Surface Modification of Organic Chromium-Free Tanned Leather Shavings and the Immobilization of Lipase
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
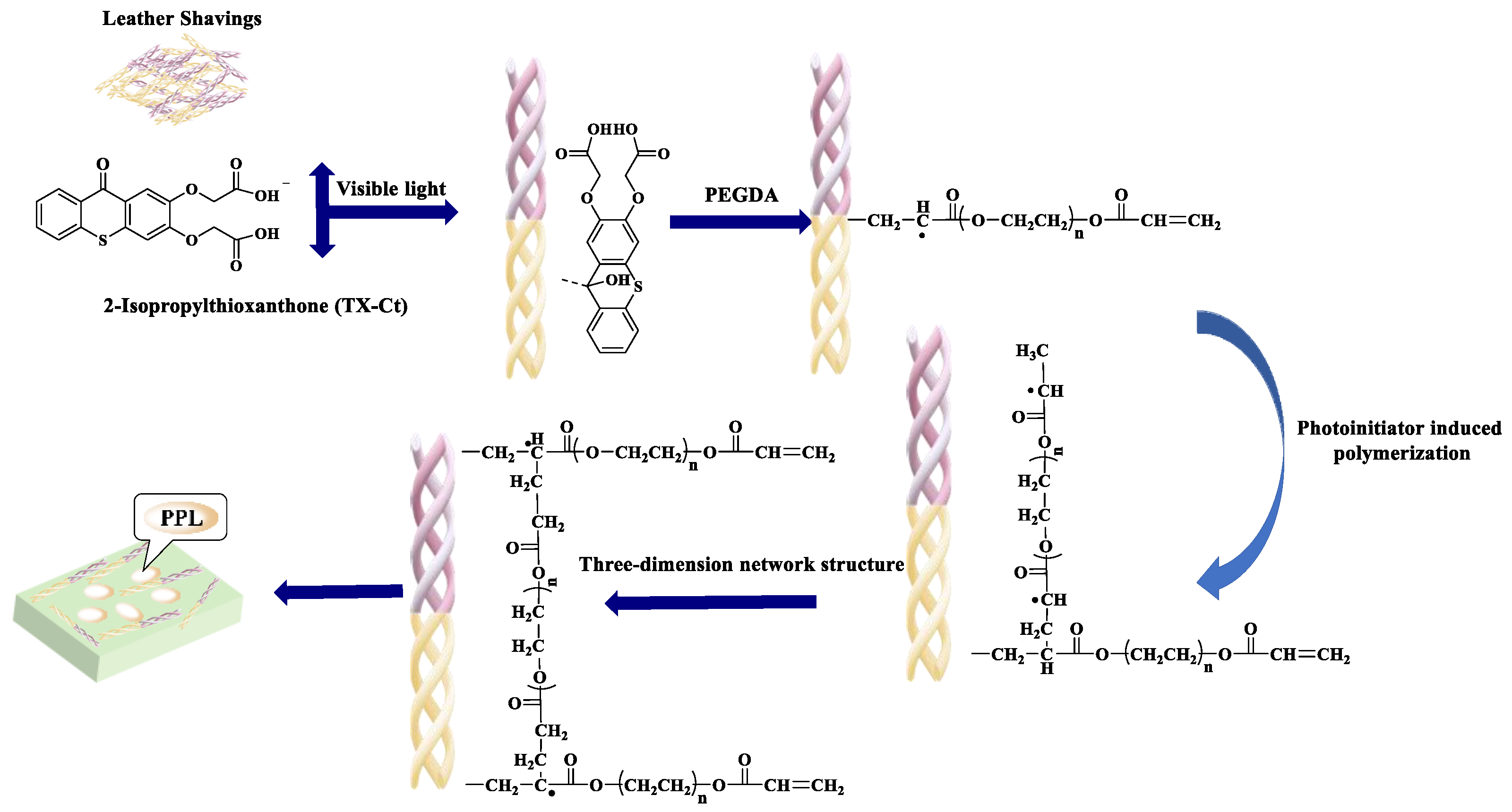
2.2.1. Synthesis of Photoinitiator TX-Ct
2.2.2. Lipase Immobilization
2.3. Testing and Characterisation
2.3.1. Scanning Electron Microscope (SEM)
2.3.2. X-Ray Photoelectron Spectroscopy Test (XPS)
2.3.3. Fourier Transform Infrared Spectroscopy Test (FT-IR)
2.3.4. Ultra-Depth-of-Field Microscope Test
2.4. Studies on the Catalytic Properties of Immobilized Lipases
2.5. Enzyme Load Testing
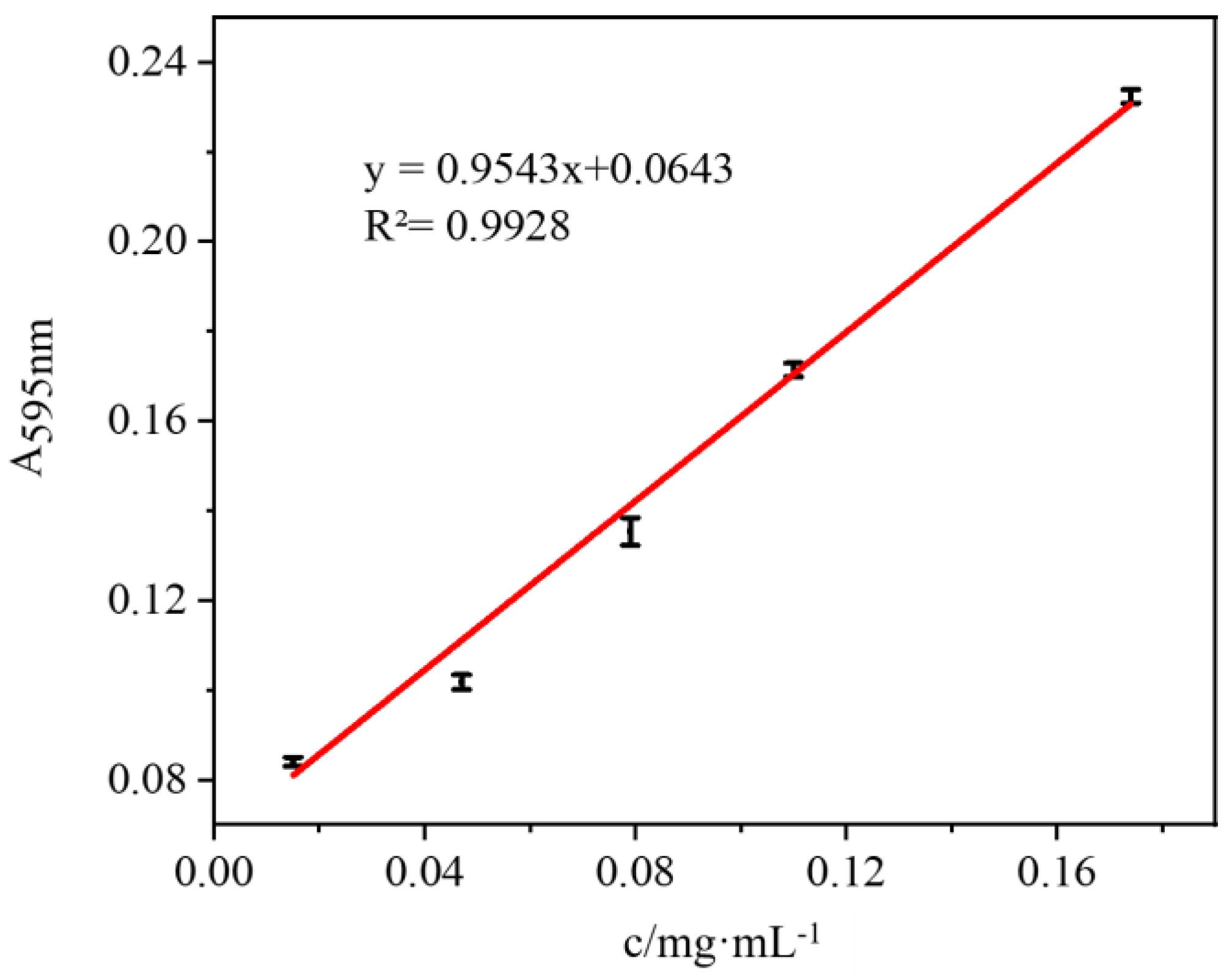
2.6. Study on the Environmental Performance of Immobilized Lipase
- (1)
- The catalytic activity test of lipase was conducted according to reference [31].
- (2)
- Biological risk testing of soil degradates
- (3)
- Hydrolysis efficiency test of immobilized enzymes
- (4)
- Determination of optimal catalytic conditions
3. Results and Discussion
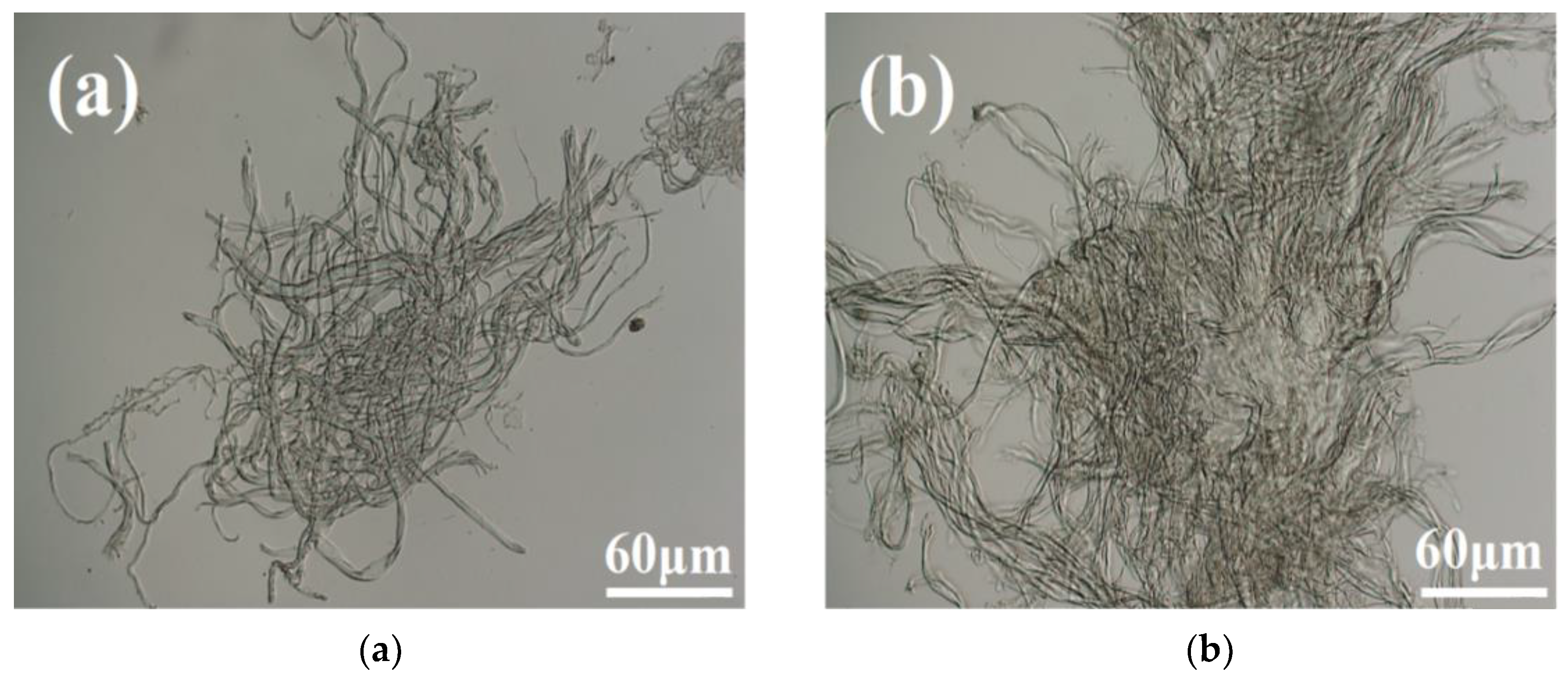
3.1. Ultra-Depth-of-Field Microscope Analysis
3.2. SEM Analysis
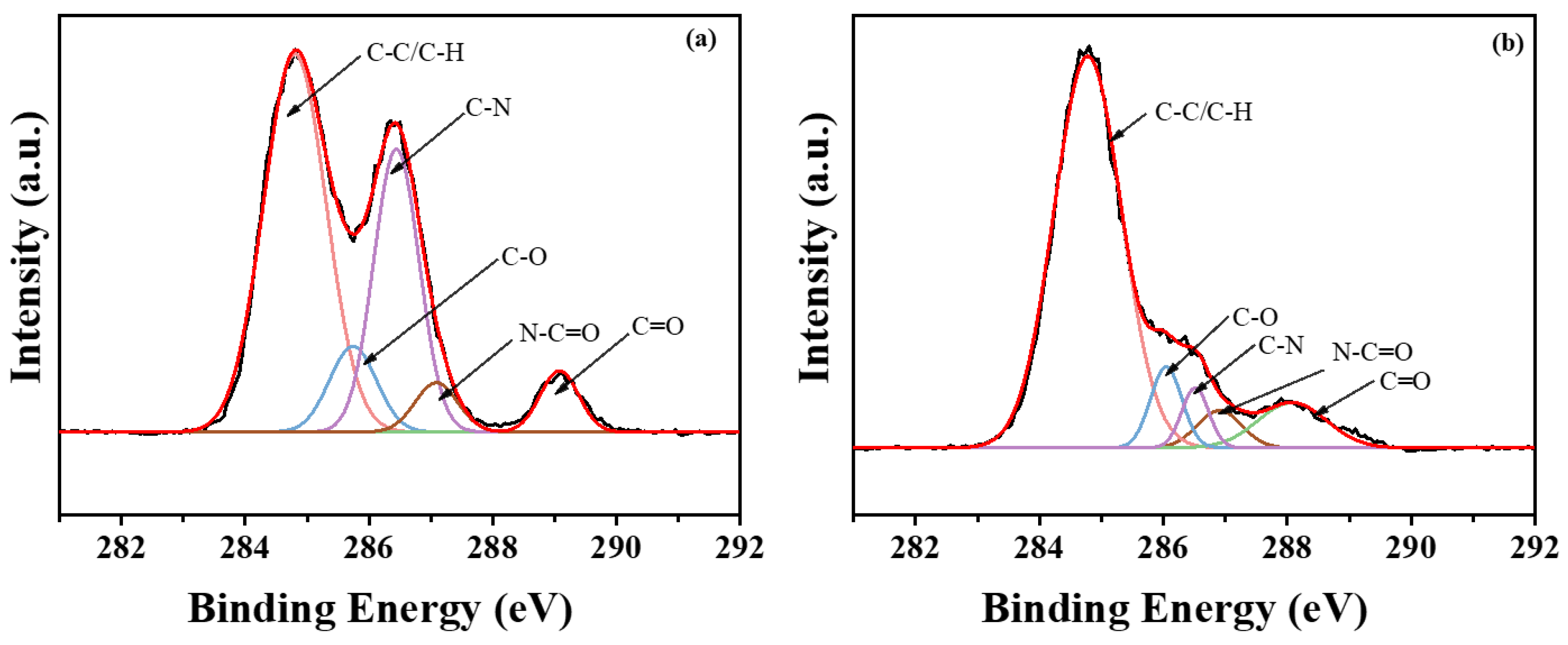
3.3. XPS Analysis
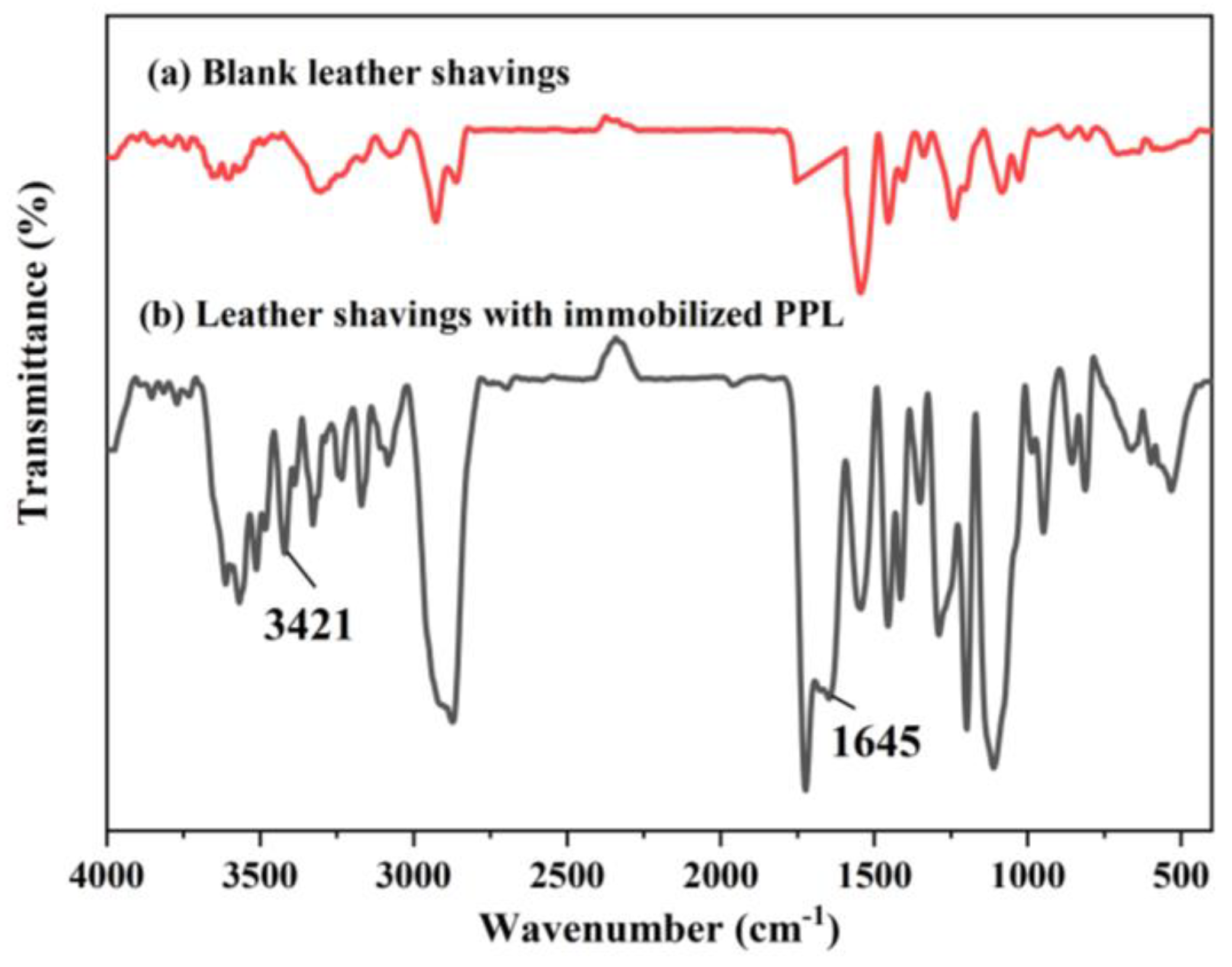
3.4. FTIR Analysis
3.5. Catalytic Properties of Immobilized Lipase
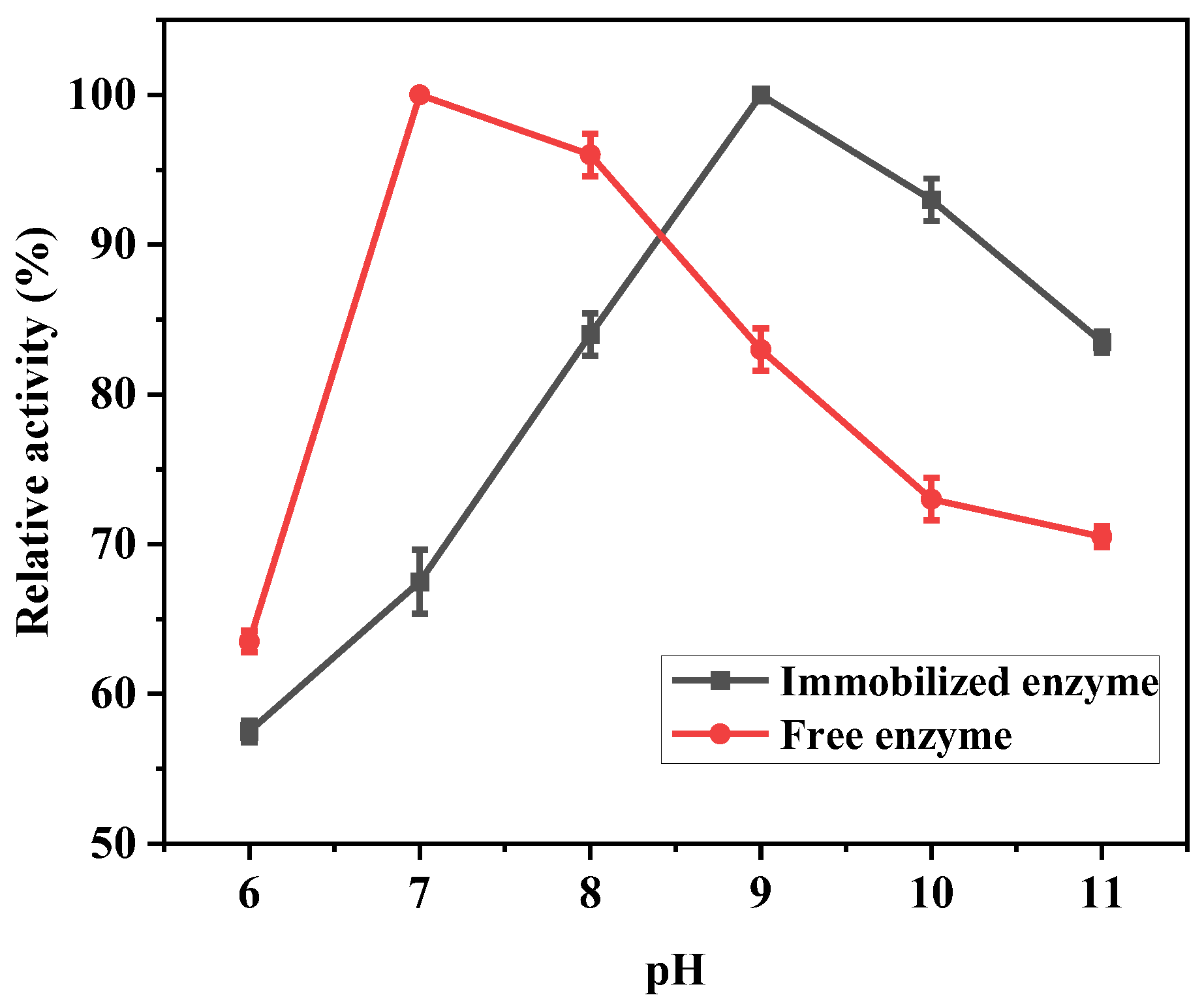
