Highly Branched Poly(Adipic Anhydride-Co-Mannitol Adipate): Synthesis, Characterization, and Thermal Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
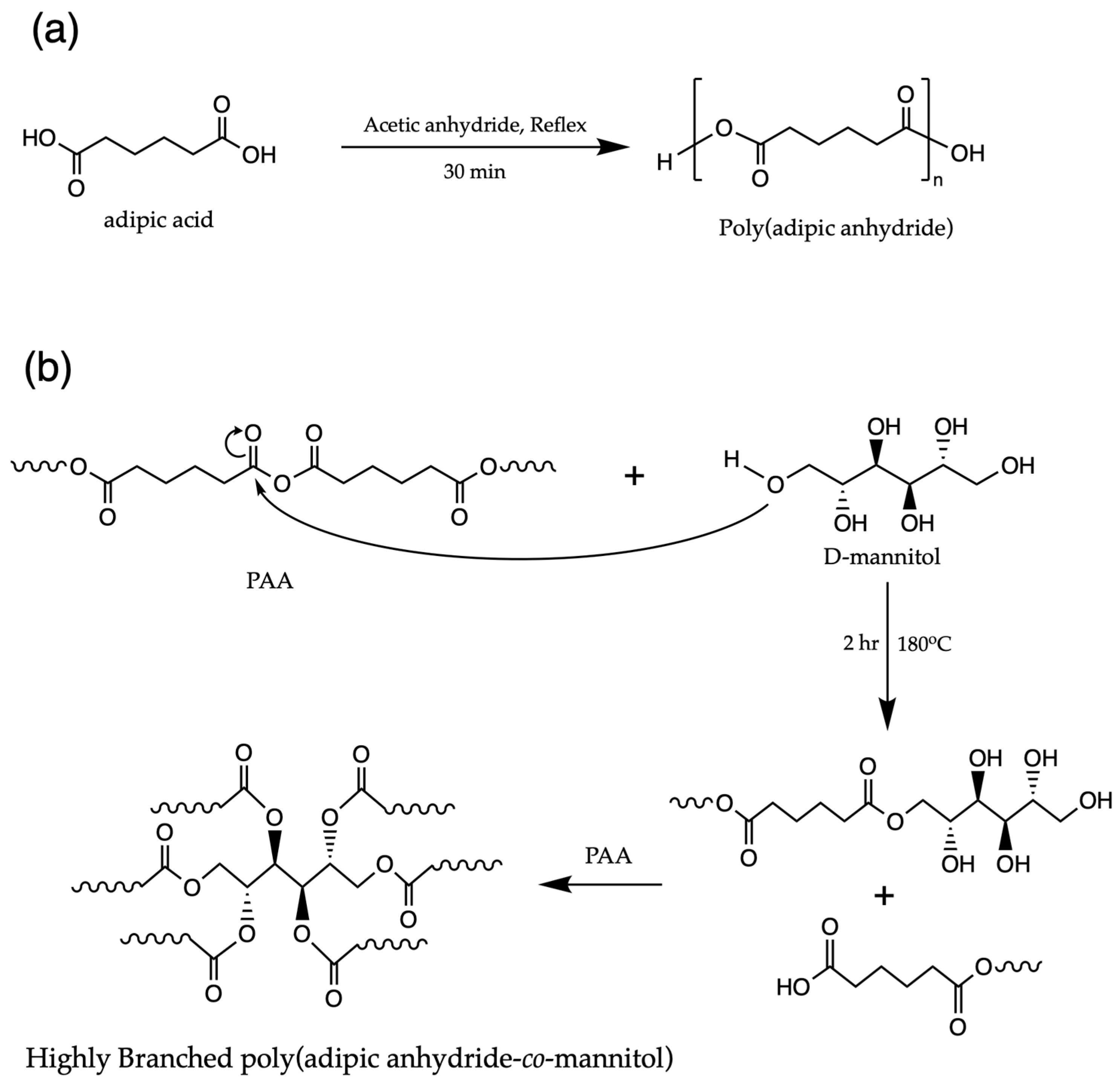
2.3. Synthesis of Poly(Adipic Anhydride) (PAA)
2.4. Synthesis of Poly(Adipic Anhydride-Co-Mannitol Adipate) (PAA-Co-M)
3. Results and Discussion
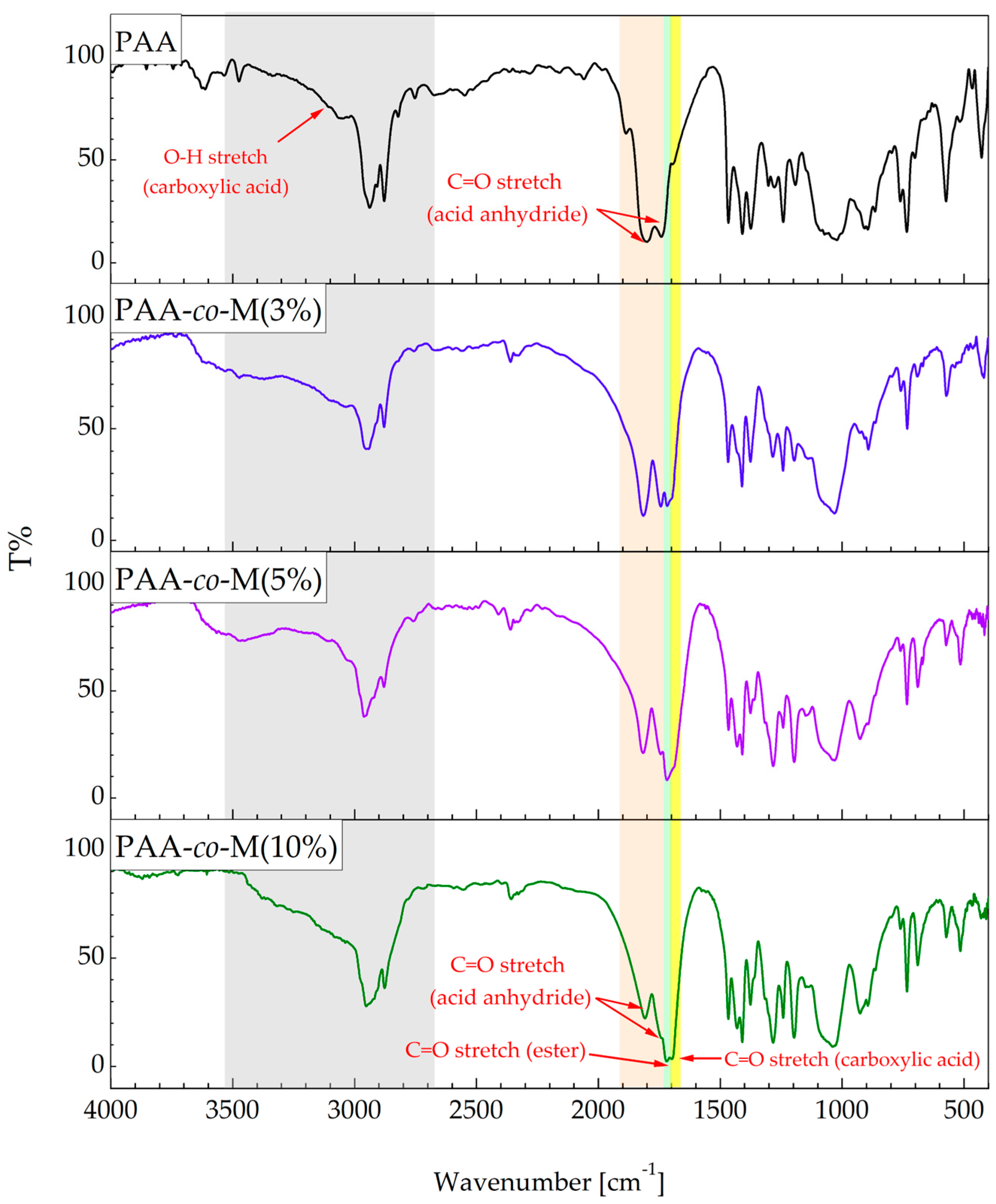
3.1. FTIR
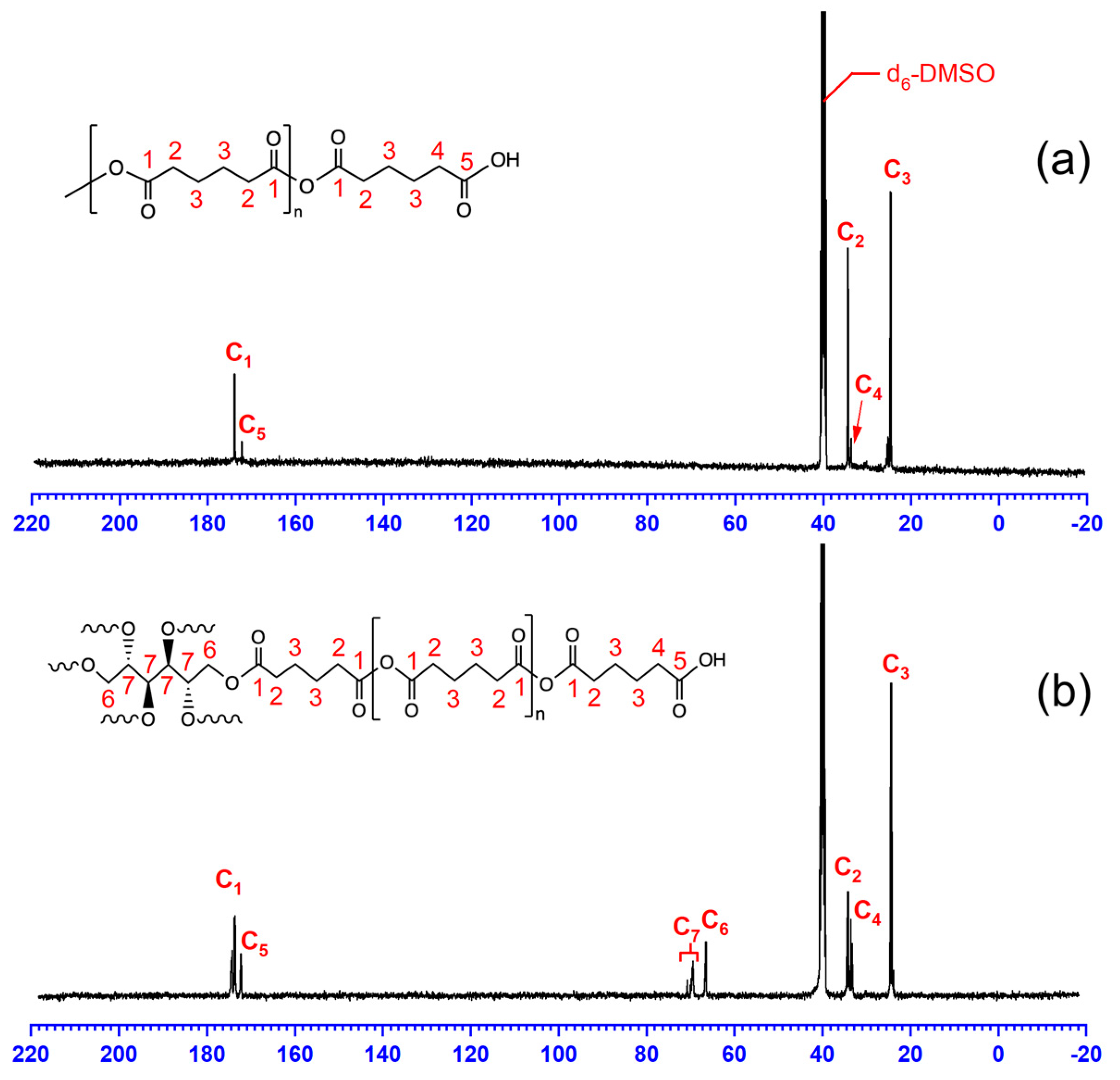
3.2. 13C-NMR
3.3. GPC Analysis
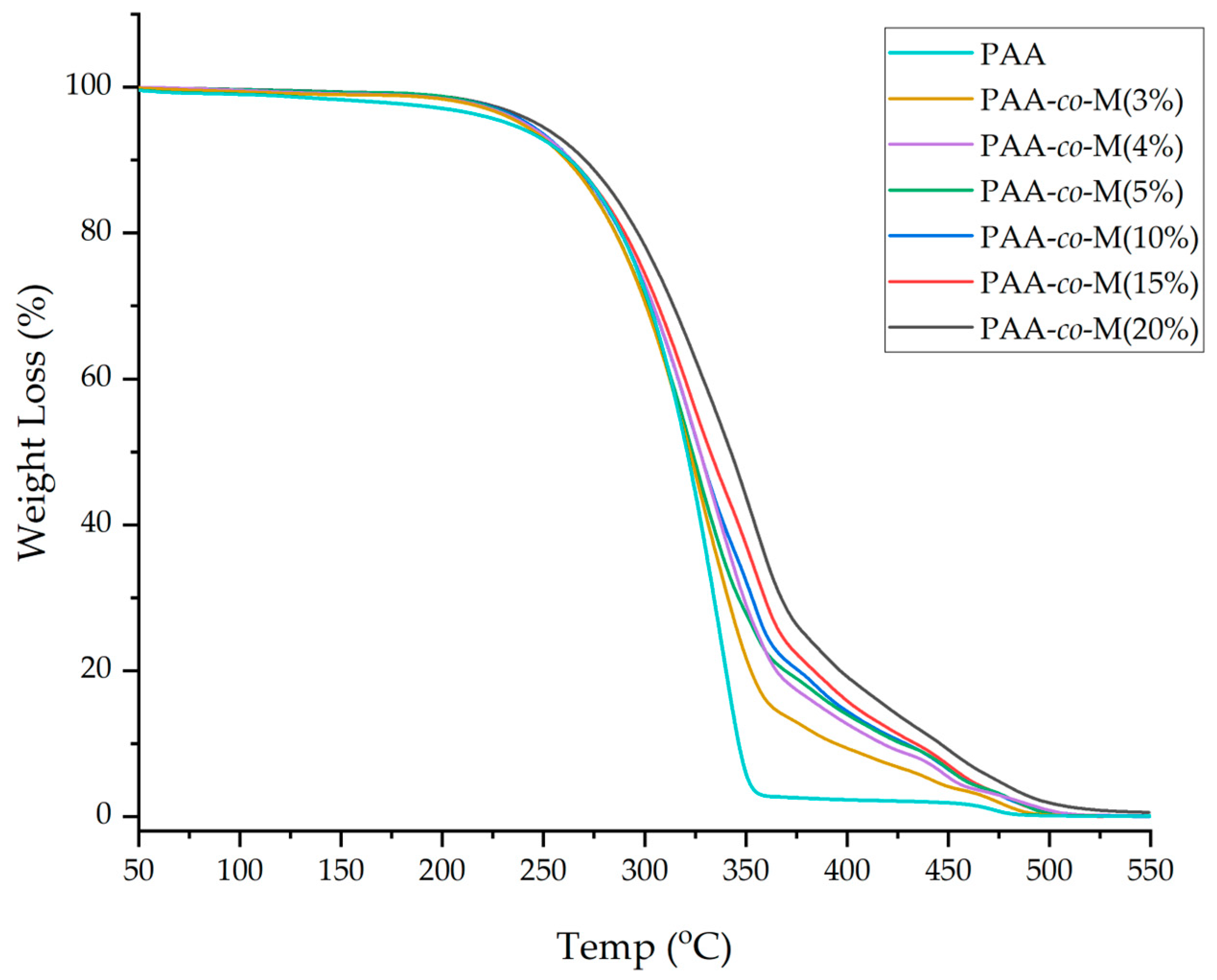
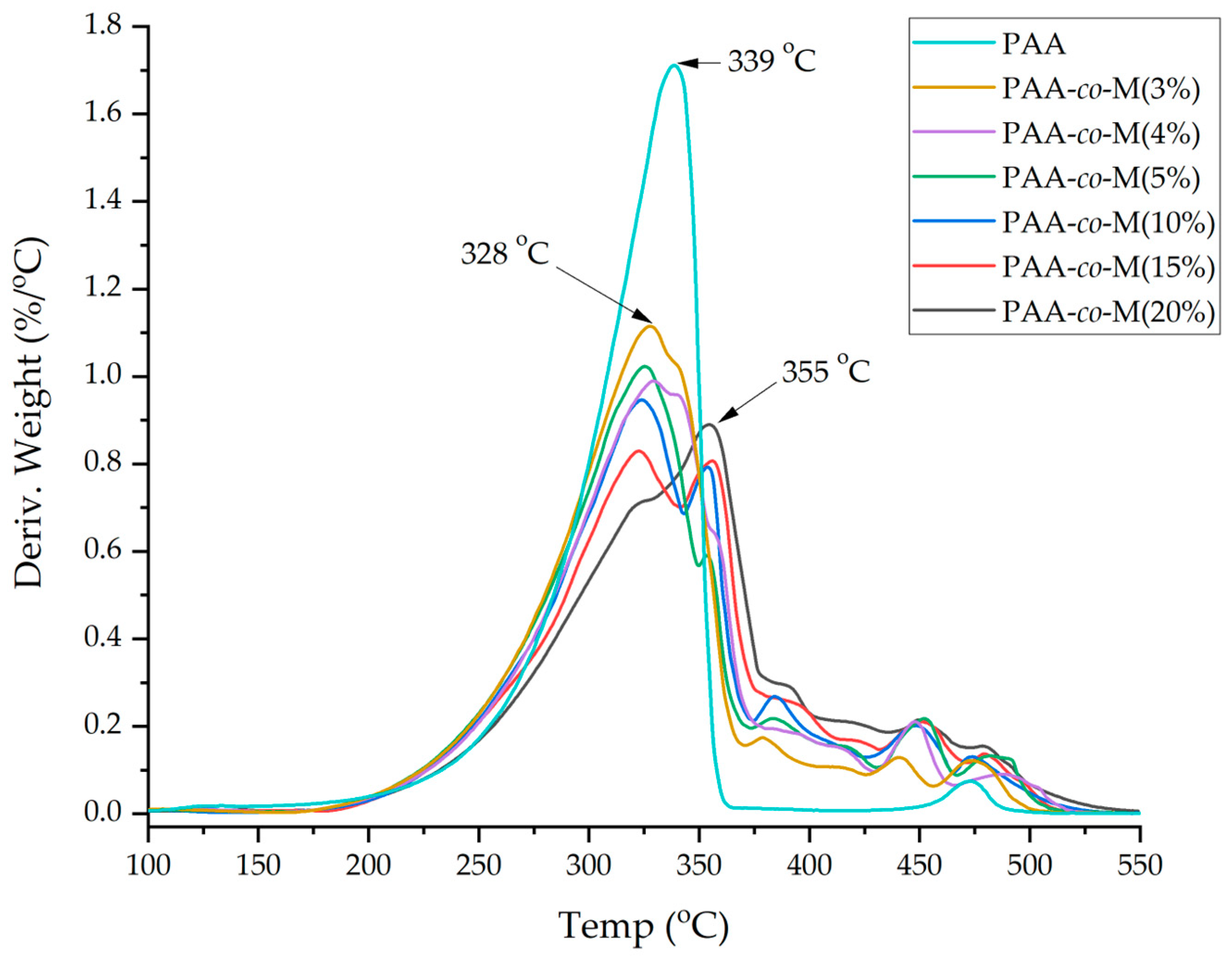
3.4. TGA and DTG Analyses
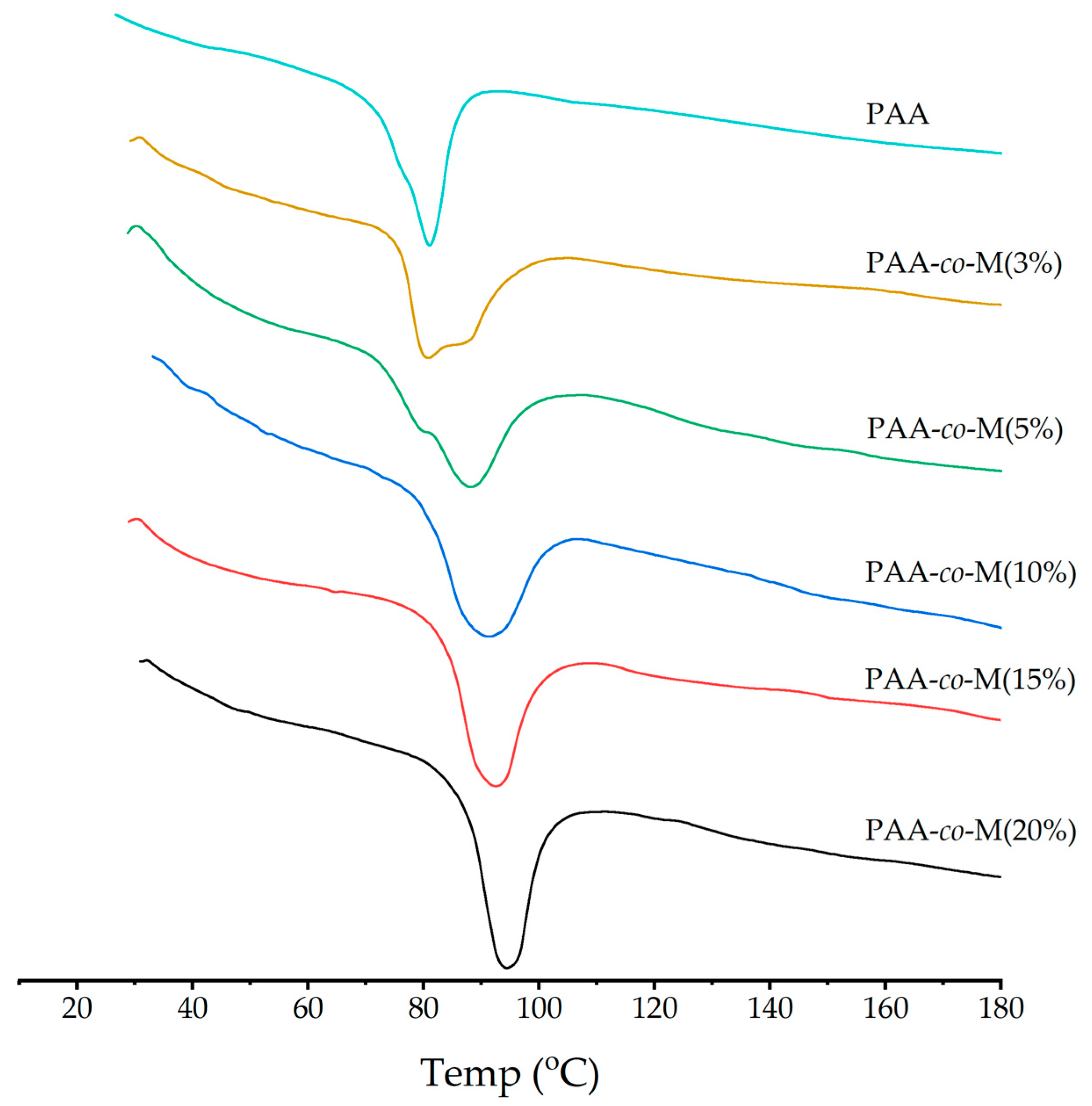
3.5. DSC Analysis
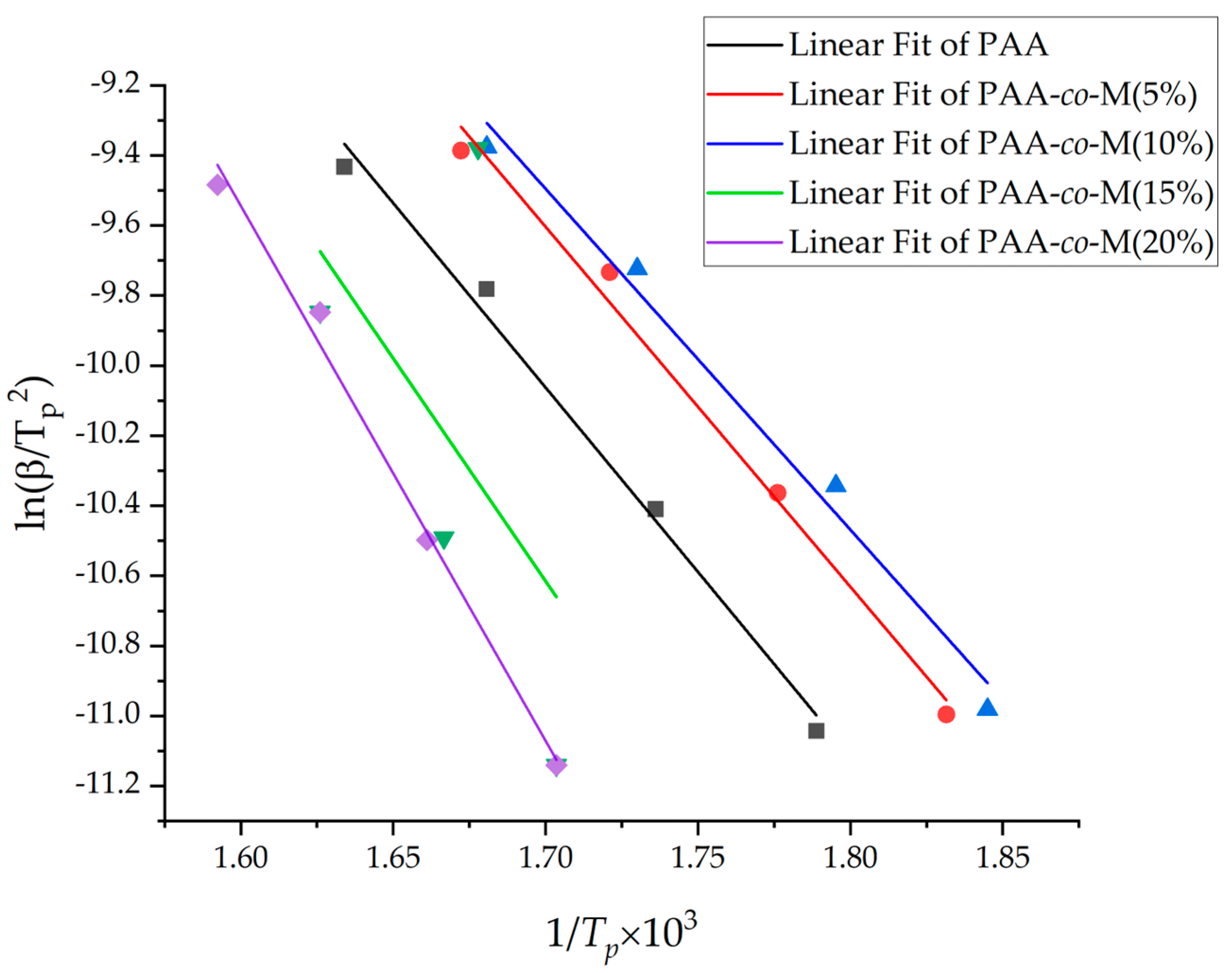
3.6. Kinetics of Thermal Decomposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dinarvand, R.; Alimorad, M.M.; Amanlou, M.; Akbari, H. In vitro release of clomipramine HCL and buprenorphine HCL from poly adipic anhydride (PAA) and poly trimethylene carbonate (PTMC) blends. J. Biomed. Mater. Res. Part A 2005, 75, 185–191. [Google Scholar] [CrossRef]
- Leśniak-Ziółkowska, K.; Śmiga-Matuszowicz, M.; Blacha-Grzechnik, A.; Student, S.; Brzychczy-Włoch, M.; Krok-Borkowicz, M.; Pamuła, E.; Simka, W.; Kazek-Kęsik, A. Antibacterial and cytocompatible coatings based on poly(adipic anhydride) T for a Ti alloy surface. Bioact. Mater. 2020, 5, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Swier, S. Controlling Morphology of Block Copolymers. U.S. Patent US20130231438A1, 5 September 2013. [Google Scholar]
