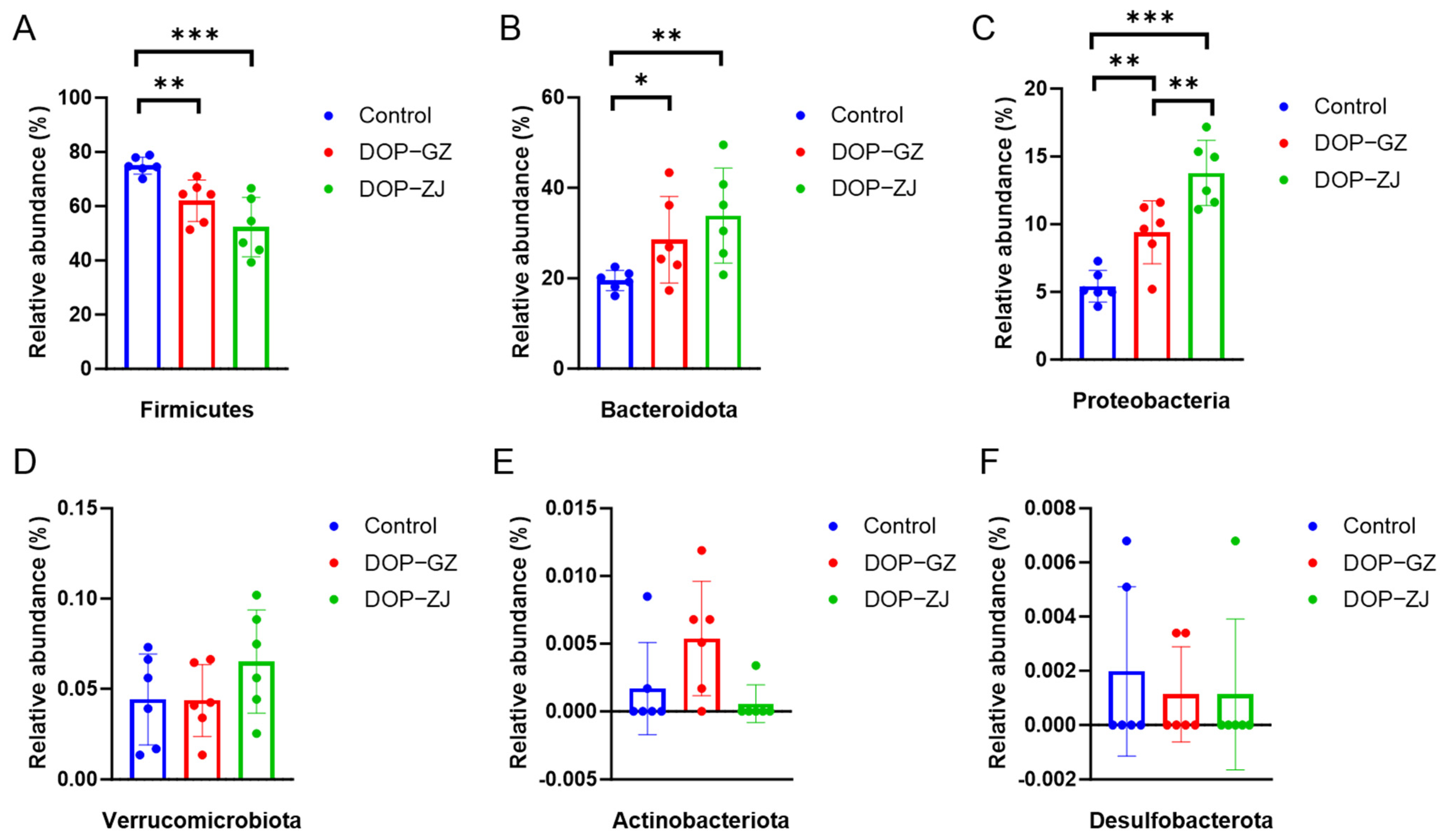
3.8. Gut Microbial Analysis
3.9. Structure-Activity Relationships
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, J.; Dang, P.; Zhao, Z.; Yuan, L.; Zhou, Z.; Wolf, D.; Luo, Y. An assessment of the Chinese medicinal Dendrobium industry: Supply, demand and sustainability. J. Ethnopharmacol. 2018, 229, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zuo, J.; Huang, Y.; Li, C.; Chen, Y. The origin and germplasm collection for cultivated Dendrobium officinale K. Kimura & Migo individuals revealed by EST-SSR markers, Genet. Resour. Crop. Evol. 2020, 67, 1209–1219. [Google Scholar]
- Xu, L.; Zeng, X.; Liu, Y.; Wu, Z.; Zheng, X.; Zhang, X. Inhibitory effect of Dendrobium officinale polysaccharide on oxidative damage of glial cells in aging mice by regulating gut microbiota. Int. J. Biol. Macromol. 2023, 247, 125787. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, Y.; Yuan, H.; Yang, Y.; Xiong, Q.; Liang, C.; Li, Z.; Li, C.; Zhang, G.; Lai, X.; et al. Dendrobium officinale polysaccharides attenuate learning and memory disabilities via anti-oxidant and anti-inflammatory actions. Int. J. Biol. Macromol. 2019, 126, 414–426. [Google Scholar] [CrossRef]
- Sun, S.; Deng, P.; Peng, C.; Ji, H.; Mao, L.; Peng, L. Extraction, structure and immunoregulatory activity of low molecular weight polysaccharide from Dendrobium officinale. Polymers 2022, 14, 2899. [Google Scholar] [CrossRef]
- Cai, M.; Zhu, H.; Xu, L.; Wang, J.; Xu, J.; Li, Z.; Yang, K.; Wu, J.; Sun, P. Structure, anti-fatigue activity and regulation on gut microflora in vivo of ethanol-fractional polysaccharides from Dendrobium officinale. Int. J. Biol. Macromol. 2023, 234, 123572. [Google Scholar] [CrossRef]
- He, Y.; Li, L.; Chang, H.; Cai, B.; Gao, H.; Chen, G.; Hou, W.; Jappar, Z.; Yan, Y. Research progress on extraction, purification, structure and biological activity of Dendrobium officinale polysaccharides. Front. Nutr. 2022, 9, 965073. [Google Scholar] [CrossRef]
- He, L.; Yan, X.; Liang, J.; Li, S.; He, H.; Xiong, Q.; Lai, X.; Hou, S.; Huang, S. Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohyd. Polym. 2018, 198, 101–108. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Nilsson, P.M. The metabolic syndrome: A glance at its history. J. Hypertens. 2006, 24, 621–626. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, M.; Meng, J.; Wang, L.; Chen, M. A review of the interaction between diet composition and gut microbiota and its impact on associated disease. J. Future Foods 2024, 4, 221–232. [Google Scholar] [CrossRef]
