Preparation of a Macromolecular Flame Retardant with a Phosphine Oxide Structure and Its Application in Polyamide 6
Abstract
1. Introduction
2. Experimental Method
2.1. Materials
2.2. Preparation of Flame-Retardant Polyamide 6
2.3. Characterization
3. Results and Discussion
3.1. Properties of the MFR
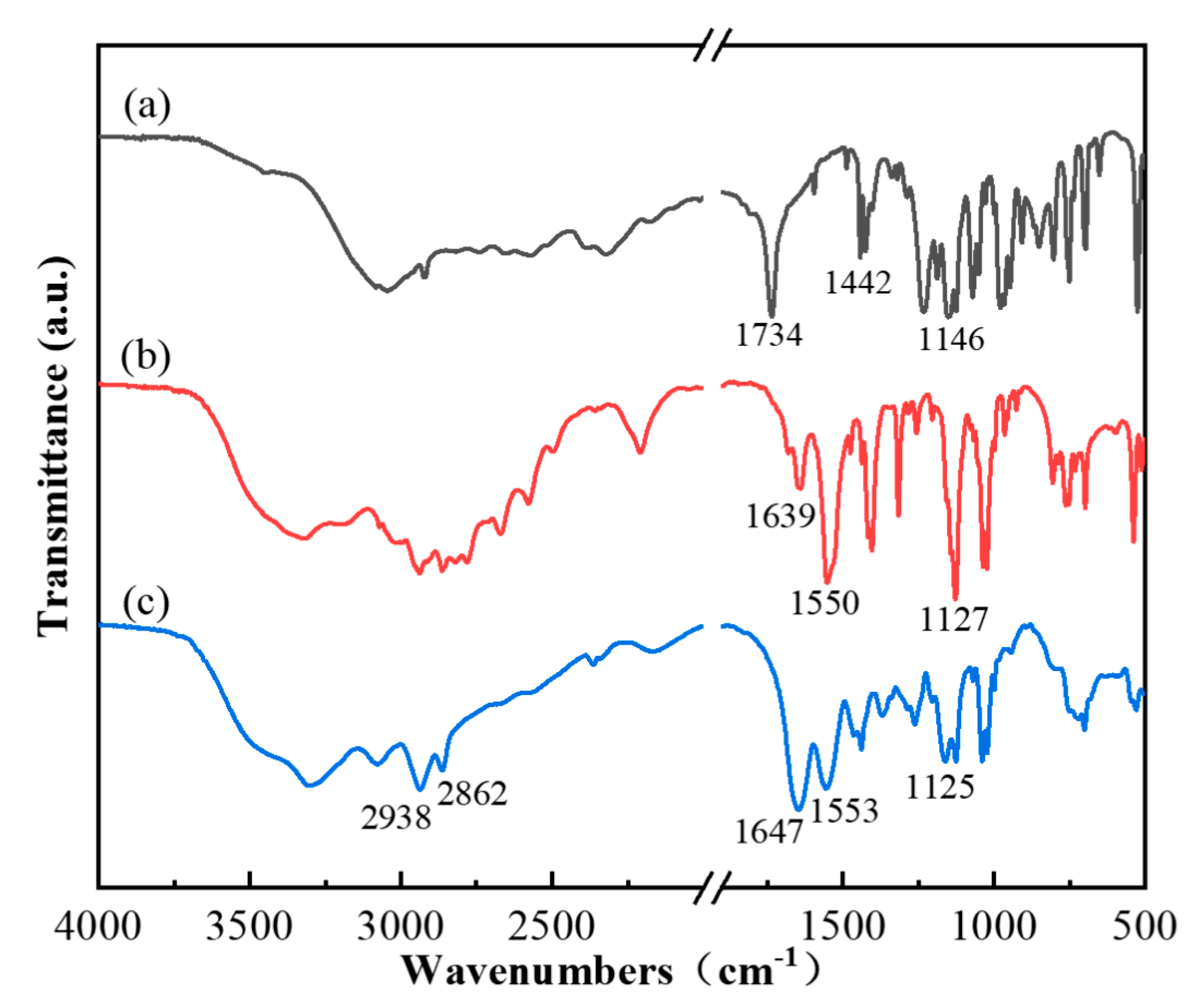
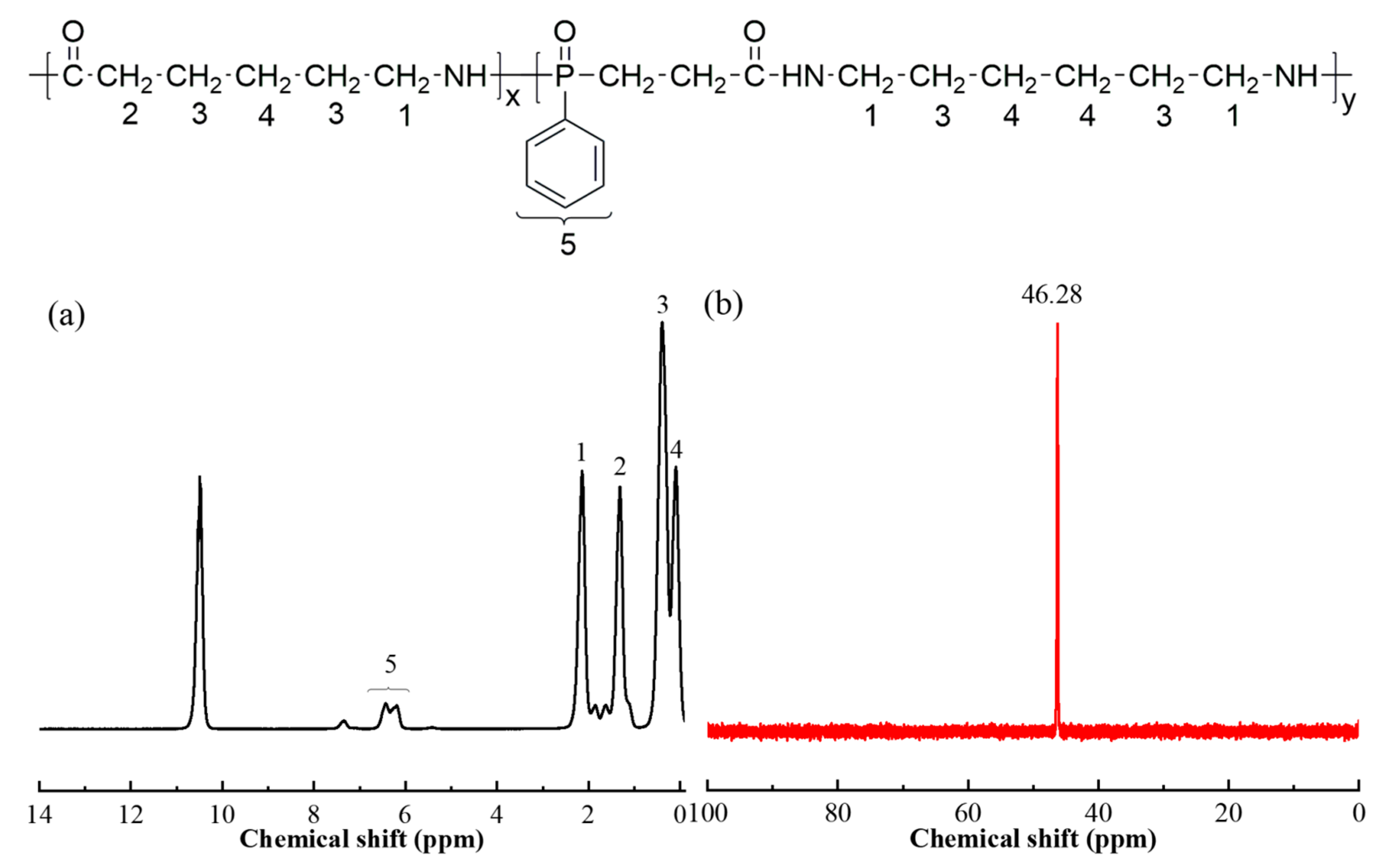
3.1.1. Chemical Structural of the MFR
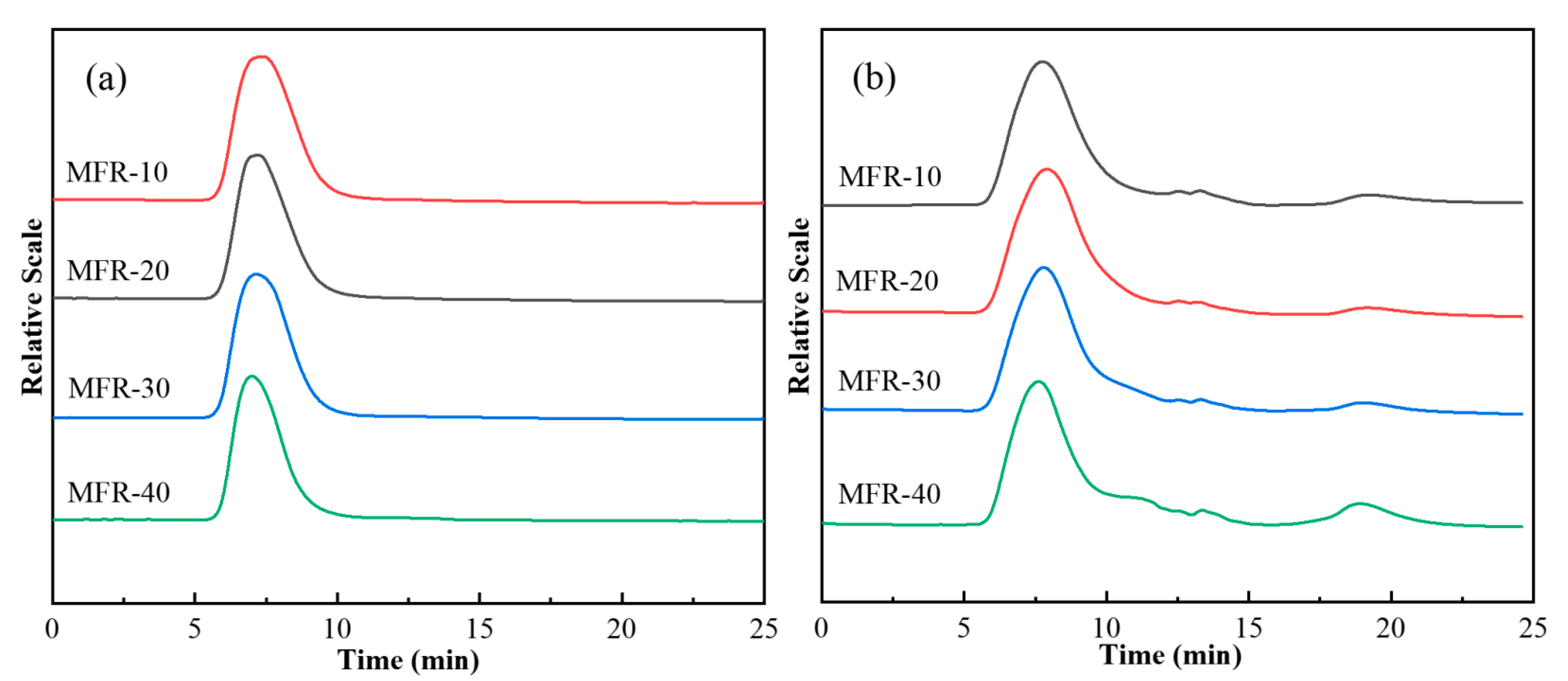
3.1.2. Molecular Weight and Molecular Weight Distribution of the MFR
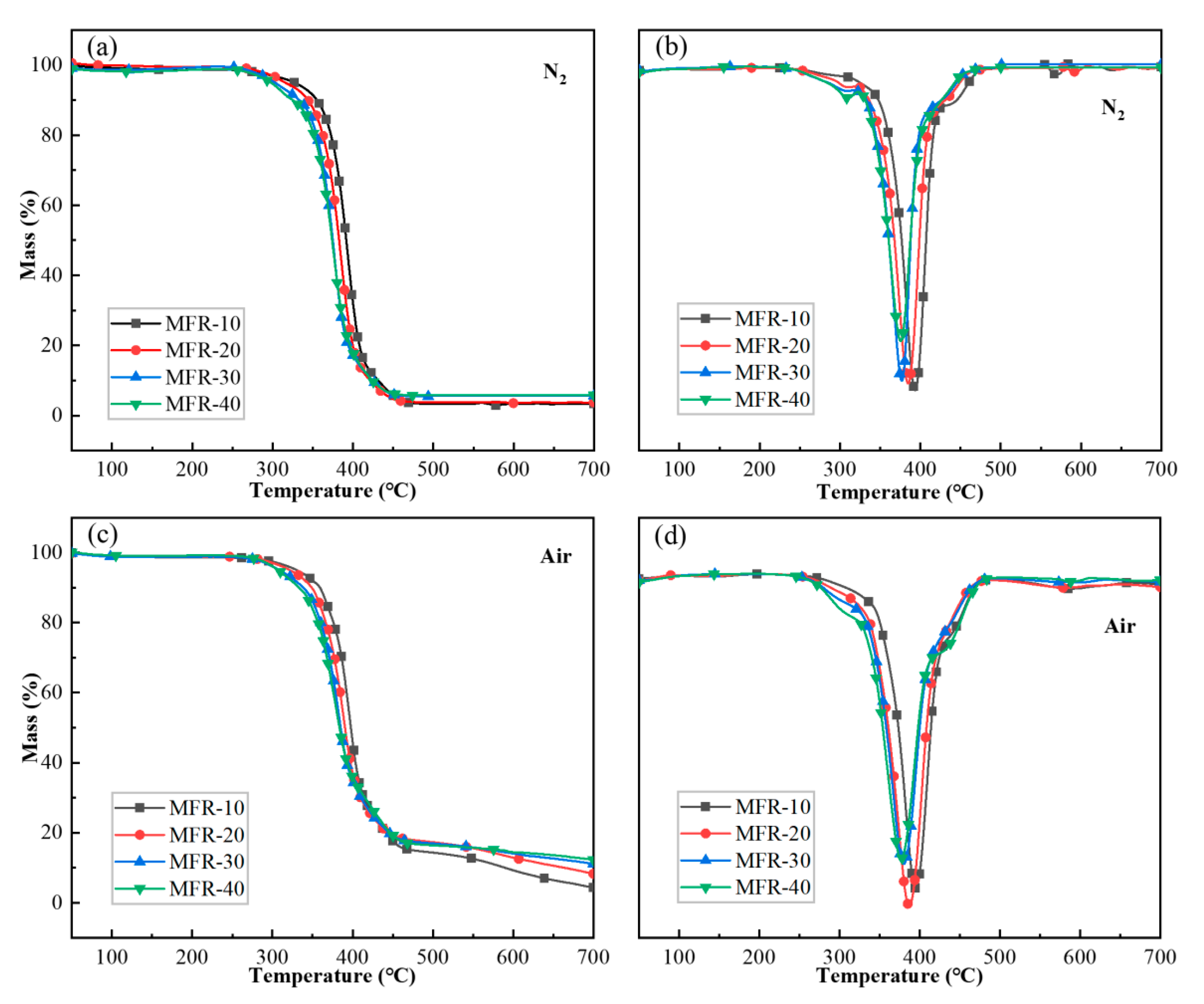
3.1.3. Thermal Properties of the MFR
3.2. Properties of FR–PA6
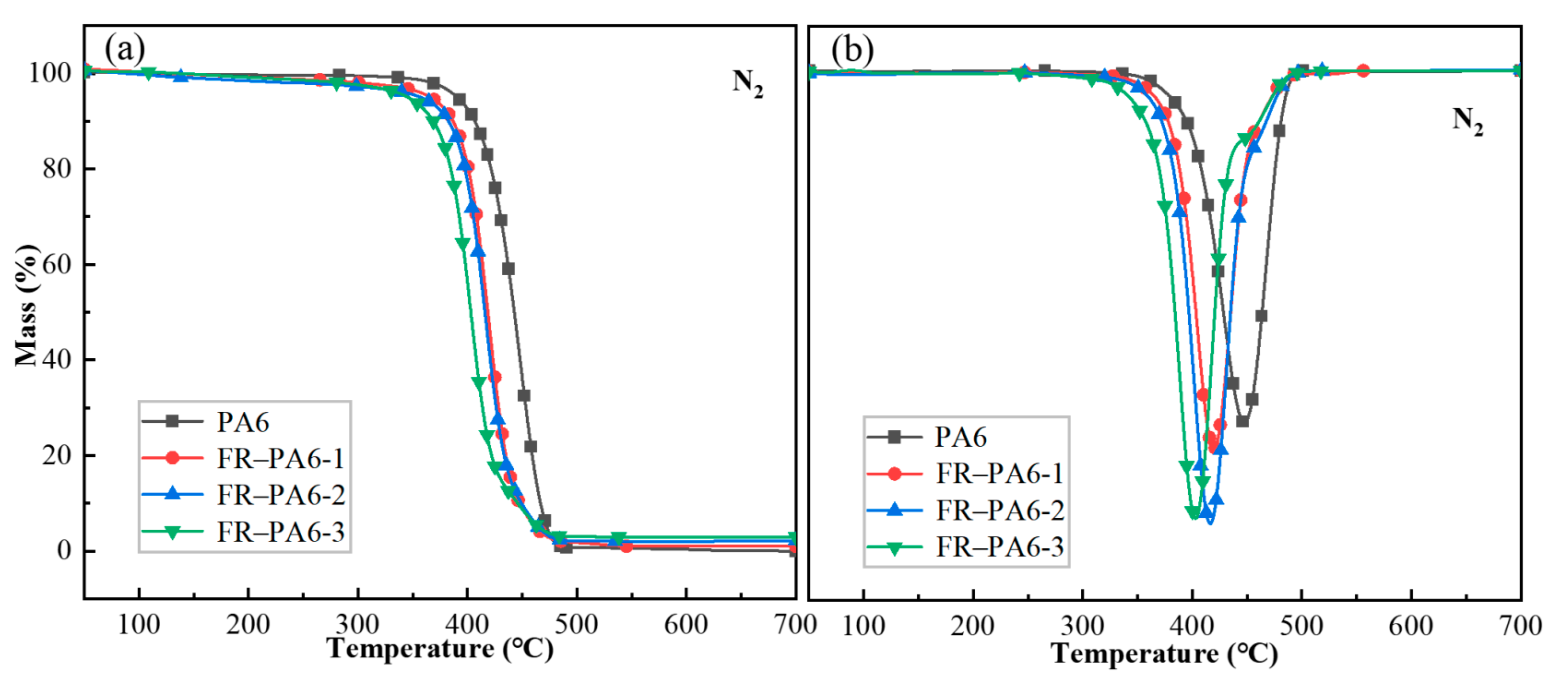
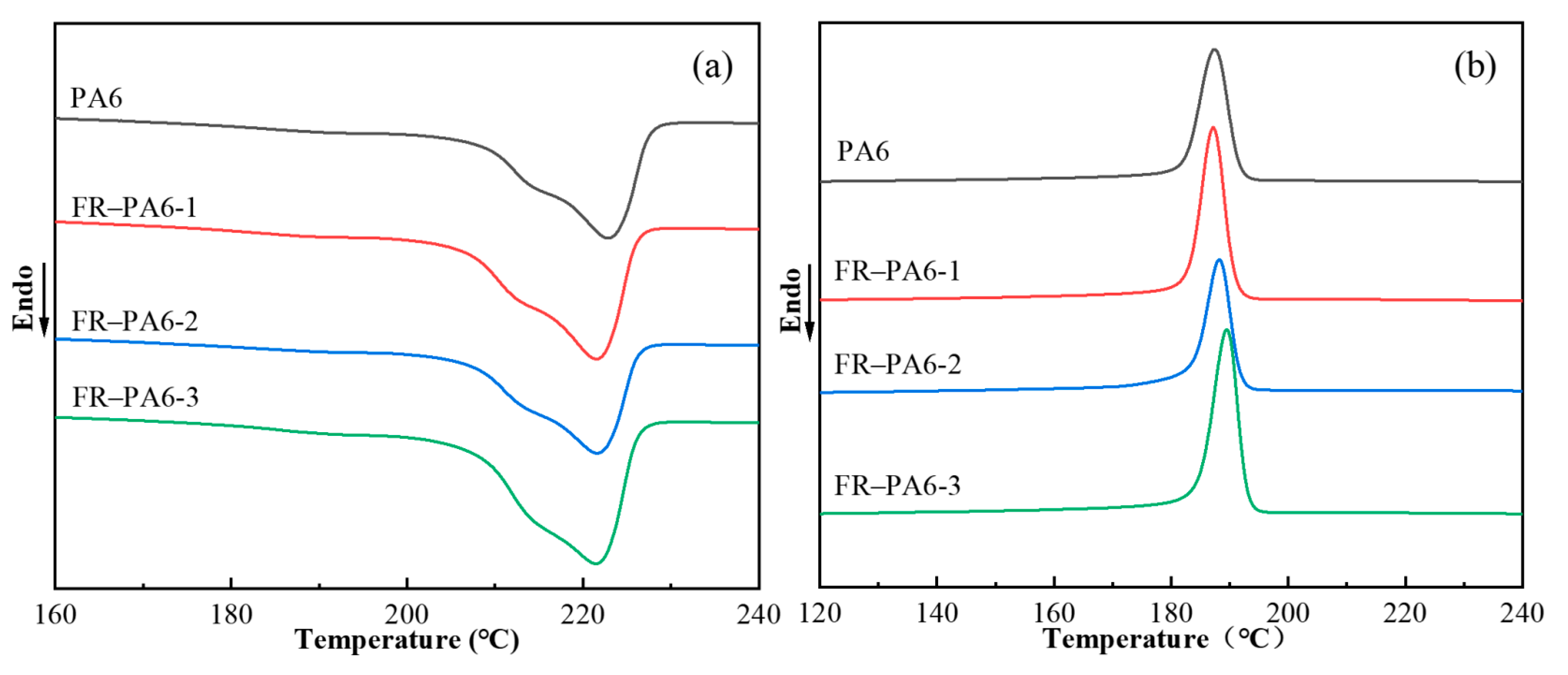
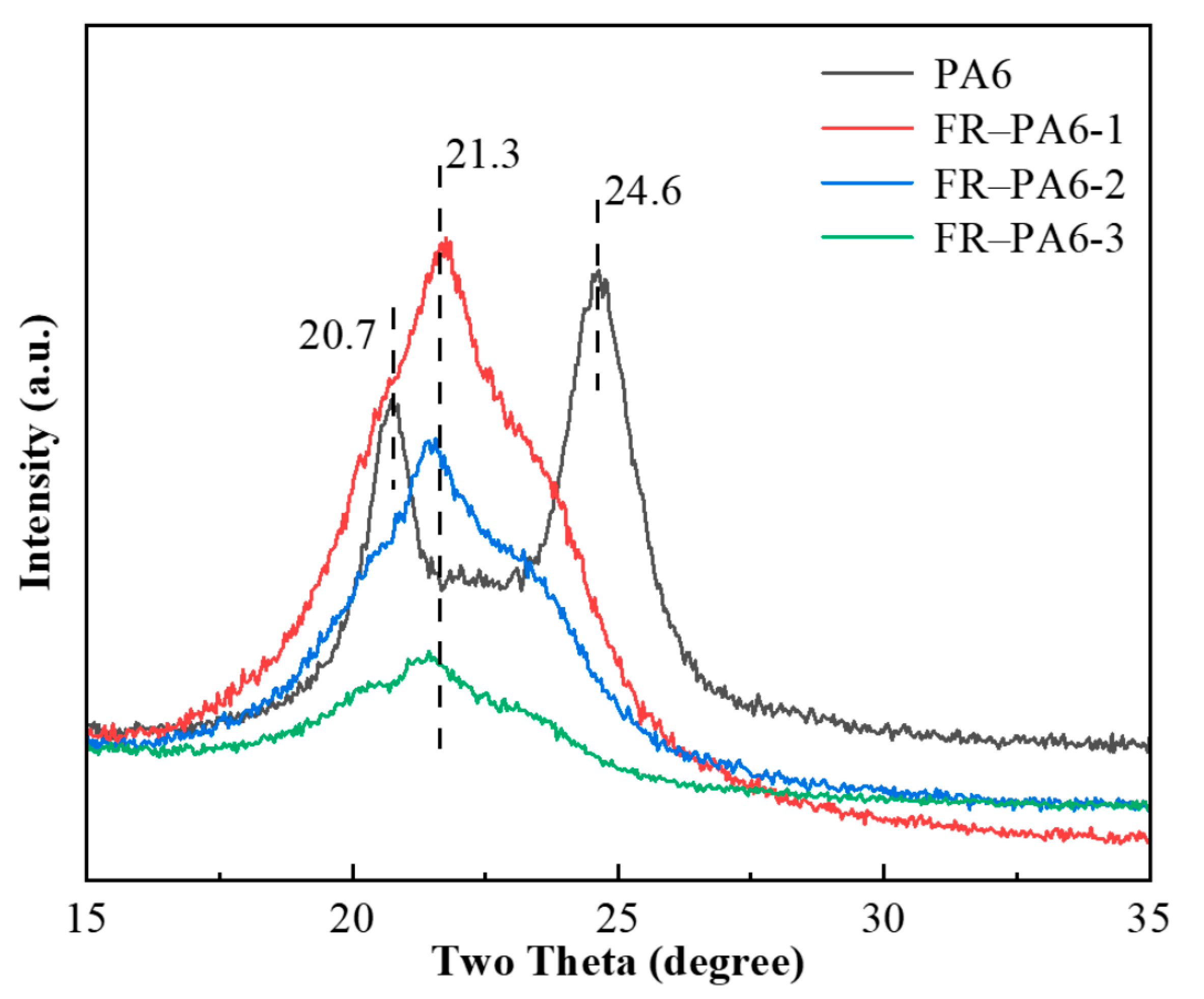
3.2.1. Thermal Properties of FR–PA6
3.2.2. Mechanical Properties of FR–PA6
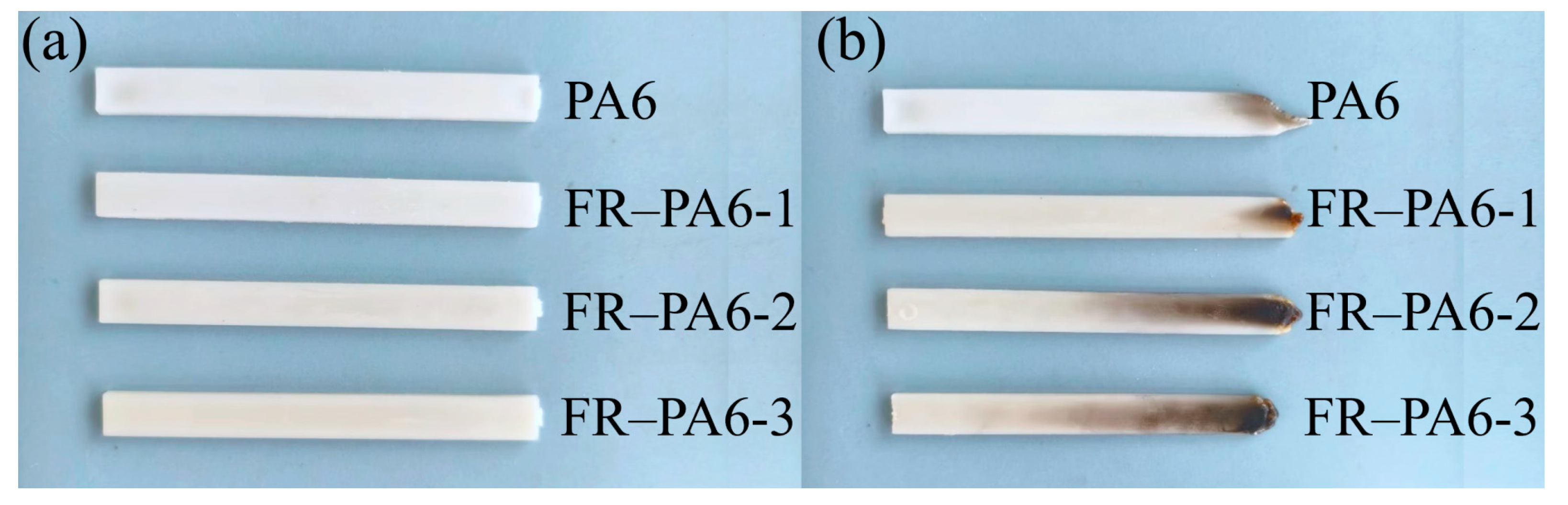
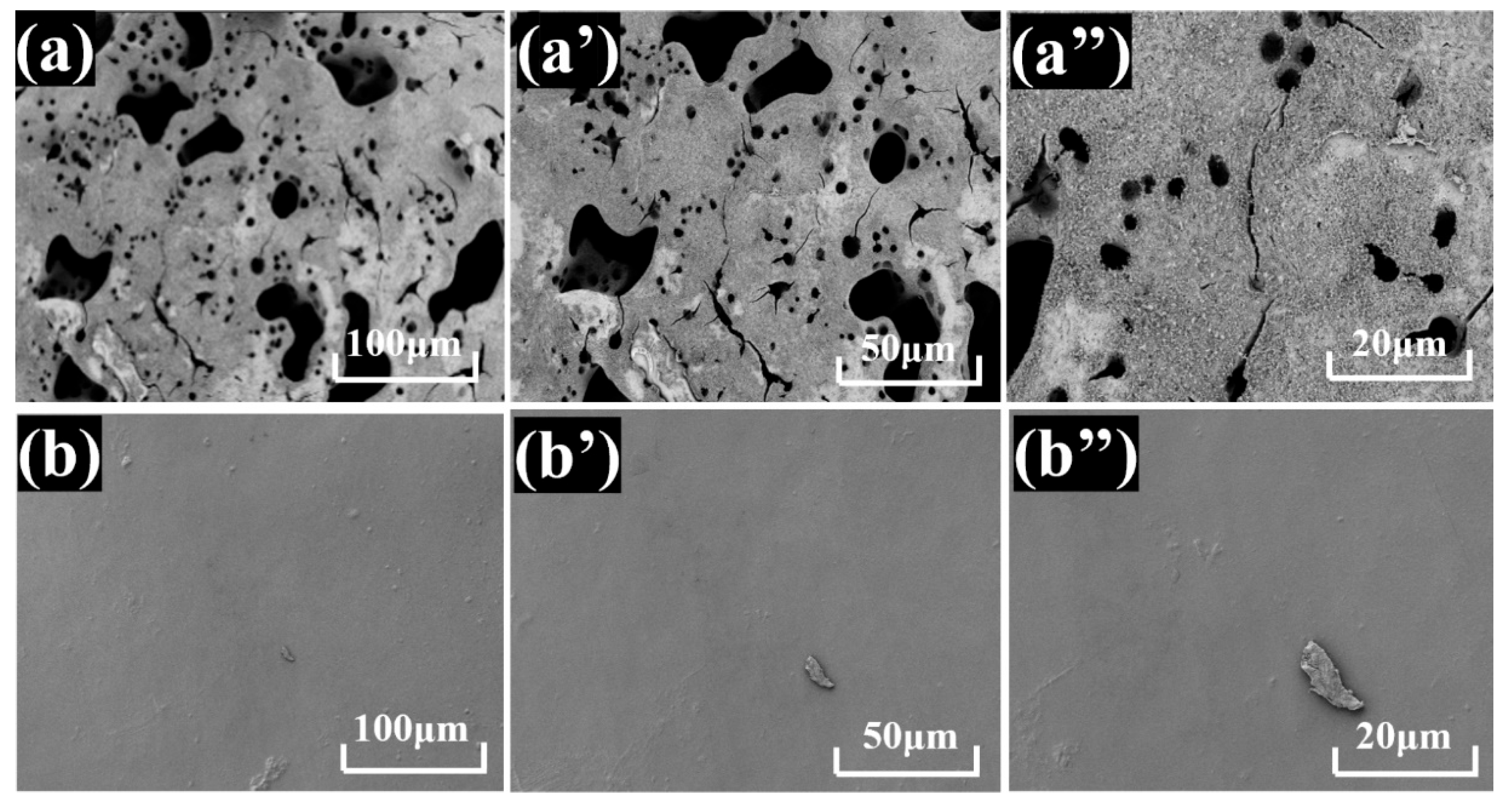
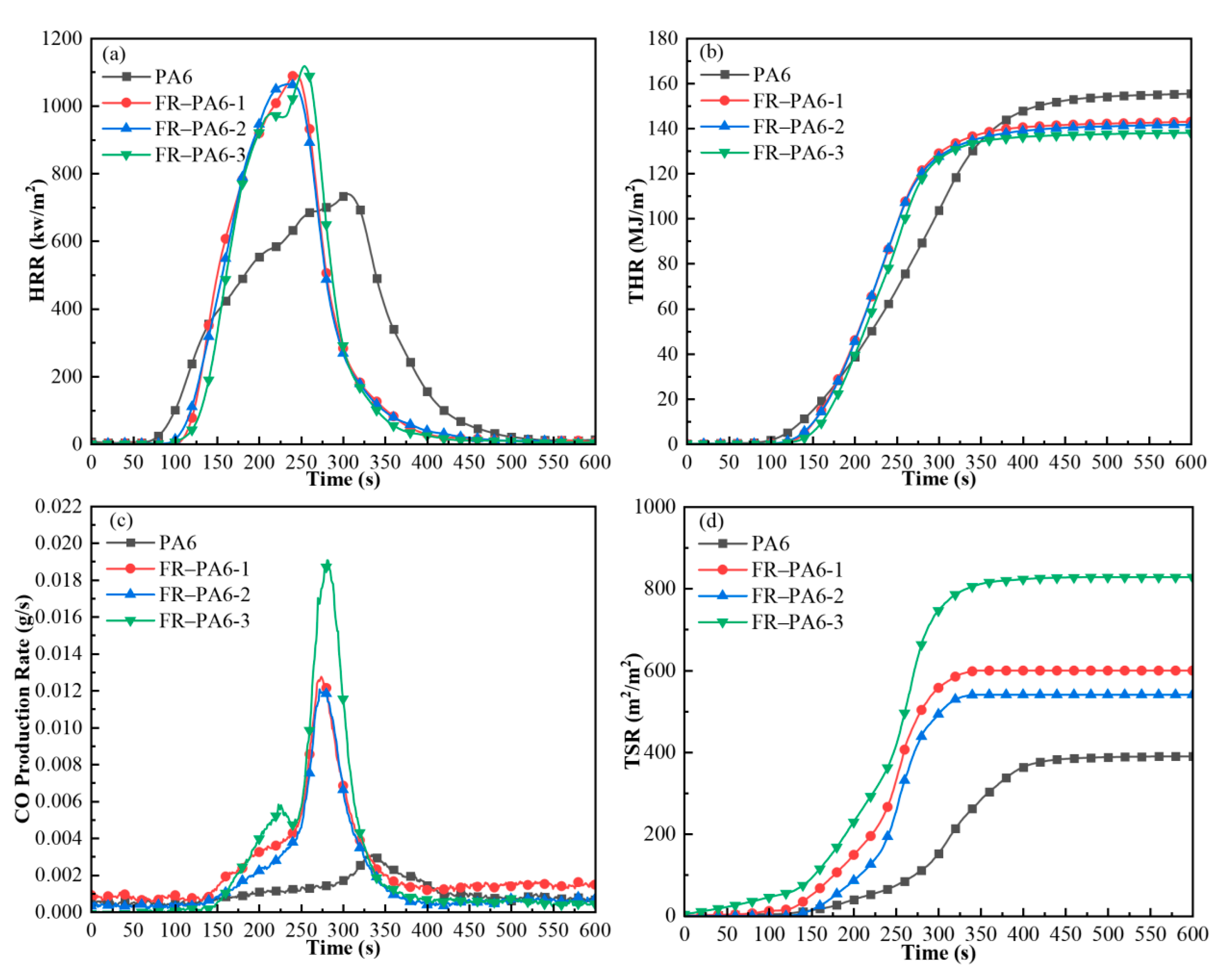
3.2.3. Flame-Retardant Property and Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Faramarzi, I.; Razzaghi-Kashani, M. Improvements in tribological properties of polyamide 6 by application of aramid pulp. Iran. Polym. J. 2015, 24, 329–335. [Google Scholar] [CrossRef]
- Crespo, J.; Parres, F.; Peydró, M.; Navarro, R. Study of rheological, thermal, and mechanical behavior of reprocessed polyamide 6. Polym. Eng. Sci. 2012, 53, 679–688. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, W.; Liu, Y. A novel phosphorus-nitrogen flame retardant for improving the flame retardancy of polyamide 6: Preparation, properties, and flame retardancy mechanism. Polym. Adv. Technol. 2021, 32, 2508–2516. