The Structural Design of a New Graftable Antioxidant and the Theoretical Study of Its Role in the Cross-Linking Reaction Process of Polyethylene
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
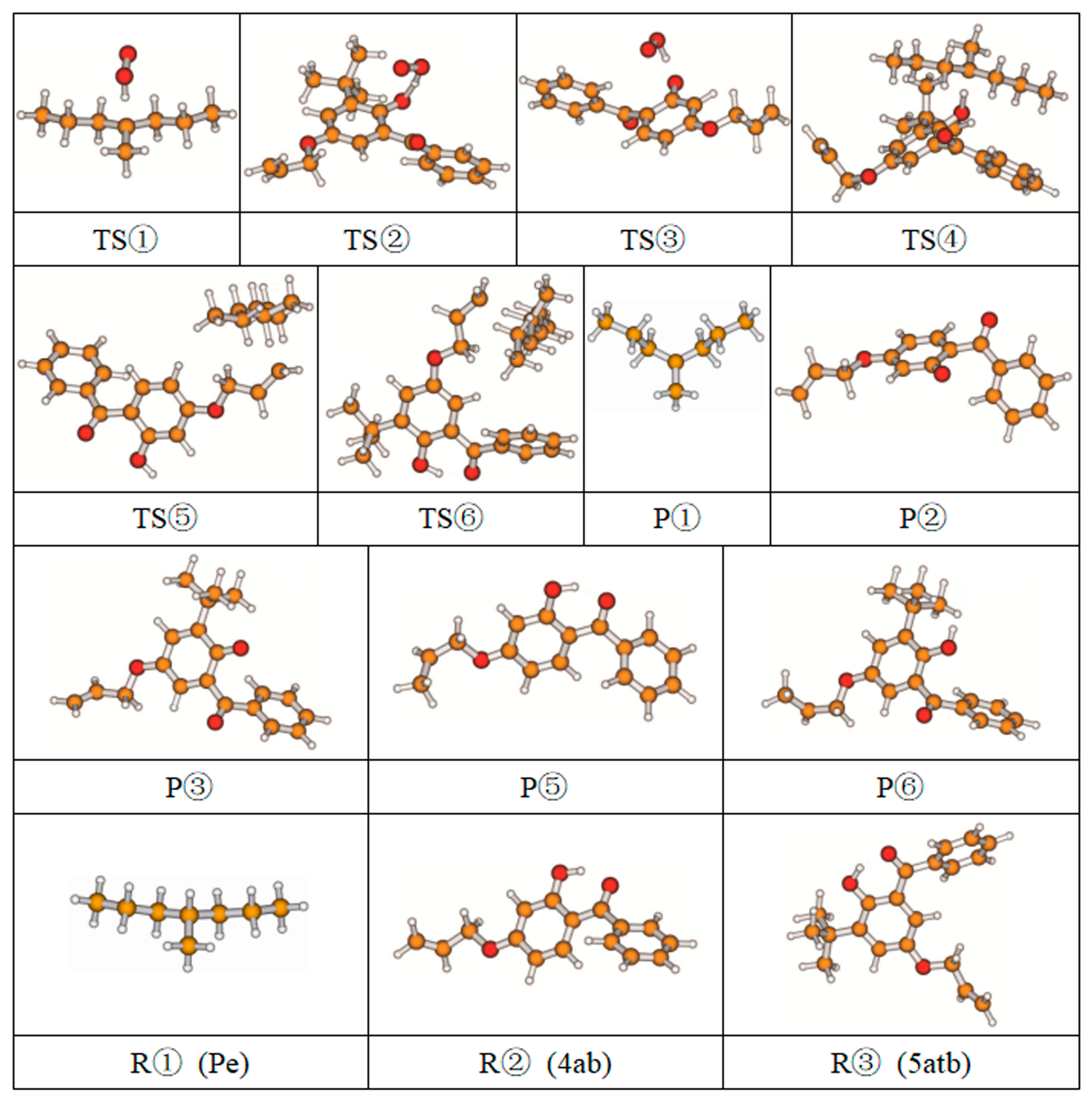
3.1. Stationary Point Geometries and NBO Charge Population
| Reaction Equation | Reactant | b/f | Product | ΔG≠ | ΔG0 | |
|---|---|---|---|---|---|---|
| ① |  | 1.100 | 1.606/1.076 | 0.977 | 1.96 | 1.72 |
| ①-1 | 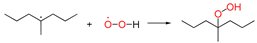 | two radicals combined | ||||
| ①-2 | 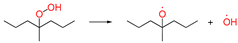 | peroxide bond homolytic cleavage | ||||
| ② | 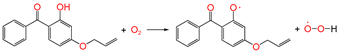 | 0.963 | 1.341/1.089 | 0.977 | 1.86 | 1.79 |
| ③ | 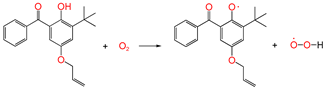 | 0.991 | 1.286/1.121 | 0.977 | 1.48 | 1.40 |
| ③-1 | 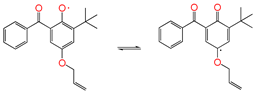 | isomerization which is the same as keto-enol tautomerism | ||||
| ④ | 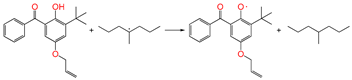 | 0.963 | 1.243/1.350 | 1.100 | 1.06 | −3.31 |
| ④-1 | 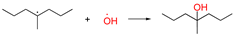 | two radicals combined (it can also be Pe radicals or PeO radicals) | ||||
| ④-2 | 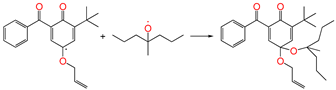 | two radicals combined (it can also be OH radicals or Pe radicals) | ||||
| ⑤ | 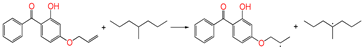 | 1.100 | 1.328/1.420 | 1.101 | 0.98 | −0.96 |
| ⑥ | 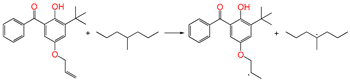 | 1.100 | 1.329/1.420 | 1.101 | 0.63 | −0.46 |
| ⑥-1 |  | two radicals combined | ||||
3.2. Frontier MOs
3.3. Energetics
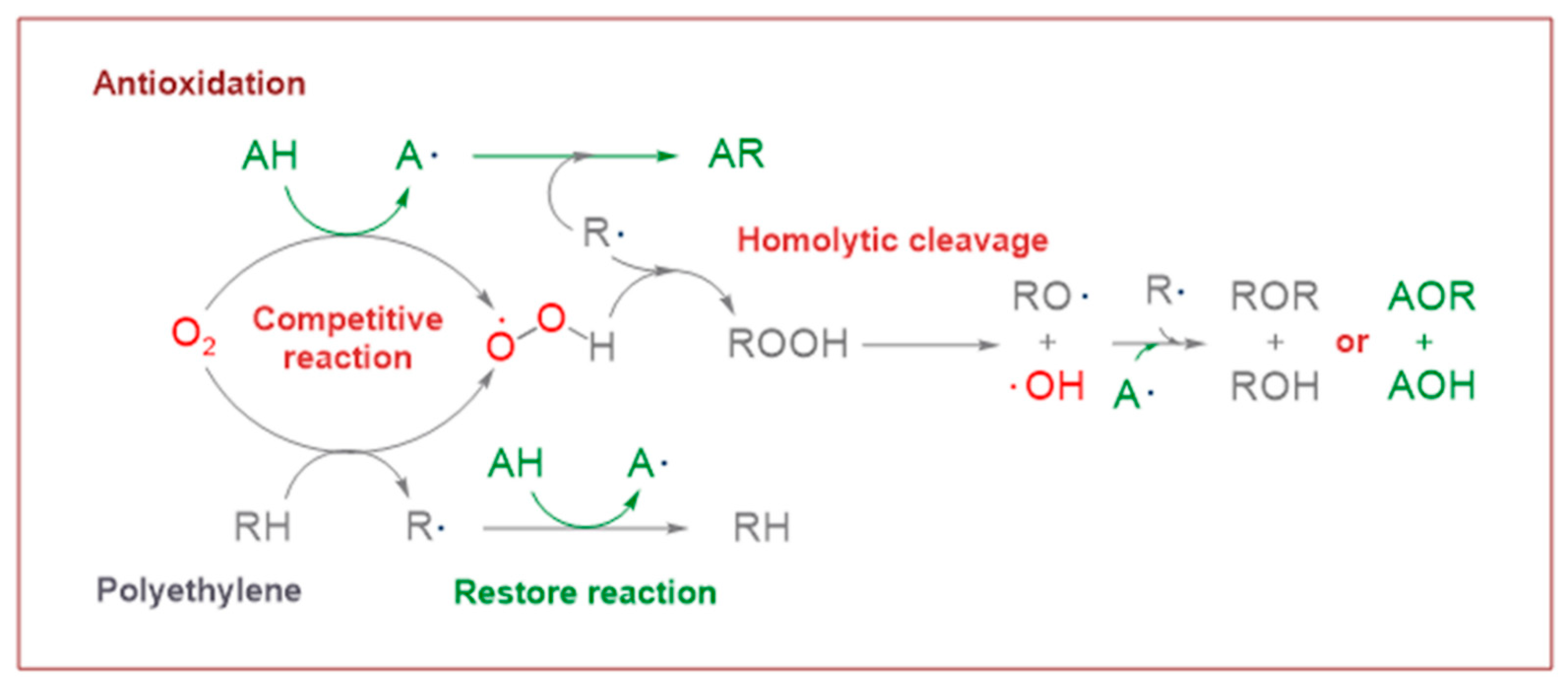
3.3.1. Oxidative Reaction
3.3.2. Restore Reaction
3.3.3. Isomerization Reaction
3.3.4. Grafting Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzanti, G.; Marzinotto, M. Extruded Cables for High-Voltage Direct-Current Transmission; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Montanari, G.C.; Morshuis, P.H.F.; Zhou, M.; Stevens, G.C.; Vaughan, A.S.; Han, Z.; Li, D. Criteria influencing the selection and design of HV and UHV DC cables in new network applications. IET High Volt. 2018, 3, 90–95. [Google Scholar] [CrossRef]
