1. Introduction
Resorbable medical devices represent a significant advancement in biomedicine designed to perform critical functions such as structural support or drug delivery for a temporary period [
1,
2,
3]. Typically, once their therapeutic purpose has been fulfilled, these devices gradually degrade into non-toxic byproducts that can be naturally absorbed or excreted by the body, eliminating the need for surgical removal. This feature not only reduces the risks associated with long-term implantation such as local infection [
4] or chronic inflammation [
5], but also minimizes the need for follow-up surgeries, enhancing patient recovery and reducing healthcare costs.
As research continues to improve the performance and biocompatibility of resorbable materials, their potential extends to treating life-threatening conditions like pulmonary embolism (PE). PE affects nearly 900,000 people every year in the United States alone [
6]. Patients at risk of this condition typically receive anticoagulant therapy (e.g., low molecular weight heparin and direct-acting oral anticoagulants) to prevent the development of blood clots [
6,
7] and mitigate the risk of subsequent complications including strokes [
7] and cardiac arrest [
8]. However, for patients with contraindications to anticoagulant therapy, the implantation of inferior vena cava filters (IVCFs) is often typically recommended as an alternative treatment option [
9]. Traditionally, IVCFs are fabricated from metals such as nitinol or stainless steel and are intended for surgical retrieval after 25–54 days post-implantation as recommended by the Food and Drug Administration (FDA) [
10]. Yet, only 17–30% of patients have these temporary filters removed despite the risk of complications such as filter migration and perforation [
11]. To address this issue, research has focused on developing resorbable IVCFs, which would eliminate the need for surgical removal.
One of the biggest challenges of making an effective resorbable IVCF is selecting a polymer that has the most optimal degradation time. It is critical that temporary IVCF remain fully functional for a minimum of 35 days following a major trauma or procedure, while also being removed prior to the onset of significant complications. Pre-clinical testing has been performed to test the efficacy of absorbable IVCFs made with polylactic-co-glycolic acid (PLGA) [
12], polyglycolic acid (PGA) [
13], poly-L-lactic acid (PLLA), and polycaprolactone (PCL) [
14]. All these studies were successfully fabricated into filters with different degradation characteristics and clot-trapping efficacies. On the other hand, Eggers et al. [
15] showed that poly-p-dioxanone (PPDO) is a promising polymer for fabricating absorbable IVCFs with a degradation time of 6 weeks. PPDO is commercially available and inexpensive because of its common application as surgical sutures. However, unlike metallic IVCFs, PPDO filters cannot be easily visualized on conventional imaging such as computed tomography (CT) or X-ray. Visualizing the filter is critical for patient safety. It allows the assessment of immediate and delayed filter complications, such as filter tilt, incomplete expansion, and device migration. Central venous access is obtained by passing a wire into the central veins. Being able to visualize the filter is critical to ensure that the catheter or wire used during the placement procedure does not displace the filter. Also, while IVC filters are designed to capture thrombi, the foreign body itself can also be thrombogenic. Depending on the severity of thrombus in situ below the filter, thrombectomy procedures could be performed which would require that the filter be visualized. Thus, for the safety of the patients, ideally resorbable IVCFs should be capable of being monitored with conventional X-ray imaging modalities. Previously, this has been addressed by the infusion of high atomic number (high-Z) NPs such as gold [
16], bismuth [
17], and tungsten [
18].
Gadolinium (Gd) is another high-Z element (Z = 64) with attractive properties for enhancing imaging. Most commonly used as a contrast agent for magnetic resonance imaging (MRI), Gd chelates function by shortening T1 (longitudinal relaxation time) and T2 (transverse relaxation time) relaxation times. T1 represents the time it takes for protons to realign with the magnetic field after being disturbed by a pulse, while T2 refers to the time it takes for protons to lose phase coherence in the transverse plane. By shortening these relaxation times, Gd enhances signal intensity, thus improving the clarity and resolution of MRI scans. Beyond MRI, Gd is also considered novel for its versatility in multiple imaging modalities such as CT imaging. In its NP form (GdNP), Gd offers additional advantages, including a significantly higher surface area due to its small size and large surface-to-volume ratio, allowing the increased loading and therefore higher X-ray attenuation. This enhanced imaging capability and safety profile makes GdNPs particularly attractive for applications like infusion with PPDO IVCFs, as they can improve both the precision of filter placement and monitoring post-deployment outcomes through dual-modality imaging.