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Feng, H.; Li, D.; Xia, Z.; Yang, R. Flame Retardancy of Nylon 6 Fibers: A Review. Polymers 2023, 15, 2161. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, L.; Li, Z.; Wang, W.; Liu, Y.; Huang, X.; Liu, Y. Synergistic effect of SiO2 doped g-C3N4 and ammonium polyphosphate on flame retardancy of PA6 composites. J. Polym. Res. 2023, 30, 386. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Fischer, A.; Zhong, Y.; Drummer, D.; Wu, W. Enhanced thermal conductivity and flame retardancy of polyamide 6/flame retardant composites with hexagonal boron nitride. J. Polym. Eng. 2018, 38, 767–774. [Google Scholar] [CrossRef]
- Vasiljević, J.; Čolović, M.; Jerman, I.; Simončič, B. Recent Advances in Production of Flame Retardant Polyamide 6 Filament Yarns. Tekstilec 2018, 61, 136–148. [Google Scholar] [CrossRef]
- Adner, D.; Helmy, M.; Otto, T.; Schellenberg, J.; Schadewald, A. A macromolecular halogen-free flame retardant and its effect on the properties of thermoplastic polyesters. Fire Mater. 2018, 43, 169–174. [Google Scholar] [CrossRef]
- Yurddaskal, M.; Celik, E. Effect of halogen-free nanoparticles on the mechanical, structural, thermal and flame retardant properties of polymer matrix composite. Compos. Struct. 2018, 183, 381–388. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 3429286. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. Engl. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Carbone, S.; Drigo, N.; Huang, K.; Lehner, S.; Jovic, M.; Bifulco, A.; Gooneie, A.; Aronne, A.; Gaan, S. Developing flame retardant solutions for partially aromatic polyamide with phosphine oxides. Mater. Des. 2024, 243, 113080. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Li, L.; Chen, W.; Yu, S.; Sun, B.; Zhu, M. Flame retardancy of polyamide 6 hybrid fibers: Combined effects of α -zirconium phosphate and ammonium sulfamate. Prog. Nat. Sci. 2017, 27, 369–373. [Google Scholar] [CrossRef]
- Huang, B.; Ma, M.; Liu, Z.; Jiang, Z.; Chen, S.; Shi, Y.; He, H.; Zhu, Y.; Wang, X. A strategy toward improving flame retardancy and thermal oxidative stability of polyamide 6 based on cuprous diethylphosphinate. Polymer 2024, 302, 127046. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Lin, S.; Ying, Z.; Hu, S.; Wang, Y.; Mo, X. Organophosphate flame retardants in Hangzhou tap water system: Occurrence, distribution, and exposure risk assessment. Sci. Total. Environ. 2022, 849, 157644. [Google Scholar] [CrossRef]
- van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, L.; Zeng, B.; Yi, X.; Huang, C.; Du, K.; Liu, X.; Xu, Y.; Yuan, C.; Dai, L. Diblock Copolymers Containing Titanium-Hybridized Polyhedral Oligomeric Silsesquioxane Used as a Macromolecular Flame Retardant for Epoxy Resin. Polymers 2022, 14, 1708. [Google Scholar] [CrossRef]
- Parcheta-Szwindowska, P.; Habaj, J.; Krzemińska, I.; Datta, J. A Comprehensive Review of Reactive Flame Retardants for Polyurethane Materials: Current Development and Future Opportunities in an Environmentally Friendly Direction. Int. J. Mol. Sci. 2024, 25, 5512. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Zhao, Z.-Y.; Lu, P.; Xiao, X.-X.; He, S.; Deng, C.; Wang, Y.-Z. Novel macromolecular flame retardant derived from sulfonated naphthalene monomer for simultaneous fire safety and high performance of polycarbonate. Polym. Degrad. Stab. 2023, 214, 110388. [Google Scholar] [CrossRef]