3.5.1. The Optimal Catalytic pH for Immobilized Lipase
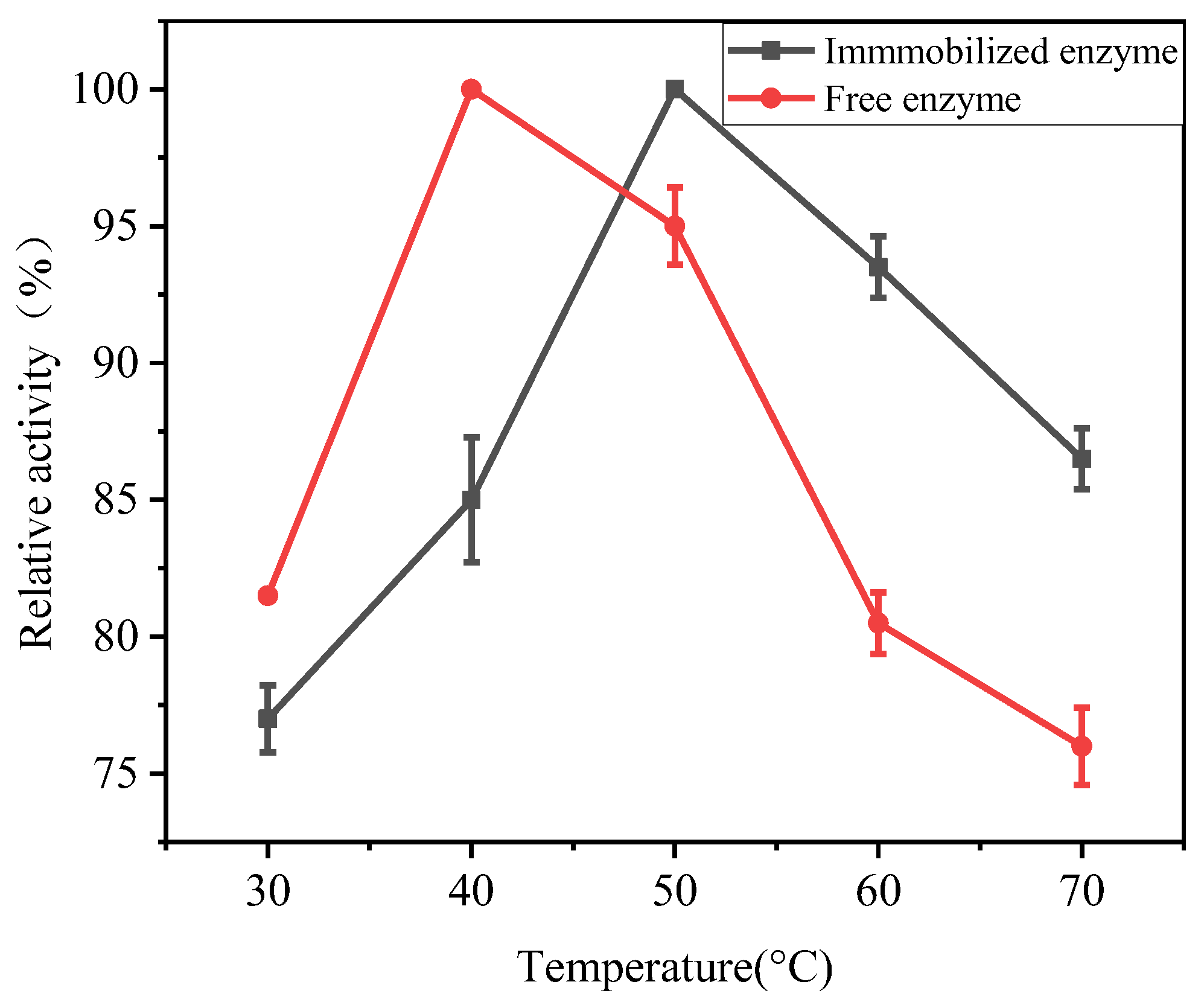
3.5.2. Optimum Catalytic Temperature
3.5.3. Determination of Storage Stability
3.5.4. Hydrolysis Efficiency of Immobilized Enzymes
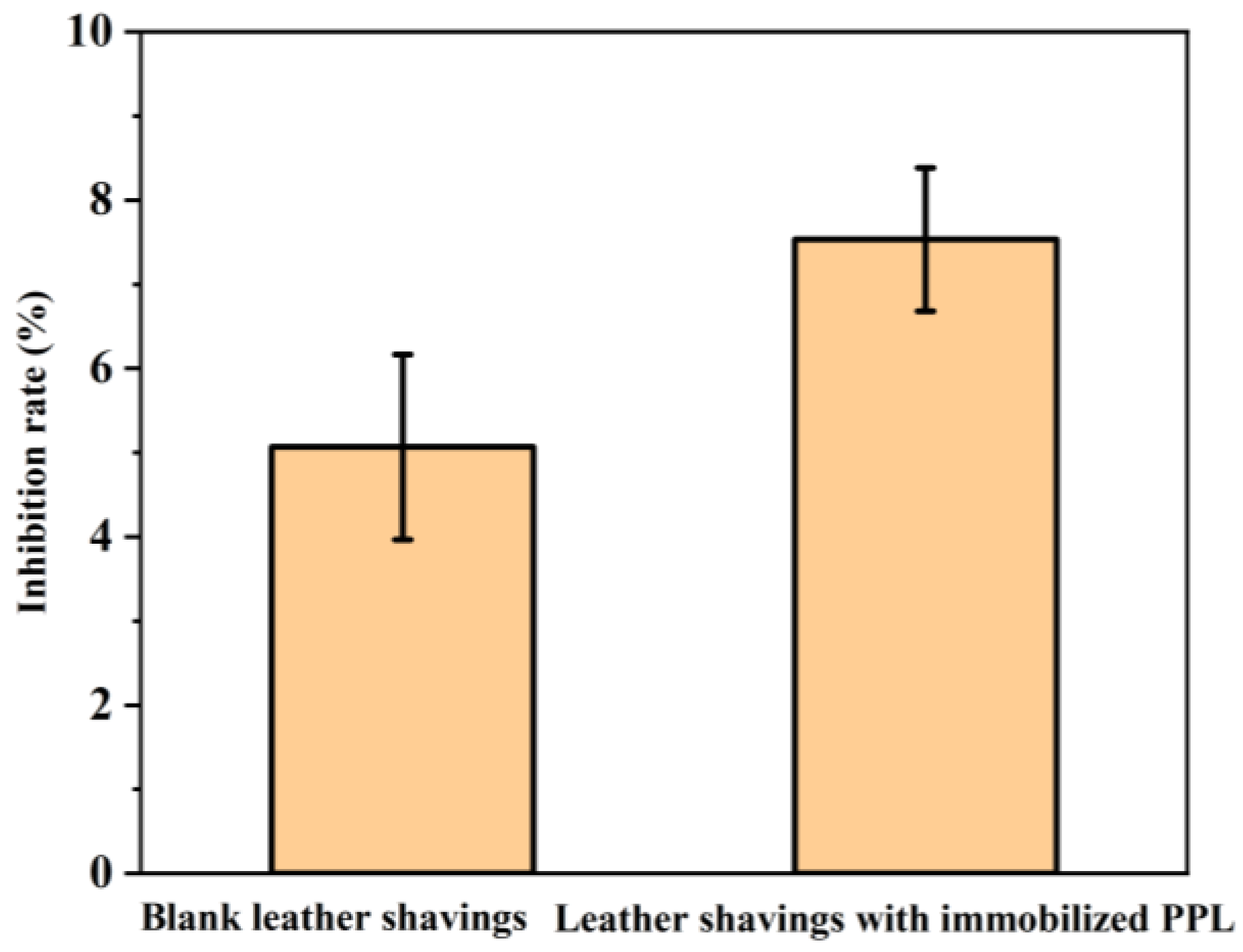
3.5.5. Soil Toxicity Testing of Immobilized Enzymes
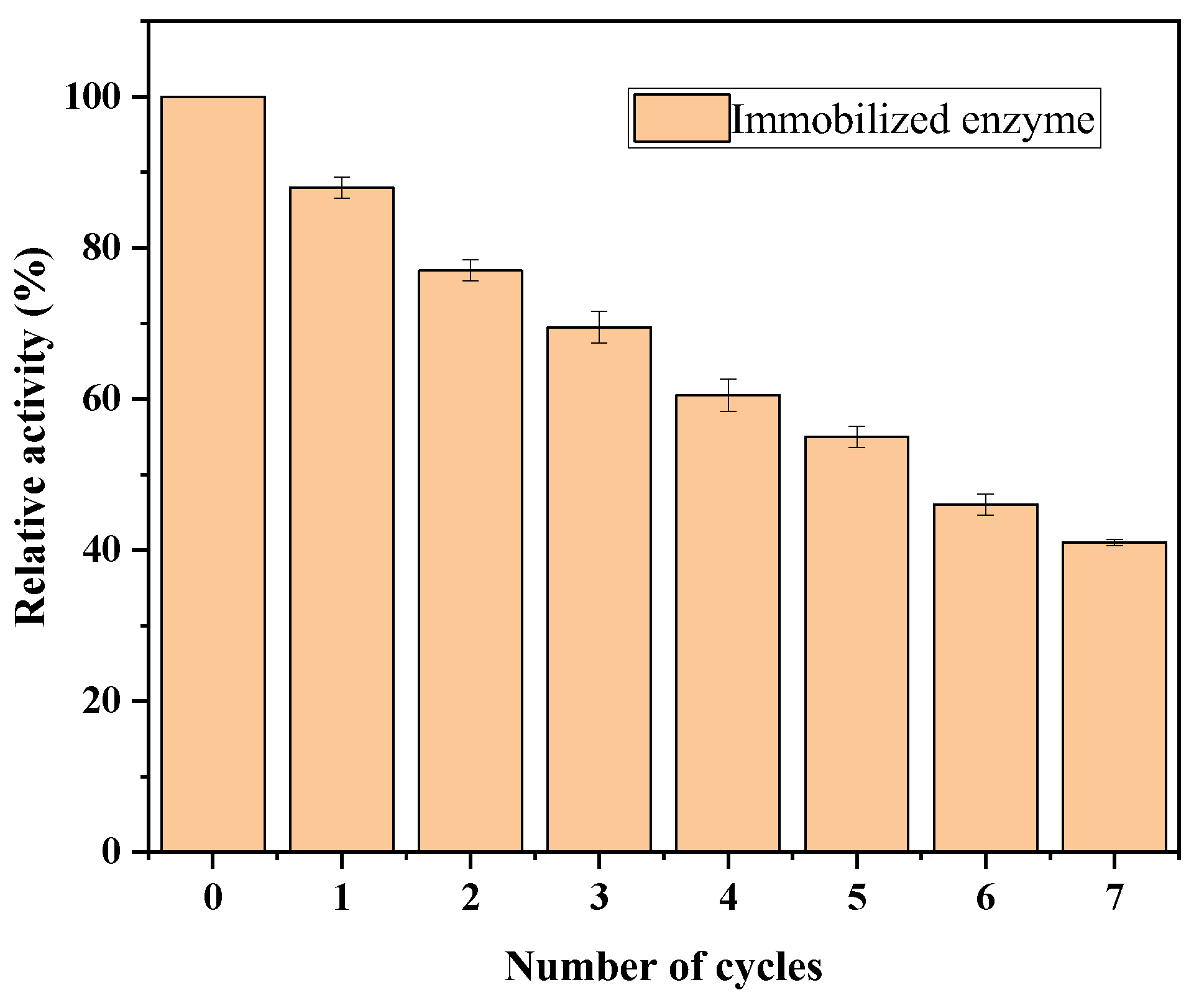
3.5.6. Circulating Stability Testing of Immobilized Lipase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Onem, E.; Heil, V.; Yesil, H.; Prokein, M.; Renner, M. Hydrocarbon fuel blendstock from tannery waste: Energy from fleshing oil via gas phase catalytic cracking. Biofuels Bioprod. Biorefining 2024, 18, 1423–1436. [Google Scholar] [CrossRef]
- Muralidharan, V.; Palanivel, S.; Balaraman, M. Turning problem into possibility: A comprehensive review on leather solid waste intra-valorization attempts for leather processing. J. Clean. Prod. 2022, 367, 133021. [Google Scholar] [CrossRef]
- Khambhaty, Y. Applications of enzymes in leather processing. Environ. Chem. Lett. 2022, 18, 747–769. [Google Scholar] [CrossRef]
- Hodaifa, G.; Gallardo, P.A.R.; García, C.A.; Kowalska, M.; Seyedsalehi, M. Chemical oxidation methods for treatment of real industrial olive oil mill wastewater. J. Taiwan Inst. Chem. E 2019, 97, 247–254. [Google Scholar] [CrossRef]
