Self-Toughened Epoxy Resin via Hybridization of Structural Isomeric Curing Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Epoxy Thermosets
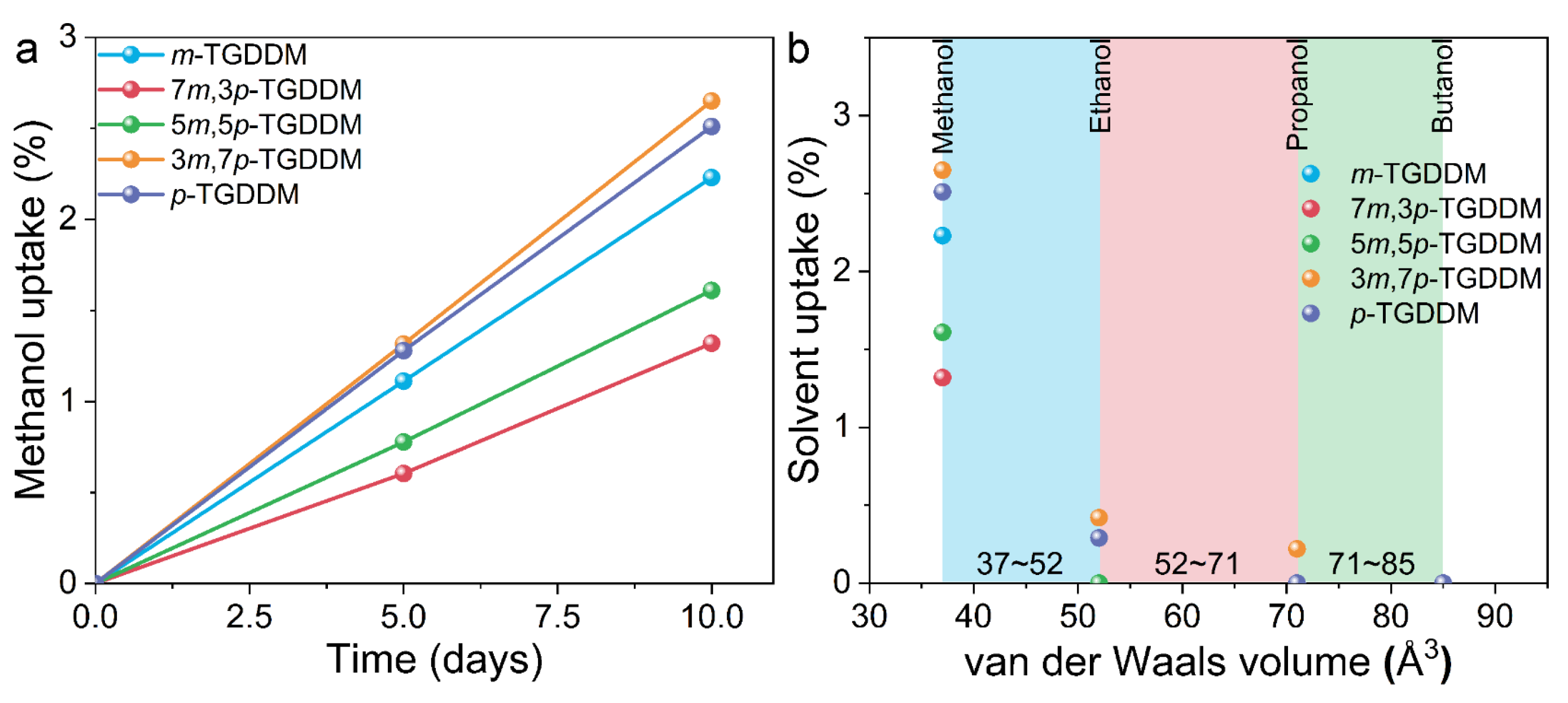
2.3. Solvent Uptake Measurements
2.4. Characterization of Prepared Samples
3. Results and Discussion
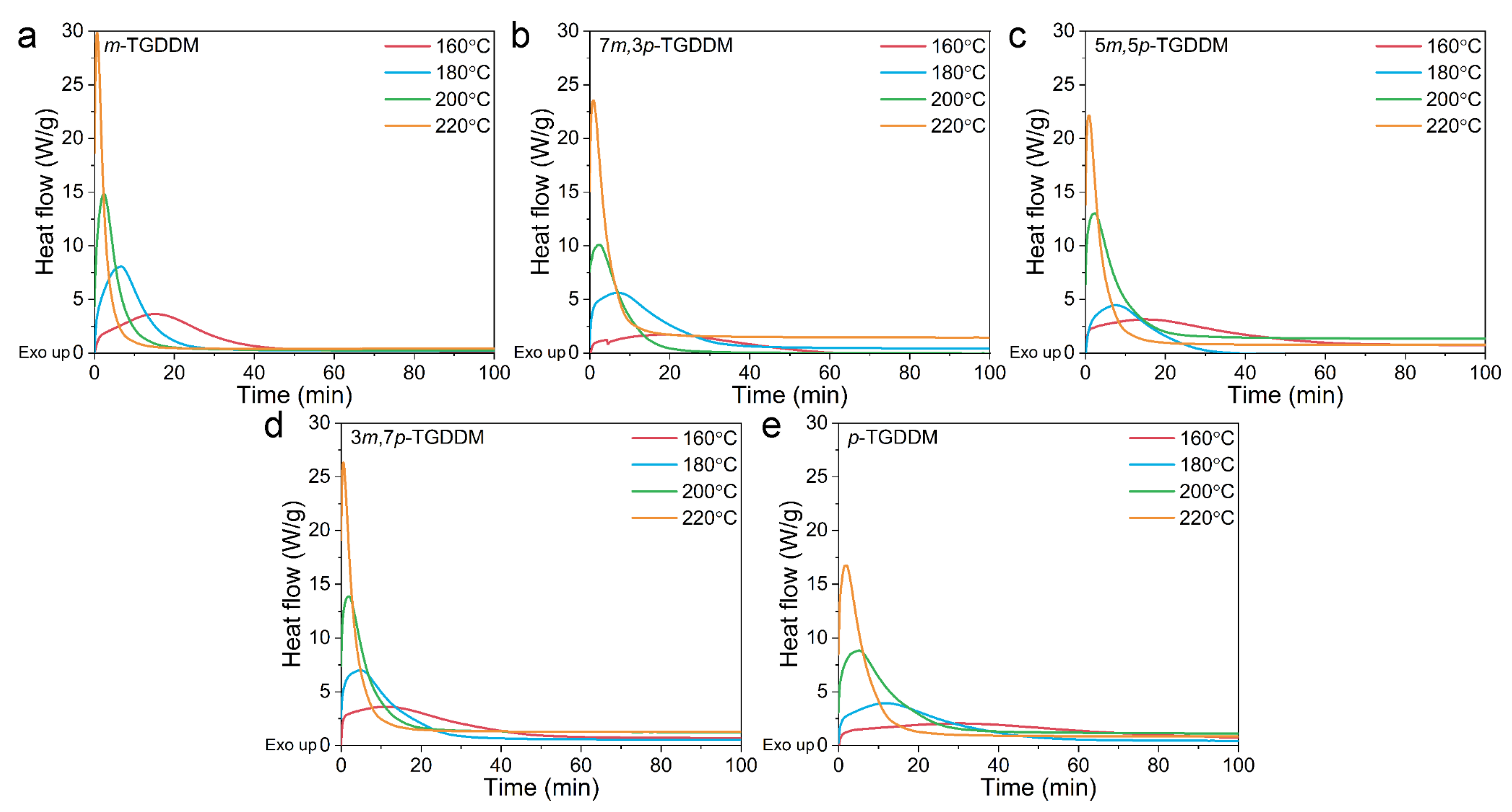
3.1. Curing Behaviors of the Prepared Samples
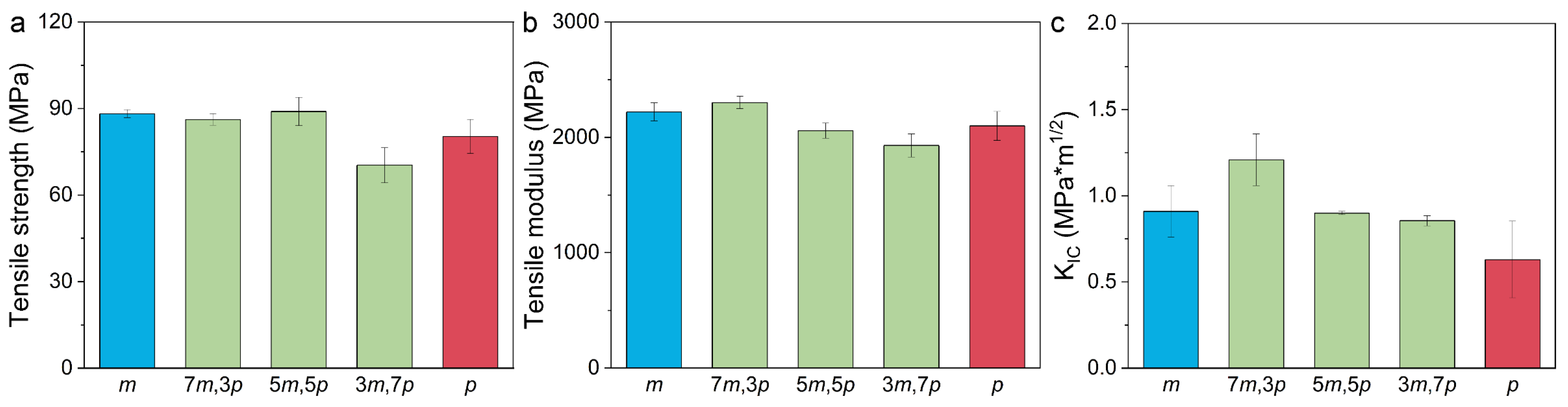
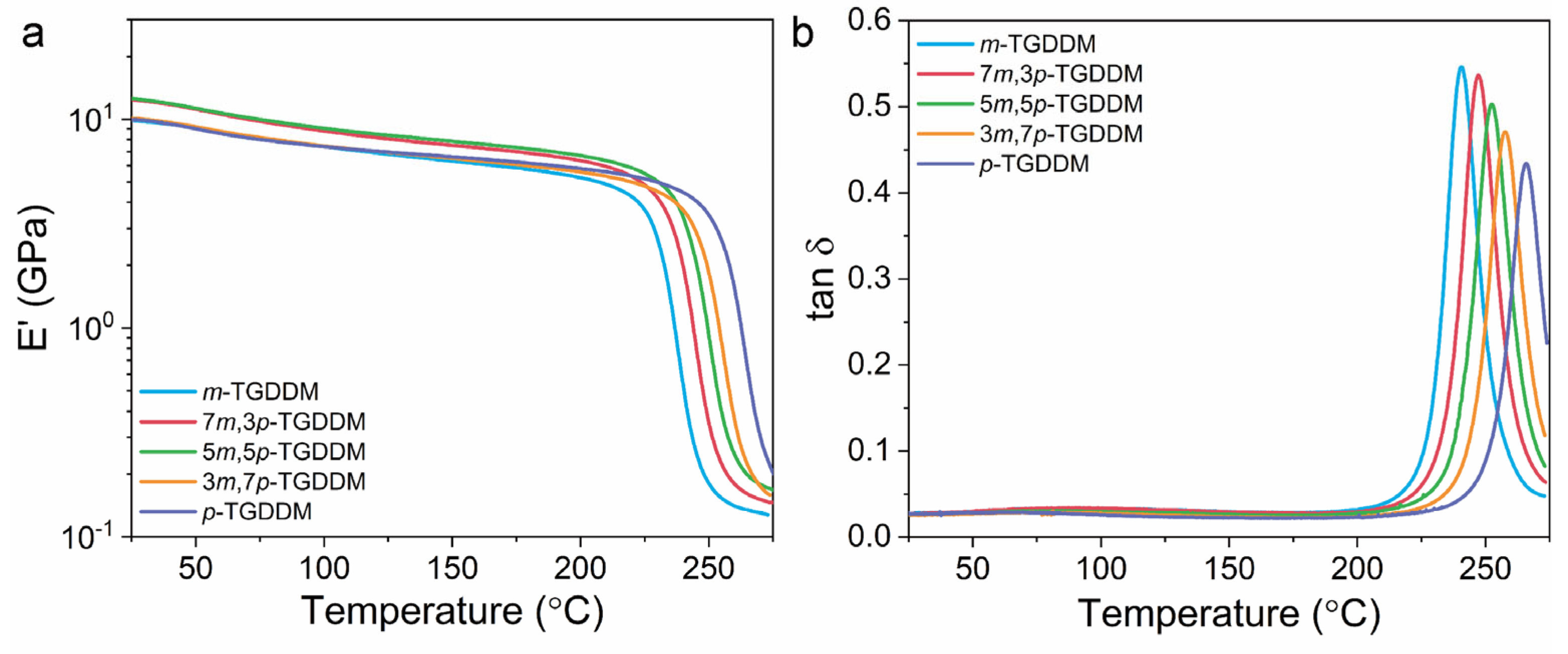
3.2. Mechanical and Thermal Properties of the Prepared Samples
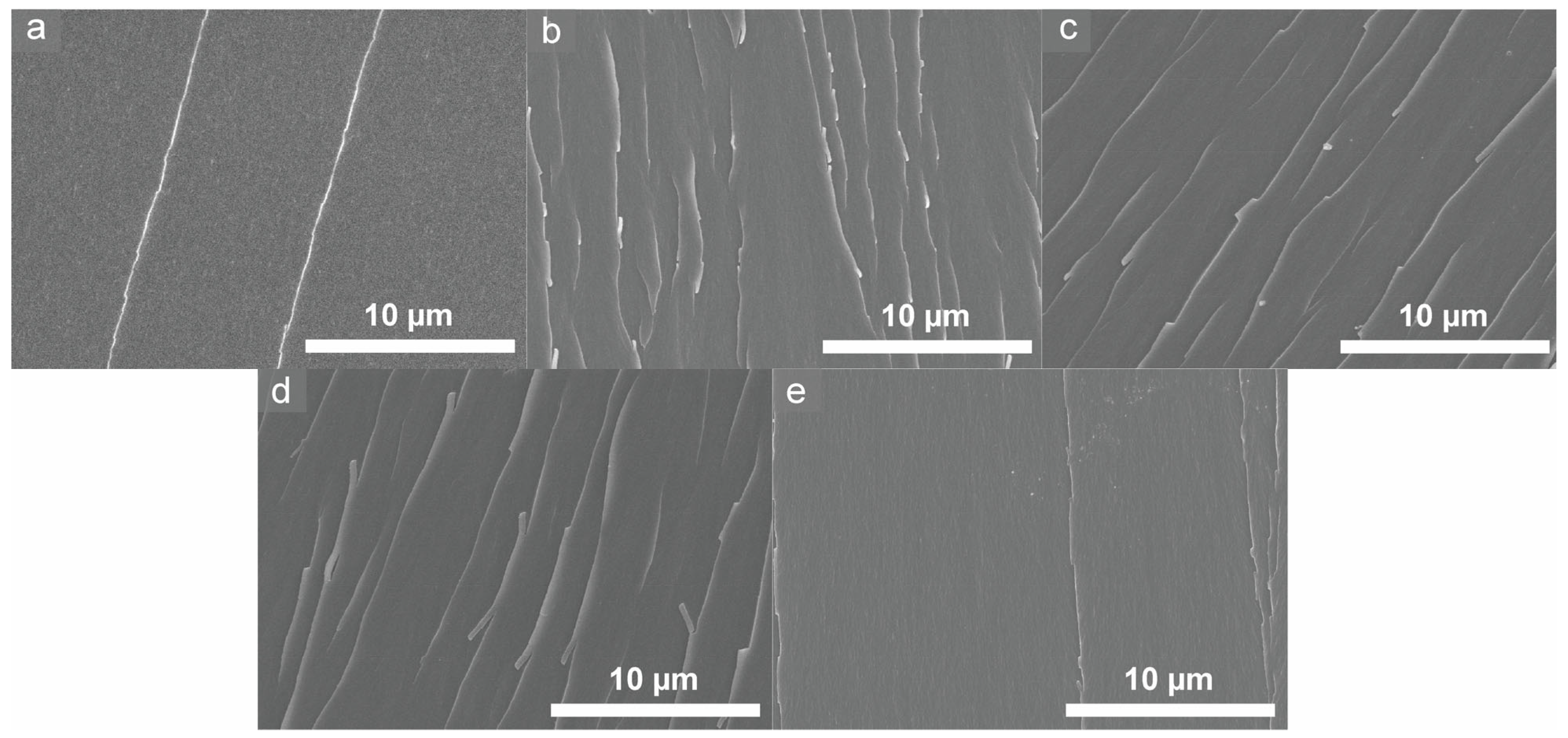
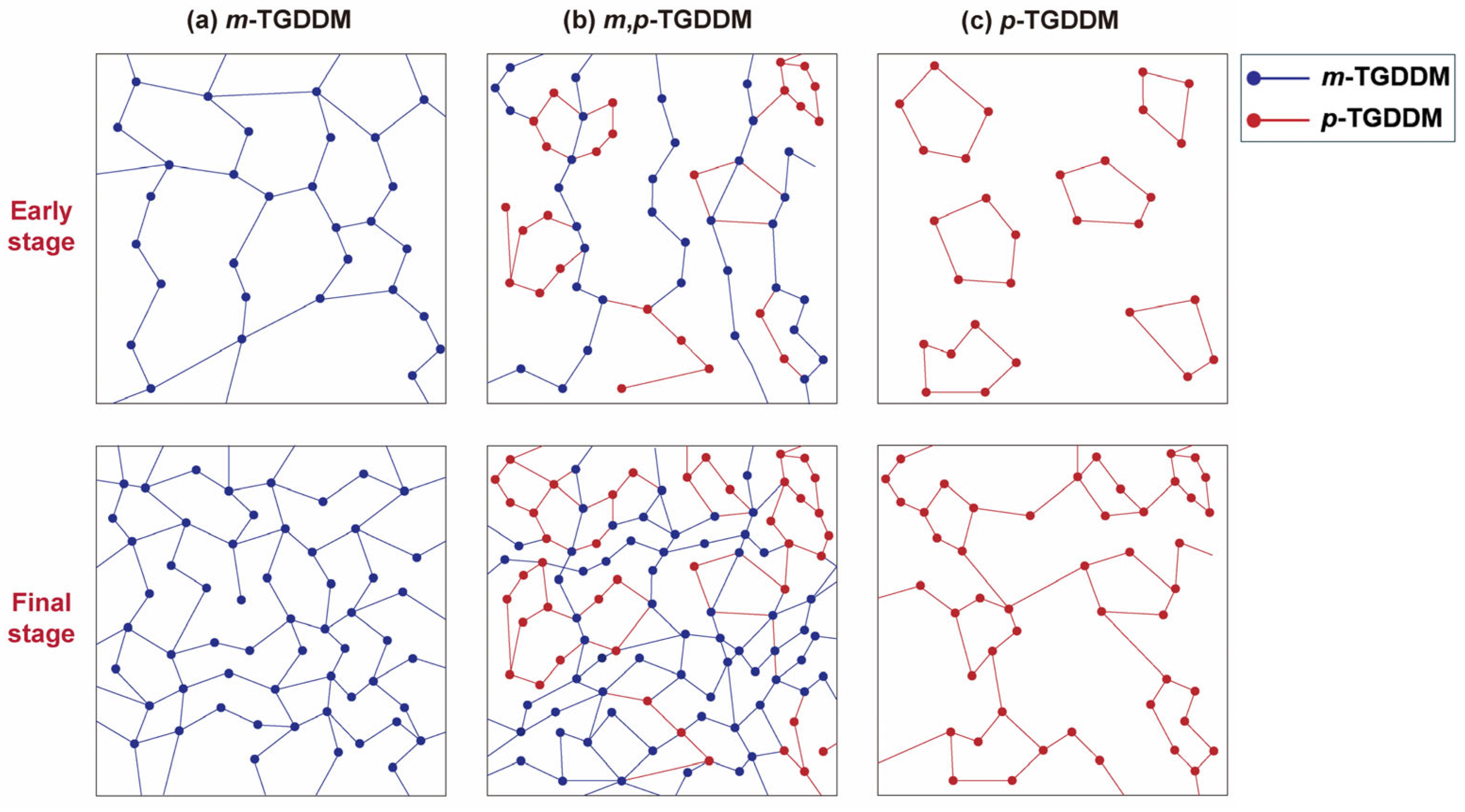
3.3. Mechanism of Property Changes in Epoxy Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Le, Q.-H.; Kuan, H.-C.; Dai, J.-B.; Zaman, I.; Luong, L.; Ma, J. Structure–property relations of 55 nm particle-toughened epoxy. Polymer 2010, 51, 4867–4879. [Google Scholar] [CrossRef]
- Marks, M.J.; Snelgrove, R.V. Effect of conversion on the structure− property relationships of amine-cured epoxy thermosets. ACS Appl. Mater. Interfaces 2009, 1, 921–926. [Google Scholar] [CrossRef]
- Stutz, H. Lifetime assessment of epoxies by the kinetics of thermal degradation. J. Appl. Polym. Sci. 2004, 91, 1881–1886. [Google Scholar] [CrossRef]
- Carra, G.; Carvelli, V. Ageing of pultruded glass fibre reinforced polymer composites exposed to combined environmental agents. Compos. Struct. 2014, 108, 1019–1026. [Google Scholar] [CrossRef]
- Johnsen, B.; Kinloch, A.; Taylor, A. Toughness of syndiotactic polystyrene/epoxy polymer blends: Microstructure and toughening mechanisms. Polymer 2005, 46, 7352–7369. [Google Scholar] [CrossRef]