- Meng, F.B.; Chen, X.R.; Hong, Z.L.; Shi, Y.W.; Zhu, H.S.; Muhammad, A.; Paramane, A.; Huang, R.B.; Han, Z. Insulation Properties and Interfacial Quantum Chemical Analysis of Cross-Linked Polyethylene Under Different Degassing Time for HVDC Cable Factory Joint Applications. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 271–278. [Google Scholar] [CrossRef]
- Mcnulty, M. Cross-Linked Polyethylene Compound for Cable Systems. In Wire & Cable Technology International: Serving Manufacturers, Specifiers and Users of Wire and Cable; Wire & Cable Technology International (WCTI): Akron, OH, USA, 2024; Volume 52. [Google Scholar]
- Pourrahimi, A.M.; Pitois, C.; Abbasi, A. XLPE high voltage insulation; A link between DC conductivity and microstructure. Polym. Test. 2024, 131, 108330. [Google Scholar] [CrossRef]
- Ahmed, M.; Lisheng, Z.; Li, F.; Xu, N.; Ren, H. DC Conductivity Fluctuation Due to Temperature Dependence and Cross-linking Byproducts of XLPE Insulation Material in HVDC Cables. In Proceedings of the 2021 IEEE Electrical Insulation Conference (EIC), Denver, CO, USA, 7–28 June 2021; pp. 173–176. [Google Scholar] [CrossRef]
- Chen, W.; Yang, Z.; Liu, X.; Wang, Y. Influence of type, content and degassing time of crosslinking agent on electrical tree characteristics of XLPE in high voltage cables. In Proceedings of the 22nd International Symposium on High Voltage Engineering, Xi’an, China, 21–26 November 2021; pp. 1623–1626. [Google Scholar] [CrossRef]
- Qu, B.; Bao, W.; Wu, Q.; Shi, W. Recent Developments on photoinitiated crosslinking of polyethylene and its applications for manufacturing insulated wire and cable. In Proceedings of the 2009 IEEE 9th International Conference on the Properties and Applications of Dielectric Materials, Harbin, China, 19–23 July 2009; pp. 33–36. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Li, M.X.; Zhao, H.; Wang, X.; Han, B.Z. Theoretical study on the radical reaction mechanism in the cross-linking process of polyethylene. RSC Adv. 2015, 5, 90343–90353. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, C.; Du, X.; Shang, Y.; Zhao, H.; Wang, X.; Han, B.Z.; Li, Z.S. Theoretical study on the grafting reaction of maleimide containing 2-hydroxy-benzophenone onto polyethylene. J. Mol. Model. 2021, 27, 259. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Si, Z.C.; Wang, S.H.; Li, S.T.; Zhou, H.; Liu, J.J. Effect of hindered phenolic antioxidants on crosslinking characteristics of low-density polyethylene initiated by peroxide. Energy Rep. 2023, 9, 159–166. [Google Scholar] [CrossRef]