- Aghel, B.; Gouran, A.; Nasirmanesh, F. Transesterification of waste cooking oil using clinoptilolite/industrial phosphoric waste as green and environmental catalysts. Energy 2022, 244, 123138. [Google Scholar] [CrossRef]
- Sabino, J.; Liborio, D.O.; Arias, S.; Gonzalez, J.F.; Barbosa, C.M.B.M.; Carvalho, F.R.; Frety, R.; Barros, I.C.L.; Pacheco, J.G.A. Hydrogen-free deoxygenation of oleic acid and industrial vegetable oil waste on CuNiAl catalysts for biofuel production. Energies 2023, 16, 6131. [Google Scholar] [CrossRef]
- Aghel, B.; Gouran, A.; Shahsavari, P. Optimizing the production of biodiesel from waste cooking oil utilizing industrial waste-derived MgO/CaO catalysts. Chem. Eng. Technol. 2022, 45, 348–354. [Google Scholar] [CrossRef]
- Welter, R.A.; Santana, H.S.; de la Torre, L.G.; Barnes, M.C.; Taranto, O.P.; Oelgemöller, M. Biodiesel Production by Heterogeneous Catalysis and Eco-friendly Routes. ChemBioEng Rev. 2023, 10, 86–111. [Google Scholar] [CrossRef]
- Liu, C.M. The Process Selection for The Production of Fatty Acid in Oil Splitting Method. Guangdong Chem. 2014, 41, 105–106. [Google Scholar]