- Li, L.; Yao, H.; Li, X.; Zhang, Q.; Wu, X.; Wong, T.; Zheng, H.; Fung, H.; Yang, B.; Ma, D.; et al. Destiny of Dendrobium officinale polysaccharide after oral administration: Indigestible and nonabsorbing, ends in modulating gut microbiota. J. Agric. Food Chem. 2019, 67, 5968–5977. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ji, H.; Fan, K.; Xu, H.; Huang, Y.; Zheng, Y.; Xu, Q.; Li, C.; Zhao, L.; Li, Y.; et al. Targeting gut microbiota and host metabolism with Dendrobium officinale dietary fiber to prevent obesity and improve glucose homeostasis in diet-induced obese mice. Mol. Nutr. Food Res. 2022, 66, e2100772. [Google Scholar] [CrossRef] [PubMed]
- Bourdeau-Julien, I.; Castonguay-Paradis, S.; Rochefort, G.; Perron, J.; Lamarche, B.; Flamand, N.; Di Marzo, V.; Eilleux, A.; Raymond, F. The diet rapidly and differentially affects the gut microbiota and host lipid mediators in a healthy population. Microbiome 2023, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Li, J.; Yang, X.; Yang, L.; Xu, J.; Yan, S.; Lv, Y.; Ren, F.; Hu, J.; Zhou, J. Structural characterization and hypoglycemic effect via stimulating glucagon-like peptide-1 secretion of two polysaccharides from Dendrobium officinale. Carbohyd. Polym. 2020, 241, 116326. [Google Scholar] [CrossRef]
- Wong, T.L.; Li, L.F.; Zhang, J.X.; Zhang, Q.W.; Zhang, X.T.; Zhou, L.S.; Fung, H.Y.; Feng, L.; Cheng, H.Y.; Huo, C.Y.; et al. Oligosaccharide analysis of the backbone structure of the characteristic polysaccharide of Dendrobium officinale. Food Hydrocolloid. 2023, 134, 108038. [Google Scholar] [CrossRef]
- Lai, C.H.; Huo, C.Y.; Xu, J.; Han, Q.B.; Li, L.F. Critical review on the research of chemical structure, bioactivities, and mechanism of actions of Dendrobium officinale polysaccharide. Int. J. Biol. Macromol. 2024, 263, 130315. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma strum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef]
- Xu, J.; Guan, J.; Chen, X.J.; Zhao, J.; Li, S.P. Comparison of polysaccharides from different dendrobium using saccharide mapping. J. Pharm. Biomed. Anal. 2011, 55, 977–983. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Wang, Y.; Liu, X.; Ren, X.; Dong, Y.; Ma, J.; Song, R.; Wei, J.; Yu, A.; et al. A systematic review on polysaccharides from Dendrobium genus: Recent advances in the preparation, structural characterization, bioactive molecular mechanisms, and applications. Am. J. Chin. Med. 2022, 50, 471–509. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; Zhou, Z.M.; Ye, Y.C.; Zhou, Y.; D’Cruze, N.C.; Macdonald, D.W. What is “TCM”? A conservation-relevant taxonomy of traditional Chinese medicine. Glob. Ecol. Conserv. 2021, 32, e01905. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, B.; Du, A.Q.; Zhou, Z.Y.; Lu, D.Z.; Zhu, Z.H.; Ke, S.Z.; Wang, S.J.; Yu, Y.L.; Chen, J.W.; et al. Saccharina japonica fucan suppresses high fat diet-induced obesity and enriches fucoidan-degrading gut bacteria. Carbohyd. Polym. 2022, 290, 119411. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lu, D.; Wang, H.; Zhou, Z.; Luo, X.; Ma, M.; Ke, S.; Wang, H.; Yu, Y.; Wei, B. Metagenomic insights into the anti-obesity effect of a polysaccharide from Saccharina japonica. Foods 2023, 12, 665. [Google Scholar] [CrossRef] [PubMed]
