1. Introduction
Metals and metalloids are ubiquitous and non-biodegradable elements present in all areas of the environment, having entered through either natural or anthropogenic pathways [
1,
2,
3]. Because only a few metals in the periodic table are essential for living organisms, and their concentration is decisive in determining their toxicity, it is very important to carefully monitor these and apply decontamination measures if the maximum permitted levels are exceeded [
4,
5]. Moreover, in the paradigm of the circular bioeconomy, valuable metals can be recovered from industrial effluents and reused [
6]. In these contexts, analytical methodologies are continuously improved to ensure their high sensitivity and selectivity for metal determination [
7], and, in the water treatment field, the facilities for the removal of metal ions are continuously technologically enhanced. As part of these efforts, in the last decades, the concept of polymer liquid membranes has been proposed as an alternative to classical solvent extraction, ion exchange, adsorption, or precipitation techniques [
8,
9].
Polymer inclusion membranes (PIMs) are a type of liquid membrane designed to eliminate several disadvantages such as inadequate mechanical stability and the risk of carrier leakage. PIMs are designed by embedding the liquid phase in a solid matrix, which increases the stability of the membrane and extends its lifetime, improving its viability for industrial applications [
10]. The production of Solvent Polymeric Membranes (SPMs) was first reported by Bloch et al. [
11]. These were prepared by pouring a carrier polymer on paper, which enhanced its mechanical and diffusion properties. Later, Sugiura [
12] upgraded this material by including a plasticizer in the membrane composition, which improved the mechanical strength properties of the polymer film, meaning paper was no longer necessary [
13]. PIMs are characterized by their ability to facilitate the selective transport of metal ions through a polymeric matrix, which can be tailored by incorporating various carriers and plasticizers. Research has shown that, compared to traditional solvent extraction methods, PIMs containing ionic liquids or surfactants as carriers exhibit enhanced selectivity for specific metal ions [
14]. PIMs consist of a base polymer that offers structure and mechanical support to the membrane, an extractant (carrier), and, if required, a plasticizer or modifier [
15]. To obtain homogeneous and flexible PIMs, the base polymers’ and carriers’ compatibility is vital, and these should be soluble in the same solvent. The solvent’s nature and quantity can influence PIMs’ performance and quality by affecting the dispersion of the carriers and base polymers [
10].
PIMs represent an important advance in membrane separation technology, mainly for metal separation. PIMs have transformed traditional separation techniques through their outstanding selectivity, cost-effectiveness, and versatility and by introducing the possibility of simultaneously performing extraction and stripping in one operation [
9]. Thus, PIMs are positioned as a sustainable choice for metal separation in analytical chemistry, water treatment, and metal recovery due to their selectivity, recyclability, and ease of use. Despite the advantages of PIMs, their production involves the use of base polymers such as poly (vinyl chloride) (PVC), cellulose triacetate (CTA), or poly (vinylidene fluoride-co-hexafluoropropylene) (PVDF HFP), which are known to be non-biodegradable and are converted into micro- and nanoplastics, causing environmental issues. Moreover, the typical casting method for preparing PIMs involves dissolving all components (base polymer, carrier, and plasticizer) into a volume of a suitable solvent such as tetrahydrofuran, chloroform, or dichloromethane. This mixture is then cast into a mold, and the solvent evaporates slowly [