- Mahieux, C. Cost effective manufacturing process of thermoplastic matrix composites for the traditional industry: The example of a carbon-fiber reinforced thermoplastic flywheel. Compos. Struct. 2001, 52, 517–521. [Google Scholar] [CrossRef]
- Okabe, T.; Oya, Y.; Tanabe, K.; Kikugawa, G.; Yoshioka, K. Molecular dynamics simulation of crosslinked epoxy resins: Curing and mechanical properties. Eur. Polym. J. 2016, 80, 78–88. [Google Scholar] [CrossRef]
- Salimi, A.; Maghsoudian, S.; Mirzataheri, M. Synthesis and characterization of the nano alumina-filled tetraglycidyl-4, 4′-diaminodiphenylmethane epoxy resin. Polym. Bull. 2017, 74, 1283–1298. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, X.; Yu, X. Reaction mechanism, cure behavior and properties of a multifunctional epoxy resin, TGDDM, with latent curing agent dicyandiamide. RSC Adv. 2018, 8, 8248–8258. [Google Scholar] [CrossRef]
- Bae, J.-S.; Bae, J.; Woo, H.; Lee, B.; Jeong, E. Novel thermoplastic toughening agents in epoxy matrix for vacuum infusion process manufactured composites. Carbon Lett. 2018, 25, 43–49. [Google Scholar]
- Drake, R.; Siebert, A. Elastomer-modified epoxy resins for structural applications. Sampe Q. 1975, 6, 11–21. [Google Scholar]
- Kinloch, A.; Shaw, S.; Tod, D.; Hunston, D. Deformation and fracture behaviour of a rubber-toughened epoxy: 1. Microstructure and fracture studies. Polymer 1983, 24, 1341–1354. [Google Scholar] [CrossRef]