A significant challenge with most implantable medical devices, including IVCFs, is the high incidence of thrombosis. To address this issue, we hypothesize that coating IVCFs with antithrombotic drugs can reduce local clotting and mitigate this common complication. Dipyridamole (DPA; 2,6-bis-diethanolamino-4,8-dipiperidinopyrimido (5,4-d)-pyrimidine), a drug known for its antiplatelet activity, shows promise in minimizing thrombosis when integrated into medical devices [
19]. This study aims to develop a novel absorbable IVCF by combining the resorbable polymer PPDO with radiopaque Gd nanoparticles (GdNPs) and the antithrombotic drug DPA. The fabricated filters were evaluated for their physicochemical properties and degradation behavior over time in an in vitro system. This work represents the first preclinical development of an absorbable IVCF that integrates both imaging capability and therapeutic functionality.
2. Materials and Methods
2.1. Materials
Gd (III) acetate hydrate (99.9%), oleic acid (technical grade, 90%), oleylamine (technical grade, 70%), ethanol (ACS reagent grade ≥99.5%), acetone (ACS reagent grade ≥99.5%), dimethyl sulfoxide (DMSO, ACS reagent grade ≥99.9%), dichloromethane (DCM, ACS reagent grade, ≥99.8%), PCL (average Mn 80,000), DPA (HPLC grade ≥98%), and phosphate-buffered saline (PBS) at pH 7.4 were purchased from Sigma-Aldrich (St. Louis, MO, USA). WebMax 2-0 PPDO sutures were purchased from Patterson Veterinary (Liberty, MO, USA). Gd standard solution (10,021 ± 35 µg/mL) in 7% (v/v) HNO3 (density, 1.053 g/mL) was obtained from Inorganic Ventures (Christiansburg, VA, USA) and used for preparing standard Gd solutions of varying concentrations from 0 to 50 ppm. Using this, a standard graph was plotted for the estimation of Gd during the release study. A stock solution of DPA (1 mg/mL) was prepared in DMSO and used to plot a standard calibration graph for quantifying DPA in the release study. A silicone vena cava model (United Biologics, Irvine, CA, USA) connected to a Flowtek 125 pump was purchased from United Biologics (Irvine, CA, USA). All chemicals were used without further purification unless otherwise mentioned.
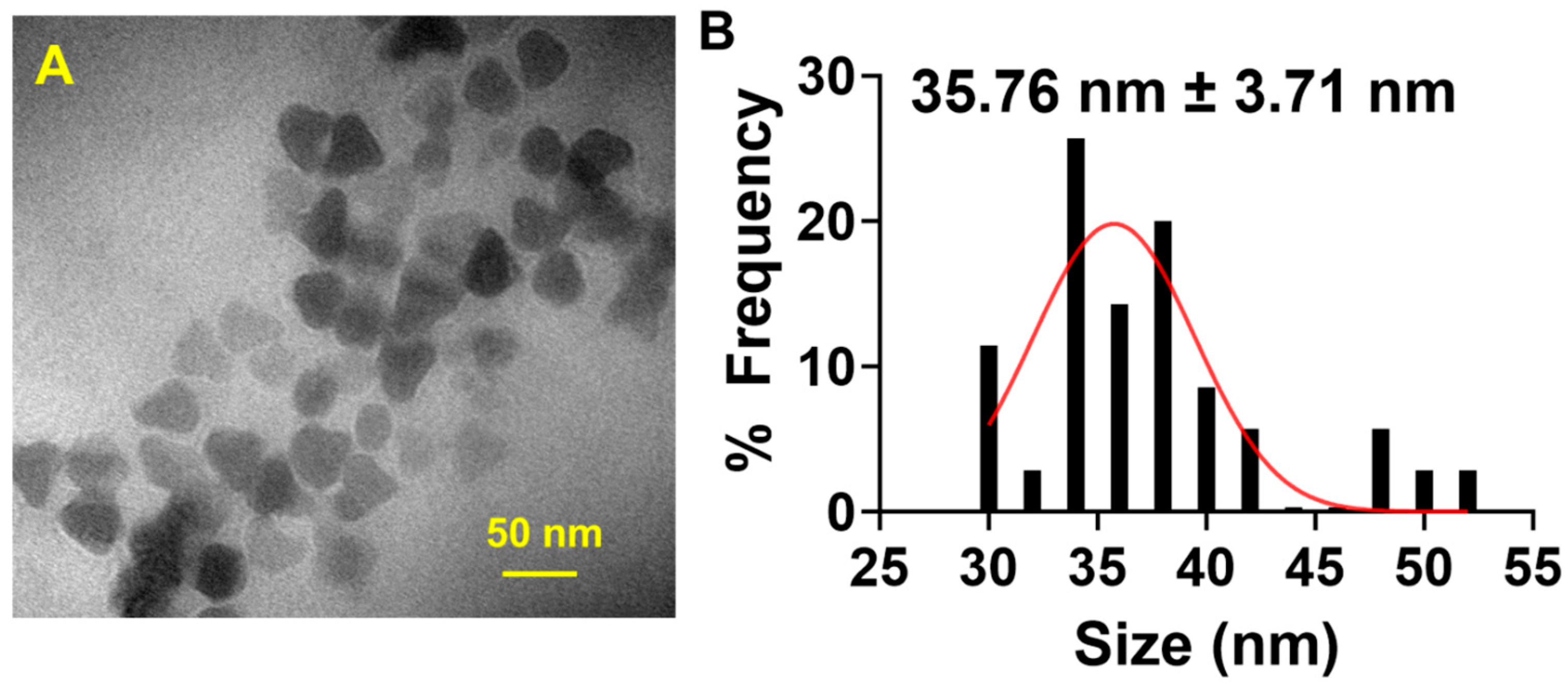