- Vescovi, V.; Rojas, M.J.; Baraldo, A.; Botta, D.C.; Santana, F.A.M.; Costa, J.P.; Machado, M.S.; Honda, V.K.; de Lima Camargo Giordano, R.; Tardioli, P.W. Lipase-catalyzed production of biodiesel by hydrolysis of waste cooking oil followed by esterification of free fatty acids. J. Am. Oil Chem. Soc. 2016, 93, 1615–1624. [Google Scholar] [CrossRef]
- Salgado, C.A.; dos Santos CI, A.; Vanetti MC, D. Microbial lipases: Propitious biocatalysts for the food industry. Food Biosci. 2022, 45, 101509. [Google Scholar] [CrossRef]
- Razack, S.A.; Duraiarasan, S. Response surface methodology assisted biodiesel production from waste cooking oil using encapsulated mixed enzyme. Waste Manag. 2016, 47, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, F.T.T.; Neto, F.S.; de Aguiar Falcão, I.R.; da Silva Souza, J.E.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Souza, G.P.; Correia, T.B.; Reis, W.S.; Bredda, E.H.; Da Rós, P.C.; Pereira, E.B. Enzymatic hydrolysis of waste cooking oil by lipase catalysis: Simplex mixture design optimization. Catal. Lett. 2023, 153, 689–697. [Google Scholar] [CrossRef]
- Pang, L.; Liu, M.; Li, X.; Guo, L.; Man, C.; Yang, X.; Jiang, Y. Effect of enzymatic hydrolysis combined with processing on allergenicity of food allergens. Trends Food Sci. Technol. 2023, 143, 104248. [Google Scholar] [CrossRef]
- Wang, T.T. Reseach Advances Inheterogeneous Acid Catalysts forreparation of Biodiesel. Biol. Chem. Eng. 2020, 6, 170–172. [Google Scholar]