- Pearson, R.A.; Yee, A.F. Toughening mechanisms in elastomer-modified epoxies: Part 2 Microscopy studies. J. Mater. Sci. 1986, 21, 2475–2488. [Google Scholar] [CrossRef]
- Rowe, E.; Siebert, A.; Drake, R. Toughening thermosets with liquid butadiene/acrylonitrile polymers. Mod. Plast. 1970, 47, 110–117. [Google Scholar]
- Esmaeili, A.; Ma, D.; Manes, A.; Oggioni, T.; Jiménez-Suárez, A.; Ureña, A.; Hamouda, A.; Sbarufatti, C. An experimental and numerical investigation of highly strong and tough epoxy based nanocomposite by addition of MWCNTs: Tensile and mode I fracture tests. Compos. Struct. 2020, 252, 112692. [Google Scholar] [CrossRef]
- Jayan, J.S.; Saritha, A.; Deeraj, B.; Joseph, K. Triblock copolymer grafted Graphene oxide as nanofiller for toughening of epoxy resin. Mater. Chem. Phys. 2020, 248, 122930. [Google Scholar] [CrossRef]
- Ma, J.; Mo, M.-S.; Du, X.-S.; Rosso, P.; Friedrich, K.; Kuan, H.-C. Effect of inorganic nanoparticles on mechanical property, fracture toughness and toughening mechanism of two epoxy systems. Polymer 2008, 49, 3510–3523. [Google Scholar] [CrossRef]
- Brooker, R.; Kinloch, A.; Taylor, A. The morphology and fracture properties of thermoplastic-toughened epoxy polymers. J. Adhes. 2010, 86, 726–741. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Partridge, I.K. Phase separation in epoxy resins containing polyethersulphone. Polymer 1983, 24, 639–644. [Google Scholar] [CrossRef]
- Kinloch, A.; Yuen, M.; Jenkins, S. Thermoplastic-toughened epoxy polymers. J. Mater. Sci. 1994, 29, 3781–3790. [Google Scholar] [CrossRef]
