Unraveling of Poly(lactic acid) (PLA)/Natural Wax/Titanium Dioxide Nanoparticle Composites for Disposable Plastic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Non-Functionalized Titanium Dioxide Nanoparticles
2.3. Synthesis of Functionalized Titanium Dioxide Nanoparticles
2.4. Formulation of PLA/Natural Wax/TiO2-NF and PLA/Natural Wax/TiO2-F Composites
Preparation of Films and Probes
2.5. Hydrolytic Degradation
2.6. Characterization of TiO2-NF and TiO2-F Nanoparticles
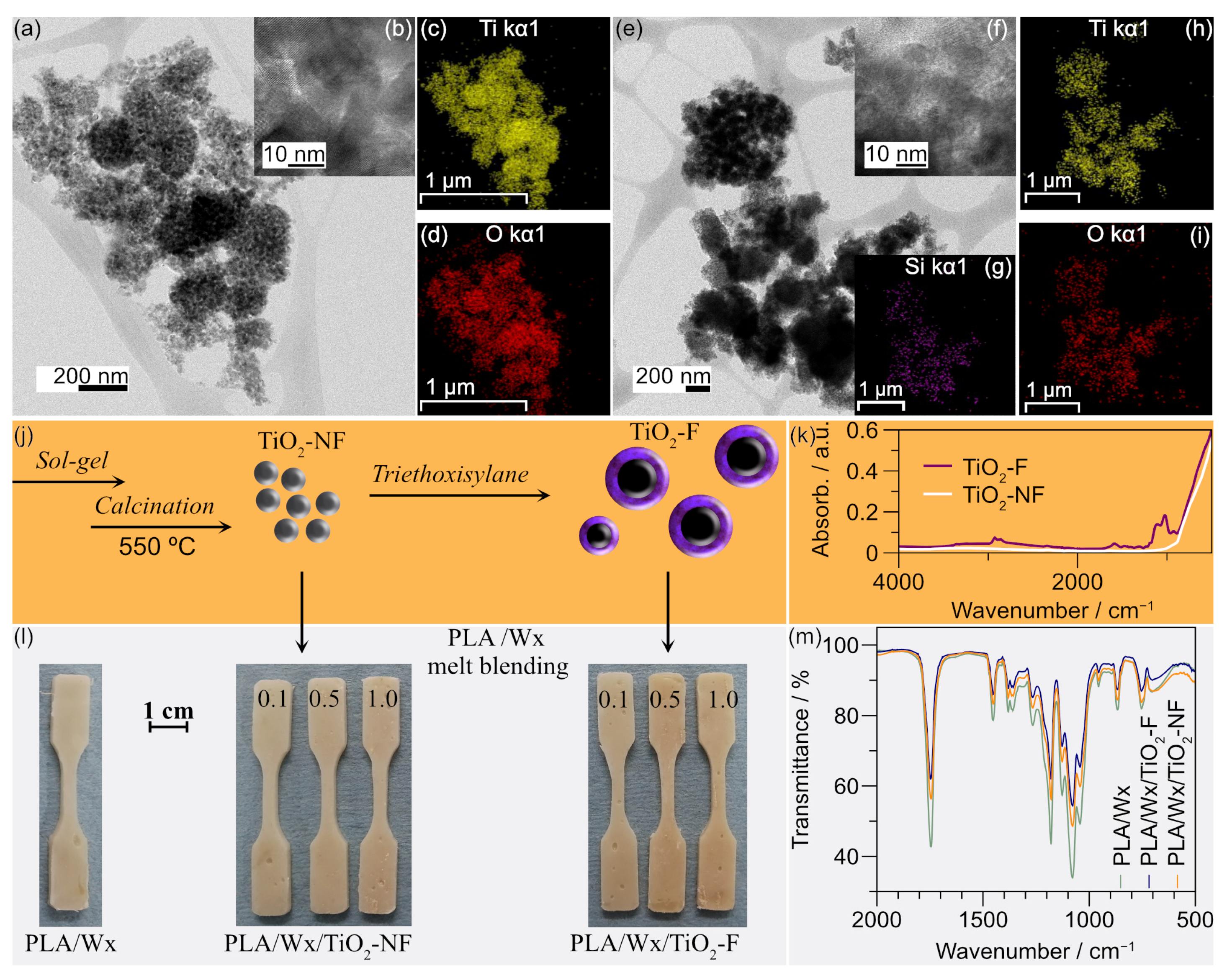
2.6.1. Morphology
2.6.2. X-Ray Diffraction
2.6.3. FTIR
2.7. Characterization of PLA/Natural Wax/TiO2-NF and PLA/Natural Wax/TiO2-F Composites
2.7.1. Chemical Structure
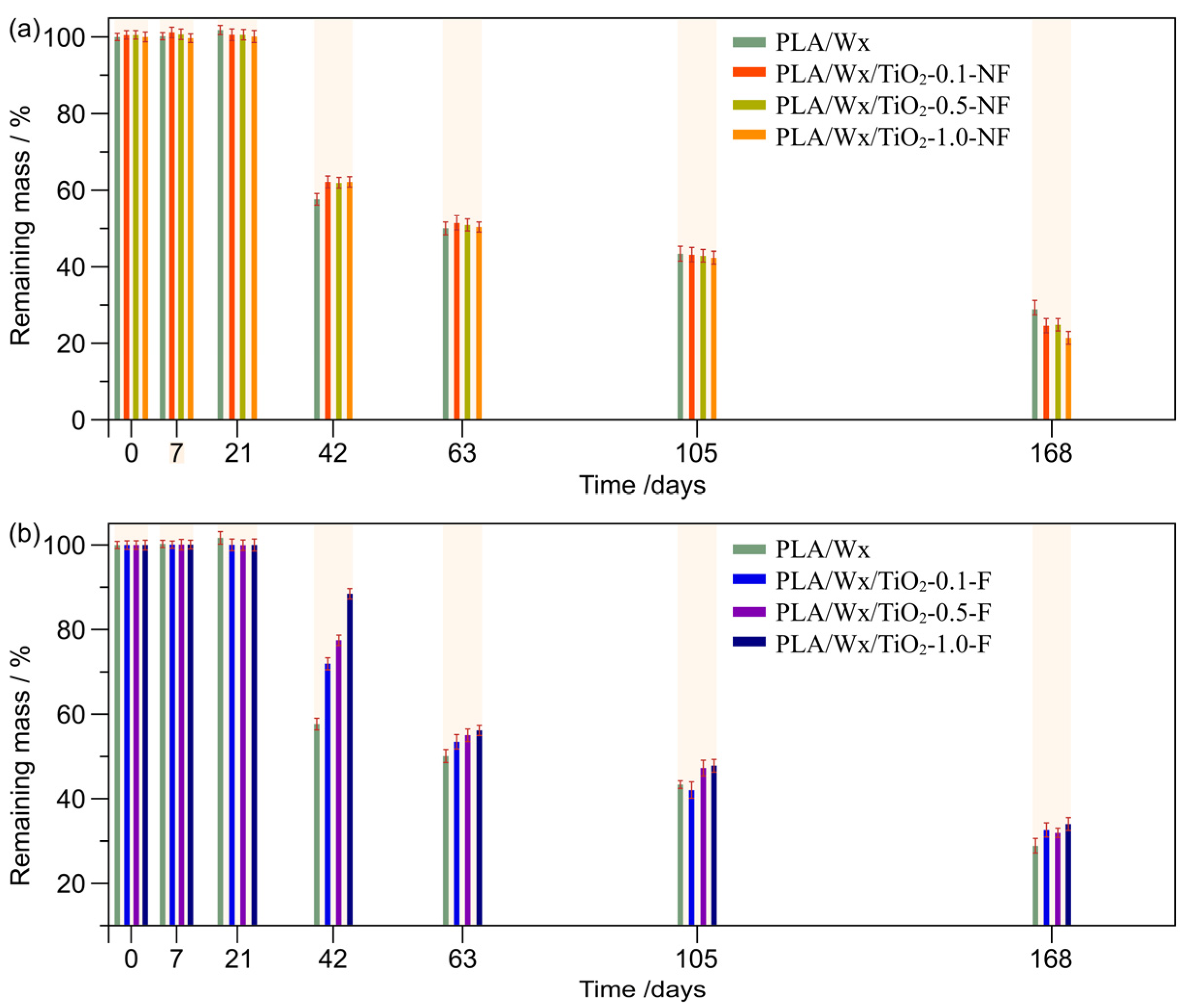
2.7.2. Water Absorption and Remaining Mass
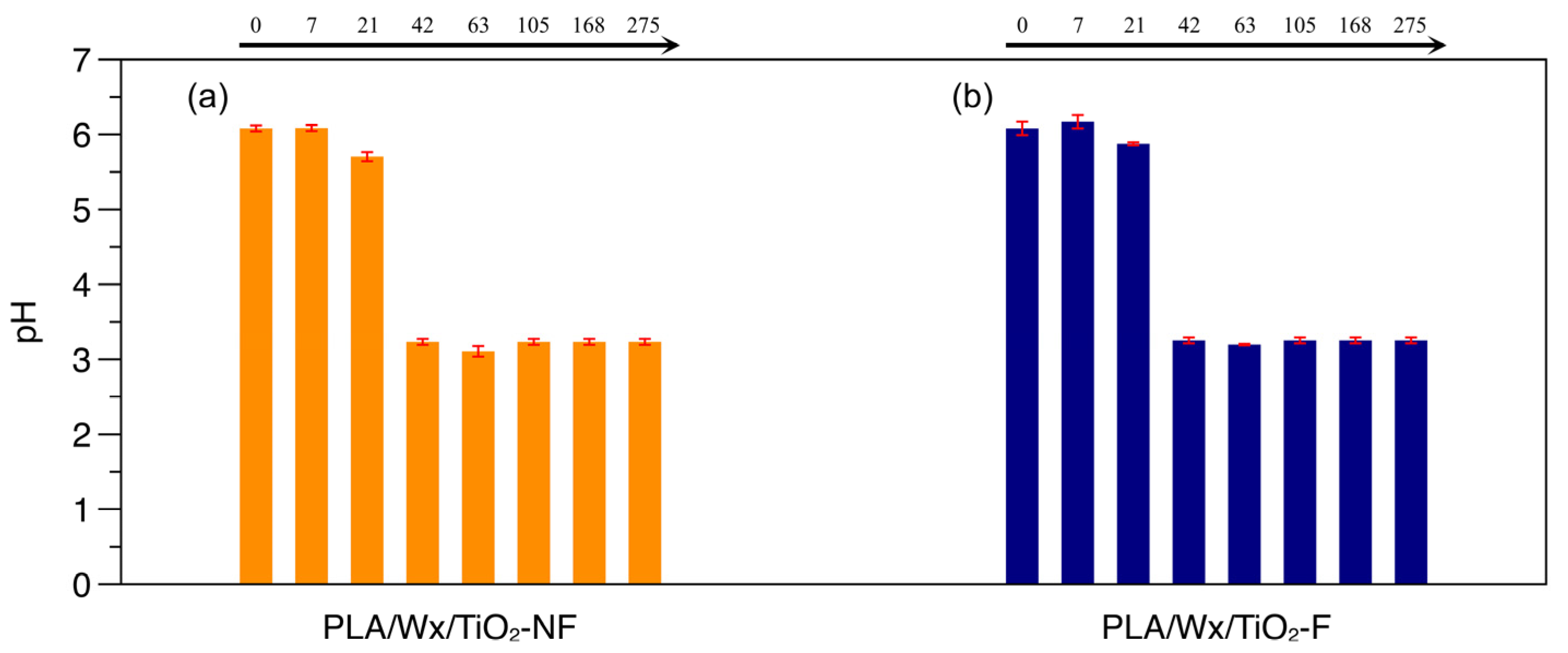
2.7.3. pH
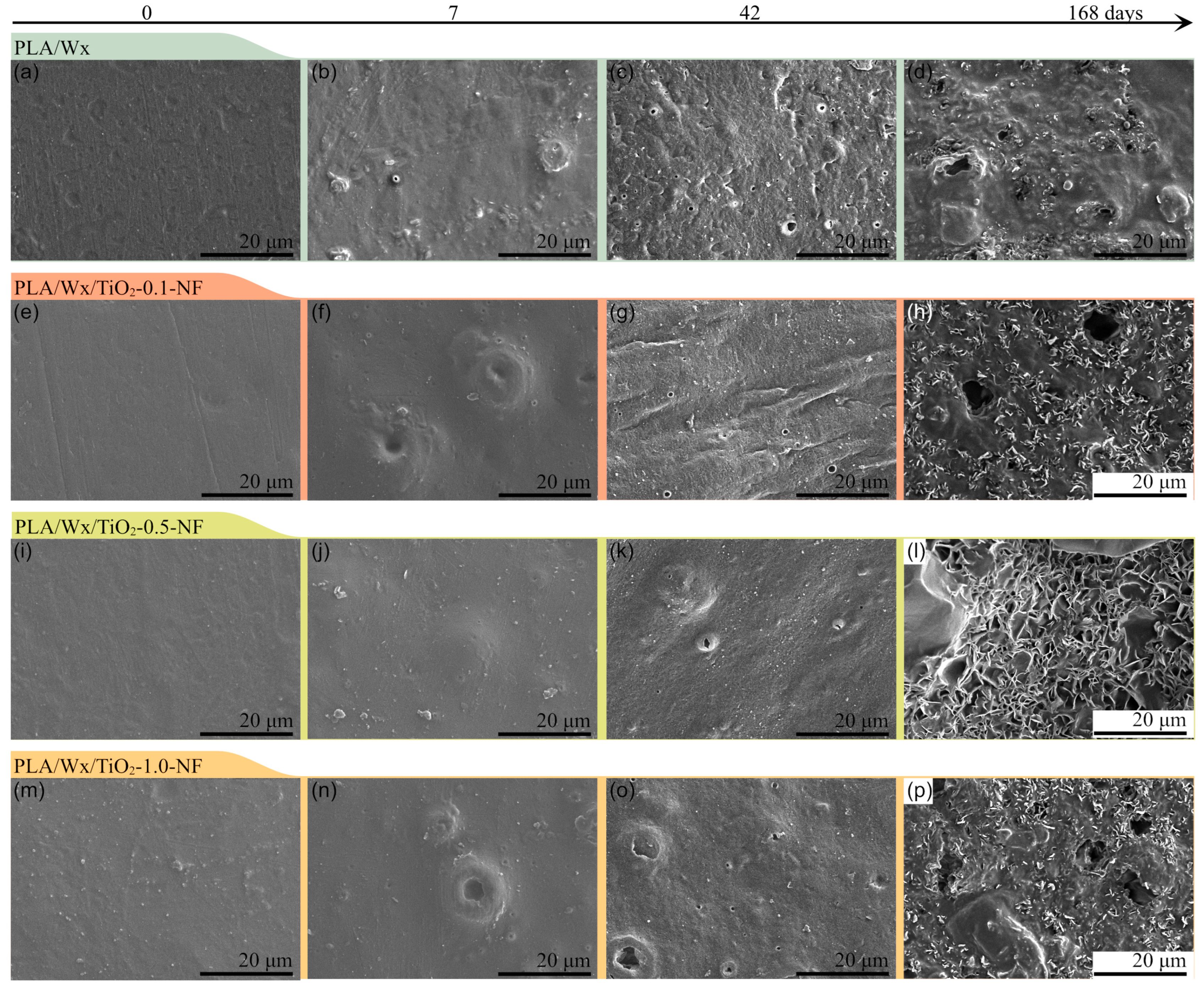
2.7.4. Scanning Electron Microscopy (SEM)
2.7.5. Melt Rheology
2.7.6. Antibacterial Activity
2.7.7. Oxygen Gas Transmission Rate
2.7.8. Optical Transparency