- Verma, M.L.; Dhanya, B.S.; Wang, B.; Thakur, M.; Rani, V.; Kushwaha, R. Bio-Nanoparticles mediated transesterification of algal biomass for biodiesel production. Sustainability 2023, 16, 295. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.Y. Enzyme immobilization on cellulose matrixes. J. Bioact. Compat. Pol. 2016, 31, 553–567. [Google Scholar] [CrossRef]
- Aydemir, D.; Gecili, F.; Özdemir, N.; Ulusu, N.N. Synthesis and characterization of a triple enzyme-inorganic hybrid nanoflower (TrpE@ ihNF) as a combination of three pancreatic digestive enzymes amylase, protease and lipase. J. Biosci. Bioeng. 2020, 129, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, immobilization methods, and industrial applications. Appl. Microbiol. Biot. 2019, 103, 7399–7423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, S.; Lv, R.; Golubev, Y.A.; Wang, K.; Dong, F.; Kotova, O.B.; Kotova, E.L. Construction and Characterization of a Nanostructured Biocatalyst Consisting of Immobilized Lipase on Mg-Amino-Clay. Clay Clay Miner. 2021, 69, 434–442. [Google Scholar] [CrossRef]
- Liu, D.M.; Chen, J.; Shi, Y.P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Venezia, V.; Sannino, F.; Costantini, A.; Silvestri, B.; Cimino, S.; Califano, V. Mesoporous silica nanoparticles for β-glucosidase immobilization by templating with a green material: Tannic acid. Microporous Mesoporous Mater. 2020, 302, 110203. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Jiang, Y.; Jiang, T.; Wang, Y.; Chen, Y.; Liu, S. Immobilizing enzymes in regular-sized gelatin microspheres through a membrane emulsification method. J. Mater. Sci. 2016, 51, 6357–6369. [Google Scholar] [CrossRef]
- Wang, K.J. Studies on the Preparation and Enzyme Immobilization of Functional Polymeric Microspheres. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2023. [Google Scholar]
- Hao, D.; Zhu, X.; Hao, D.; Wei, C.; Hu, Y.; Wang, X. Synthesis of Ionic Liquid-Based Amphiphilic Polymer Surfactant and its Application in Leather Fatliquoring. J. Soc. Leather Technol. Chem. 2023, 107, 60–65. [Google Scholar]
- Sun, S.; Wang, X.; Zhu, X.; Liu, X.; Guo, P.; Tian, Y. Synthesis of an amphiphilic amphoteric peptide-based polymer for organic chrome-free ecological tanning. J. Clean. Prod. 2022, 330, 129880. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, B.; Chang, J.; Fu, Y.; Lv, P.; Wang, X. Synthesis of biodiesel from waste cooking oil using immobilized lipase in fixed bed reactor. Energy Convers. Manag. 2009, 50, 668–673. [Google Scholar] [CrossRef]
