Formation of PEG-PLGA Microspheres for Controlled Release of Simvastatin and Carvacrol: Enhanced Lipid-Lowering Efficacy and Improved Patient Compliance in Hyperlipidemia Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SIV-CAV-Loaded MS (SIV-CAV-PP-MS)
2.3. Characterisation of SIV-CAV-PP-MS Formulations
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. The Size, Zeta Potential and Polydispersity Index (PDI) of Microspheres
2.3.3. Determination of SIV-CAV-PP-MS Entrapment Efficiency
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Stability Studies
2.4. In Vitro Drug Release
2.5. In Vivo Release Kinetics
2.6. In Vivo Studies
2.6.1. Lipid-Lowering Research
2.6.2. Side Effect Mitigation Research
2.6.3. Histopathologic Examination
2.6.4. Behavioral Testing of Exercise Tolerance
2.6.5. Biochemical Analysis
2.6.6. Statistical Analysis
3. Results and Discussion
3.1. Morphology of SIV-CAV-PP-MS
3.2. The Size, Zeta Potential and Polydispersity Index (PDI) of Microspheres
3.3. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.4. Differential Scanning Calorimetry (DSC)
3.5. Stability Study
3.6. In Vitro Release Studies
3.7. Bioavailability Study
3.8. In Vivo Evaluation
3.8.1. Investigation of the Lipid-Lowering Effects of SIV-CAV-PP-MS
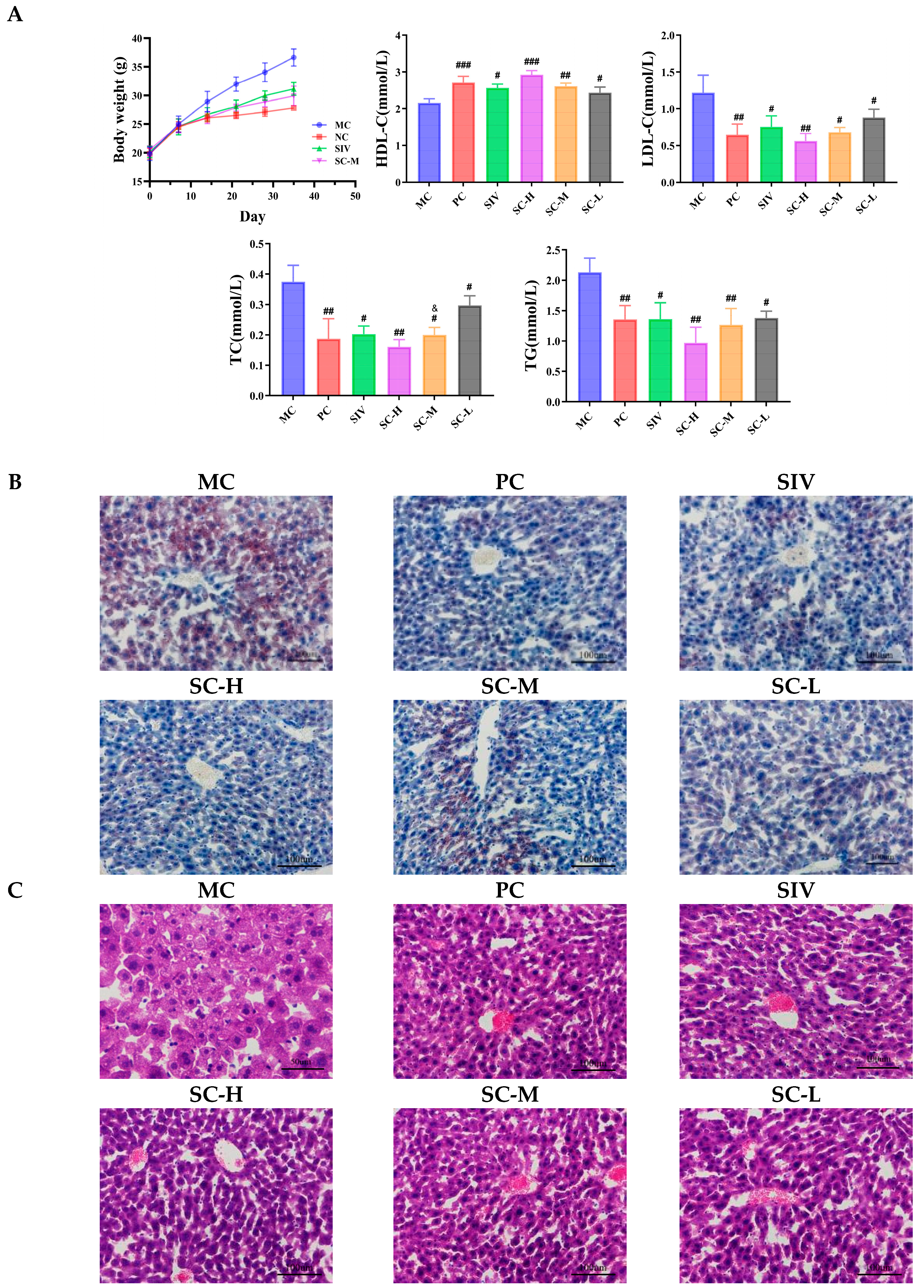
The Body Weight and the Levels of TC, TG, HDL-C, and LDL-C Detection
Histological Examinations
3.8.2. Investigation of the Adverse Effects Mitigation of SIV-CAV-PP-MS
Behavioral Parameters
The Levels of CK, LDH, AST and ALT Detection
Histological Examinations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Maksoud, M.; Sazonov, V.; Gutkin, S.W.; Hokanson, J.E. Effects of modifying triglycerides and triglyceride-rich lipoproteins on cardiovascular outcomes. J. Cardiovasc. Pharmacol. 2008, 51, 31–51. [Google Scholar]
- Li, H.; Rabearivony, A.; Zhang, W.; Chen, S.; An, X.; Liu, C. Chronopharmacology of simvastatin on hyperlipidaemia in high-fat diet-fed obese mice. J. Cell. Mol. Med. 2020, 24, 11024–11029. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chen, C.; Yang, Y.; Fang, J.; Cao, L.; Liu, Y. Musculoskeletal Adverse Events Associated with PCSK9 Inhibitors: Disproportionality Analysis of the FDA Adverse Event Reporting System. Cardiovasc. Ther. 2022, 25, 1–9. [Google Scholar] [CrossRef]
- Stewart, J.; McCallin, T.; Martinez, J.; Chacko, S.; Yusuf, S. Hyperlipidemia . Pediatr. Rev. 2020, 41, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Fujita, Y.; Ieiri, I. An updated review of pharmacokinetic drug interactions and pharmacogenetics of statins. Expert Opin. Drug Metabolsim Toxicol. 2020, 16, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Backes, J.M.; A Howard, P.; Ruisinger, J.F.; Moriarty, P.M. Does simvastatin cause more myotoxicity compared with other statins? Ann. Pharmacother. 