16], which may lead to environmental and health issues. Consequently, it is of great interest to replace the classical chemicals used in PIM preparation with sustainable and greener alternatives. Since several previous reviews presented PIMs’ functioning principles, components, and applications [
6,
8,
9,
10,
15,
17,
18,
19], this paper aimed to present a thorough overview of the recent research on the advances in PIM applications related to the aqueous solutions for metal separation, with a special focus on sustainable and environmentally friendly materials and methods used to produce PIMs.
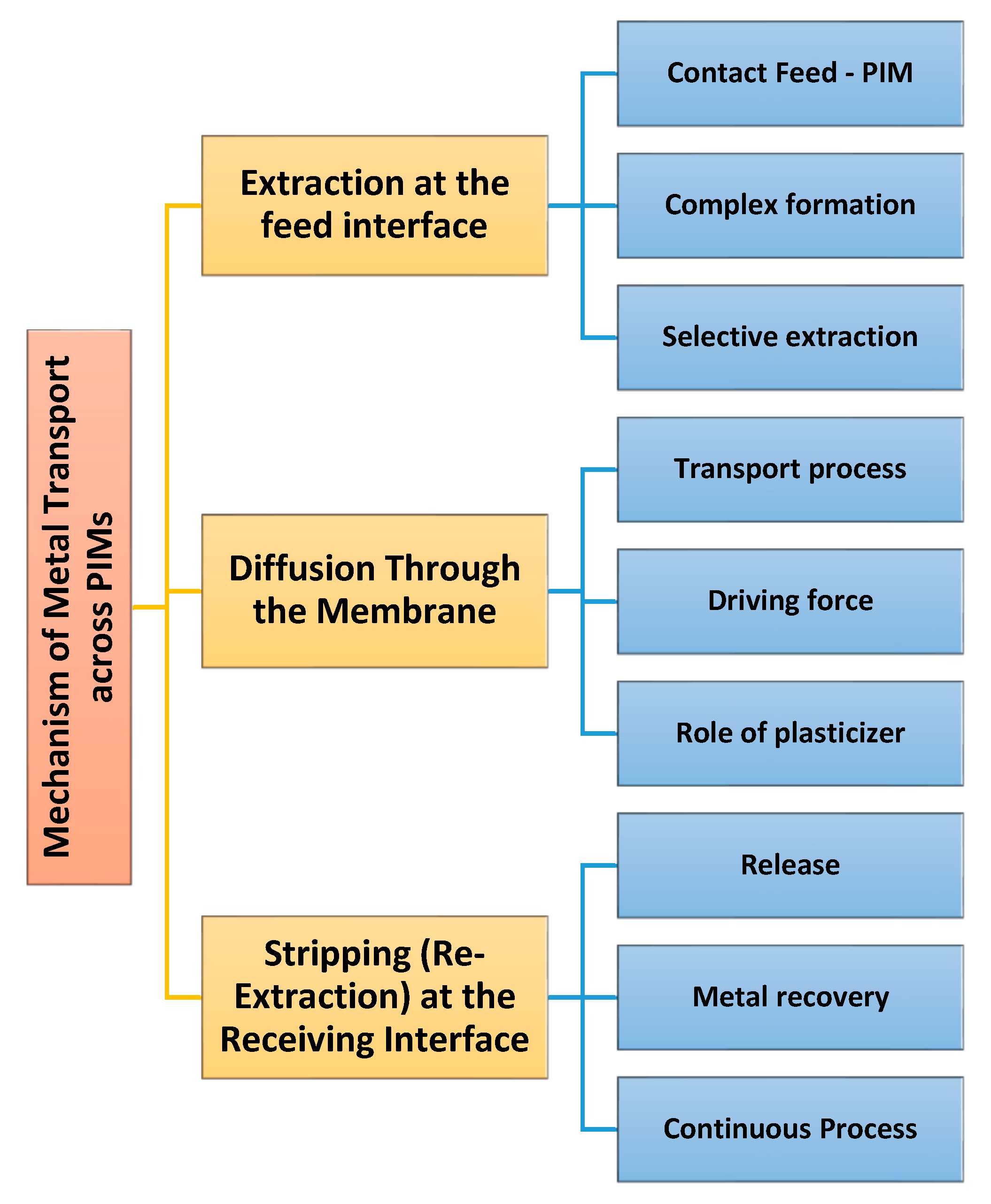
3. Functioning Principle of Polymer Inclusion Membranes
As stated in the Introduction, the components of PIMs are a base polymer, a carrier, and a plasticizer/modifier. Understanding the functioning principles and transport mechanisms for PIMs is a key element of appraising their efficiency in selective extraction processes. The principal transport mechanism for metals through PIMs is based on carrier-mediated diffusion [
9]. The mechanism of metal ions passing through a PIM usually occurs via the steps shown in
Figure 1.
Typically, PIMs have been used to separate two aqueous media, whereas ions were transported through the membrane. As presented in
Figure 1, the transport of metal ions across the PIM usually occurs via three main steps [
6,
10]. The first step consists of the extraction of metal ions at the feed interface. Therefore, the PIM is placed in contact with a feed solution containing the metal ions to be extracted. The carrier molecules interact with it and bind the target ions, making a metal–carrier complex at the feed solution–membrane interface. The selectivity depends on the chemical affinity of the carrier, and thus only specific metal ions are extracted into the membrane phase [
6]. The second step is represented by the diffusion through the membrane. The developed metal–carrier complexes diffuse through the polymer matrix due to a concentration gradient between the feed solution and the receiving side of the membrane. In general, this transport follows Fick’s law of diffusion [
9,
20], represented in Equation (1):
where
J is the flux,
D is the metal ion diffusion coefficient across the membrane, and
is the gradient of concentration. However, this model does not satisfactorily account for the effect of carriers in the transport through PIMs, since these may increase the transport efficiency. Thus, mathematical models analogous to those related to the reaction kinetics have been developed to define the transport across membrane systems considering the experimental data of metal ions transported by several carriers, and the volume of the feed and stripping solutions [
21].
Generally, the plasticizer improves the fluidity of the membrane and assists in the faster and more efficient diffusion of the metal–carrier complexes. In the third step, the metal ions are stripped (re-released) at the receiving interface. Upon arrival at the interface with the receiving solution, physicochemical conditions such as pH, ionic strength, or the existence of a stripping agent aid the dissociation of the metal–carrier complexes. The metal ions are released into the receiving phase, and the carrier is regenerated inside the membrane; thus, it can participate in a new extraction process [
18]. This operation is cyclic and enables the continuous extraction and separation of metals and their preconcentration [
22].