- Fischer, F.; Beier, U.; Wolff-Fabris, F.; Altstädt, V. Toughened high performance epoxy resin system for aerospace applications. Sci. Eng. Compos. Mater. 2011, 18, 209–215. [Google Scholar] [CrossRef]
- Zubeldia, A.; Larranaga, M.; Remiro, P.; Mondragon, I. Fracture toughening of epoxy matrices with blends of resins of different molecular weights and other modifiers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3920–3933. [Google Scholar] [CrossRef]
- Levita, G.; De Petris, S.; Marchetti, A.; Lazzeri, A. Crosslink density and fracture toughness of epoxy resins. J. Mater. Sci. 1991, 26, 2348–2352. [Google Scholar] [CrossRef]
- Kwon, D.; Lee, M.; Kwon, W.; Lee, E.; Jeong, E. Influence of stereoisomerism of epoxy hardeners on fracture toughness of carbon fiber/epoxy composites. Carbon Lett. 2019, 29, 449–453. [Google Scholar] [CrossRef]
- Sahagun, C.M. Molecular Network Development of a Thermosetting Epoxy-Amine Polymer. Ph.D. Dissertation, The University of Southern Mississippi, Hattiesburg, MS, USA, 2012. [Google Scholar]
- Ramsdale-Capper, R.; Foreman, J.P. Internal antiplasticisation in highly crosslinked amine cured multifunctional epoxy resins. Polymer 2018, 146, 321–330. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Chen, R.; Wang, L.; Han, B.; Meng, Y.; Li, X. Nitrogen-free tetrafunctional epoxy and its DDS-cured high-performance matrix for aerospace applications. Ind. Eng. Chem. Res. 2017, 56, 7708–7719. [Google Scholar] [CrossRef]