3. Results and Discussion
3.1. Water Absorption and Remaining Mass During Hydrolytic Degradation
3.2. pH Determined During Hydrolytic Degradation
3.3. Scanning Electron Microscopy
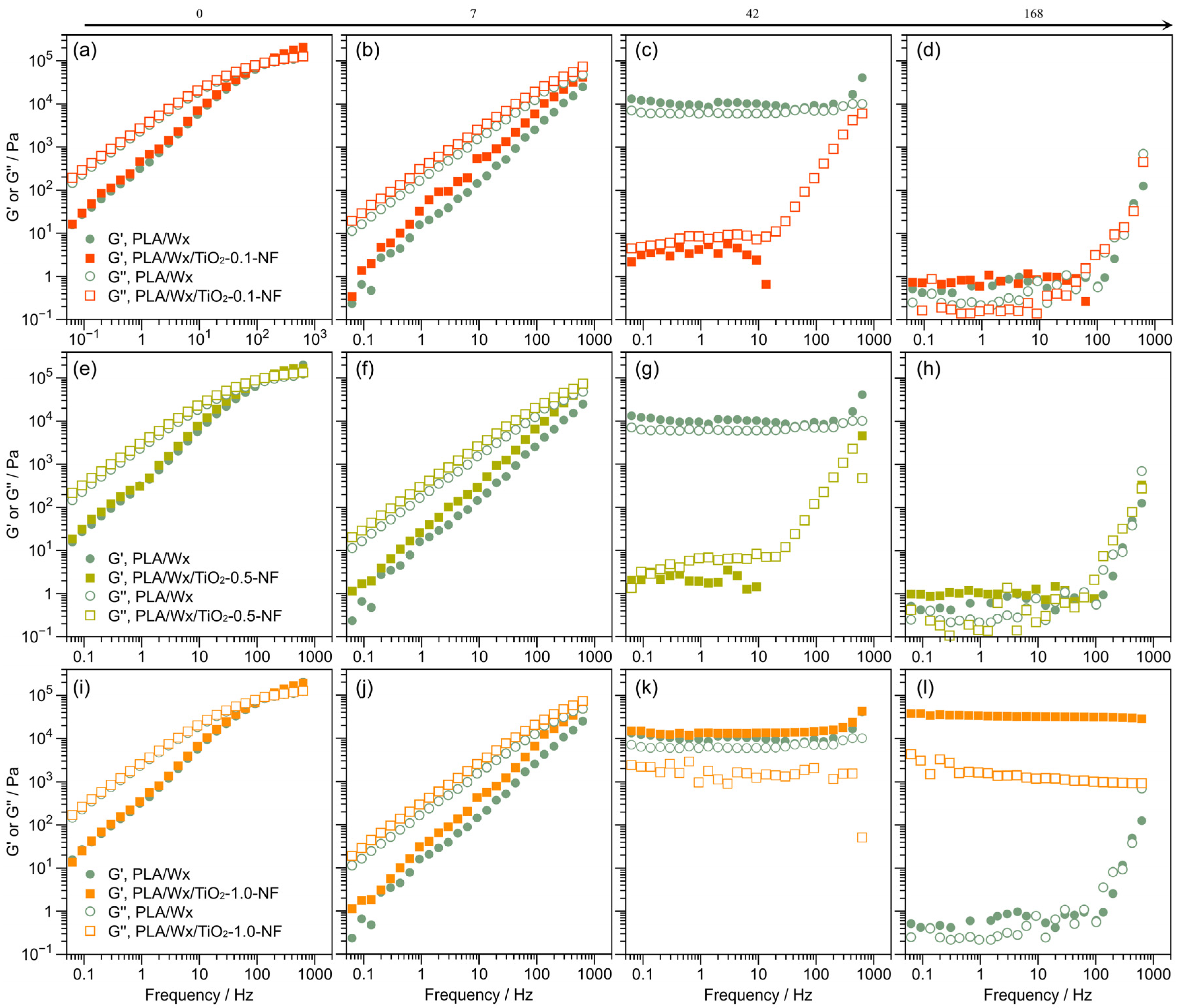
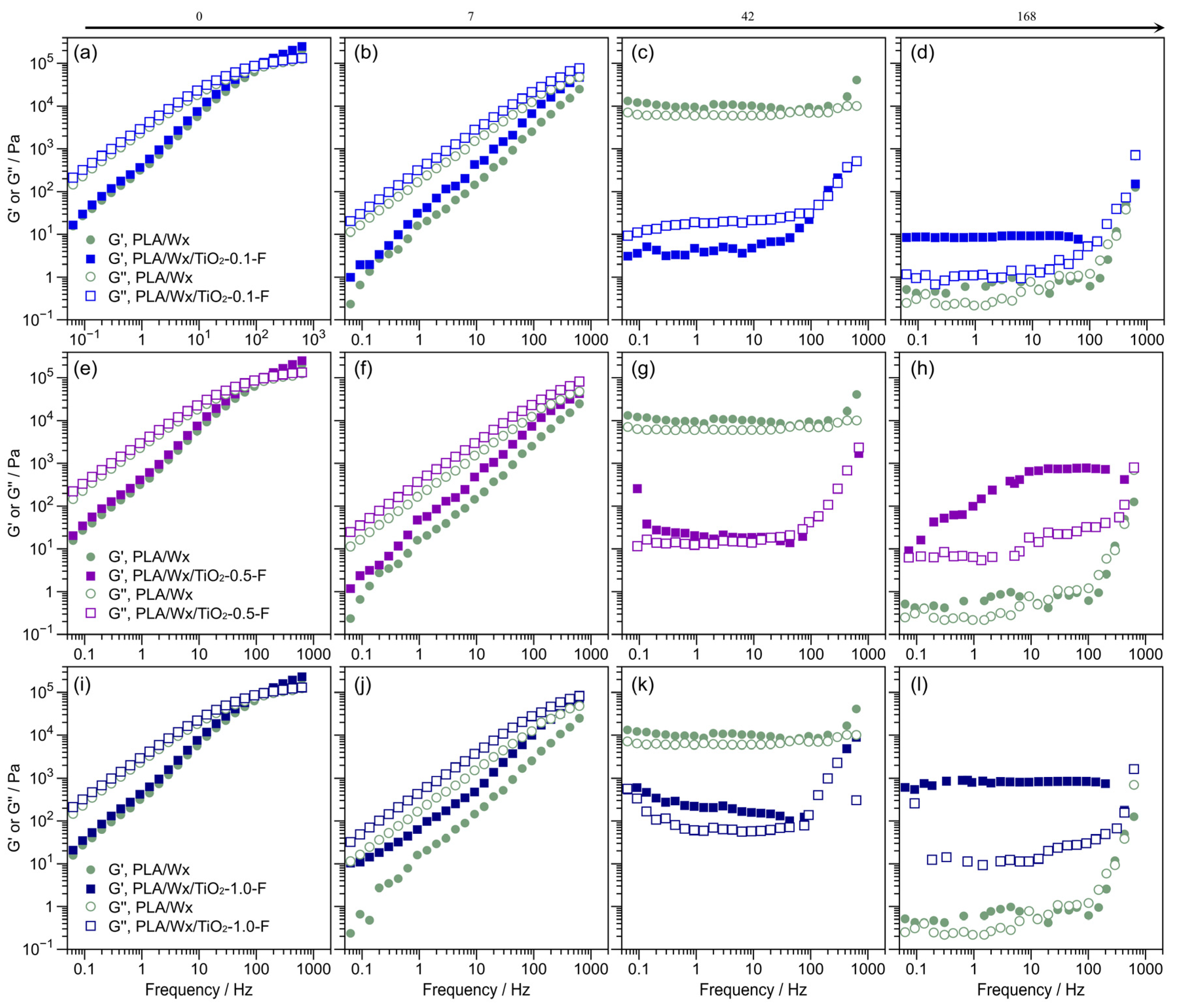
3.4. Rheological Evaluation
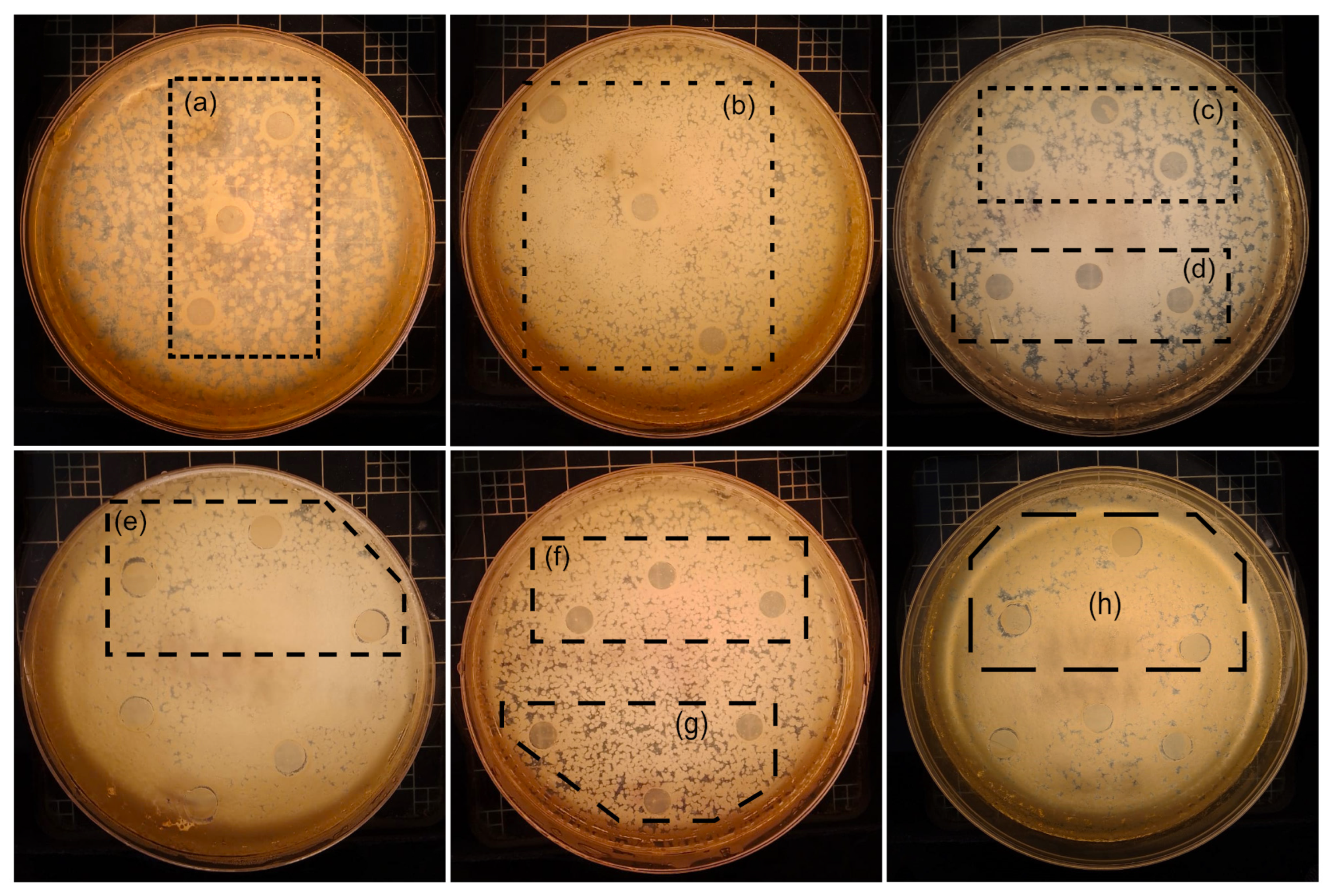
3.5. Assay of Antibacterial Activity
3.6. Evaluation of Oxygen Gas Transmission Rate
3.7. Film Transparency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLA | Polylactic acid |
| Wx | Natural waxes |
| TiO2-NF | Non-functionalized TiO2 nanoparticles |
| TiO2-F | Functionalized TiO2 nanoparticles |
References
- Mendoza-Duarte, M.E.; Estrada-Moreno, I.A.; López-Martínez, E.I.; Vega-Rios, A. Effect of the Addition of Different Natural Waxes on the Mechanical and Rheological Behavior of PLA—A Comparative Study. Polymers 2023, 15, 305. [Google Scholar] [CrossRef] [PubMed]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Ashby, M.F. Chapter 15—Material Profiles. In Materials and the Environment, 2nd ed.; Ashby, M.F., Ed.; Butterworth-Heinemann: Boston, MA, USA, 2013; pp. 459–595. ISBN 978-0-12-385971-6. [Google Scholar]