- Luoma, E.; Rokkonen, T.; Tribot, A.; Nättinen, K.; Lahtinen, J. Polybutylene succinate-co-adipate)/poly(hydroxybutyrate) blend films and their thermal, mechanical and gas barrier properties. Polym. Renew. Resour. 2022, 13, 83–101. [Google Scholar] [CrossRef]
- Song, P.; Li, M.; Wang, H.; Cheng, Y.; We, Z. Partially bio-based and biodegradable poly(propylene terephthalate-co-Adipate) copolymers: Cynthesis, thermal properties, and enzymatic degradation behavior. Polymers 2024, 16, 2588. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.-C.; Liu, Y. Comparison between physical blending and copolymerization of poly(trimethylene carbonate) and poly(adipic anhydride) with special regard to compatibility, morphology and degradation. J. Macromol. Sci. Part A 1997, 34, 1457–1482. [Google Scholar] [CrossRef]
- Deng, Z.; Riga, E.K.; Lienkamp, K. Degradable polymer films made from poly(salicylic-acid-co-sebacic acid) and poly(sebacic anhydride)/poly(adipic anhydride) blends: Degradation kinetics and use as sacrificial layers for polymer multilayer systems. Macromol. Chem. Phys. 2020, 221, 2000106. [Google Scholar] [CrossRef]
- Aguilar, J.A.D.; Rincon, J.J.; Petriciolet, A.B.; Garcia, J.R. Synthesis and characterization of aminated copolymers of polyacrylonitrile-graft-chitosan and their application for the removal of heavy metal from aqueous solution. J. Chil. Chem. Soc. 2015, 60, 2876–2880. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, H.; Deng, X. Block copolymerization of ε-caprolactone and adipic anhydride initiated with aluminum complex catalyst. J. Macromol. Sci. Part A Pure Appl. Chem. 2004, 41, 77–84. [Google Scholar] [CrossRef]