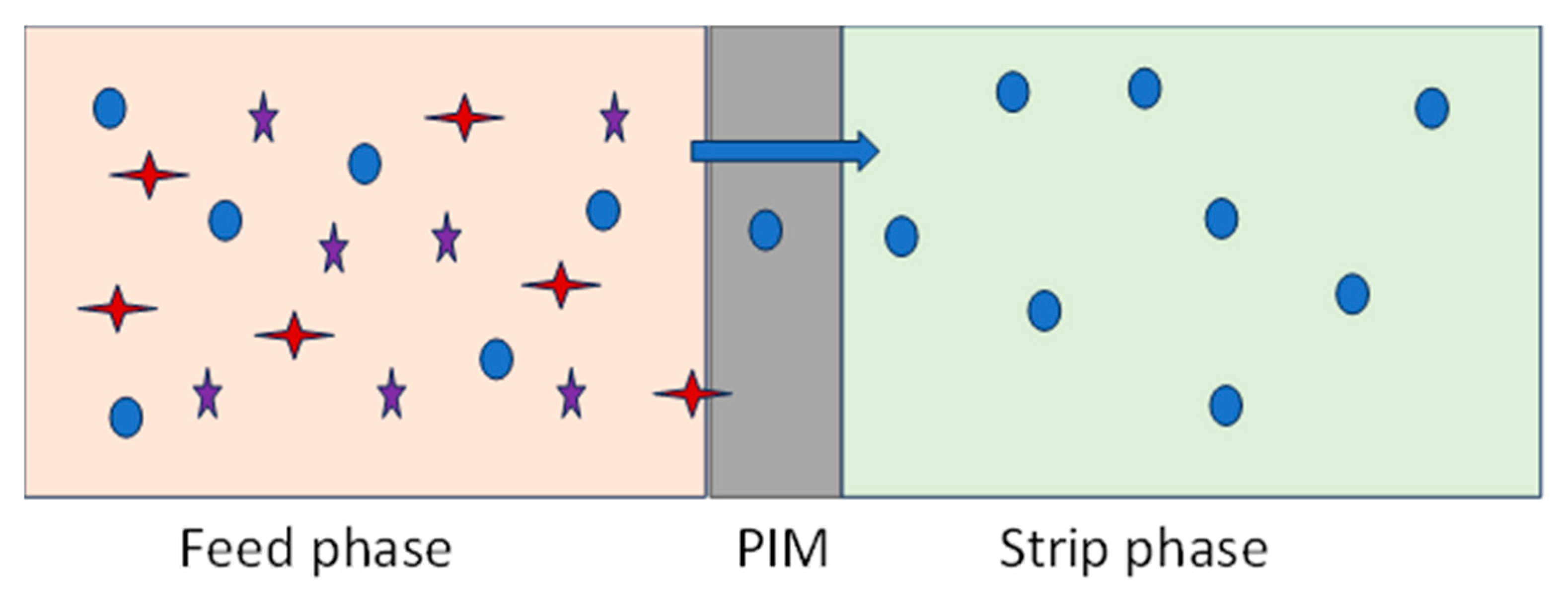
Very importantly, the transport of the analyte through the PIM occurs even if the analyte concentration in the receiving phase becomes higher than in the feed phase, explaining its potential use for analyte preconcentration. An example of the transport process of a bivalent metal Me(II) through a PIM containing an acidic diprotic carrier (H2L) and a mineral acid as a stripping reagent is schematically illustrated in Equation (2) and
Figure 2 [
23].
The carrier accomplishes the metal ion extraction from the aqueous media and their transfer inside the membrane assembly. Therefore, the carrier characteristics are significant, since they affect the process of the metal’s transport. Depending on the carrier class, metal ions are transported through the membrane in several ways: (
i) simple transport due to metal solubility in the liquid membrane; (
ii) supported transport caused by partitioning, complexation, or diffusion reaction; (
iii) counter-transport, produced by the concentration gradient; or (
iv) co-transport, which implies that a liquid substance is co-transported with an associated component in a process that ends when the concentrations between the receiving phase and the feeding phase are equal [
6].
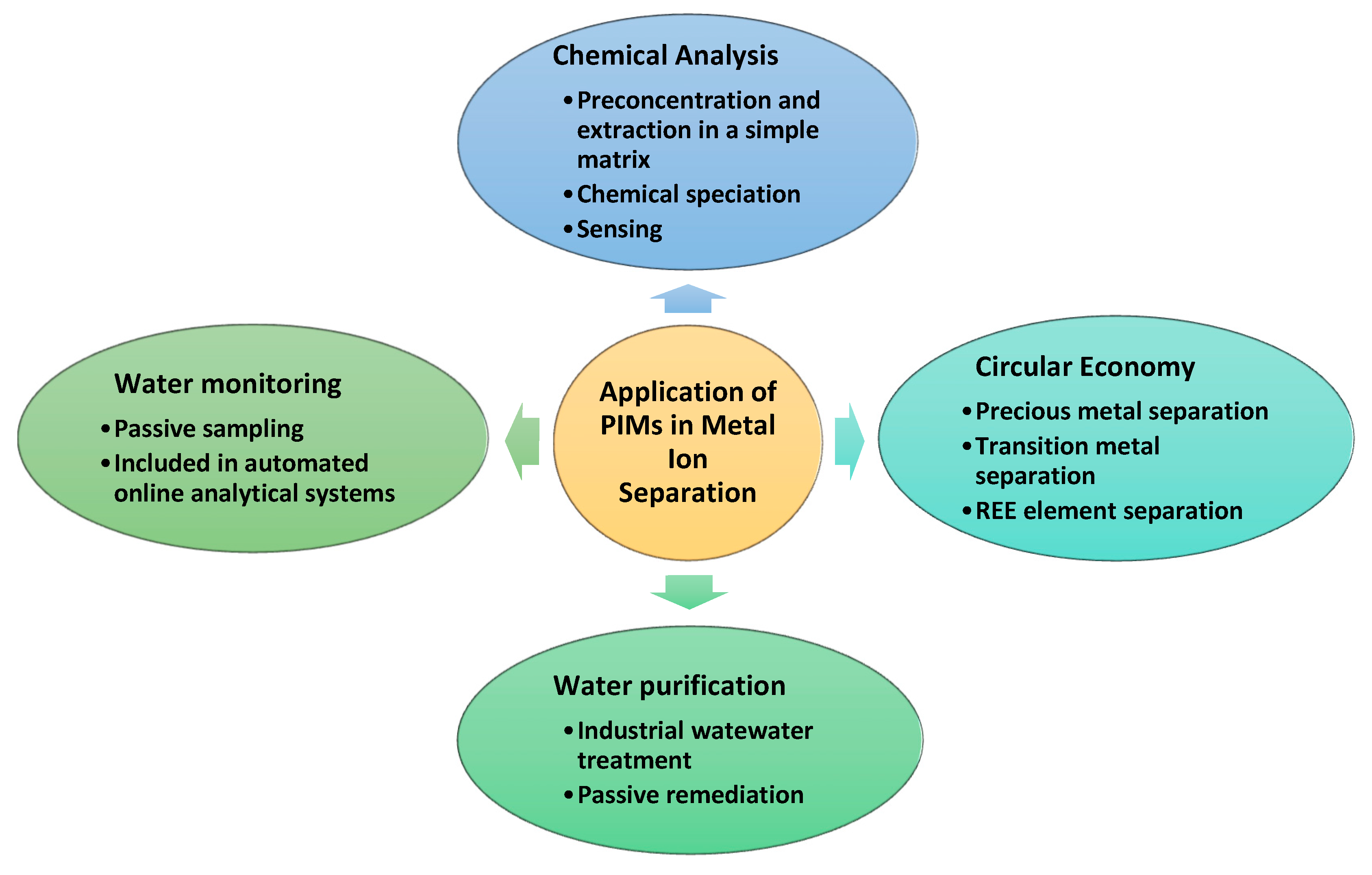
Figure 3 presents a simple representation of the selective separation of a metal ion species from the feed solution, which contains a complex matrix of other chemical species, to the strip phase, through a PIM at their interface [
6,
22].
Most frequently, the metal flux is calculated based on equations derived from Fick’s first law, considering that the concentration of the complex is negligible at the membrane-receiving phase interface [
24]. The flux
J can be correlated with the variations in metal ion concentration, the volume (
V) of the feed solution, and the area (
A) of the exposed membrane (cm
2), using Equation (3) [
25]:
The integrated flux can be accounted for using Equation (4):
where
C is the metal ion concentration (mg/L) in the donor solution at time
t,
C0 is the initial metal ion concentration (mg/L),
k represents the rate constant (s
−1), and
t represents the extraction time (s) [
25].
The permeability coefficient (
P) (cm s
−1) can be calculated using Equation (5) [
26]:
Using the permeability coefficient, the initial flux (
Ji) can be calculated (Equation (6)):
Nevertheless, in some cases, the experimental results show that the dependency of ln(c/c0) versus time is not always linear, and some authors recommend modified equations to explain the transport of metal ions. For instance, Szczepański [