- Sharma, S.; Byrne, M.; Perera, K.Y.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Active Film Packaging Based on Bio-Nanocomposite TiO2 and Cinnamon Essential Oil for Enhanced Preservation of Cheese Quality. Food Chem. 2023, 405, 134798. [Google Scholar] [CrossRef] [PubMed]
- Buzarovska, A. PLA Nanocomposites with Functionalized TiO2 Nanoparticles. Polym. Plast. Technol. Eng. 2013, 52, 280–286. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Ur Rehman, Z. Review of Recent Advances in Polylactic Acid/TiO2 Composites. Materials 2019, 12, 3659. [Google Scholar] [CrossRef]
- Nayak, J.K.; Behera, L.; Jali, B.R. TiO2 Strengthened PLA Nanocomposites: A Prospective Material for Packaging Application. J. Mol. Struct. 2024, 1316, 138892. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Li, J.; Sun, X.S. Synthesis and Characterization of Bionanocomposites of Poly (Lactic Acid) and TiO2 Nanowires by in Situ Polymerization. Polymer 2011, 52, 2367–2375. [Google Scholar] [CrossRef]
- Hou, X.; Xue, Z.; Liu, J.; Yan, M.; Xia, Y.; Ma, Z. Characterization and Property Investigation of Novel Eco-friendly Agar/Carrageenan/TiO2 Nanocomposite Films. J. Appl. Polym. Sci. 2019, 136, 47113. [Google Scholar] [CrossRef]
- Athanasoulia, I.-G.; Mikropoulou, M.; Karapati, S.; Tarantili, P.; Trapalis, C. Study of Thermomechanical and Antibacterial Properties of TiO2/Poly (Lactic Acid) Nanocomposites. Mater. Today Proc. 2018, 5, 27553–27562. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Titanium Dioxide (TiO2) for the Manufacture of Multifunctional Active Food Packaging Films. Food Packag. Shelf Life 2022, 31, 100806. [Google Scholar] [CrossRef]
- Sarker, T.; Tahmid, I.; Sarker, R.K.; Dey, S.C.; Islam, M.T.; Sarker, M. ZIF-67-Based Materials as Adsorbent for Liquid Phase Adsorption-a Review. Polyhedron 2024, 260, 117069. [Google Scholar] [CrossRef]