- McKee, L.S. Measuring enzyme kinetics of glycoside hydrolases using the 3,5-dinitrosalicylic acid assay. In Protein-Carbohydrate Interactions: Methods in Molecular Biology; Abbott, D., Lammerts van Bueren, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; p. 1588. [Google Scholar]
- Xu, G.; Amicucci, M.J.; Cheng, Z.; Galermo, A.G.; Lebrilla, C.B. Revisiting monosaccharide analysis-quantitation of a comprehensive set of monosaccharides using dynamic multiple reaction monitoring. Analyst 2018, 143, 200. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Yoshimoto, C.; Osajima, K.; Oki, T.; Osajima, Y. In vitro survey of α-glucosidase inhibitory food components. Biosci. Biotechnol. Biochem. 1996, 60, 2019–2022. [Google Scholar] [CrossRef]
- Dreher, T.W.; Hawthorne, D.B.; Grant, B.R. Comparison of open-column and high-performance gel permeation chromatography in the separation and molecular-weight estimation of polysaccharides. J. Chromatogr. A 1979, 174, 443–446. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food. Sci. F 2016, 15, 237–250. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.; Wang, L.; Wang, X.; Li, Z.; Gao, M.; Yang, Y.; Wang, Q.; et al. Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: A review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, Y.; Wu, Z.; Peng, X.; Yan, M.; Chen, S.; Lv, G. Ultrasound-assisted enzyme extraction of Dendrobium officinale polysaccharides: Extraction process, characterization, immunomodulatory effects. Ultrason. Sonochem. 2025, 114, 107248. [Google Scholar] [CrossRef]
- Xing, X.; Cui, S.W.; Nie, S.; Phillips, G.O.; Goff, H.D.; Wang, Q. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan): Part I. Extraction, purification, and partial structural characterization. Bioact. Carbohydr. Diet. Fibre 2014, 4, 74–83. [Google Scholar] [CrossRef]
- Needs, P.W.; Selvendran, R.R. Avoiding oxidative degradation during sodium hydroxide/methyl iodide-mediated carbohydrate methylation in dimethyl sulfoxide. Carbohyd. Res. 1993, 245, 1–10. [Google Scholar] [CrossRef]
- He, T.B.; Huang, Y.P.; Yang, L.; Liu, T.T.; Gong, W.Y.; Wang, X.J.; Sheng, J.; Hu, J.M. Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 83, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, X.; Wang, K.; Cao, X. Structural characterization and bioactivity of polysaccharides isolated from fermented Dendrobium officinale. J. Sci. Food. Agric. 2022, 102, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, Y.; Wang, Q.; Zheng, B.; Miao, S.; Lu, X. Structural characterization and in vitro analysis of the prebiotic activity of oligosaccharides from lotus (Nelumbo nucifera Gaertn.) seeds. Food Chem. 2022, 388, 133045. [Google Scholar] [CrossRef] [PubMed]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert. Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef]