2.2. Synthesis and Characterization of GdNPs
GdNPs were synthesized using a thermal decomposition method. In a 500 mL round-bottom three-neck flask, Gd acetate (1.67 g, 5 mmol) was added with oleic acid (30 mL, 90 mmol) and oleylamine (100 mL, 300 mmol). The mixture was stirred using a magnetic stirrer (Isotemp, ThermoFisher Scientific, Waltham, MA, USA) and heated at 120 °C using a Digi-Sense Temperature Controller R/S (Boston, MA, USA) for 1 h under an argon atmosphere. Afterward, the temperature was increased to 280 °C and maintained for 6 h. The resulting metallic white solution was allowed to cool down at room temperature and then washed three times with an ethanol–acetone (1:1) solution, followed by centrifugation. The obtained precipitate was redispersed in DCM and stored until further use.
The size and morphology of synthesized GdNPs were visualized and characterized by transmission electron microscopy (TEM) using a JEM 1010 microscope (JEOL USA, Peabody, MA, USA) and the size and distribution of the nanoparticles were estimated using ImageJ software, version 1.54 (National Institutes of Health, Bethesda, MD, USA).
2.3. Fabrication of Radiopaque, Drug-Loaded Resorbable IVCFs
The overall schema for the fabrication of the radiopaque, drug-loaded resorbable IVCFs is shown in
Supplementary Figure S1. First, IVCFs were braided by modifying an agglomerate cork (32.6 mm diameter × 36.6 mm height) with 15 zinc finishing nails (Everbilt #19 × ½ in) placed equidistantly (2.17 mm apart) at the top and bottom circumferences of the cork. WebMax PPDO sutures were wrapped around the cork by alternately looping through a nail on top and then at the bottom with 8 nail intervals. After every two turns, the suture goes up and through a round 3D-printed resorbable tip (polypropylene, 6.2 mm diameter × 1.2 mm height), printed using an S7 Ultimaker 3D-printer and designed using UltiMaker Cura 5.5.0 (Ultimaker, Utrecht, The Netherlands). This forms the conical portion of the IVCF. After braiding, the filters were annealed at 150 °C using Nuwave Bravo XL Pro Smart Oven (Vernon Hills, IL, USA). The resulting filters measured 60 mm (length) × 30 mm (diameter) and included a stent portion and a conical portion (
Supplementary Figure S2).