2009, 43, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.U.; Abd-Elkareem, M.; Kamel, A.A.; Abou Khalil, N.S.; Hamad, D.; Nasr, N.E.H.; Hassan, M.A.; El Faham, T.H. Impact of porous microsponges in minimizing myotoxic side effects of simvastatin. Sci. Rep. 2023, 13, 5790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, B.; Yang, Y.J.; Dang, W.Z.; Li, H.; Feng, G.Z.; Yu, X.C.; Shen, X.Y.; Hu, X.G. Astragaloside IV reverses simvastatin-induced skeletal muscle injury by activating the AMPK-PGC-1α signalling pathway. Phytother. Res. 2020, 34, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- González-Iglesias, E.; Ochoa, D.; Navares-Gómez, M.; Zubiaur, P.; Aldama, M.; de la Torre, T.; Ríos-Rodríguez, M.L.; Soria-Chacartegui, P.; Rodríguez-Lopez, A.; Abad-Santos, F.; et al. Evaluation of the role of metabolizing enzymes and transporter variants in ezetimibe pharmacokinetics. Front. Pharmacol. 2024, 15, 1414059. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed Shaikh, N.; Khakid, S.; Alam, M.; Hasan, S.S.; Rizvi, F.; Mobeen, K. Comparative and combination study of simvastatin alone and in combination with Beta vulgaris in hyperlipidemia patients. Pak. J. Pharm. Sci. 2024, 37, 1033–1041. [Google Scholar] [PubMed]
- Gholijani, N.; Abolmaali, S.-S.; Kalantar, K.; Ravanrooy, M.-H. Therapeutic Effect of Carvacrol-loaded Albumin Nanoparticles on Arthritic Rats. Iran. J. Pharm. Res. 2020, 19, 312–320. [Google Scholar]
- Khalil, M.; Serale, N.; Diab, F.; Baldini, F.; Portincasa, P.; Lupidi, G.; Vergani, L. Beneficial Effects of Carvacrol on In Vitro Models of Metabolically-Associated Liver Steatosis and Endothelial Dysfunction: A Role for Fatty Acids in Interfering with Carvacrol Binding to Serum Albumin. Curr. Med. Chem. 2022, 29, 5113–5129. [Google Scholar] [CrossRef]
- Cho, S.; Choi, Y.; Park, S.; Park, T. Carvacrol prevents diet-induced obesity by modulating gene expressions involved in adipogenesis and inflammation in mice fed with high-fat diet. J. Nutr. Biochem. 2012, 23, 192–201. [Google Scholar] [CrossRef] [PubMed]
- El Aal, H.A.A.; Ahmed, L.A.; Hassan, W.A.; Fawzy, H.M.; Moawad, H. Combination of carvacrol with simvastatin improves the lipid-lowering efficacy and alleviates simvastatin side effects. J. Biochem. Mol. Toxicol. 2017, 31, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.K.; Moore, W.D. Simvastatin. In Analytical Profiles of Drug Substances and Excipients; Elsevier: Amsterdam, The Netherlands, 1999; Volume 22, pp. 359–388. [Google Scholar]
- Kelley, W.J.; Fromen, C.A.; Lopez-Cazares, G.; Eniola-Adefeso, O. PEGylation of model drug carriers enhances phagocytosis by primary human neutrophils. Acta Biomater. 2018, 79, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Webster, T.J. Recent progress and challenges for polymeric microsphere compared to nanosphere drug release systems: Is there a real difference? Bioorganic Med. Chem. 2021, 33, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Jusu, S.M.; Obayemi, J.D.; Salifu, A.A.; Nwazojie, C.C.; Uzonwanne, V.; Odusanya, O.S.; Soboyejo, W.O. Drug-encapsulated blend of PLGA-PEG microspheres: In vitro and in vivo study of the effects of localized/targeted drug delivery on the treatment of triple-negative breast cancer. Sci. Rep. 2020, 10, 14188. [Google Scholar] [CrossRef]
