Use of Natamycin for the Development of Polymer Systems with Antifungal Activity for Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Ethylene-vinyl acetate (EVA) with the trade name Greenflex ML 60 was purchased from Versalis (Versalis, Milan, Italy). It is an ethylene-vinyl acetate copolymer with a melt flow index of 2.5 dg/min, a melting temperature of 74 °C, 28% vinyl acetate, and a density of 0.952 g/cm3.
- Polycaprolactone (PCL) was purchased from Sigma Aldrich (Sigma Aldrich, St. Louis, MO, USA) with a molecular weight of 80 kDa and a density of 1.145 g/cm3.
- Natamycin (NAT) also known as Pimaricin was supplied by Handary (Handary, Brussels, Belgium). Figure 1 shows an observation by SEM images at different magnifications of Natamycin used in this work.
2.2. Development of Antifungal Films for Packaging Applications
2.3. Characterization
2.3.1. Rheological Characterization
2.3.2. Morphological Characterization
2.3.3. Mechanical Characterization
2.3.4. Differential Scanning Calorimetry Analysis
2.3.5. Surface Analysis
2.3.6. Natamycin Release Kinetic
2.3.7. Determination of Antifungal Activity
3. Results and Discussion
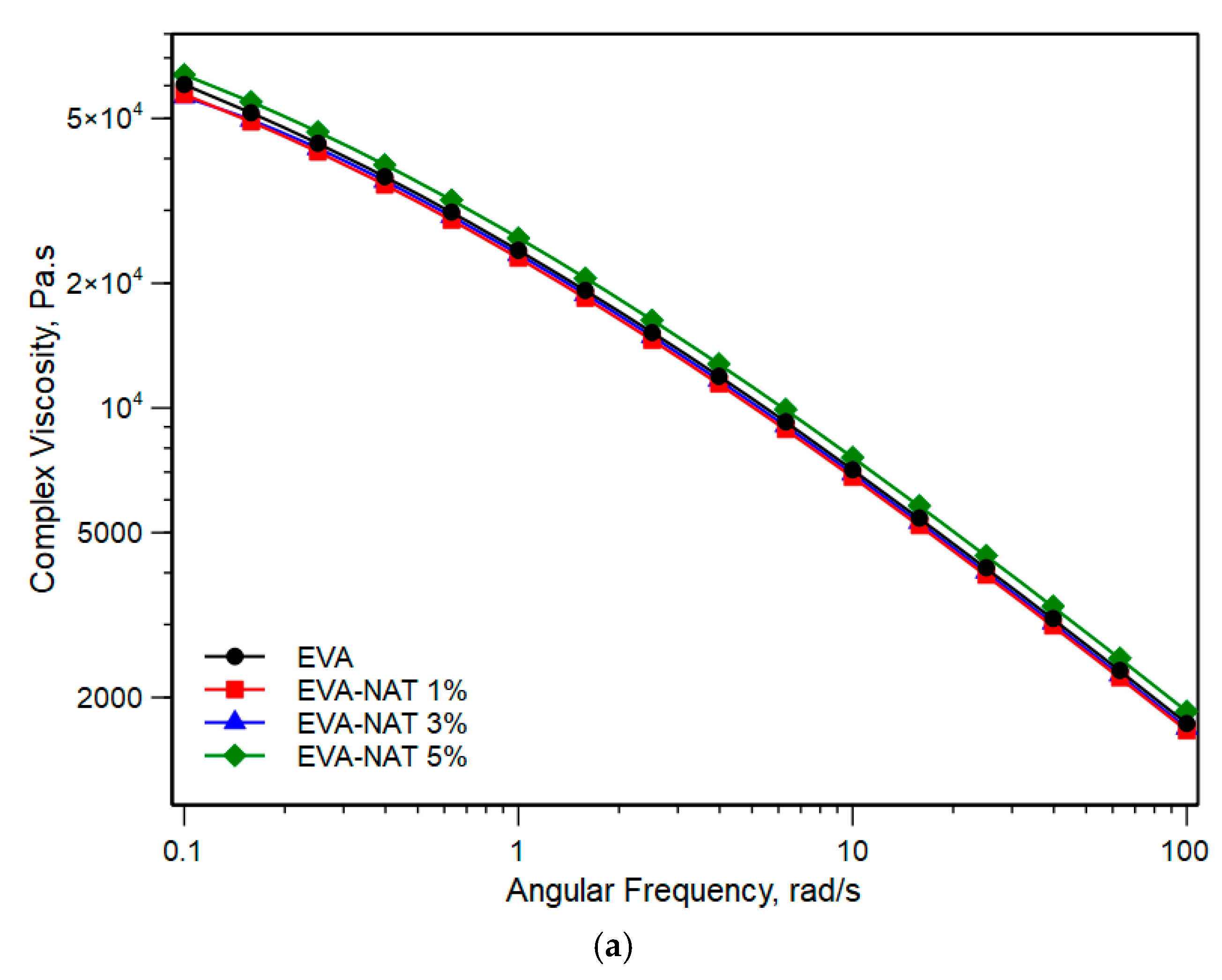
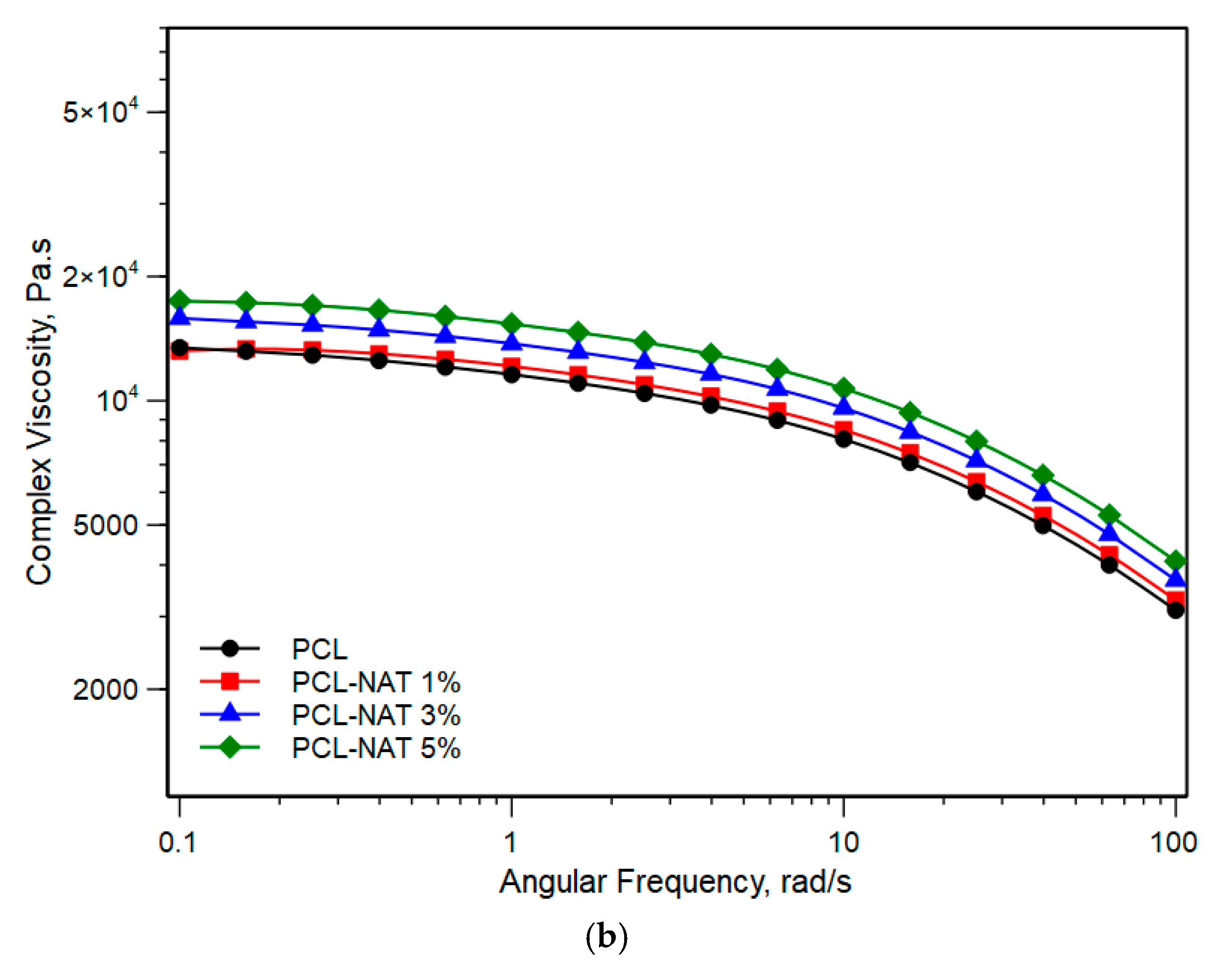