- Sun, Y.; Pei, X.; Wang, Z.; Wu, D.; Wang, X.; Yu, J.; Yuan, R.; Li, F. A macromolecular flame retardant for polyamide 6 and its filaments with enhanced fire safety, tensile and UV-blocking performance. Compos. Part B Eng. 2024, 283, 111631. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Tao, L.; Xiao, R. Preparation and characterization of polyamide 6 fibre based on a phosphorus-containing flame retardant. RSC Adv. 2018, 8, 9261–9271. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, Y.; Tao, L.; Liu, C.; Xiao, R. Synthesis and characterization of inherently flame retardant polyamide 6 based on a phosphine oxide derivative, Polym. Degrad. Stab 2019, 163, 151–160. [Google Scholar] [CrossRef]
- Liang, B.; Liu, K.; Dai, J.; Chen, W.; Lu, W. Polymer-type flame retardants based on a DOPO derivative for improving the flame retardancy of polyamide 6: Preparation, properties and flame retardancy mode of action. Polym. Degrad. Stab. 2024, 225, 110807. [Google Scholar] [CrossRef]
- Chen, X.; Xu, D.; Zhang, H.; Feng, X.; Deng, J.; Pan, K. In situ polymerization of flame retardant modification polyamide 6,6 with 2-carboxy ethyl (phenyl) phosphinic acid. J. Appl. Polym. Sci. 2019, 137, 48687. [Google Scholar] [CrossRef]
- Yang, T.; Gao, Y.; Liu, X.; Wang, X.; Ma, B.; He, Y. Flame-retardant polyamide 56 with high fire safety and good thermal performance. Polym. Adv. Technol. 2022, 33, 2807–2819. [Google Scholar] [CrossRef]
- ISO527-1:2019; Plastics—Determination of Tensile Properties. The International Organization for Standardization: Vernier, Switzerland, 2019.