- Deng, X.; Li, Z.; Yuan, M.; Hao, J. Anionic ring-opening polymerization of adipic anhydride initiated by potassium poly(ethylene glycol)ate. J. Appl. Polym. Sci. 2003, 88, 2194–2201. [Google Scholar] [CrossRef]
- Jaszcz, K.; Qukaszczyk, J. Studies on hydrolytic degradation of poly(ester-anhydride)s based on oligosuccinate and aliphatic diacids. Polym. Degrad. Stab. 2011, 96, 1973–1983. [Google Scholar] [CrossRef]
- Pfeifer, B.A.; Burdick, J.A.; Little, S.R.; Langer, R. Poly(ester-anhydride): Poly(B-amino ester) micro- and nanospheres: DNA encapsulation and cellular transfection. Pharm. Nanotechnol. 2005, 304, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Francesco, D.D.; Rigo, D.; Baddigam, K.R.; Mathew, A.P.; Hedin, N.; Selva, M.; Samec, J.S.M. A new family of renewable thermosets: Kraft lignin poly-adipates. ChemSusChem 2022, 15, e202200326. [Google Scholar] [CrossRef]
- Edgar, K.J.; Kar, N. Esters of Cellulosic Materials and Diacids and Method of Making Thereof. U.S. Patent US9708415B2, 18 July 2017. [Google Scholar]
- Zakeri, S.M.; Alimi, M.; Shokoohi, S.; Shahidi, S.A. Physicochemical and functional properties of modified quinoa starch with adipic acid and acetic anhydride mixture. Iran J. Food Sci. Ind. 2023, 20, 113–128. [Google Scholar] [CrossRef]
- Liu, H.; Kar, N.; Edgar, K.J. Direct synthesis of cellulose adipate derivatives using adipic anhydride. Cellulose 2012, 19, 1279–1293. [Google Scholar] [CrossRef]