27] used two kinetic models to improve the description of the transport kinetics, which were fitted to the experimental results of Zn(II), Cd(II), Cu(II), and Pb(II) transport through PIMs produced from CTA (base polymer), D2EHPA (carrier), and NPOE (plasticizer). The proposed model, based on an equation such as the first-order chemical reaction equation with equilibrium, provided an improved nonlinear fit to the experimental data and more accurately estimated values of the initial maximum fluxes and permeation coefficients. In a later study, Szczepański [
28] compared five mathematical models to describe the transport of Zn(II), Cd(II), Pb(II), and Cu(II) in PIMs prepared with different carriers (tri-n-octylphosphine oxide (TOPO), trihexyl(tetradecyl)phosphonium chloride (Cyphos IL 101), di-(2-ethylhexyl) phosphoric acid (D2EHPA), methyl trioctyl ammonium chloride (Aliquat336), and 3-(1,3-diethoxy-1,3-dioxopropan-2-yl)-1-octylimidazolium bromide (RILC8_Br)). These carriers were mixed in different ratios with CTA (base polymer) and NPOE (plasticizer). A comparison of the initial maximum transport fluxes of Zn(II), Cd(II), Pb(II), and Cu(II) obtained with different carriers is presented in
Table 1 [
21]. As can be observed, the carrier type strongly influences the initial maximum fluxes.
Temperature is a parameter affecting the transport process. The activation energy of the transport process can be calculated at various temperatures using Equation (7) [
26]:
where
k is the reaction rate constant,
A is the pre-exponential factor,
Ea represents the activation energy,
R is the gas constant, and
T is the absolute temperature. The activation energy calculation is important to assess if the transport of a metal ion is a process controlled by diffusion.
In the context of PIM studies, it is important to ascertain the occurrence of interactions at the interfaces and to determine whether these interactions are regular or not in terms of thermodynamics. Consequently, in addition to calculating the activation energy, the thermodynamic parameters, namely the activation entropy change (ΔS
#) and activation enthalpy change (ΔH
#), should be calculated using the Eyring equation (Equation (8)):
where
T is the absolute temperature,
k is the reaction rate constant,
R means the gas constant,
h is Planck’s constant, and
kB is the Boltzmann constant.
ΔS# and
ΔH# can be established from the kinetic dataset achieved from a ln
k/
T against 1/
T plot. [
26,
29].