- Sahoo, R.; Karmakar, S. Impact of Accelerated Thermal Aging on Electrical Tree Structure and Physicochemical Characteristics of XLPE Insulation. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 429–438. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, H.; Du, Y.; Du, X.; Shang, Y.; Yang, H.D.; Wang, X.; Chen, Q.G.; Li, Z.S. Theoretical Study of the Grafting Reaction of a New Antioxidant to Cross-Linked Polyethylene and the Antioxidation Mechanism. Int. J. Quantum Chem. 2024, 124, e27492. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhao, H.; Han, B.Z.; Zhang, H.; Zhang, C.C.; Ai, Y. The Effect of Voltage Stabilizers on the DC Insulation Performance of XLPE. Chin.S Oc Elec. Eng. 2018, 38, 7071–7079. [Google Scholar]
- Allen, N.S. Photostabilising action of ortho-hydroxy aromatic compounds: A critical review. Polym. Photochem. 1983, 3, 167–187. [Google Scholar] [CrossRef]
- Zhang, C.C.; Wang, T.T.; Sun, W.F.; Li, C.Y.; Zhao, H. Grafting of antioxidant onto polyethylene to improve DC dielectric and thermal aging properties. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 541–549. [Google Scholar] [CrossRef]
- Dobashi, Y.; Kondou, J.; Ohkatsu, Y. Photo-antioxidant abilities of 2-hydroxybenzoyl compounds. Polym. Degrad. Stab. 2005, 89, 140–144. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, C.C.; Zhao, H.; Zhang, H.; Wang, X.; Han, B.Z. Grafted UV absorber as voltage stabilizer against electrical degradation and breakdown in cross-linked polyethylene for high voltage cable insulation. Polym. Degrad. Stab. 2021, 185, 109498. [Google Scholar] [CrossRef]
- Truong, T.N.; Duncan, W.T.; Bell, R.L. Chemical Applications of Density Functional Theory; American Chemical Society: Washington, DC, USA, 1996. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti conelation energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Zangwill, A.; Soven, P. Density-functional approach to local-field effects in finite systems: Photoabsorption in the rare gases. Phys. Rev. A 1980, 21, 1561–1572. [Google Scholar] [CrossRef]