- Azócar, L.; Ciudad, G.; Heipieper, H.J.; Muñoz, R.; Navia, R. Improving fatty acid methyl ester production yield in a lipase-catalyzed process using waste frying oils as feedstock. J. Biosci. Bioeng. 2010, 109, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H.; Shih, Y.H.; Liu, W.L.; Lin, C.H.; Huang, H.Y. Enzyme immobilized on nano porous carbon derived from metal-organic framework: Anew support for biodiesel synthesis. ChemSusChem 2017, 10, 1364–1369. [Google Scholar] [CrossRef]
- Meany, J.E. Carbonic anhydrase catalysis: An experiment on enzyme kinetics. J. Chem. Educ. 1985, 62, 1124–1125. [Google Scholar]
- GB/T 5009.37-2003; Analysis Method for Hygiene Standards of Edible Vegetable Oils. China Standard Press: Beijing, China, 2003.
- Li, Y.T. Synthesis of Polyethyleneimine-Grafted Silica Nanoparticles And Its Application in Immobilized Enzyme Field. Ph.D. Thesis, Tianjin University, Tianjin, China, 2018. [Google Scholar]
- Wang, X.; Liu, Y.; Liu, X.; You, X.; Zhang, H.J. Degradable gelatin-based supramolecular coating for green paper sizing. ACS Appl. Mater. Interfaces 2020, 13, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Ren, X.Y.; Liu, Y.M. Covalent immobilization of porcine pancreatic lipase on carboxylactivated magnetic nanoparticles: Characterization and application for enzymatic inhibition assays. Mater. Sci. Eng.-C 2014, 38, 278–285. [Google Scholar] [CrossRef]