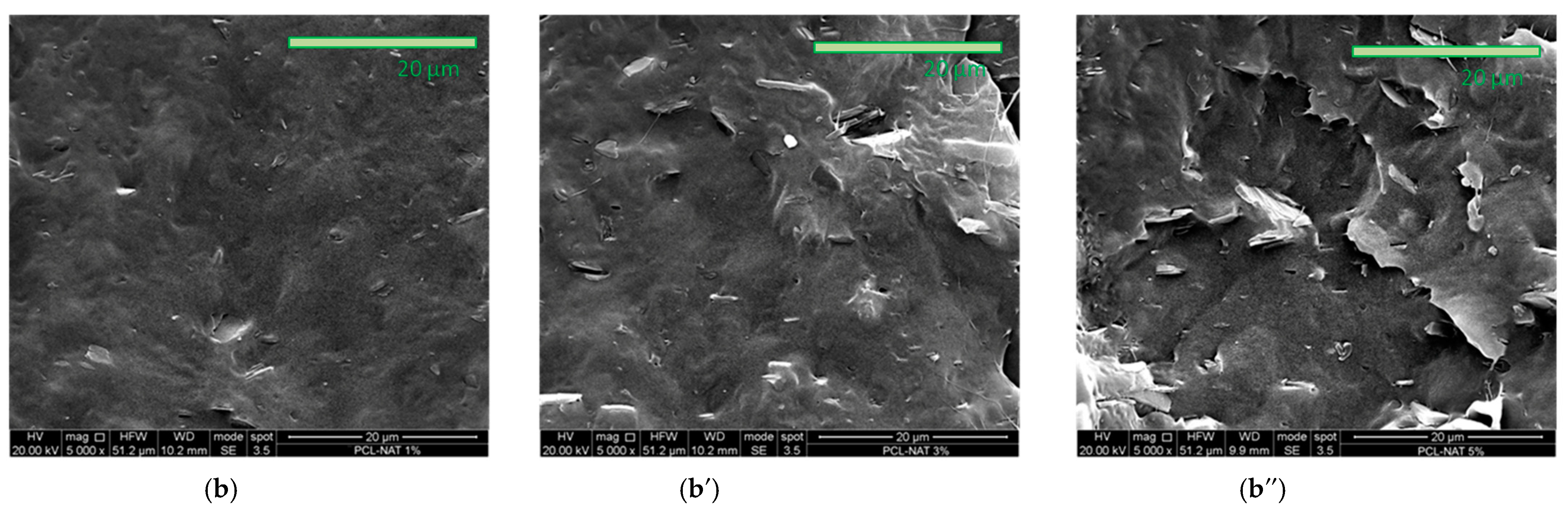
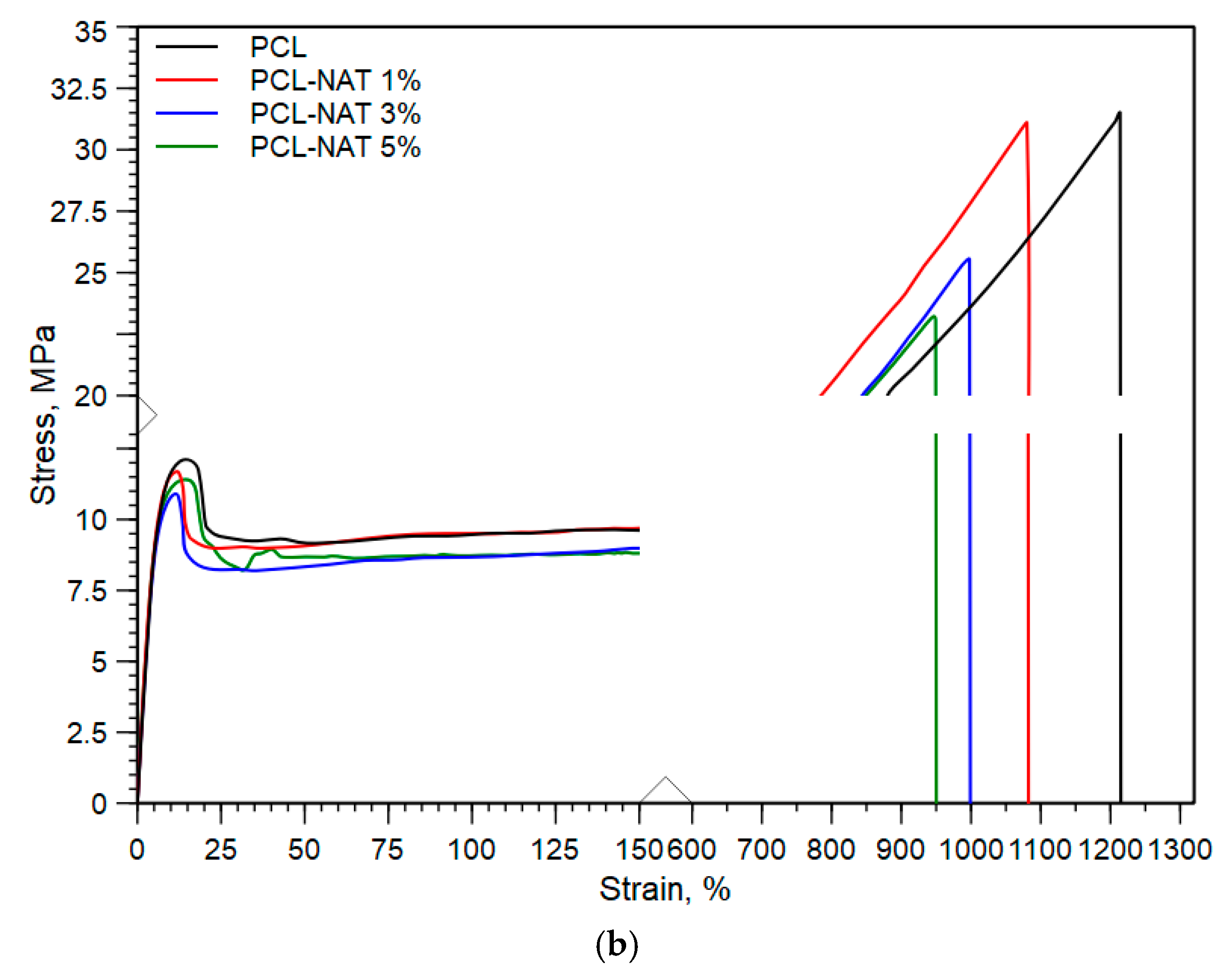
3.1. Physical and Structural Characterization
3.2. Natamycin Release Kinetics
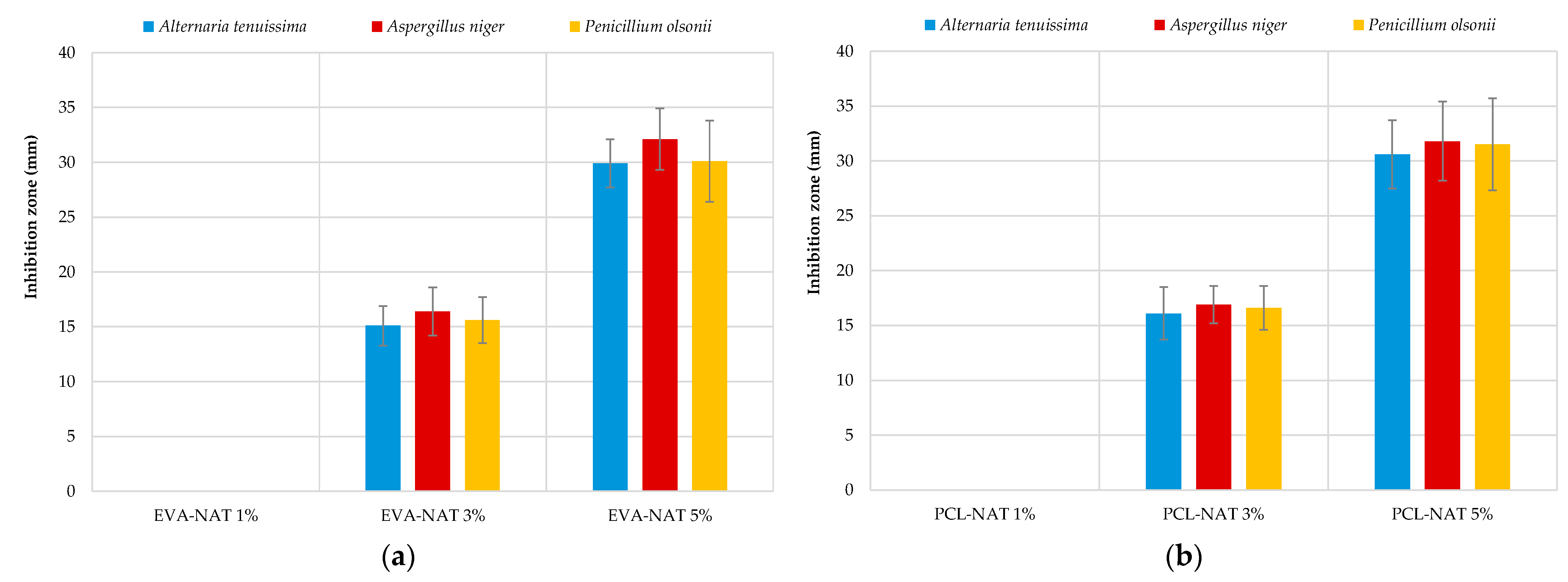
3.3. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics—The Facts 2024. Available online: https://plasticseurope.org/knowledge-Hub/plastics-the-Fast-Facts-2024/ (accessed on 5 December 2024).