- ASTM D3801; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D2863; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index). ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- Wang, L.-S.; Kang, H.-B.; Wang, S.-B.; Liu, Y.; Wang, R. Solubilities, thermostabilities and flame retardance behaviour of phosphorus-containing flame retardants and copolymers. Fluid Phase Equilibria 2007, 258, 99–107. [Google Scholar] [CrossRef]
- Wei, Z.; Zhou, C.; Yu, Y.; Li, Y. Poly(hexamethylene succinate) copolyesters containing phosphorus pendent group: Retarded crystallization and solid-state microstructure. Polymer 2015, 71, 31–42. [Google Scholar] [CrossRef]
- McAdam, C.P.; Hudson, N.E.; Liggat, J.J.; Pethrick, R.A. Synthesis and characterization of nylon 6/clay nanocomposites prepared by ultrasonication and in situ polymerization. J. Appl. Polym. Sci. 2008, 108, 2242–2251. [Google Scholar] [CrossRef]
- Sag, J.; Goedderz, D.; Kukla, P.; Greiner, L.; Schönberger, F.; Döring, M. Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins. Molecules 2019, 24, 3746. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Y.; Sha, K.; Xiao, R. Preparation and characterizations of flame retardant melamine cyanurate/polyamide 6 composite fibers via in situ polymerization. Text. Res. J. 2016, 87, 561–569. [Google Scholar] [CrossRef]
- El Khatib, W.; Youssef, B.; Bunel, C.; Mortaigne, B. Fireproofing of polyurethane elastomers by reactive organophosphonates. Polym. Int. 2003, 52, 146–152. [Google Scholar] [CrossRef]
- Li, J.; Qian, L.; Xi, W.; Wang, J.; Qiu, Y.; Chen, Y.; Tang, W. Alloying synergistic flame retardant effect on PA6 by polyimide containing alkyl hypophosphate structure. Eur. Polym. 2024, 211, 113033–113046. [Google Scholar]