- Hosseini, S.V.; Dastgerdi, H.E.; Tahergorabi, R. Marine mannitol: Extraction, structures, properties, and applications. Processes 2024, 12, 1613. [Google Scholar] [CrossRef]
- Hevilla, V.; Sonseca, Á.; Echeverría, C.; Muñoz-Bonilla, A.; Fernández-García, M. Photocured poly(mannitol sebacate) with functional methacrylic monomer: Analysis of physical, chemical, and biological properties. Polymers 2023, 15, 1561. [Google Scholar] [CrossRef]
- Hevilla, V.; Sonseca, Á.; Gimenez, E.; Muñoz-Bonilla, C.E.A.; Fernández-García, M. The incorporation of low-molecular weightpoly(mannitol sebacate)s on PLA electrospun fibers: Effects on the mechanical properties and surface chemistry. Polymers 2022, 14, 3342. [Google Scholar] [CrossRef]
- Stebbins, N.D.; Yu, W.; Uhrich, K.E. Linear, mannitol-based poly(anhydride-esters) with high ibuprofen loading and anti-inflammatory activity. Biomacromolecules 2015, 16, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.A.; Al-Lami, H.S. Biodegradation studies in vitro of novel poly(adipic anhydride-co-mannitol)-N-maleoyl chitosan networks. Baghdad Sci. J. 2015, 13, 210–220. [Google Scholar] [CrossRef]
- Ickowicz, D.E.; Abtew, E.; Khan, W.; Golovanevski, L.; Steinman, N.; Weiniger, C.F.; Domb, A.J. Poly(ester-anhydride) for controlled delivery of hydrophilic drugs. J. Bioact. Compat. Polym. 2015, 31, 127–139. [Google Scholar] [CrossRef]
- Ghosh, R.; Siman, P.; Domb, A.J. Poly(ester-anhydrides) with controlled molecular weight and structure. Polym. Adv. Technol. 2022, 33, 3774–3781. [Google Scholar] [CrossRef]