- Dalod, A.R.M.; Henriksen, L.; Grande, T.; Einarsrud, M.-A. Functionalized TiO2 Nanoparticles by Single-Step Hydrothermal Synthesis: The Role of the Silane Coupling Agents. Beilstein J. Nanotechnol. 2017, 8, 304–312. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety Assessment of Titanium Dioxide (E171) as a Food Additive. Efsa J. 2021, 19, e06585. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, L.; Zhang, H.; Yuan, M.; Qin, Y. Evaluation of PLA Nanocomposite Films on Physicochemical and Microbiological Properties of Refrigerated Cottage Cheese. J. Food Process. Preserv. 2018, 42, e13362. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Xue, W.; Zhu, J.; Sun, P.; Yang, F.; Wu, H.; Li, W. Permeability of Biodegradable Film Comprising Biopolymers Derived from Marine Origin for Food Packaging Application: A Review. Trends Food Sci. Technol. 2023, 136, 295–307. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic Degradation of Polylactic Acid (PLA) and Its Composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Luo, Y.-B.; Wang, X.-L.; Wang, Y.-Z. Effect of TiO2 Nanoparticles on the Long-Term Hydrolytic Degradation Behavior of PLA. Polym. Degrad. Stab. 2012, 97, 721–728. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Properties and Morphology of Poly(l-Lactide) 4. Effects of Structural Parameters on Long-Term Hydrolysis of Poly(l-Lactide) in Phosphate-Buffered Solution. Polym. Degrad. Stab. 2000, 67, 179–189. [Google Scholar] [CrossRef]
- Paul, M.-A.; Delcourt, C.; Alexandre, M.; Degée, P.; Monteverde, F.; Dubois, P. Polylactide/Montmorillonite Nanocomposites: Study of the Hydrolytic Degradation. Polym. Degrad. Stab. 2005, 87, 535–542. [Google Scholar] [CrossRef]
- Zhou, Q.; Xanthos, M. Nanoclay and Crystallinity Effects on the Hydrolytic Degradation of Polylactides. Polym. Degrad. Stab. 2008, 93, 1450–1459. [Google Scholar] [CrossRef]
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. ASTM Standards: West Conshohocken, PA, USA, 2014.
- ASTM D3985; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM Standards: West Conshohocken, PA, USA, 2024.