- Mustafa, M.F.; Cook, W.D.; Schiller, T.L.; Siddiqi, H.M. Curing behavior and thermal properties of TGDDM copolymerized with a new pyridine-containing diamine and with DDM or DDS. Thermochim. Acta 2014, 575, 21–28. [Google Scholar] [CrossRef]
- Levchik, S.V.; Camino, G.; Luda, M.P.; Costa, L.; Costes, B.; Henry, Y.; Muller, G.; Morel, E. Mechanistic study of thermal behaviour and combustion performance of epoxy resins. II. TGDDM/DDS system. Polym. Degrad. Stab. 1995, 48, 359–370. [Google Scholar] [CrossRef]
- Jackson, M.; Kaushik, M.; Nazarenko, S.; Ward, S.; Maskell, R.; Wiggins, J. Effect of free volume hole-size on fluid ingress of glassy epoxy networks. Polymer 2011, 52, 4528–4535. [Google Scholar] [CrossRef]
- Deng, Y.; Martin, G.C. Diffusion and diffusion-controlled kinetics during epoxy-amine cure. Macromolecules 1994, 27, 5147–5153. [Google Scholar] [CrossRef]
- Camargo, R.; Gonzalez, V.; Macosko, C.; Tirrell, M. Bulk polymerization kinetics by the adiabatic reactor method. Rubber. Chem. Technol. 1983, 56, 774–783. [Google Scholar] [CrossRef]
- Barton, J.M. The application of differential scanning calorimetry (DSC) to the study of epoxy resin curing reactions. In Epoxy Resins and Composites I; Springer: Berlin/Heidelberg, Germany, 2005; pp. 111–154. [Google Scholar]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Flynn, J. The isoconversional method for determination of energy of activation at constant heating rates: Corrections for the Doyle approximation. J. Therm. Anal. 1983, 27, 95–102. [Google Scholar] [CrossRef]