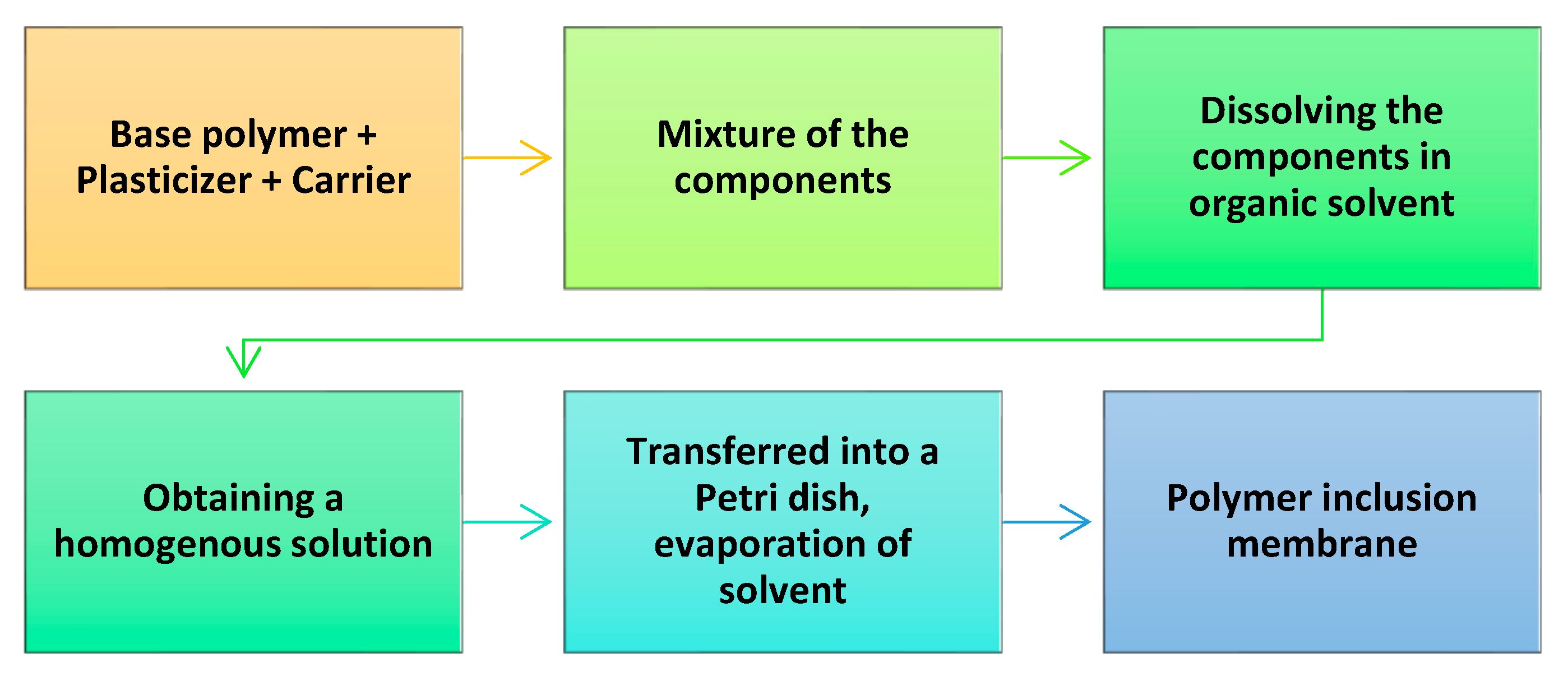
6. Components of Polymer Inclusion Membranes Obtained from Sustainable Sources
In order to ensure the efficient transport of metals from the feed solution, the PIMs should have several components that provide them stability, flexibility, and selectivity in extracting the target ions. Each of these main properties is provided by specific components, which need to be mixed in specific conditions. A general scheme for the production of PIMs is indicated in
Figure 5 [
93,
94].
Usually, PIMs comprise three main components: (
a) a base polymer, which provides support and mechanical strength ensuring stability; (
b) a plasticizer, which enables membrane elasticity; and (
c) a carrier, which constitutes the “core” of the membrane, providing the transport of metal ions across the membrane and selective extraction [
95,
96]. PIMs can be produced by dissolving all the components—the base polymer, carriers, and plasticizers—in an organic solvent. After homogenization, the solvent is evaporated to obtain the targeted membrane.
In the production of PIMs, according to the published literature, PVC and CTA have been selected as the base polymers since these are commercially available and possess well-established properties. Another advantage is that they are compatible with the most widely used carriers, such as D2EHPA and Aliquat 336. PVC is a moderately polar polymer with a low grade of crystallinity; thus, the membranes produced by casting PVC are rigid by nature, and typically a plasticizing agent is required. On the other hand, CTA is highly polar and has a high degree of crystallinity, which offers outstanding mechanical strength for the membrane [
97].
An alternative to producing less waste of polymers in PIMs is the utilization of polymers with an increased mechanical strength, reusability, and transport rate. In this sense, poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF HFP) has been proposed as a promising option for use as a base polymer [
15]. This polymer has higher flexibility, which supports improved PIM permeability [
63,
95,
96].