- Zhu, R.; Yang, C.; Li, K.; Yu, R.; Liu, G.; Peng, B. A smart high chrome exhaustion and chrome-less tanning system based on chromium (III)-loaded nanoparticles for cleaner leather processing. J. Clean. Prod. 2020, 277, 123278. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, D.; Wang, X.; Shi, J.; Wang, Z.; Zhu, X. Surface Modification of Organic Chromium-Free Tanned Leather Shavings and the Immobilization of Lipase. Polymers 2025, 17, 688. https://doi.org/10.3390/polym17050688
Hao D, Wang X, Shi J, Wang Z, Zhu X. Surface Modification of Organic Chromium-Free Tanned Leather Shavings and the Immobilization of Lipase. Polymers. 2025; 17(5):688. https://doi.org/10.3390/polym17050688
Chicago/Turabian StyleHao, Dongyan, Xuechuan Wang, Jiajia Shi, Zhisheng Wang, and Xing Zhu. 2025. "Surface Modification of Organic Chromium-Free Tanned Leather Shavings and the Immobilization of Lipase" Polymers 17, no. 5: 688. https://doi.org/10.3390/polym17050688
APA StyleHao, D., Wang, X., Shi, J., Wang, Z., & Zhu, X. (2025). Surface Modification of Organic Chromium-Free Tanned Leather Shavings and the Immobilization of Lipase. Polymers, 17(5), 688. https://doi.org/10.3390/polym17050688