- Pramanik, M.; Fowler, E.W.; Rawlins, J.W. Another look at epoxy thermosets correlating structure with mechanical properties. Polym. Eng. Sci. 2014, 54, 1990–2004. [Google Scholar] [CrossRef]
- Whittaker, M.B.; Foreman, J.P. Identifying the Influences on Network Formation in Structural Isomers of Multifunctional Epoxies Using Near-Infrared Spectroscopy. Macromolecules 2024, 57, 3438–3450. [Google Scholar] [CrossRef]
- Tucker, S.J. Study of 3,3′ vs. 4,4′DDS Isomer Curatives on Physical Properties and Phenyl Ring Motions of DGEBA Epoxy via Molecular Dynamics, Deuterium NMR, and Dielectric Spectroscopy. Ph.D. Thesis, The University of Southern Mississippi, Hattiesburg, MS, USA, 2010. [Google Scholar]
- Cheng, X.; Wu, Q.; Morgan, S.E.; Wiggins, J.S. Morphologies and mechanical properties of polyethersulfone modified epoxy blends through multifunctional epoxy composition. J. Appl. Polym. Sci. 2017, 134, 44775. [Google Scholar] [CrossRef]
- Fernandez, B.; Arbelaiz, A.; Diaz, E.; Mondragon, I. Influence of polyethersulfone modification of a tetrafunctional epoxy matrix on the fracture behavior of composite laminates based on woven carbon fibers. Polym. Compos. 2004, 25, 480–488. [Google Scholar] [CrossRef]
- Hourston, D.J.; Lane, J.M.; Macbeath, N.A. Toughening of epoxy resins with thermoplastics. Ii. Tetrafunctional epoxy resin-polyetherimide blends. Polym. Int. 1991, 26, 17–21. [Google Scholar] [CrossRef]
- Biolley, N.; Pascal, T.; Sillion, B. Polyimide-modified epoxy system: Time-temperature-transformation diagrams, mechanical and thermal properties. Polymer 1994, 35, 558–564. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, C.; Yang, X.; Zhao, X.; Huang, W. Morphology and properties of TGDDM/DDS epoxy systems toughened by amino-bearing phenyl silicone resins. J. Mater. Sci. 2012, 47, 4415–4427. [Google Scholar] [CrossRef]