Prior to coating with NPs, the IVCFs were soaked in DCM to wash off the purple coating. The filters were then infused with one of the following solutions using a wet-dipping method: GdNPs only (0.2 g/mL DCM) (PPDO-Gd), DPA only (1 mg/mL DMSO) (PPDO-DPA), or a combination of GdNPs and DPA (PPDO-Gd + DPA). The wet dipping procedure followed a method previously described by Damasco et al. [
17]. Specifically, IVCFs were soaked in a solution of GdNPs or DPA for 5 min, air-dried, immersed in PCL for another 5 min, and air-dried again. This cycle was repeated 20 times (
Supplementary Figure S1). For PPDO-Gd + DPA, the filters were alternately infused with GdNPs for 5 min, air-dried, immersed in PCL for 5 min, air-dried, immersed in DPA for 5 min, and then air-dried again, repeating this cycle for 20 cycles (
Supplementary Figure S1).
2.4. Characterization and Monitoring of Radiopacity, Mechanical Strength, and Gd/DPA Release over Time
The surface morphology and elemental composition of the coated IVCFs were characterized in field-emission scanning electron microscope (Nova NanoSEM 230, FEI, Hillsboro, OR, USA), energy-dispersive X-ray spectroscopy (EDAX Element EDS, Ametek, Berwyn, PA, USA) and analyzed using TEAM WDS Analysis System software (version V4.5.1-RC13 (Ametek). Radiopacity was assessed using micro-computed tomography (Micro-CT, Skyscan 1276, Bruker, Billerica, MA, USA), which contains a high-resolution, flat-panel detector-based system. The system features a tungsten source X-ray tube operating at 80 kV and 450 μA. The X-ray source and detector rotate around the sample, acquiring projections at 1.0-degree increments. The mechanical strength in terms of load-at-break was measured using an Expert 7601 Tension Testing System (ADMET, Norwood, MA, USA). The ADMET universal testing system was operated at a crosshead speed of 304.80 mm/min, using a high-resolution 250-lb load cell and 2KN pneumatic grippers to ensure accurate testing without slippage and precise measurements. Data acquisition and analysis were performed using MTESTQuattro™ software, version 6.02.03. Samples were analyzed in triplicate and data are presented as mean ± standard deviation (SD).
The amount of Gd and DPA was quantified using an atomic emission spectrometer (Agilent MP-AES; Agilent Technologies, Santa Clara, CA, USA) and a UV-Vis spectrophotometer (Cary WinUV 60 SW, Agilent Technologies, Stanta Clara, CA, USA), respectively. Gd solutions were prepared for elemental analysis by dissolving 1 mL aliquot with 1 mL HNO3, and shaken at room temperature overnight. The samples were then diluted to 15 mL with 2% HNO3 for quantification of Gd, while the DPA in solution was quantified at 295 nm using a UV-Vis spectrophotometer. Samples were analyzed in triplicate and data were presented as mean ± SD.
To monitor the radiopacity, mechanical strength, and release of Gd/DPA in vitro, IVCFs were soaked in 20 mL phosphate-buffered saline (PBS, pH 7.4, 37 °C). The PBS solution was collected and replaced on days 1, 3, 5, and weekly up to 12 weeks. Micro-CT imaging was performed weekly, followed by mechanical strength testing using the universal testing system described above. Gd and DPA concentrations were analyzed from the collected PBS.
The percent (%) Gd/DPA remaining was calculated as:
where total Gd/DPA was the sum of released Gd/DPA and residual Gd/DPA in the IVCF. The Gd/DPA content was normalized to IVCF weight:
All samples were analyzed in triplicate, with data presented as mean ± SD.