- Stoett, P.; Scrich, V.M.; Elliff, C.I.; Andrade, M.M.; Grilli, N.d.M.; Turra, A. Global Plastic Pollution, Sustainable Development, and Plastic Justice. World Dev. 2024, 184, 106756. [Google Scholar] [CrossRef]
- Morreale, M.; La Mantia, F.P. Current Concerns about Microplastics and Nanoplastics: A Brief Overview. Polymers 2024, 16, 1525. [Google Scholar] [CrossRef] [PubMed]
- Titone, V.; Botta, L.; La Mantia, F.P. Mechanical Recycling of New and Challenging Polymer Systems: A Brief Overview. Macromol. Mater. Eng. 2024, 310, 2400275. [Google Scholar] [CrossRef]
- Kim, M.S.; Chang, H.; Zheng, L.; Yan, Q.; Pfleger, B.F.; Klier, J.; Nelson, K.; Majumder, E.L.-W.; Huber, G.W. A Review of Biodegradable Plastics: Chemistry, Applications, Properties, and Future Research Needs. Chem. Rev. 2023, 123, 9915–9939. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent Advances in the Sustainable Design and Applications of Biodegradable Polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers That Are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Comprehensive Review on Polylactic Acid (PLA)—Synthesis, Processing and Application in Food Packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, T.; Zhang, W.; Rhim, J.W. Recent Progress in PBAT-Based Films and Food Packaging Applications: A Mini-Review. Food Chem. 2024, 437, 137822. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(Butylene Succinate) (PBS): Materials, Processing and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-Caprolactone Based Formulations for Drug Delivery and Tissue Engineering: A Review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Majid, I.; Hussain, S.; Nanda, V. Poly(ε-caprolactone): A Potential Polymer for Biodegradable Food Packaging Applications. Packag. Technol. Sci. 2021, 34, 449–461. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.-S.; Han, J. Development of a Biodegradable Polycaprolactone Film Incorporated with an Antimicrobial Agent via an Extrusion Process. Sci. Rep. 2019, 9, 20236. [Google Scholar] [CrossRef]
- Yahiaoui, F.; Benhacine, F.; Ferfera-Harrar, H.; Habi, A.; Hadj-Hamou, A.S.; Grohens, Y. Development of Antimicrobial PCL/nanoclay Nanocomposite Films with Enhanced Mechanical and Water Vapor Barrier Properties for Packaging Applications. Polym. Bull. 2015, 72, 235–254. [Google Scholar] [CrossRef]
- Lopresti, F.; Botta, L.; Pernice, G.; Garofalo, G.; Gaglio, R. Influence of Biochar on the Properties of Antibacterial PBAT/Carvacrol Films. J. Polym. Environ. 2024, 32, 2780–2796. [Google Scholar] [CrossRef]
- Lopresti, F.; Botta, L.; La Carrubba, V.; Attinasi, G.; Settanni, L.; Garofalo, G.; Gaglio, R. Physical and Antibacterial Properties of PLA Electrospun Mats Loaded with Carvacrol and Nisin. Express Polym. Lett. 2022, 16, 1083–1098. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, Y.; Wang, D.; Jiao, S.; Zhang, L.; Sun, J. Improving the Production of Natamycin in Streptomyces Natalensis HW-2 by L-Valine Feeding. Food Sci. Biotechnol. 2024, 33, 3323–3333. [Google Scholar] [CrossRef]
- Wen, M.; Lin, X.; Yu, Y.; Wu, J.; Xu, Y.; Xiao, G. Natamycin Treatment Reduces the Quality Changes of Postharvest Mulberry Fruit during Storage. J. Food Biochem. 2019, 43, e12934. [Google Scholar] [CrossRef]