- Fang, Q.; Hu, J.; Nie, Q.; Nie, S. Effects of polysaccharides on glycometabolism based on gut microbiota alteration. Trends Food. Sci. Tech. 2019, 92, 65–70. [Google Scholar] [CrossRef]
- Malard, F.; Vekhoff, A.; Lapusan, S.; Isnard, F.; D’incan-Corda, E.; Rey, J.; Saillard, C.; Thomas, X.; Ducastelle-Lepretre, S.; Paubelle, E.; et al. Gut microbiota diversity after autologous fecal microbiota transfer in acute myeloid leukemia patients. Nat. Commun. 2021, 12, 3084. [Google Scholar] [CrossRef]
- Wilmanski, T.; Rappaport, N.; Earls, J.C.; Magis, A.T.; Manor, O.; Lovejoy, J.; Omenn, G.S.; Hood, L.; Gibbons, S.M.; Price, N.D. Blood metabolome predicts gut microbiome α-diversity in human. Nat. Commun. 2019, 37, 1217–1228. [Google Scholar] [CrossRef]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Jenior, M.L.; Keenan, O.; Hart, J.L.; Specker, J.; Abbas, A.; Rangel, P.C.; Di, C.; Green, J.; Bustin, K.A.; et al. Enterococci enhance Clostridioides difficile pathogenesis. Nature 2022, 611, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae family in the gut microbiota: Diversity, metabolism, and function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, Z.; Zhao, Z.; Zhang, D.; Zhang, Q.; Zhang, J.; Fang, Z.; Bai, Y.; Guo, X. Structure, health benefits, mechanisms, and gut microbiota of dendrobium officinale polysaccharides: A review. Nutrients 2023, 15, 4901. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Yin, J.; Nie, S. Multiomics approach to explore the amelioration mechanisms of glucomannans on the metabolic disorder of type 2 diabetic rats. J. Agric. Food Chem. 2021, 69, 2632–2645. [Google Scholar] [CrossRef]
- Yu, C.; Ahmadi, S.; Shen, S.; Wu, D.; Xiao, H.; Ding, T.; Liu, D.; Ye, X.; Chen, S. Structure and fermentation characteristics of five polysaccharides sequentially extracted from sugar beet pulp by different methods. Food Hydrocoll. 2022, 126, 107462. [Google Scholar] [CrossRef]
- Liu, Q.; An, X.; Chen, Y.; Deng, Y.; Niu, H.; Ma, R.; Zhao, H.; Cao, W.; Wang, X.; Wang, M. Effect of Auricularia auricula polysaccharides on gut microbiota and metabolic phenotype in mice. Foods 2022, 11, 2700. [Google Scholar] [CrossRef]


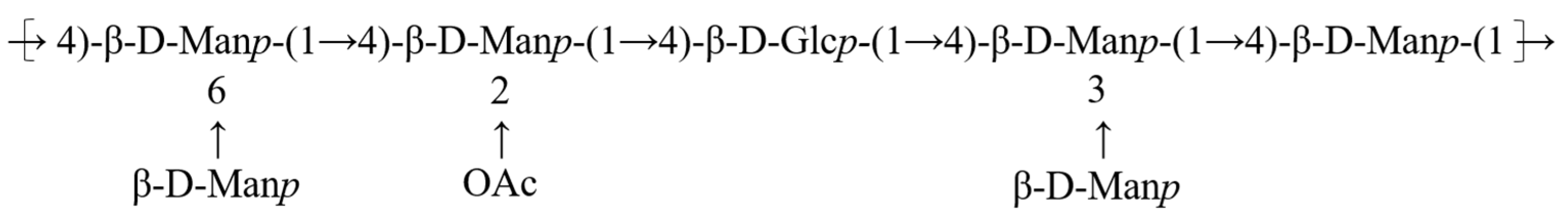


| Sample | Purity (%) | Protein (%) | Starch (%) | Uronic Acid (%) | Mw (kDa) | Monosaccharide (Molar Ratio) | |
|---|---|---|---|---|---|---|---|
| Man | Glc | ||||||
| DOP-GZ | 91.3 | 1.45 | 0.21 | 2.31 | 356 | 2.51 | 1 |
| DOP-ZJ | 89.4 | 1.54 | 0.40 | 2.44 | 544 | 2.66 | 1 |
| Reside Linkage | Retention Time (min) | Detected Mass Fraction (m/z) | Relative Content (%) | |
|---|---|---|---|---|
| DOP-GZ | DOP-ZJ | |||
| Terminal-Manp | 18.87 | 43, 57, 71, 87, 101, 117, 129, 145, 161, 191, 205 | 11.5 | 8.6 |
| 1,4-linked Manp | 20.64 | 43, 45,71, 87, 99, 101, 113, 117, 129, 131, 143, 161, 173, 203, 233 | 61.0 | 56.5 |