- Poetz, K.L.; Shipp, D.A. Polyanhydrides: Synthesis, properties, and applications. Aust. J. Chem. 2016, 69, 1223–1239. [Google Scholar] [CrossRef]
- Pavelkova, A.; Kucharczyk, P.; Zednik, J.; Sedlarik, V. Synthesis of poly(sebacic anhydride): Effect of various catalysts on structure and thermal properties. J. Polym. Res. 2014, 21, 426. [Google Scholar] [CrossRef]
- Arun, Y.; Ghosh, R.; Domb, A.J. Poly(ester-anhydrides) derived from esters of hydroxy acid and cyclic anhydrides. Biomacromolecules 2022, 23, 3417–3428. [Google Scholar] [CrossRef]
- Pietro, M.E.D.; Mannu, A.; Mele, A. NMR determination of free fatty acids in vegetable oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef]
- Monaco, A.; Drain, B.; Becer, C.R. Detailed GPC analysis of poly(N-isopropylacrylamide) with core cross-linked star architecture. Polym. Chem. 2021, 12, 5229–5238. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Jose, S.; Thomas, S.; Biju, P.K.; Koshy, P.; Karger-Kocsis, J. Thermal degradation and crystallisation studies of reactively compatibilised polymer blends. Polym. Degrad. Stab. 2008, 93, 1176–1187. [Google Scholar] [CrossRef]
- Ziolo, A.; Mossety-Leszczak, B.; Walczak, M.; Strachota, B.; Strachota, A.; Awsiuk, K.; Janiszewska, N.; Raczkowska, J. Synthesis and morphology characteristics of new highly branched polycaprolactone PCL. Molecules 2024, 29, 991. [Google Scholar] [CrossRef]
- Kavimani, V.; Lakkaboyana, S.K.; Trilaksana, H.; Atanase, L.I. Mechanical properties and degradation rate of poly(sorbitol adipate-co-dioladipate) copolymers obtained with a catalyst-free melt polycondensation method. Polymers 2024, 16, 499. [Google Scholar] [CrossRef]