- Asif, A.; Shi, W.; Shen, X.; Nie, K. Physical and thermal properties of UV curable waterborne polyurethane dispersions incorporating hyperbranched aliphatic polyester of varying generation number. Polymer 2005, 46, 11066–11078. [Google Scholar] [CrossRef]
- Hübner, F.; Brückner, A.; Dickhut, T.; Altstädt, V.; de Anda, A.R.; Ruckdäschel, H. Low temperature fatigue crack propagation in toughened epoxy resins aimed for filament winding of type V composite pressure vessels. Polym. Test. 2021, 102, 107323. [Google Scholar] [CrossRef]
- Thitsartarn, W.; Fan, X.; Sun, Y.; Yeo, J.C.C.; Yuan, D.; He, C. Simultaneous enhancement of strength and toughness of epoxy using POSS-Rubber core–shell nanoparticles. Compos. Sci. Technol. 2015, 118, 63–71. [Google Scholar] [CrossRef]
- Wang, K.; Chen, L.; Wu, J.; Toh, M.L.; He, C.; Yee, A.F. Epoxy nanocomposites with highly exfoliated clay: Mechanical properties and fracture mechanisms. Macromolecules 2005, 38, 788–800. [Google Scholar] [CrossRef]


| Epoxy Sample | Amount of Curing Agent (phr) | |
|---|---|---|
| 3,3′-DDS | 4,4′-DDS | |
| m-TGDDM | 33.0 | 0 |
| 7m,3p-TGDDM | 23.1 | 9.9 |
| 5m,5p-TGDDM | 16.5 | 16.5 |
| 3m,7p-TGDDM | 9.9 | 23.1 |
| p-TGDDM | 0 | 33.0 |
| Epoxy Sample | Activation Energy (kJ/mol) | |
|---|---|---|
| Kissinger | Flynn–Wall–Ozawa (Average) | |