- Aparicio, J.F.; Barreales, E.G.; Payero, T.D.; Vicente, C.M.; de Pedro, A.; Santos-Aberturas, J. Biotechnological Production and Application of the Antibiotic Pimaricin: Biosynthesis and Its Regulation. Appl. Microbiol. Biotechnol. 2016, 100, 61–78. [Google Scholar] [CrossRef]
- Te Welscher, Y.M.; ten Napel, H.H.; Balagué, M.M.; Souza, C.M.; Riezman, H.; de Kruijff, B.; Breukink, E. Natamycin Blocks Fungal Growth by Binding Specifically to Ergosterol without Permeabilizing the Membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A Natural Preservative for Food Applications—A Review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Dalhoff, A.A.H.; Levy, S.B. Does Use of the Polyene Natamycin as a Food Preservative Jeopardise the Clinical Efficacy of Amphotericin B? A Word of Concern. Int. J. Antimicrob. Agents 2015, 45, 564–567. [Google Scholar] [CrossRef][Green Version]
- Stark, J.; Tan, H.S. Natamycin. In Food Preservatives; Russell, N.J., Gould, G.W., Eds.; Springer: Boston, MA, USA, 2003; pp. 179–195. ISBN 978-1-4757-1006-9. [Google Scholar]
- Lule, V.K.; Garg, S.; Gosewade, S.C.; Khedkar, C.D. Natamycin. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 56–62. ISBN 978-0-12-384953-3. [Google Scholar]
- Dong, H.; Xu, Y.; Zhang, Q.; Li, H.; Chen, L. Activity and Safety Evaluation of Natural Preservatives. Food Res. Int. 2024, 190, 114548. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-Loaded Nanocarriers for Food Packaging Applications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef] [PubMed]
- Grafia, A.L.; Vázquez, M.B.; Bianchinotti, M.V.; Barbosa, S.E. Development of an Antifungal Film by Polyethylene Surface Modification with Natamycin. Food Packag. Shelf Life 2018, 18, 191–200. [Google Scholar] [CrossRef]
- Naderi Bab Anari, H.; Majdinasab, M.; Shaghaghian, S.; Khalesi, M. Development of a Natamycin-Based Non-Migratory Antimicrobial Active Packaging for Extending Shelf-Life of Yogurt Drink (Doogh). Food Chem. 2022, 366, 130606. [Google Scholar] [CrossRef]
- Wu, M.; Deng, Z.-A.; Shen, C.; Yang, Z.; Cai, Z.; Wu, D.; Chen, K. Fabrication of Antimicrobial PCL/EC Nanofibrous Films Containing Natamycin and Trans-Cinnamic Acid by Microfluidic Blow Spinning for Fruit Preservation. Food Chem. 2024, 442, 138436. [Google Scholar] [CrossRef]
- Zheng, Y.; Jia, X.; Zhao, Z.; Ran, Y.; Du, M.; Ji, H.; Pan, Y.; Li, Z.; Ma, X.; Liu, Y.; et al. Innovative Natural Antimicrobial Natamycin Incorporated Titanium Dioxide (nano-TiO2)/Poly (butylene Adipate-Co-Terephthalate) (PBAT)/poly (lactic Acid) (PLA) Biodegradable Active Film (NTP@PLA) and Application in Grape Preservation. Food Chem. 2023, 400, 134100. [Google Scholar] [CrossRef]
- Colak, B.Y.; Peynichou, P.; Galland, S.; Oulahal, N.; Prochazka, F.; Degraeve, P. Antimicrobial Activity of Nisin and Natamycin Incorporated Sodium Caseinate Extrusion-Blown Films: A Comparative Study with Heat-Pressed/Solution Cast Films. J. Food Sci. 2016, 81, E1141–E1150. [Google Scholar] [CrossRef]
- Mistretta, M.C.; Titone, V.; La Mantia, F.P.; Pellitteri, V.; Botta, L. Recycling of a Multilayer Barrier Food Packaging through the Use of a Nanofiller: Effect of Post-Consumer Plastic Bag Conditions. Polym. Test. 2023, 128, 108224. [Google Scholar] [CrossRef]
- D638; Standard Test Method for Tensile Properties of Plastics. ASTM international: West Conshohocken, PA, USA, 2014.