- Anwar, A.; Sun, P.; Rong, X.; Arkin, A.; Elham, A.; Yalkun, Z.; Li, X.; Iminjan, M. Process analytical technology as in-process control tool in semi-continuous manufacturing of PLGA/PEG-PLGA microspheres. Heliyon 2023, 9, e15753. [Google Scholar] [CrossRef]
- Wang, P.; Zhuo, X.; Chu, W.; Tang, X. Exenatide-loaded microsphere/thermosensitive hydrogel long-acting delivery system with high drug bioactivity. Int. J. Pharm. 2017, 528, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Buske, J.; König, C.; Bassarab, S.; Lamprecht, A.; Mühlau, S.; Wagner, K.G. Influence of PEG in PEG-PLGA microspheres on particle properties and protein release. Eur. J. Pharm. Biopharm. 2012, 81, 57–63. [Google Scholar] [CrossRef]
- Andhariya, J.V.; Burgess, D.J. Recent advances in testing of microsphere drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, B. Preparation of chitosan microcarriers by high voltage electrostatic field and freeze drying. J. Biosci. Bioeng. 2019, 128, 504–509. [Google Scholar]
- Hakiem, A.F.A.; Mohamed, N.A.; Ali, H.R. FTIR spectroscopic study of two isostructural statins: Simvastatin and Lovastatin as authentic and in pharmaceuticals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 120045. [Google Scholar]
- Suriyaamporn, P.; Sahatsapan, N.; Patrojanasophon, P.; Opanasopit, P.; Kumpugdee-Vollrath, M.; Ngawhirunpat, T. Optimization of In Situ Gel-Forming Chlorhexidine-Encapsulated Polymeric Nanoparticles Using Design of Experiment for Periodontitis. AAPS PharmSciTech 2023, 24, 161. [Google Scholar] [CrossRef]
- Narita, A.; Nakano, Y.; Okada, H.; Yamamoto, T.; Matsunaga, N.; Ikeda, S.; Izumi, Y.; Kitagawa, A.; Ota, T.; Suzuki, K. In Vitro Characterization of Drug-Loaded Superabsorbent Polymer Microspheres: Absorption and Release Capacity of Contrast Material, Antibiotics and Analgesics. Cardiovasc. Interv. Radiol. 2023, 46, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Z.; Wang, S.; Cheng, C.; Sun, X.; Liu, Z.; Wei, J.; Jiang, J.; Lan, H.; Zhou, M.; et al. Long-Circulating Lipid Nanospheres Loaded with Flurbiprofen Axetil for Targeted Rheumatoid Arthritis Treatment. Int. J. Nanomed. 2023, 18, 5159–5181. [Google Scholar] [CrossRef]
- Gao, S.; Tian, B.; Han, J.; Zhang, J.; Shi, Y.; Lv, Q.; Li, K. Enhanced transdermal delivery of lornoxicam by nanostructured lipid carrier gels modified with polyarginine peptide for treatment of carrageenan-induced rat paw edema. Int. J. Nanomed. 2019, 14, 6135–6150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhang, M.; Zheng, Y.; Wang, M.; Gao, Y.; Wang, X.; Liu, X.; Lv, W.; Zeng, X.; Belosludtsev, K.N.; et al. α-Ketoglutarate prevents hyperlipidemia-induced fatty liver mitochondrial dysfunction and oxidative stress by activating the AMPK-pgc-1α/Nrf2 pathway. Redox Biol. 2024, 74, 103230. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.C.; Fenning, A.S.; Ryan, K.R.; Vella, R.K. Validation of a clinically-relevant rodent model of statin-associated muscle symptoms for use in pharmacological studies. Toxicol. Appl. Pharmacol. 2018, 360, 78–87. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Zhang, J.; Wang, Y.; Lu, H.; Zhang, Y.; Song, G.; Niu, F.; Shen, Y.; Midgley, A.C.; et al. Elastic porous microspheres/extracellular matrix hydrogel injectable composites releasing dual bio-factors enable tissue regeneration. Nat. Commun. 2024, 15, 1377. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L.; Wan, F.; Bera, H.; Cun, D.; Rantanen, J.; Yang, M. Quality by design thinking in the development of long-acting injectable PLGA/PLA-based microspheres for peptide and protein drug delivery. Int. J. Pharm. 2020, 585, 119441. [Google Scholar]
- Harisa, G.I.; Alomrani, A.H.; Badran, M.M. Simvastatin-loaded nanostructured lipid carriers attenuate the atherogenic risk of erythrocytes in hyperlipidemic rats. Eur.J. Pharm. Sci. 2017, 96, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Li, K. Preparation, Evaluation, and Pharmacodynamic Study of Long-Acting Sustained-Release Microspheres of Dopamine Receptor Agonists; Yantai University: Yantai, China, 2020. [Google Scholar]
- Zhang, S.; Zhou, X.; Qin, Q.; Na, Q.I. Process Optimization, Characterization, and In Vitro Release Study of Quercetin Sustained-Release Microspheres Prepared by SPG Membrane Emulsification Method. Her. Med. 2021, 40, 502–508. [Google Scholar]
- Su, Y.; Liu, J.; Tan, S.; Liu, W.; Wang, R.; Chen, C. PLGA sustained-release microspheres loaded with an insoluble small-molecule drug: Microfluidic-based preparation, optimization, characterization, and evaluation in vitro and in vivo. Drug Deliv. 2022, 29, 1437–1446. [Google Scholar] [CrossRef]
- Wang, M.; Kong, X.P.; Li, H.; Ge, J.C.; Han, X.Z.; Liu, J.H.; Yu, S.L.; Li, W.; Li, D.L.; Wang, J. Coprecipitation-based synchronous chlorantraniliprole encapsulation with chitosan: Carrier-pesticide interactions and release behavior. Pest. Manag. Sci. 2023, 79, 3757–3766. [Google Scholar] [CrossRef]
- Wu, B.; Wu, L.; He, Y.; Yin, Z.; Deng, L. Engineered PLGA microspheres for extended release of brexpiprazole: In vitro and in vivo studies. Drug Dev. Ind. Pharm. 2021, 47, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Yeom, M.; Park, J.; Lee, B.; Lee, H.S.; Park, H.J.; Won, R.; Lee, H.; Hahm, D.H. Electroacupuncture ameliorates poloxamer 407-induced hyperlipidemia through suppressing hepatic SREBP-2 expression in rats. Life Sci. 2018, 203, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, S.N.; Li, L.Y.; Chen, Z.Y.; Lei, L.; Zhang, N.; Shen, Z.F. A dyslipidemia animal model induced by poloxamer 407 in golden hamsters and pilot study on the mechanism. Acta Pharm. Sin. 2011, 46, 406–411. (In Chinese) [Google Scholar]