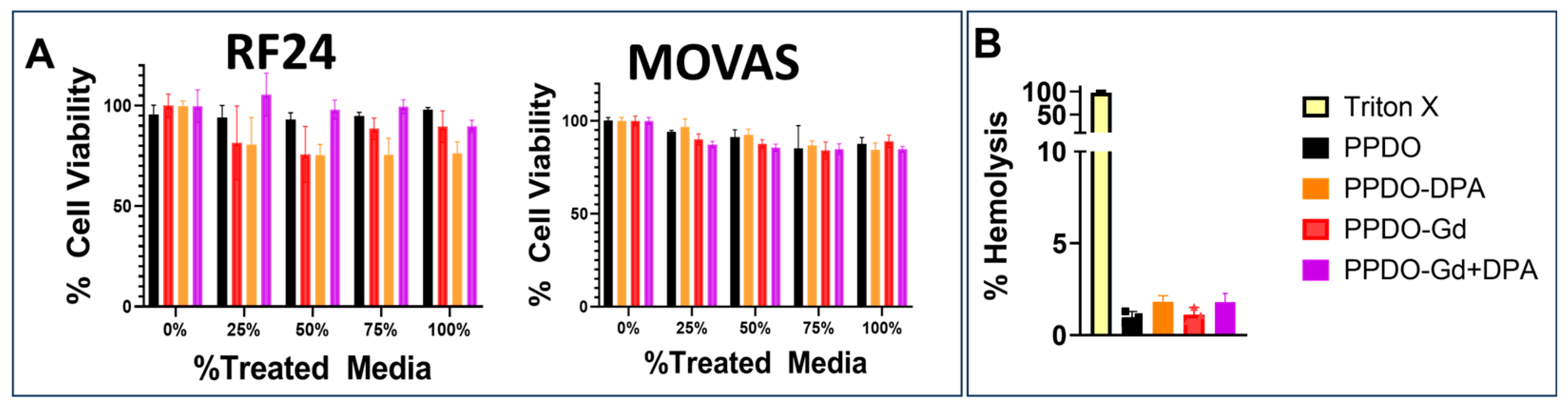
2.5. Biocompatibility of the Materials
Cytotoxic effects of GdNPs were tested against mouse vascular aortic smooth muscle cells (MOVAS, American Type Culture Collection [ATCC], Manassas, VA, USA) and immortalized human vascular endothelial cells (EC-RF24, ATCC) using standard protocol for biological evaluation of medical devices, ISO 10993-5:2009 [
20]. Cells were grown in 96-well plates with complete media for 24 h before incubating with GdNPs, DPA, and Gd-DPA-treated sutures at varying extract concentrations. Cell viability was measured after 24 h with alamarBlue cell viability assay (ThermoFisher Scientific). To prepare the IVCF extracts, control PPDO, PPDO-Gd, PPDO-DPA, and PPDO-Gd + DPA were sterilized by soaking in absolute ethanol for 1 h. Ethanol was subsequently removed by soaking the IVCFs in PBS for 15 min, followed by extensive rinsing with PBS and MEM-alpha culture media. The IVCFs (30 cm in length) were then incubated in 5 mL of MEM-alpha culture media for 24 h to generate the extracts.
Following incubation, the IVCFs were removed, and the resulting conditioned media were used for cell viability testing. EC-RF24 cells (5000 cells/well) were seeded into a 96-well flat-bottom plate in MEM-alpha culture media supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin and allowed to adhere overnight at 37 °C in a humidified incubator with 5.5% CO2. The media were then replaced with the suture extract media at varying concentrations (0, 25, 50, 75, and 100%), with 0% extract (untreated MEM-alpha culture media) serving as the viability reference (100%). Cells were incubated with the treated media for 72 h before replacing it with fresh media containing 10% alamarBlue reagent (ThermoFisher Scientific) and further incubating for 4 h. Fluorescence intensity was measured at 590 nm with excitation at 540 nm using a microplate reader (Cytation5, Biotek, Winooski, VT, USA). Each condition was tested in six replicates (n = 6) to ensure statistical robustness.