- Levine, Z.H.; Soven, P. Time-dependent local-density theory of dielectric effects in small molecules. Phys. Rev. A 1984, 29, 625–635. [Google Scholar] [CrossRef]
- Hratchian, H.P.; Schlegel, H.B. Accurate reaction paths using a hessian based predictor-corrector integrator. J. Chem. Phys. 2004, 120, 9918–9924. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Rubio-Pons, Ó.; Loboda, O.; Minaev, B.; Schimmelpfennig, B.; Vahtras, O.; Ågren, H. CASSCF calculations of triplet-state properties applications to benzene derivatives. Mol. Phys. 2003, 101, 2103–2114. [Google Scholar] [CrossRef]
- Minaev, B.F.; Knuts, S.; Ågren, H.; Vahtras, O. The vibronically induced phosphorescence in benzene. Chem. Phys. 1993, 175, 245–254. [Google Scholar] [CrossRef]
- Fang, W.H. Ab initio determination of dark structures in radiationless transitions for aromatic carbonyl compounds. Acc. Chem. Res. 2008, 41, 452–457. [Google Scholar] [CrossRef]
- Hammond, G.S. A correlation of reaction rates. J. Am. Chem. Soc. 1955, 77, 334–338. [Google Scholar] [CrossRef]
- Lias, S.G.; Levin, R.D.; Kafafi, S.A.; Bartmess, J.E. Nist Chemistry Web Book, Nist Standard Reference Database Number 69; NIST: Gaithersburg, MA, USA, 1998. [CrossRef]
- Yamano, Y. Roles of polycyclic compounds in increasing breakdown strength of LDPE film. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 773–781. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Zhao, H.; Wang, X.; Han, B.Z.; Li, Z.S. Theoretical study on the tailored side-chain architecture of benzil-like voltage stabilizers for enhanced dielectric strength of cross-linked polyethylene. RSC Adv. 2016, 6, 11618. [Google Scholar] [CrossRef]
| ab. | Molecular Formula | Molecular Name |
|---|---|---|
| Pe | 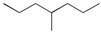 | 4-methylheptane (model molecule of polyethylene) |
| 4OB | 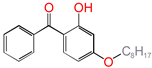 | 2-hydroxyl-4-n-octoxybenzophenone (ultraviolet absorber UV-531) |