- Zhang, Y.; Zheng, W.; Xiao, Y.; Yi, C. Preparation of high-efficient flame retardant PA6 via DOPO-ITA initiated ring-opening polymerization of caprolactam. J. Polym. Sci. 2023, 62, 547–553. [Google Scholar] [CrossRef]
- Ma, C.; Qiu, S.; Wang, J.; Sheng, H.; Zhang, Y.; Hu, W.; Hu, Y. Facile synthesis of a novel hyperbranched poly(urethane-phosphine oxide) as an effective modifier for epoxy resin. Polym. Degrad. Stab. 2018, 154, 157–169. [Google Scholar] [CrossRef]
- Carja, I.-D.; Serbezeanu, D.; Vlad-Bubulac, T.; Hamciuc, C.; Coroaba, A.; Lisa, G.; López, C.G.; Soriano, M.F.; Pérez, V.F.; Sánchez, M.D.R. A straightforward, eco-friendly and cost-effective approach towards flame retardant epoxy resins. J. Mater. Chem. A 2014, 2, 16230–16241. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Qian, X.; Guo, N.; Lu, L.; Wang, X.; Wang, H.; Shao, G. Effect of Phosphorus-Based Flame Retardants and PA6 on the Flame Retardancy and Thermal Degradation of Polypropylene. Polym. Technol. Eng. 2018, 57, 1567–1575. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Pan, Y.-T.; Xu, X.; Song, Y.; Yang, R. Mitigation the release of toxic PH3 and the fire hazard of PA6/AHP composite by MOFs. J. Hazard. Mater. 2020, 395, 122604. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Effect of Red Phosphorus and Melamine Polyphosphate on the Fire Behavior of HIPS. J. Fire Sci. 2005, 23, 5–30. [Google Scholar] [CrossRef]



| Sample | CEPPA–HMDA Salt (g) | CPL (g) | H2O (g) | Ratio of CEPPA–HMDA Salt (%) |
|---|---|---|---|---|
| MFR-10 | 120 | 1080 | 130 | 10 |
| MFR-20 | 240 | 960 | 130 | 20 |
| MFR-30 | 360 | 840 | 130 | 30 |
| MFR-40 | 480 | 720 | 130 | 40 |