A hemolysis assay was performed by modifying the protocol by Evans et al. [
21]. Erythrocytes were collected by placing fresh rat blood in green-top heparinized vacutainers (BD Vacutainer, Franklin Lakes, NJ, USA), washed with cold PBS, and centrifuged to collect erythrocytes. Positive controls were added with 50 µL of 10% Triton X-100, while negative controls were added with 50 µL of PBS. The remaining wells were added with three sterilized 1 cm strands of the control PPDO, PPDO-Gd, PPDO-DPA, and PPDO-Gd + DPA each. The percent lysis was calculated using the optical densities (OD) of the samples against samples containing Triton-X 100 solution. Samples were analyzed in triplicates and percent (%) hemolysis was calculated by subtracting the absorbance of the PBS control from the absorbance of the sample, dividing the difference by the difference between the absorbances of the positive and negative controls. The samples were analyzed in triplicate and data were presented as mean ± SD.
2.6. In Vitro Clot-Trapping Efficacy Experiment
First, alginate-based clots were synthesized using 2% sodium alginate solution and iron nanoparticles (0.1 mg/mL)/fluorescent dye rhodamine B (0.1 mg/mL). Once thoroughly homogenized, 1 mL suspension solution was mixed with 100 mM CaCl2 solution in a 2 mL Eppendorf tube and allowed to solidify overnight at room temperature. Solidified clots were washed with MilliQ water (Merck Millipore, Burlington, MA, USA), cut (5 mm × 5 mm × 20 mm) and stored at 4 °C until further application.
A silicone vena cava model connected to a Flowtek 125 pump was used to study clot-trapping efficacy of the fabricated IVCFs. IVCFs were first implanted by inserting it through a designated insertion pouch. This was securely fastened using a screw clamp (United Biologics, Irvine, CA, USA) before setting the flow pump. The pump was then filled with about 1 L of water and allowed to flow through the model while removing air bubbles. To ensure a constant flow of water throughout the model, the pump was set at a continuous pulse at a 40% flow rate.
Clots (5 mm × 5 mm × 20 mm) were deployed through the right posterior access site in the model (representing the right iliac vein) which is secured with a hemostatic valve. The ratio of the maximum diameter of the clot to the filter is 0.33. The clots were allowed to flow towards the IVCF and clot-trapping efficiency was evaluated whether the clots were captured by the filters or not. Each cylindrical clot was only introduced once in the flow pump.
Clot-trapping efficiency experiments were also observed under fluoroscopy using a Siemens SOMATOM Definition Edge scanner (Siemens Healthineers, Erlangen, Germany). To replicate soft tissue in an animal or human model, saline bags were placed over the IVC silicone model.
2.7. Statistical Analysis
All studies were conducted in triplicate (n = 3) or three independent experiments. The data were presented as mean ± SD. GraphPad Prism, version 10.3.1 (464) software (GraphPad, San Diego, CA, USA) was used to perform all statistical analyses. A two-tailed t-test or one-way analysis of variance (ANOVA) was used where appropriate, and differences were considered significant at p < 0.05.
4. Discussion
Temporary medical devices are critical in providing short-term support to patients with various medical conditions, with the intent of removal once the condition improves or resolves. A significant advantage of using absorbable materials, such as resorbable polymers, is that they can provide the necessary functionality during their active period and then gradually dissolve, being safely absorbed by the body, thus eliminating the need for surgical extraction. This approach not only minimizes the risks associated with additional surgeries but also enhances patient comfort and recovery. In this study, we introduced a multifunctional, polymeric IVCF fabricated from PPDO, which is not only absorbable but also imageable, incorporating GdNPs for radiopacity and serving as a depot for DPA to reduce clot formation. This combination allows real-time imaging and controlled drug delivery, potentially improving the therapeutic efficacy and safety of temporary implants.
GdNPs have been previously shown as an imaging enhancer for various medical devices [
22,
23]. Although more known as an MRI agent due to its paramagnetic properties, Gd is also a heavy metal like iodine which attenuates X-rays [
24]. With a higher atomic number (Z = 64) compared to iodine (Z = 53), Gd has a stronger ability to absorb X-ray energy. It also features a higher K-edge energy (50 keV), which is better aligned with the peak intensity of the X-ray spectrum generated during CT scans, typically ranging from 50 to 60 keV at 80–140 kVp settings in modern CT systems. As a result, Gd effectively absorbs more of the X-ray energy, making it a more efficient attenuator during clinical CT imaging [
24].