| 4AB | 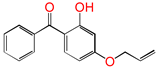 | 4-allyloxy-2-hydroxylbenzophenone (graftable UV absorber as voltage stabilizer) |
| 5ATB | 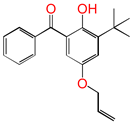 | 5-allyloxy-2-hydroxyl-3-tert-butylbenzophenone (designed as a new graftable antioxidant) |
| States | Molecular Formula | Natural Charge Population | |||
|---|---|---|---|---|---|
| H of OH | O of OH | C of CH on C=C | C of CH2 on C=C | ||
| T1 |  | 0.472 | −0.638 | −0.196 | −0.348 |
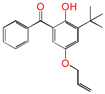 | 0.479 | −0.606 | −0.197 | −0.348 | |
| S0 |  | 0.504 | −0.671 | −0.193 | −0.353 |
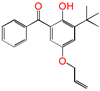 | 0.503 | −0.686 | −0.189 | −0.359 | |
| ab. | Molecular Formula | Eg | IP(a) | EA(a) |
|---|---|---|---|---|
| Pe | 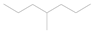 | 8.38 | 9.41 | −1.09 |
| Bp |  | 4.90 | 8.64(9.05) | 0.73(0.69 ± 0.05) |
| 4OB |  | 4.32 | 7.76 | 0.85 |
| 4AB | 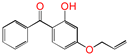 | 4.34 | 7.82 | 0.88 |
| 5ATB |  | 3.66 | 7.18 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Zhang, H.; Deng, C.; Du, X.; Shang, Y.; Wang, X.; Chen, Q.; Li, Z. The Structural Design of a New Graftable Antioxidant and the Theoretical Study of Its Role in the Cross-Linking Reaction Process of Polyethylene. Polymers 2025, 17, 546. https://doi.org/10.3390/polym17040546
Du Y, Zhang H, Deng C, Du X, Shang Y, Wang X, Chen Q, Li Z. The Structural Design of a New Graftable Antioxidant and the Theoretical Study of Its Role in the Cross-Linking Reaction Process of Polyethylene. Polymers. 2025; 17(4):546. https://doi.org/10.3390/polym17040546
Chicago/Turabian StyleDu, Yang, Hui Zhang, Chi Deng, Xia Du, Yan Shang, Xuan Wang, Qingguo Chen, and Zesheng Li. 2025. "The Structural Design of a New Graftable Antioxidant and the Theoretical Study of Its Role in the Cross-Linking Reaction Process of Polyethylene" Polymers 17, no. 4: 546. https://doi.org/10.3390/polym17040546
APA StyleDu, Y., Zhang, H., Deng, C., Du, X., Shang, Y., Wang, X., Chen, Q., & Li, Z. (2025). The Structural Design of a New Graftable Antioxidant and the Theoretical Study of Its Role in the Cross-Linking Reaction Process of Polyethylene. Polymers, 17(4), 546. https://doi.org/10.3390/polym17040546