- Gu, X.; Wang, Z.; Wang, H.; Zhou, G.; Zhou, Y. Synthesis of thermally robust benzimidazolone-based wholly aromatic polyketones. RSC Adv. 2021, 11, 5444–5450. [Google Scholar] [CrossRef] [PubMed]
- Ujcic, M.; Levak, M.; Penava, N.V.; Bajaic, E.G.; Kosar, V. Thermal degradation and thermal kinetic of SEBS block copolymer compatibilized PS/HDPE blends. J. Elastomers Plast. 2023, 55, 82–98. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger method in kinetics of materials: Things to beware and be aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef] [PubMed]
- Gola, A.; Knysak, T.; Mucha, I.; Musial, W. Synthesis, thermogravimetric analysis, and kinetic study of poly-N-isopropylacrylamide with varied initiator content. Polymers 2023, 15, 2427. [Google Scholar] [CrossRef]
- Pal, A.K.; Katiyar, V. Theoretical and analyzed data related to thermal degradation kinetics of poly (L-lactic acid)/chitosan-grafted-oligo L-lactic acid (PLA/CH-g-OLLA) bionanocomposite films. Data Brief 2017, 10, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, R.; Liu, L. Study on the thermal degradation kinetics of the common wooden boards. Procedia Eng. 2016, 135, 72–82. [Google Scholar] [CrossRef]
- Demir, P. Investigation of thermal degradation kinetics of poly(Ɛ-caprolactone) grafted onto PEMA-co-PHEMA. AKU J. Sci. Eng. 2017, 17, 37–85. [Google Scholar] [CrossRef]