However, considering the environmental problems created by the use of polymers of petroleum origin, their replacement with polymers derived from sustainable sources is of great interest, considering their characteristics and biodegradability [
98,
99]. According to recent studies, biodegradable polymers represent sustainable alternatives for membrane production. Based on the ways they are obtained, biopolymers can be divided into three groups: (
a) polymerization of monomers; (
b) conversion by microbial fermentation; (
c) chemical modification of natural products [
99]. Among these, polylactic acid (PLA) is considered one of the emergent biopolymers that can rapidly replace petroleum-origin polymers. PLA has several features that are similar to well-known polymers such as polyvinylchloride, polypropylene, polystyrene, etc., in terms of flexural strength, elongation, yield strength, or tensile modulus. Moreover, PLA can be easily molded and reshaped in diverse forms using processes such as extrusion or injection molding [
100]. In comparison with fossil-based polymers, PLA has higher permeability and a lower melting point and thermal stability, which may require its modification for some applications [
101]. Surface modification of PLA expands its domains of applications by adapting its characteristics to specific requirements [
102]. Also, to enhance PLA characteristics, many recent investigations have been carried out to produce PLA nanocomposites. The addition of nanoparticles reveals a notable improvement in the mechanical and thermal properties of PLA [
103,
104,
105,
106]. A major advantage of PLA over other bioplastics is its high production capacity, as it can be produced from a variety of bio-sources. Thus, this is a green and cost-effective alternative to fossil-based plastics and is commercially available [
107,
108].
Due to its favorable properties, PLA has been found to be suitable for the fabrication of membranes with applications in separation, water treatment, ion exchange, and adsorption [
89]. Gardner et al. [
109] investigated the performance of several cellulose derivative polymers such as cellulose acetate propionate (CAP), cellulose acetate butyrate (CAB), and cellulose tributyrate (CTB) in the production of PIMs encompassing bis-tert-butylcyclohexano 18-crown-6 as a carrier for K
+ transport. The tested PIMs showed an improved performance in terms of extraction degree and robustness in acidic and alkaline environments. Kunene et al. [
110] created a new PIM using polysulfone (recognized for its stability, hydrophobicity, and durability) as the base polymer and Aliquat 336 as the extractant. They reported that a growth in carrier concentration improves the membrane’s hydrophilicity and that the produced PIMs were stable up to 180 °C.
Keawsupsak et al. [
111] investigated the use of PLA or biodegradable polymer combinations to produce filtration membranes. The results showed that the PLA membrane efficiently removed contaminants and improved tensile properties. Sellami et al. [
112] reported that a combination of CTA and poly(butylene adipate-co-terephthalate) (PBAT) was effective in Cr(VI) separation, with efficiency improving as the proportion of PBAT was increased. Later, the same research group developed PIMs for Cr(VI) using PVDF modified by montmorillonites [
48]. Scaffaro et al. [
113] created a PLA with a polyethylene oxide (PEO) electrospun membrane for oil adsorption.
Recently, Hammadi et al. [
25] tested a biodegradable polymer blend consisting of 54% PLA and 13% PBAT as base polymers. Aliquat 336 at a concentration of 30% was used as the ion carrier next to hybrid nanofillers such as 3% graphene oxide (GO) and/or 3% modified montmorillonite. The combination produces synergistic benefits from the addition of the modifiers, resulting in a higher extraction efficiency of Cr(VI). PIMs based on biodegradable cellulose and Algerian clay were produced and tested for metal removal from wastewater [
114].
Darvishi et al. [
115] developed a new PIM with cross-linked high-molecular-weight green polyol (GPO) made from castor oil as the polymer base, as an alternative to the “classical” base polymers such as PVC, PVDF, or CTA. The PIM was tested for selective extraction of Ca
2+ over competitive ions like Na
+, K
+, and Mg
2+. The tests indicated an improved selectivity and flux of Ca
2+. To improve the PIM performance, Ershad et al. [
116] proposed the use of purified dinonylnaphthalene sulfonic acid (DNNS). The PIMs obtained in this way show an improved extraction performance and better stability. A sustainable non-plasticized PIM containing an immobilizing optode ligand has been developed for in situ colorimetric determination and pre-concentration of Be
2+ in biological and environmental samples by the encapsulation in PVC of selective ligand (E)-6-(4-((2,5-dihydroxyphenyl)diazenyl)phenyl)-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile [
117].
Maiphetlho et al. [
118] developed new PIMs, which included silver nanoparticles (AgNPs), for the extraction of divalent cations (Cd
2+, Co
2+, Cu
2+, and Ni
2+) from contaminated water. The PIMs containing AgNPs displayed an improved transportation capacity compared to simple PIMs. In another study [
70], the separation of Cd
2+, Co
2+, Cu
2+, and Ni
2+ was investigated using PIMs containing ethylenediamine-bis-acetylacetone as the carrier. Hu et al. [
119] proposed a chemical modification of PIMs containing PVC, NPOE, and 2 hydroxy-5-nonylacetophenoneoxime (LIX
®84I), using modifiers with polar groups. The PIMs were tested for Cu(II) transportation and presented significantly improved permeability and transport efficiency compared to the unmodified PIMs.
According to the literature, PIMs utilizing petroleum-based polymers such as PVC and CTA are confirmed as having a commendable stability, robustness, and extraction efficiency [
120]. The shift towards sustainable materials brings environmental benefits, including a reduced carbon footprint. It has been estimated that PLA production uses around 55% less fossil energy and releases significantly less CO
2 compared to traditional polymers [
121]. However, despite their environmentally friendly features, the use of biodegradable polymers in PIMs presents some challenges. The robustness of PIMs for applications at a large scale still seems to be limited, mainly due to their degradability. However, limited data are available in the literature offering a comprehensive analysis regarding the stability and extraction efficiency between PIMs based on biodegradable polymers and those based on petroleum-based polymers, though several studies present the stability and extraction efficiency of PIMs based on biodegradable polymers, mainly PLA. This is one of the desirable biodegradable polymers because it is soluble in many organic solvents, and thus PLA-based PIMs can be prepared by the casting method. Despite some advantages of PLA membranes, their impact resistance is inferior to that of conventional polymers used for PIM fabrication.