- Cargnello, M.; Gordon, T.R.; Murray, C.B. Solution-Phase Synthesis of Titanium Dioxide Nanoparticles and Nanocrystals. Chem. Rev. 2014, 114, 9319–9345. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-H.; Hsiao, S.-T.; Wu, Y.-F.; Zeng, S.-J.; Li, S.-M.; Wang, Y.-S.; Ma, C.-C.M. Effect of Silane-Functionalized TiO2 on the Optical Properties and Moisture Barrier of Silicone Resin Nanocomposites. RSC Adv. 2014, 4, 38614–38622. [Google Scholar] [CrossRef]
- Riccio, M.; Montanari, T.; Castellano, M.; Turturro, A.; Negroni, F.M.; Busca, G. An IR Study of the Chemistry of Triethoxysilane at the Surface of Metal Oxides. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 181–190. [Google Scholar] [CrossRef]
- Marrone, M.; Montanari, T.; Busca, G.; Conzatti, L.; Costa, G.; Castellano, M.; Turturro, A. A Fourier Transform Infrared (FTIR) Study of the Reaction of Triethoxysilane (TES) and Bis[3-Triethoxysilylpropyl]Tetrasulfane (TESPT) with the Surface of Amorphous Silica. J. Phys. Chem. B 2004, 108, 3563–3572. [Google Scholar] [CrossRef]
- Zou, J.; Lu, M.; Chen, S.; Cai, C.; Yao, Z.; Cui, W.; Fan, C.; Liu, S. Beeswax-Inspired Superhydrophobic Electrospun Membranes for Peritendinous Anti-Adhesion. Mater. Sci. Eng. C 2020, 116, 111166. [Google Scholar] [CrossRef]
- Palacios, A.Y.; Jiménez, C.R.; Aguirre, E.; Rodríguez, G.; Ortecho-Llanos, R.; Piscoche-Chinchay, R.H.; Pantoja-Tirado, L.R.; Hurtado-Soria, B.; Villanueva, E. Optimization of Cocoa Butter (Theobroma cacao L.) Extraction with Ethanol Using Response Surface Methodology. J. Food Nutr. Res. 2024, 12, 482–488. [Google Scholar] [CrossRef]
- Marra, A.; Cimmino, S.; Silvestre, C. Effect of TiO2 and ZnO on PLA Degradation in Various Media. Adv. Mater. Sci. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Buzarovska, A.; Grozdanov, A. Biodegradable Poly(L-Lactic Acid)/TiO2 Nanocomposites: Thermal Properties and Degradation. J. Appl. Polym. Sci. 2012, 123, 2187–2193. [Google Scholar] [CrossRef]
- Wang, W.; Ye, G.; Bian, X.; Lin, P.; Wang, D.; Wang, X. Enhanced Photocatalytic Degradation of Polylactide (PLA) through TiO2-Based up-Conversion Nanoparticles. Polym. Degrad. Stab. 2024, 228, 110927. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, M.; Nagappan, S.; Lee, W.; Ha, C. Preparation and Properties of Poly (Lactic Acid)/Lipophilized Graphene Oxide Nanohybrids. Polym. Int. 2018, 67, 91–99. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Hua, K.; Duan, C.; Zhang, W.; Ji, J.; Yang, X. Enhanced Mechanical Properties and Degradability of Poly (Butylene Succinate) and Poly(Lactic Acid) Blends. Iran. Polym. J. 2013, 22, 267–275. [Google Scholar] [CrossRef]
- Williams, D.F. Enzymic Hydrolysis of Polylactic Acid. Eng. Med. 1981, 10, 5–7. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation Assessment of Poly (Lactic Acid) Filled with Functionalized Titania Nanoparticles (PLA/TiO2) under Compost Conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef]
- Bhattacharya, S.N.; Kamal, M.R.; Gupta, R.K. Rheology of Nanocomposites. In Polymeric Nanocomposites; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2007; pp. 145–231. ISBN 978-3-446-40270-6. [Google Scholar]
- Galgali, G.; Ramesh, C.; Lele, A. A Rheological Study on the Kinetics of Hybrid Formation in Polypropylene Nanocomposites. Macromolecules 2001, 34, 852–858. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Liu, J.; Qiu, X.; Lin, L. Preparation and Characterization of Tea Tree Essential Oil Microcapsule-Coated Packaging Paper with Bacteriostatic Effect. Food Chem. X 2024, 23, 101510. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A.; Yu, D.; Wang, H. Potential Nano Bacteriostatic Agents to Be Used in Meat-Based Foods Processing and Storage: A Critical Review. Trends Food Sci. Technol. 2023, 131, 77–90. [Google Scholar] [CrossRef]