- Park, S.; Yang, L.; Park, B.D.; Du, G. Thermal cure kinetics of highly branched polyurea resins at different molar ratios. J. Therm. Anal. Calorim. 2023, 148, 6389–6405. [Google Scholar] [CrossRef]

| D-Mannitol %Wt. | Copolymer Symbol | Yield (%) |
|---|---|---|
| 3 | PAA-co-M(3%) | 94 |
| 4 | PAA-co-M(4%) | 91 |
| 5 | PAA-co-M(5%) | 90 |
| 10 | PAA-co-M(10%) | 86 |
| 15 | PAA-co-M(15%) | 85 |
| 20 | PAA-co-M(20%) | 81 |
| Sample Name | Mn (g/mol) | Mw (g/mol) | PDI | PPA to Copolymer Conv. (%) |
|---|---|---|---|---|
| PAA | 11,424 | 27,211 | 2.38 | 0 |
| PAA-co-M(5%) | 21,181 | 68,821 | 3.24 | 33.51 |
| PAA-co-M(10%) | 36,477 | 122,173 | 3.34 | 69.5 |
| PAA-co-M(15%) | 72,875 | 216,616 | 2.97 | 88.04 |
| PAA-co-M(20%) | 106,652 | 314,178 | 2.94 | 92.99 |
| Sample Name | Tp (°C) β = 5 °C/min | Tp (°C) β = 10 °C/min | Tp (°C) β = 20 °C/min | Tp (°C) β = 30 °C/min | Ea (KJ/mol) | R2 (%) |
|---|---|---|---|---|---|---|
| PAA | 286 | 303 | 322 | 339 | 87.52 | 98.68 |
| PAA-co-M(5%) | 274 | 290 | 308 | 325 | 85.43 | 98.57 |
| PAA-co-M(10%) | 269 | 284 | 304 | 322 | 80.9 | 97.9 |
| PAA-co-M(15%) | 314 | 327 | 342 | 323 | 0.1 | 7.06 |
| PAA-co-M (20%) | 314 | 329 | 342 | 355 | 126.69 | 98.81 |
| Sample Name | Tp (°C) β = 5 °C/min | Tp (°C) β = 10 °C/min | Tp (°C) β = 20 °C/min | Tp (°C) β = 30 °C/min | Ea (KJ/mol) | R2 (%) |
|---|---|---|---|---|---|---|
| First DTG peak (first stage) | ||||||
| PAA | 286 | 303 | 322 | 339 | 97.24 | 98.96 |
| PAA-co-M(5%) | 274 | 290 | 308 | 325 | 94.39 | 98.8 |
| PAA-co-M(10%) | 269 | 284 | 304 | 322 | 90.29 | 98.3 |