Semicrystalline PLA has a better stability than amorphous PLA due to its higher shear viscosity and differences in macromolecular structure. Furthermore, the mechanical characteristics of PLA can be adjusted by incorporating different mixtures [
107]. For instance, the incorporation of zeolites or graphene oxide in biodegradable polymers enhanced membrane strength and functionality [
122]. A PIM based on a biodegradable PLA/PBAT polymer blend, filled with Cloisite 30B (C30B) and/or graphene oxide (GO) fillers and Aliquat 336 carrier, was tested for Cr(VI) transport and reached an extraction efficiency of 79.5%. Moreover, it achieved an 84.4% extraction efficiency when 3% GO was added to the PIM composition [
25].
7. Green Solvents and Green Methods for the Preparation of PIMs
Although PIM-based metal extraction is a green alternative to classical solvent extraction because it significantly decreases the use of toxic solvents, these are still widely used in PIM production. To reduce this drawback, efforts have been made in the last years to replace toxic solvents with greener ones, or to drastically reduce or eliminate the use of solvents by improving the preparation technologies [
123].
In a recent paper, the suitability of several non-toxic and “green” solvents obtained from renewable sources, such as ethyl acetate, acetone, 2-methyltetrahydrofuran, and dihydrolevoglucosenone (CyreneTM), was tested for the manufacture of PIMs comprising the most commonly used polymers (PVC, CTA, and PVDF-HFP) and extractants (Aliquat 336 and D2EHPA) [
16]. The authors reported a similar extraction efficiency and membrane stability when PIMs were prepared using THF as the solvent or green solvents. Even though imaging techniques revealed changes in the surface morphology of PIMs made using classical or green solvents, the extraction performances were not substantially affected.
The scientific literature indicates that the choice of solvent in manufacturing PIMs can significantly influence their porosity, morphology, and selectivity. The use of less hazardous solvents can lead to the formation of membranes with different morphologies, often resulting in more asymmetric structures, depending on the solvent–nonsolvent exchange rates. The kinetics of phase inversion can be sluggish, leading to slower pore formation [
124]. Conventional solvents (THF, chloroform) enable fast phase separation, resulting in porous membranes with a dense and uniform polymer distribution, which have a good separation efficiency. Nevertheless, the high volatility of these solvents can occasionally cause pore reduction post-fabrication, which can be a disadvantage. Green solvents can produce membranes with higher porosity than conventional solvents. For example, when dialkyl carbonates were used as solvents for PIM fabrication, the results showed that porosity tendencies can vary during the fabrication process depending on specific conditions [
125]. In regard to PIM selectivity, some green solvents enhance hydrophilicity, improving antifouling properties and selectivity [
126]. Consequently, the choice of solvent has a decisive role in determining the porosity, morphology, and selectivity of PIMs. Green solvents can be an alternative to classical solvents but produce membranes with a less predictable morphology and higher porosity, requiring process optimization depending on the intended use.
An alternative green approach to producing PIMs entails the elimination of solvents through the implementation of a thermal compression technique. This technique involves melting the PIM components and the subsequent application of high pressure to the molten specimen, resulting in the formation of a flat-sheet film [
124]. A variety of polymers were evaluated in the study, including two cellulose derivatives and two thermoplastic polymers: polyurethane (TPU) and poly-caprolactone (PCL). The composition of PIMs also incorporated the ionic liquid Aliquat 336. The investigation revealed that TPU and PCL membranes exhibited non-oily, translucent, whitish, and flexible characteristics. In contrast, cellulose-derivative polymers resulted in films that did not meet the desired standards. Notably, PCL-based membranes exhibited enhanced stability compared to those prepared by solvent casting. Furthermore, PIMs derived from TPU and PCL membranes demonstrated a remarkable 90% extraction efficiency for the separation of Cr(VI). Other authors produced micropolymer inclusion beads (μPIBs) [
127]. The μPIBs tested contained PVC or PVDF-HFP as the polymer and D2EHPA as the extractant.
To mitigate the necessity of producing a substantial quantity of PIMs, some authors have directed their attention toward the fabrication of membranes that exhibit high stability, enabling their repeated utilization. Innovative composite PIMs were prepared by a modified solvent evaporation method using a porous polytetrafluoroethylene as the base membrane, PVC polymer dibutyl phthalate as the plasticizer, and 2-ethylhexyl phosphonate mono-2-ethylhexyl ester as a carrier [
128]. The obtained PIMs had a higher tensile strength (36.86 MPa to 52.77 MPa) and bursting strength (>0.2 MPa) than conventional PIMs. The authors concluded that composite PIMs could be reused multiple times due to their consistent mechanical properties and separation performance, indicating their industrial application potential.