| m-TGDDM | 64.8 | 75.9 |
| 7m,3p-TGDDM | 64.6 | 85.3 |
| 5m,5p-TGDDM | 64.7 | 67.8 |
| 3m,7p-TGDDM | 68.1 | 72.5 |
| p-TGDDM | 66.6 | 89.2 |
| Epoxy Sample | Storage Modulus (GPa) | Tg (°C) | Crosslink Density (mol/m3) | |
|---|---|---|---|---|
| 25 °C | Tg + 20 °C | |||
| m-TGDDM | 9.9 | 0.124 | 241 | 9.28 × 103 |
| 7m,3p-TGDDM | 12.5 | 0.146 | 247 | 10.8 × 103 |
| 5m,5p-TGDDM | 12.6 | 0.138 | 253 | 10.1 × 103 |
| 3m,7p-TGDDM | 10.1 | 0.125 | 258 | 9.06 × 103 |
| p-TGDDM | 10.0 | 0.122 | 266 | 8.77 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, W.; Cheon, J.; Jeong, H.J.; Won, J.S.; Kim, B.-J.; Lee, M.Y.; Lee, S.G.; Jeong, E. Self-Toughened Epoxy Resin via Hybridization of Structural Isomeric Curing Agents. Polymers 2025, 17, 695. https://doi.org/10.3390/polym17050695
Kwon W, Cheon J, Jeong HJ, Won JS, Kim B-J, Lee MY, Lee SG, Jeong E. Self-Toughened Epoxy Resin via Hybridization of Structural Isomeric Curing Agents. Polymers. 2025; 17(5):695. https://doi.org/10.3390/polym17050695
Chicago/Turabian StyleKwon, Woong, Jiyeon Cheon, Hei Je Jeong, Jong Sung Won, Byeong-Joo Kim, Man Young Lee, Seung Geol Lee, and Euigyung Jeong. 2025. "Self-Toughened Epoxy Resin via Hybridization of Structural Isomeric Curing Agents" Polymers 17, no. 5: 695. https://doi.org/10.3390/polym17050695
APA StyleKwon, W., Cheon, J., Jeong, H. J., Won, J. S., Kim, B.-J., Lee, M. Y., Lee, S. G., & Jeong, E. (2025). Self-Toughened Epoxy Resin via Hybridization of Structural Isomeric Curing Agents. Polymers, 17(5), 695. https://doi.org/10.3390/polym17050695