- Shieh, Y.-T.; Lin, Y.-G. Equilibrium Solubility of CO2 in Rubbery EVA over a Wide Pressure Range: Effects of Carbonyl Group Content and Crystallinity. Polymer 2002, 43, 1849–1856. [Google Scholar] [CrossRef]
- Simão, J.A.; Bellani, C.F.; Branciforti, M.C. Thermal Properties and Crystallinity of PCL/PBSA/cellulose Nanocrystals Grafted with PCL Chains. J. Appl. Polym. Sci. 2017, 134, 44493. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-Wetting Characterization Using Contact-Angle Measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, A.; Lo Piccolo, S.; Conigliaro, G.; Ventorino, V.; Burruano, S.; Moschetti, G. Antifungal Peptides Produced by Bacillus Amyloliquefaciens AG1 Active against Grapevine Fungal Pathogens. Ann. Microbiol. 2012, 62, 1593–1599. [Google Scholar] [CrossRef]
- Lopresti, F.; Botta, L.; La Carrubba, V.; Di Pasquale, L.; Settanni, L.; Gaglio, R. Combining Carvacrol and Nisin in Biodegradable Films for Antibacterial Packaging Applications. Int. J. Biol. Macromol. 2021, 193, 117–126. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Maio, A.; Mistretta, M.; La Mantia, F. Effect of Graphene Nanoplatelets on the Physical and Antimicrobial Properties of Biopolymer-Based Nanocomposites. Materials 2016, 9, 351. [Google Scholar] [CrossRef]
- Tazibt, N.; Kaci, M.; Dehouche, N.; Ragoubi, M.; Atanase, L.I. Effect of Filler Content on the Morphology and Physical Properties of Poly(Lactic Acid)-Hydroxyapatite Composites. Materials 2023, 16, 809. [Google Scholar] [CrossRef]
- Suaduang, N.; Ross, S.; Ross, G.M.; Pratumshat, S.; Mahasaranon, S. Effect of Spent Coffee Grounds Filler on the Physical and Mechanical Properties of Poly(lactic Acid) Bio-Composite Films. Mater. Today Proc. 2019, 17, 2104–2110. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, X.; Qian, F.; Zhang, T.; Mu, G. Influence of Natamycin Loading on the Performance of Transglutaminase-induced Crosslinked Gelatin Composite Films. Int. J. Food Sci. Technol. 2019, 54, 2425–2436. [Google Scholar] [CrossRef]
- Nakae, H.; Inui, R.; Hirata, Y.; Saito, H. Effects of Surface Roughness on Wettability. Acta Mater. 1998, 46, 2313–2318. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, J.; He, Y.; Wang, L. Droplet and Bubble Wetting Behaviors: The Roles of Surface Wettability and Roughness. Colloids Surf. Physicochem. Eng. Asp. 2022, 653, 130008. [Google Scholar] [CrossRef]
- Snyder, A.B.; Worobo, R.W. Fungal Spoilage in Food Processing. J. Food Prot. 2018, 81, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhong, C.; Xia, L.; Li, W.; Wang, Z.; Deng, L.; Li, L.; Long, C. Antifungal Activity of Natamycin against Kiwifruit Soft Rot Caused by Botryosphaeria Dothidea and Potential Mechanisms. Sci. Hortic. 2022, 305, 111344. [Google Scholar] [CrossRef]