Ouchun et al. [
129] prepared PIMs from methyltrioctylammonium oleate fixed in a mixture of PVDF and polysulfone, characterized by long-term stability [
104]. The inclusion of the ionic liquid with a percentage of 33% conferred the PIM a highly hydrophobic and porous character, thereby rendering it suitable for use in desalination processes. Recently, a novel hydrophobic PIM was developed for direct-contact membrane distillation (DCMD) application [
130]. PIMs were obtained using PVDF loaded with the ionic liquid methyltrioctylammonium bis(2-ethylhexyl) phosphate. The resulting membrane proved to have outstanding stability, with negligible degradation over multiple cycles and prolonged operation in water desalination.
8. Scalability of PIM-Based Separation
PIMs have emerged as a promising technology for metal ion separation processes in a variety of applications, including water treatment, metal recovery, and tools in analytical chemistry for water quality assessment and monitoring. Nevertheless, for a successful scaling up to industrial applications from laboratory-scale research, understanding the scalability of PIMs is critical. The production cost of PIMs can vary depending on factors such as material selection and manufacturing processes. Remarkably, a recent study reported that producing 1 m
2 of PIM may cost approximately USD 0.08 to 0.16 [
33].
PVC and CTA are polymers that are commonly used and affordable. The newly tested polymeric component PLA is also a promising alternative. The common carrier agents that showed good selectivity and stability are Aliquat 336 and D2EHPA. As manufacturing techniques, transitioning from casting production to continuous roll-to-roll processing represents a solution for large-scale PIM production.
Next to PIM production costs, other expenditures associated with PIM-based processes include energy consumption [
131]. Ghaffour et al. [
132] calculated for membrane-base processes that energy consumption represents around 69% of the total costs, membrane replacement accounts for about 21%, while material costs constitute approximately 10% of the overall expenses. Compared to other separation processes based on pressure-driven systems such as reverse osmosis, PIM-based separation characteristically operates at a lower energy consumption. PIMs normally operate at atmospheric pressure, which reduces energy needs.
Considering the two main aspects, the cost of producing PIMs and the energy required for functioning, it can be stated that PIM-based separation technology represents a promising option for industrial-scale applications. Still, challenges in PIMs’ sustainability, stability, and large-scale production must be addressed. Future research is necessary on optimizing PIM formulations, improving manufacturing techniques, and conducting life cycle analyses to increase economic viability.
9. Conclusions and Future Perspectives
This review has summarized the recent literature on the developments of polymer inclusion membranes (PIMs) for metal ion separation, with a particular focus on the trend of pursuing alternative, greener PIM production. PIMs have found applicability in various fields such as analytical chemistry, water quality monitoring, water treatment, and metal recovery. PIMs typically include a base polymer, a carrier, and, if necessary, a plasticizer. PIMs can be formed by dissolving all the components—base polymer, carriers, and plasticizers—in an appropriate solvent. After homogenization, the solvent is evaporated to achieve the desired membrane. Consequently, numerous studies have been carried out to develop PIMs tailored toward specific analytes and specific matrices, and various authors have contributed to the improvement of PIMs by including new materials for these components.
Although PVC and CTA continue to be the most commonly used base polymers in PIMs due to their wide availability, stability, and compatibility with an extensive range of plasticizers and carriers, they are of petroleum origin, which can cause environmental issues. A possible substitute to produce less polymer waste is to use polymers with a better mechanical strength, reusability, and transport rate, and, in this respect, PVDF HFP has been reported as a promising option. Moreover, biodegradable polymers obtained from sustainable sources are increasingly studied for membrane production. Thus, it is expected that future studies will focus on the replacement of petroleum-based polymers with biodegradable and sustainable sources.
The carriers ensure the metal ion transport across the membrane and thus are the main component responsible for PIM selectivity and the transportation rate. Among these, Aliquat 336 and D2EHPA have been the most studied, but on this topic, many more alternatives have also been studied to ensure selectivity towards target metals. Derivates of pyridine, calix[4]arene, and Kelex 100 were among the carriers tested in recent studies. However, further research is required to find novel carriers with improved characteristics.
The plasticizer is mainly used to provide membrane elasticity, and in some cases, it is not included in the PIM composition. NPPE and NPOE were largely used as plasticizers in the studies reported in the literature. Since the number of known plasticizers is still limited, future research should be conducted to discover new appropriate types of plasticizers.
One of the drawbacks of PIM fabrication is the need to use volatile and toxic organic solvents. To avoid this, several papers present the possibility of their replacement by greener solvents or to produce PIMs using solvent-free methods. This is clearly a topic of interest for future research.
Nevertheless, challenges in PIMs’ sustainability, stability, and large-scale production must be addressed. Future research is necessary to optimize PIM formulations, improve manufacturing techniques, and conduct life cycle analyses to increase economic viability. While specific studies focusing exclusively on PIMs under real wastewater conditions are limited, insights from broader research on polymeric membranes provide valuable guidance. Enhancing biofouling resistance through material modifications and understanding the impacts of fouling and cleaning on long-term performance are critical steps. Future research should prioritize long-term, real-world testing of PIMs to validate their practical utility in wastewater treatment applications.
PIMs can be used as a passive sampling tool, as they are well-integrated in the trend in analytical chemistry of working toward greener sample preparation. This can improve the performance parameters of analytical methods, reduce the chemical and physical interferences in metal determination in complex matrices, and reduce the number of sample preparation steps. In this field of research, the production of membranes capable of extracting more components simultaneously may be a future development. Passive sampling is also a useful instrument for mimicking metal bioavailability in soil and evaluating the uptake by crops. Future studies linking PIM separation with plant bioaccumulation will provide a more complete understanding of metals’ bioavailability and mobility in soil.