- Pušnik Črešnar, K.; Aulova, A.; Bikiaris, D.N.; Lambropoulou, D.; Kuzmič, K.; Fras Zemljič, L. Incorporation of Metal-Based Nanoadditives into the PLA Matrix: Effect of Surface Properties on Antibacterial Activity and Mechanical Performance of PLA Nanoadditive Films. Molecules 2021, 26, 4161. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Baskar, R.; Anusuya, T.; Seshan, C.A.; Chelliah, R. Toxicity Mechanism of Titanium Dioxide and Zinc Oxide Nanoparticles against Food Pathogens. Colloids Surf. B Biointerfaces 2016, 148, 600–606. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez De Aberasturi, D.; Larramendi, I.R.D.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial Properties of Nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and Mechanisms of Action of Titanium Dioxide Nanoparticles in Living Organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Yang, L.; Chen, R.; Zou, W.; Lin, X.; Qu, J. Preparation, Characterization and Properties of PLA/TiO2 Nanocomposites Based on a Novel Vane Extruder. RSC Adv. 2015, 5, 4639–4647. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of Polymeric Food Packaging Materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Puyol, S.; Hierrezuelo, J.; Benítez, J.J.; Tedeschi, G.; Porras-Vázquez, J.M.; Heredia, A.; Athanassiou, A.; Romero, D.; Heredia-Guerrero, J.A. Transparent, UV-Blocking, and High Barrier Cellulose-Based Bioplastics with Naringin as Active Food Packaging Materials. Int. J. Biol. Macromol. 2022, 209, 1985–1994. [Google Scholar] [CrossRef]



| Sample | PLA/wt% | Beeswax/wt% | Cocoa Wax/wt% | TiO2-NF/wt% | TiO2-F/wt% |
|---|---|---|---|---|---|
| PLA/Wx | 85.0 | 7.5 | 7.5 | 0 | 0 |
| PLA/Wx/TiO2-0.1-NF | 84.9 | 7.5 | 7.5 | 0.1 | 0 |
| PLA/Wx/TiO2-0.5-NF | 84.9 | 7.5 | 7.5 | 0.5 | 0 |
| PLA/Wx/TiO2-1.0-NF | 84.5 | 7.5 | 7.5 | 1.0 | 0 |
| PLA/Wx/TiO2-0.1-F | 84.5 | 7.5 | 7.5 | 0 | 0.1 |
| PLA/Wx/TiO2-0.5-F | 84.0 | 7.5 | 7.5 | 0 | 0.5 |
| PLA/Wx/TiO2-1.0-F | 84.0 | 7.5 | 7.5 | 0 | 1.0 |
| Sample | Conc. TiO2/wt% | E. coli (Inhibition Zone (mm)) | S. aureus (Inhibition Zone (mm)) | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | ||
| PLA/Wx | 0.0 | No antibacterial activity | No antibacterial activity | ||
| PLA/Wx/TiO2-0.1-NF | 0.1 | Bacteriostatic | Bacteriostatic | Bacteriostatic | Bacteriostatic |
| PLA/Wx/TiO2-0.5-NF | 0.5 | 8.83 ± 0.40 | 9.44 ± 0.85 | 8.67 ± 0.33 | 8.74 ± 0.14 |
| PLA/Wx/TiO2-1.0-NF | 1.0 | 8.74 ± 0.60 | 8.93 ± 0.36 | 8.62 ± 0.18 | 8.59 ± 0.58 |
| PLA/Wx/TiO2-0.1-F | 0.1 | Bacteriostatic | Bacteriostatic | Bacteriostatic | Bacteriostatic |
| PLA/Wx/TiO2-0.5-F | 0.5 | 9.12 ± 0.52 | 9.29 ± 0.34 | 8.64 ± 0.40 | 8.50 ± 0.17 |
| PLA/Wx/TiO2-1.0-F | 1.0 | 9.16 ± 0.27 | 9.47 ± 0.42 | 8.40 ± 0.47 | 8.67 ± 0.72 |
| Sample | Oxygen Permeability (cm3 cm/cm2 s Pa) | Reference |
|---|---|---|
| HDPE films | 4.56 × 10−14–8.33 × 10−14 | [46] |
| LDPE films | 18.2 × 10−14–23.9 × 10−14 | [46] |
| Oriented PET | 0.12 × 10−14 | [46] |
| PLA, PLA/1% TiO2 | 2.0 × 10−14, 1.8 × 10−14 | [7] |
| PLA/Wx | 2.5 × 10−14 | In this work |
| PLA/Wx/TiO2-0.1-NF | 23.0 × 10−14 | |
| PLA/Wx/TiO2-0.5-NF | 23.7 × 10−14 | |
| PLA/Wx/TiO2-1.0-NF | 33.4 × 10−14 | |
| PLA/Wx/TiO2-1.0-F | 25.9 × 10−14 |
| Sample | Transmittance600 nm/% | Reference |
|---|---|---|
| PHBV (Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)) | 85 | [48] |
| LDPE (low-density polyethylene) | 87 | [48,49] |
| PVC (Polyvinyl chloride) | 90 | [48] |
| PLLA (Poly-L-lactide) | 89 | [48] |
| PLA/Wx | 93 | In this work |
| PLA/Wx/TiO2-0.1-NF | 94 | |
| PLA/Wx/TiO2-0.5-NF | 95 | |
| PLA/Wx/TiO2-1.0-NF | 92 | |
| PLA/Wx/TiO2-0.1-F | 96 | |
| PLA/Wx/TiO2-0.5-F | 93 | |
| PLA/Wx/TiO2-1.0-F | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocarando-Chacón, J.G.; Estrada-Moreno, I.A.; Olivas-Armendáriz, I.; Vega-Rios, A.; Mendoza-Duarte, M.E. Unraveling of Poly(lactic acid) (PLA)/Natural Wax/Titanium Dioxide Nanoparticle Composites for Disposable Plastic Applications. Polymers 2025, 17, 685. https://doi.org/10.3390/polym17050685
Bocarando-Chacón JG, Estrada-Moreno IA, Olivas-Armendáriz I, Vega-Rios A, Mendoza-Duarte ME. Unraveling of Poly(lactic acid) (PLA)/Natural Wax/Titanium Dioxide Nanoparticle Composites for Disposable Plastic Applications. Polymers. 2025; 17(5):685. https://doi.org/10.3390/polym17050685
Chicago/Turabian StyleBocarando-Chacón, Jacqueline Guadalupe, Iván Alziri Estrada-Moreno, Imelda Olivas-Armendáriz, Alejandro Vega-Rios, and Mónica Elvira Mendoza-Duarte. 2025. "Unraveling of Poly(lactic acid) (PLA)/Natural Wax/Titanium Dioxide Nanoparticle Composites for Disposable Plastic Applications" Polymers 17, no. 5: 685. https://doi.org/10.3390/polym17050685
APA StyleBocarando-Chacón, J. G., Estrada-Moreno, I. A., Olivas-Armendáriz, I., Vega-Rios, A., & Mendoza-Duarte, M. E. (2025). Unraveling of Poly(lactic acid) (PLA)/Natural Wax/Titanium Dioxide Nanoparticle Composites for Disposable Plastic Applications. Polymers, 17(5), 685. https://doi.org/10.3390/polym17050685