The infusion of GdNPs onto PPDO-Gd is significantly higher when applied alone compared to the combined application of GdNPs and DPA onto PPDO-Gd + DPA. This difference arises because diffusion is the primary mechanism governing the wet dipping method, where mass transport follows Fick’s laws of diffusion [
25]. In the alternating layer-by-layer deposition on PPDO-Gd-DPA, surface saturation occurs as both GdNPs and DPA are sequentially adsorbed. This saturation reduces the availability of free surface sites for additional GdNP infusion, thereby limiting its overall incorporation. The lower amount of infused Gd in PPDO-Gd + DPA resulted in lower initial radiopacity measured. In addition, to efficiently incorporate Gd into absorbable polymers, such as PPDO, we utilized a nanoparticle form of Gd and employed a wet-dipping method alternating with PCL. GdNPs have a high surface area that allows them to easily integrate into the polymeric matrix of PPDO. The addition of PCL enhances the surface roughness of PPDO, improving the entrapment of Gd within the polymer, as seen in the studies of Damasco et al. [
17] and San Valentin et al. [
18]. Our study confirmed that adding PCL to PPDO increased the surface roughness, facilitating greater Gd infusion, as observed in the SEM images while maintaining the mechanical properties (
Figure 2 and
Figure 4B). This modification also enabled sustained release of Gd over time, with 7.88% remaining in PPDO-Gd and 7.36% in PPDO-Gd + DPA at week 12 (
Figure 4A). However, the release of Gd did not correspond directly to a decrease in radiopacity. Even with approximately 7% of Gd remaining, the radiopacity was still intense. For instance, the radiopacity of PPDO-Gd decreased from 2594 ± 222 HU at week 0 to 1406 ± 147 HU at week 12 (approximately 46% reduction), and for PPDO-Gd + DPA, it decreased from 1589 ± 233 HU at week 0 to 1078 ± 227 HU at week 12 (approximately 32% reduction). This discrepancy may be due to the high initial radiopacity, meaning that even with a relatively small amount of Gd remaining, it still produced a strong signal in micro-CT imaging. Fine-tuning the concentration to start with less Gd and lower radiodensity could allow more precise monitoring of the Gd levels.
Beyond GdNPs, this study also focused on DPA, an antiplatelet drug known to inhibit platelet aggregation and prevent thrombosis. DPA’s incorporation into the IVCF offers the potential for enhancing the filter’s antithrombotic properties, minimizing clot formation, and improving device efficacy. Previous studies [
26] have demonstrated that DPA-loaded devices resist platelet adhesion without compromising hemocompatibility, suggesting that its inclusion could further improve the IVCF’s ability to prevent thrombosis. DPA works by inhibiting platelet aggregation, thus preventing the formation of blood clots, which is vital for minimizing the risk of thrombosis in the IVCF. By reducing platelet activation, DPA enhances the overall anti-thrombotic properties of the filter, promoting its effectiveness in capturing and preventing the propagation of thrombi. Similarly, Dominguez-Robles et al. [
27] developed a 3D-printed drug-loaded cardiovascular graft wherein they showed resistance of the device to platelet adhesion without affecting hemocompatibility and cytocompatibility in HUVEC cells. Their work also showed that increased loading of DPA onto the device also corresponded to a more effective antiplatelet activity, suggesting that our study can be further improved by exploring different concentrations of loaded DPA. However, concerns about the risk of excessive local DPA concentration following implantation remain. In our study, 0.98 mg of DPA is released within a week (
Figure 4D). While burst release can sometimes lead to excessive local drug concentrations, the total DPA released in the initial phase remains below the reported cytotoxic threshold. Previous studies have indicated that acute toxicity is observed at concentrations above 200 mg in a single dose, which can lead to mild symptoms, such as dizziness, headache, and nausea. Higher doses, such as 1250 mg, have been reported in cases of overdose [
28]. In our system, the initial release does not reach this level, suggesting that the burst phase is unlikely to pose a significant cytotoxic risk. Moreover, while several studies have investigated drug-coated metallic IVCFs (e.g., rapamycin, heparin, and paclitaxel) primarily to combat neointimal hyperplasia [
29,
30], this is the first study on DPA-loaded absorbable IVCFs. The systemic effect of the DPA resorbs was not evaluated during this study but we plan to assess plasma drug concentration levels in future work to evaluate systemic DPA exposure. Furthermore, this study evaluated the performance of the device in vitro while in quiescent conditions. In future studies, a more dynamic setup simulating the complex components and behavior of the circulatory system will enhance the analysis of the device. This will allow for a more in-depth assessment of the interactions between the device and the blood microenvironment. Moreover, surface and molecular interactions that may or may not interfere with the release or mechanism of DPA should also be considered in future experiments to provide a more comprehensive evaluation of combination therapies.