| Sample | MFR-20 (wt%) | PA6 (wt%) | Theoretical P (ppm) |
|---|---|---|---|
| FR–PA6-1 | 18.8 | 100 | 3000 |
| FR–PA6-2 | 26.7 | 100 | 4000 |
| FR–PA6-3 | 35.7 | 100 | 5000 |
| Sample | Mn (g·mol−1) | Mw (g·mol−1) | PDI |
|---|---|---|---|
| MFR-10 | 2.47 × 104 | 3.38 × 104 | 1.37 |
| MFR-20 | 1.95 × 104 | 3.00 × 104 | 1.54 |
| MFR-30 | 1.56 × 104 | 2.51 × 104 | 1.61 |
| MFR-40 | 1.53 × 104 | 2.40 × 104 | 1.57 |
| Sample | N2 Atmosphere | Air Atmosphere | ||||
|---|---|---|---|---|---|---|
| T5% (°C) | Tmax (°C) | Char (%) | T5% (°C) | Tmax (°C) | Char (%) | |
| MFR-10 | 327.0 | 453.6 | 3.3 | 330.4 | 458.5 | 4.3 |
| MFR-20 | 309.0 | 441.2 | 4.0 | 312.4 | 445.1 | 5.2 |
| MFR-30 | 308.2 | 439.1 | 6.4 | 308.5 | 440.3 | 6.1 |
| MFR-40 | 302.9 | 433.0 | 6.6 | 304.4 | 439.9 | 6.9 |
| Sample | Tc (°C) | Tm (°C) | ∆Hm (J·g−1) |
|---|---|---|---|
| MFR-10 | 179 | 211 | 32 |
| MFR-20 | 166 | 200 | 33 |
| MFR-30 | 147 | 189 | 27 |
| MFR-40 | 136 | 180 | 35 |
| Sample | T5% (°C) | Tmax (°C) | Char (%) |
|---|---|---|---|
| PA6 | 386.5 | 469.2 | 0.7 |
| FR–PA6-1 | 367.7 | 464.8 | 1.8 |
| FR–PA6-2 | 358.6 | 463.3 | 2.2 |
| FR–PA6-3 | 340.1 | 460.9 | 2.3 |
| Sample | Tc (°C) | Tm (°C) | ∆Hm (J·g−1) | Xc (%) |
|---|---|---|---|---|
| PA6 | 187 | 223 | 50 | 22 |
| FR–PA6-1 | 187 | 222 | 50 | 22 |
| FR–PA6-2 | 188 | 222 | 49 | 21 |
| FR–PA6-3 | 189 | 221 | 70 | 31 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Moduli (MPa) |
|---|---|---|---|
| PA6 | 63.2 ± 3.5 | 26.5 ± 4.2 | 1968.8 ± 203.8 |
| FR–PA6-1 | 57.9 ± 3.4 | 3.7 ± 1.3 | 1564.9 ± 338.9 |
| FR–PA6-2 | 49.5 ± 2.4 | 3.2 ± 1.1 | 1546.9 ± 339.9 |
| FR–PA6-3 | 46.2 ± 3.2 | 3.0 ± 1.2 | 1540.0 ± 363.8 |