- Wei, J.; Huan, Y.; Heng, Z.; Zhao, C.; Jia, L.; Yu, Y.; Gao, Y. Dynamic urine proteome changes in a rat model of simvastatin-induced skeletal muscle injury. J. Proteom. 2022, 254, 104477. [Google Scholar] [CrossRef]
- Hareedy, M.S.; Ahmed, E.A.; Ali, M.F. Montelukast modifies simvastatin-induced myopathy and hepatotoxicity. Drug Dev. Res. 2019, 80, 1000–1009. [Google Scholar] [CrossRef]



| Solvents | Solubility (mg/L) | |
|---|---|---|
| SIV | PBS (pH7.4) + 0.5% Tween-80 | 315.57 |
| PBS (pH7.4) + 0.5% SDS | 346.17 | |
| CAV | PBS (pH7.4) + 0.5% Tween-80 | 6830.00 |
| PBS (pH7.4) + 0.5% SDS | 6230.00 |
| Model | Equation | r | |
|---|---|---|---|
| SIV | Zero-order kinetics | Mt = 3.32k + 11.82 | 0.9775 |
| First-order kinetics | Mt = 104.69(1 − e−0.07) | 0.9043 | |
| Higuchi equation | Mt = 17.33x1/2 − 4.43 | 0.9642 | |
| Ritger–Peppas equation | Qt = 11.69x0.62 | 0.9886 | |
| CAV | Zero-order kinetics | Mt = 5.61k + 3.94 | 0.9747 |
| First-order kinetics | Mt = 143.45(1 − e−0.04) | 0.9807 | |
| Higuchi equation | Mt = 20.45x1/2 − 13.45 | 0.9654 | |
| Ritger–Peppas equation | Qt = 8.07x0.77 | 0.9889 |
| Parameter | SIV-CAV-PP-MS | SIV-CAV-Suspensions | ||
|---|---|---|---|---|
| SIV | CAV | SIV | CAV | |
| t1/2 (h) | 206.48 ± 55.07 * | 156.75 ± 48.66 * | 1.87 ± 0.12 | 0.95 ± 0.06 |
| Tmax (h) | 87 ± 93.53 * | 100 ± 94.80 * | 0.25 ± 0.00 | 0.5 ± 0.00 |
| Cmax (ng/mL) | 6.302 ± 1.313 * | 15.01 ± 1.29 * | 350.71 ± 42.57 | 2346.52 ± 231.05 |
| AUClast (h ng/mL) | 1745.23 ± 195.81 | 5120.41 ± 518.27 | 1213.97 ± 106.35 | 4631.42 ± 753.51 |
| AUCinf (h ng/mL) | 1875.54 ± 293.86 | 5327.48 ± 655.86 | 1228.72 ± 112.97 | 4647.68 ± 749.42 |
| MRTlast (h) | 191.42 ± 12.78 * | 211.86 ± 10.09 * | 2.45 ± 0.15 | 2.17 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Ren, H.; Wang, C.; Zhao, Y.; Zou, B.; Zhang, X. Formation of PEG-PLGA Microspheres for Controlled Release of Simvastatin and Carvacrol: Enhanced Lipid-Lowering Efficacy and Improved Patient Compliance in Hyperlipidemia Therapy. Polymers 2025, 17, 574. https://doi.org/10.3390/polym17050574
Fu L, Ren H, Wang C, Zhao Y, Zou B, Zhang X. Formation of PEG-PLGA Microspheres for Controlled Release of Simvastatin and Carvacrol: Enhanced Lipid-Lowering Efficacy and Improved Patient Compliance in Hyperlipidemia Therapy. Polymers. 2025; 17(5):574. https://doi.org/10.3390/polym17050574
Chicago/Turabian StyleFu, Lin, Hengxin Ren, Chaoxing Wang, Yaxin Zhao, Bohang Zou, and Xiangyu Zhang. 2025. "Formation of PEG-PLGA Microspheres for Controlled Release of Simvastatin and Carvacrol: Enhanced Lipid-Lowering Efficacy and Improved Patient Compliance in Hyperlipidemia Therapy" Polymers 17, no. 5: 574. https://doi.org/10.3390/polym17050574
APA StyleFu, L., Ren, H., Wang, C., Zhao, Y., Zou, B., & Zhang, X. (2025). Formation of PEG-PLGA Microspheres for Controlled Release of Simvastatin and Carvacrol: Enhanced Lipid-Lowering Efficacy and Improved Patient Compliance in Hyperlipidemia Therapy. Polymers, 17(5), 574. https://doi.org/10.3390/polym17050574