Although there were no significant differences in load-at-break among the groups tested at week 0, the thickness and melting temperature (T
m) of PPDO slightly decreased with the addition of GdNPs and DPA. This decrease in T
m is likely due to modifications in the crystalline and amorphous regions of PPDO caused by the incorporation of GdNPs and DPA, a phenomenon observed in previous studies of polymer composites [
31,
32]. Despite these initial differences in T
m, our in vitro evaluation demonstrated that all fabricated IVCFs, including those loaded with GdNPs and/or DPA, degraded over time without negatively affecting the material’s inherent degradation properties. Although we expected to have a sharp decrease in load-at-break starting at week 6 as shown by Eggers et al. [
33], we did not see this trend in all of our IVCFs. This is due to the in vitro system that was used in the study where IVCFs were soaked in PBS at room temperature with shaking compared to Eggers’ system that used a more elaborate closed-loop venous flow simulator with controlled flow, pressure, pH, temperature, viscosity and density to mimic blood [
15,
33]. On the other hand, the release profiles of GdNPs and DPA followed a predictable trend, with a rapid initial release phase transitioning into a steady release phase, consistent with the degradation kinetics observed for radiopaque resorbable IVCFs previously developed [
17,
18].
Additionally, the IVCFs demonstrated excellent biocompatibility in vitro, as evidenced by the lack of cytotoxic effects and the maintenance of cell viability in endothelial and smooth muscle cell lines. One of the most significant advantages of this approach is the clot-trapping capability of the IVCF, which was demonstrated in a controlled vena cava flow model. The 40% flow rate in a vena cava flow model represents a controlled percentage of the system’s maximum flow capacity, used to simulate blood flow under reproducible, physiological conditions. In the human inferior vena cava (IVC), the average blood flow rate is typically around 2 to 3 L per minute. Setting the flow rate to 40% of the system’s maximum capacity mimics this normal blood flow at approximately 1.2 L per minute, providing a moderate yet accurate representation of IVC dynamics [
34]. This flow rate ensures that the experiment avoids excessive turbulence while still reflecting realistic blood flow, allowing for effective testing of IVC filters and other medical devices in a controlled environment. We opted for alginate because of its ability to mimic the mechanical properties of blood clots, providing a stable and reproducible model for testing medical devices [
35,
36]. Alginate’s gel-like properties allow for precise control over clot size and consistency, ensuring standardized experimental conditions. It is non-biological, reducing safety concerns, and is easy to handle in laboratory settings. Additionally, alginate clots can be dyed to improve visibility under imaging techniques and may also be loaded with imaging agents for fluoroscopy and CT. The results in
Figure 6B show the enhanced visibility of the IVCFs containing Gd (PPDO-Gd and PPDO-Gd + DPA), even the single strands using the axial micro-CT images. The same is true for fluoroscopy images which are helpful for device implantation since usually this is the imaging modality that is used when the device is placed.
Although the results from this study are promising, there are several limitations. First, the in vitro degradation testing may not fully replicate in vivo conditions, where biological factors, such as blood flow and pressure, pH, and the presence of enzymes, could influence the degradation rate and performance. Second, the absence of in vitro and in vivo testing for DPA’s efficacy and long-term biological effects leaves some uncertainties regarding its performance in a living organism. Future studies involving large animal models are needed to further assess the degradation, efficacy, and overall performance of the PPDO-Gd-DPA IVCF in a dynamic biological environment. Additionally, the design and findings from this study could be translated to other absorbable medical devices, such as stents and sutures, with temporary functionality and long-term risks, offering the potential for improvements in patient care across multiple clinical areas.