| PAA-co-M(15%) | 268 | 285 | 303 | 323 | 88.76 | 97.68 |
| PAA-co-M (20%) | 264 | 281 | 303 | 322 | 82.3 | 98.52 |
| Second DTG peak (second stage) | ||||||
| PAA-co-M(5%) | 310 | 326 | 340 | 353 | 129.1 | 99.3 |
| PAA-co-M(10%) | 310 | 325 | 337 | 353 | 130.04 | 97.14 |
| PAA-co-M(15%) | 314 | 327 | 342 | 355 | 135.09 | 98.83 |
| PAA-co-M(20%) | 314 | 329 | 342 | 355 | 137.14 | 98.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalal, M.A.; Abood, E.A.; Sweah, Z.J.; Al-Lami, H.S.; Abdulkarem, A.A.; Abdulelah, H. Highly Branched Poly(Adipic Anhydride-Co-Mannitol Adipate): Synthesis, Characterization, and Thermal Properties. Polymers 2025, 17, 684. https://doi.org/10.3390/polym17050684
Jalal MA, Abood EA, Sweah ZJ, Al-Lami HS, Abdulkarem AA, Abdulelah H. Highly Branched Poly(Adipic Anhydride-Co-Mannitol Adipate): Synthesis, Characterization, and Thermal Properties. Polymers. 2025; 17(5):684. https://doi.org/10.3390/polym17050684
Chicago/Turabian StyleJalal, Mahir A., Einas A. Abood, Zainab J. Sweah, Hadi S. Al-Lami, Alyaa Abdulhasan Abdulkarem, and Haider Abdulelah. 2025. "Highly Branched Poly(Adipic Anhydride-Co-Mannitol Adipate): Synthesis, Characterization, and Thermal Properties" Polymers 17, no. 5: 684. https://doi.org/10.3390/polym17050684
APA StyleJalal, M. A., Abood, E. A., Sweah, Z. J., Al-Lami, H. S., Abdulkarem, A. A., & Abdulelah, H. (2025). Highly Branched Poly(Adipic Anhydride-Co-Mannitol Adipate): Synthesis, Characterization, and Thermal Properties. Polymers, 17(5), 684. https://doi.org/10.3390/polym17050684