- Sani, M.A.; Dabbagh-Moghaddam, A.; Jahed-Khaniki, G.; Ehsani, A.; Sharifan, A.; Khezerlou, A.; Tavassoli, M.; Maleki, M. Biopolymers-Based Multifunctional Nanocomposite Active Packaging Material Loaded with Zinc Oxide Nanoparticles, Quercetin and Natamycin; Development and Characterization. J. Food Meas. Charact. 2023, 17, 2488–2504. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. Fabrication of Quercetin-Loaded Biopolymer Films as Functional Packaging Materials. ACS Appl. Polym. Mater. 2021, 3, 2131–2137. [Google Scholar] [CrossRef]
- Shen, C.; Cao, Y.; Rao, J.; Zou, Y.; Zhang, H.; Wu, D.; Chen, K. Application of Solution Blow Spinning to Rapidly Fabricate Natamycin-Loaded Gelatin/zein/polyurethane Antimicrobial Nanofibers for Food Packaging. Food Packag. Shelf Life 2021, 29, 100721. [Google Scholar] [CrossRef]





| Sample Code | E, MPa | TS, MPa | EB, % |
|---|---|---|---|
| EVA | 9.5 ± 0.6 | 14.3 ± 0.9 | 1172 ± 69 |
| EVA—NAT 1% | 9.8 ± 0.7 | 14.6 ± 1.0 | 1170 ± 60 |
| EVA—NAT 3% | 11.1 ± 0.8 | 14.9 ± 1.4 | 1219 ± 71 |
| EVA—NAT 5% | 11.9 ± 0.9 | 14.2 ± 1.2 | 1124 ± 102 |
| PCL | 216 ± 15 | 31.5 ± 2.2 | 1214 ± 95 |
| PCL—NAT 1% | 226 ± 7.2 | 31.1 ± 2.3 | 1080 ± 60 |
| PCL—NAT 3% | 234 ± 19 | 26.0 ± 2.4 | 1046 ± 103 |
| PCL—NAT 5% | 256 ± 20 | 21.1 ± 2.1 | 960 ± 27 |
| Sample Code | Tm, °C | ΔH, J/g | Xc, % |
|---|---|---|---|
| EVA | 75.2 ± 0.1 | 12.7 ± 0.9 | 4.50 ± 0.32 |
| EVA—NAT 1% | 75.3 ± 0.2 | 12.6 ± 1.0 | 4.52 ± 0.35 |
| EVA—NAT 3% | 75.3 ± 0.2 | 12.4 ± 1.4 | 4.54 ± 0.51 |
| EVA—NAT 5% | 75.5 ± 0.3 | 12.2 ± 1.2 | 4.57 ± 0.44 |
| PCL | 58.7 ± 0.1 | 67.8 ± 2.1 | 49.8 ± 1.61 |
| PCL—NAT 1% | 58.8 ± 0.2 | 67.8 ± 2.2 | 50.3 ± 1.63 |
| PCL—NAT 3% | 58.6 ± 0.1 | 66.7 ± 2.5 | 51.9 ± 1.89 |
| PCL—NAT 5% | 58.5 ± 0.2 | 68.7 ± 2.7 | 53.1 ± 2.08 |
| Sample Code | WCA, % |
|---|---|
| EVA | 72 ± 2 |
| EVA—NAT 1% | 74 ± 1 |
| EVA—NAT 3% | 75 ± 2 |
| EVA—NAT 5% | 77 ± 2 |
| PCL | 67 ± 2 |
| PCL—NAT 1% | 75 ± 1 |
| PCL—NAT 3% | 76 ± 2 |
| PCL—NAT 5% | 77 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titone, V.; Ceraulo, M.; Lopresti, F.; Garofalo, G.; Gaglio, R.; Mistretta, M.C.; Botta, L. Use of Natamycin for the Development of Polymer Systems with Antifungal Activity for Packaging Applications. Polymers 2025, 17, 686. https://doi.org/10.3390/polym17050686
Titone V, Ceraulo M, Lopresti F, Garofalo G, Gaglio R, Mistretta MC, Botta L. Use of Natamycin for the Development of Polymer Systems with Antifungal Activity for Packaging Applications. Polymers. 2025; 17(5):686. https://doi.org/10.3390/polym17050686
Chicago/Turabian StyleTitone, Vincenzo, Manuela Ceraulo, Francesco Lopresti, Giuliana Garofalo, Raimondo Gaglio, Maria Chiara Mistretta, and Luigi Botta. 2025. "Use of Natamycin for the Development of Polymer Systems with Antifungal Activity for Packaging Applications" Polymers 17, no. 5: 686. https://doi.org/10.3390/polym17050686
APA StyleTitone, V., Ceraulo, M., Lopresti, F., Garofalo, G., Gaglio, R., Mistretta, M. C., & Botta, L. (2025). Use of Natamycin for the Development of Polymer Systems with Antifungal Activity for Packaging Applications. Polymers, 17(5), 686. https://doi.org/10.3390/polym17050686