| 1,4-linked Glcp | 20.75 | 43, 45, 71, 87, 99, 101, 113, 117, 129, 131, 143, 161, 173, 203, 233 | 16.7 | 19.1 |
| 3,4-linked Manp | 21.86 | 43, 58, 87, 97, 117, 129, 149, 172, 185, 203, 231, 305 | 1.0 | 2.6 |
| 4,6-linked Manp | 22.00 | 43, 71, 85, 101, 117, 127, 142, 161, 171, 187, 201, 231, 261 | 9.8 | 13.2 |
| Glycosyl Residue (DOP-GZ) | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 |
|---|---|---|---|---|---|---|
| →4)-β-Glcp-(1→ | 4.45/102.50 | 3.28/72.89 | 3.29/72.85 | 3.64/78.60 | 3.49/75.01 | 3.85/60.43 |
| →4)-β-Manp-(1→ | 4.69/100.21 | 4.06/70.05 | 3.75/71.45 | 3.76/76.59 | 3.49/75.01 | 3.69/60.46 |
| →4)-2-O-acetyl-β-Manp-(1→ | 4.88/99.13 | 5.45/71.59 | 3.95/73.19 | 3.79/76.66 | 3.57/74.85 | 3.80/60.25 |
| →4,6)-β-Man-(1→ | 4.93/93.27 | 3.85/70.48 | 3.51/66.70 | 3.42/69.87 | 3.89/71.02 | 3.60/65.21 |
| →4,3)-β-Man-(1→ | 4.96/98.48 | 3.99/71.69 | 3.98/73.17 | 3.79/75.28 | 3.56/74.00 | 3.62/60.21 |
| Glycosyl residue (DOP-ZJ) | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 |
| →4)-β-Glcp-(1→ | 4.45/102.55 | 3.28/72.89 | 3.29/72.83 | 3.63/78.70 | 3.49/75.00 | 3.85/60.43 |
| →4)-β-Manp-(1→ | 4.69/100.14 | 4.06/69.98 | 3.75/71.46 | 3.76/76.57 | 3.51/75.00 | 3.69/60.45 |
| →4)-2-O-acetyl-β-Manp-(1→ | 4.88/99.11 | 5.44/71.67 | 3.96/73.20 | 3.75/76.59 | 3.53/75.00 | 3.80/60.12 |
| →4,6)-β-Man-(1→ | 4.93/93.27 | 3.86/70.50 | 3.52/66.78 | 3.42/70.01 | 3.90/71.01 | 3.60/65.31 |
| →4,3)-β-Man-(1→ | 4.99/99.56 | 3.99/71.69 | 3.98/73.17 | 3.80/75.00 | 3.57/73.91 | 3.60/60.52 |
| Groups | Average Inhibition Rate (%) |
|---|---|
| Acarbose | 98.2 ± 1.0 |
| DOP-GZ | 41.5 ± 2.4 |
| DOP-ZJ | 57.3 ±5.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, H.; Jin, X.; Huang, W.; Zhao, Y.; Wang, N.; Lu, D.; Wei, B.; Wang, H. Structural Characterization of Dendrobium officinale Polysaccharides and Their Regulation Effect on Intestinal Microbiota During In Vitro Fermentation. Polymers 2025, 17, 727. https://doi.org/10.3390/polym17060727
Yu Y, Wang H, Jin X, Huang W, Zhao Y, Wang N, Lu D, Wei B, Wang H. Structural Characterization of Dendrobium officinale Polysaccharides and Their Regulation Effect on Intestinal Microbiota During In Vitro Fermentation. Polymers. 2025; 17(6):727. https://doi.org/10.3390/polym17060727
Chicago/Turabian StyleYu, Yanlei, Honggang Wang, Xiaoshu Jin, Wenjing Huang, Yunjie Zhao, Ningning Wang, Dongze Lu, Bin Wei, and Hong Wang. 2025. "Structural Characterization of Dendrobium officinale Polysaccharides and Their Regulation Effect on Intestinal Microbiota During In Vitro Fermentation" Polymers 17, no. 6: 727. https://doi.org/10.3390/polym17060727
APA StyleYu, Y., Wang, H., Jin, X., Huang, W., Zhao, Y., Wang, N., Lu, D., Wei, B., & Wang, H. (2025). Structural Characterization of Dendrobium officinale Polysaccharides and Their Regulation Effect on Intestinal Microbiota During In Vitro Fermentation. Polymers, 17(6), 727. https://doi.org/10.3390/polym17060727