| Sample | Vertical Combustion Test | LOI Value (%) | |
|---|---|---|---|
| Cotton Ignited or Not | Rating | ||
| PA6 | Yes | V-2 | 21.8 ± 0.1 |
| FR–PA6-1 | No | V-0 | 25.7 ± 0.1 |
| FR–PA6-2 | No | V-0 | 27.2 ± 0.1 |
| FR–PA6-3 | No | V-0 | 28.2 ± 0.1 |
| Sample | p-HRR (kw/m2) | THR (MJ/m2) | av-EHC (MJ/kg) | SEA (m2/kg) | av-CO (kg/kg) | TSR (m2/m2) | Char (%) |
|---|---|---|---|---|---|---|---|
| PA6 | 753 | 158 | 25.8 | 84 | 0.0280 | 390 | 0.01 |
| FR–PA6-1 | 1116 | 144 | 20.0 | 84 | 0.0597 | 599 | 0.33 |
| FR–PA6-2 | 1098 | 142 | 19.1 | 78 | 0.0404 | 541 | 0.50 |
| FR–PA6-3 | 1197 | 138 | 17.5 | 140 | 0.0528 | 827 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Liang, B.; Zhang, S.; Li, R.; Dai, J.; Lu, W. Preparation of a Macromolecular Flame Retardant with a Phosphine Oxide Structure and Its Application in Polyamide 6. Polymers 2025, 17, 475. https://doi.org/10.3390/polym17040475
Liu K, Liang B, Zhang S, Li R, Dai J, Lu W. Preparation of a Macromolecular Flame Retardant with a Phosphine Oxide Structure and Its Application in Polyamide 6. Polymers. 2025; 17(4):475. https://doi.org/10.3390/polym17040475
Chicago/Turabian StyleLiu, Ke, Bohan Liang, Shujuan Zhang, Ruyi Li, Junming Dai, and Wangyang Lu. 2025. "Preparation of a Macromolecular Flame Retardant with a Phosphine Oxide Structure and Its Application in Polyamide 6" Polymers 17, no. 4: 475. https://doi.org/10.3390/polym17040475
APA StyleLiu, K., Liang, B., Zhang, S., Li, R., Dai, J., & Lu, W. (2025). Preparation of a Macromolecular Flame Retardant with a Phosphine Oxide Structure and Its Application in Polyamide 6. Polymers, 17(4), 475. https://doi.org/10.3390/polym17040475








