Novel Biobased Double Crystalline Poly(butylene succinate)-b-poly(butylene 2,5-thiophenedicarboxylate) Multiblock Copolymers with Excellent Thermal and Mechanical Properties and Enhanced Crystallization Behavior
Abstract
1. Introduction
2. Experimental
2.1. Materials
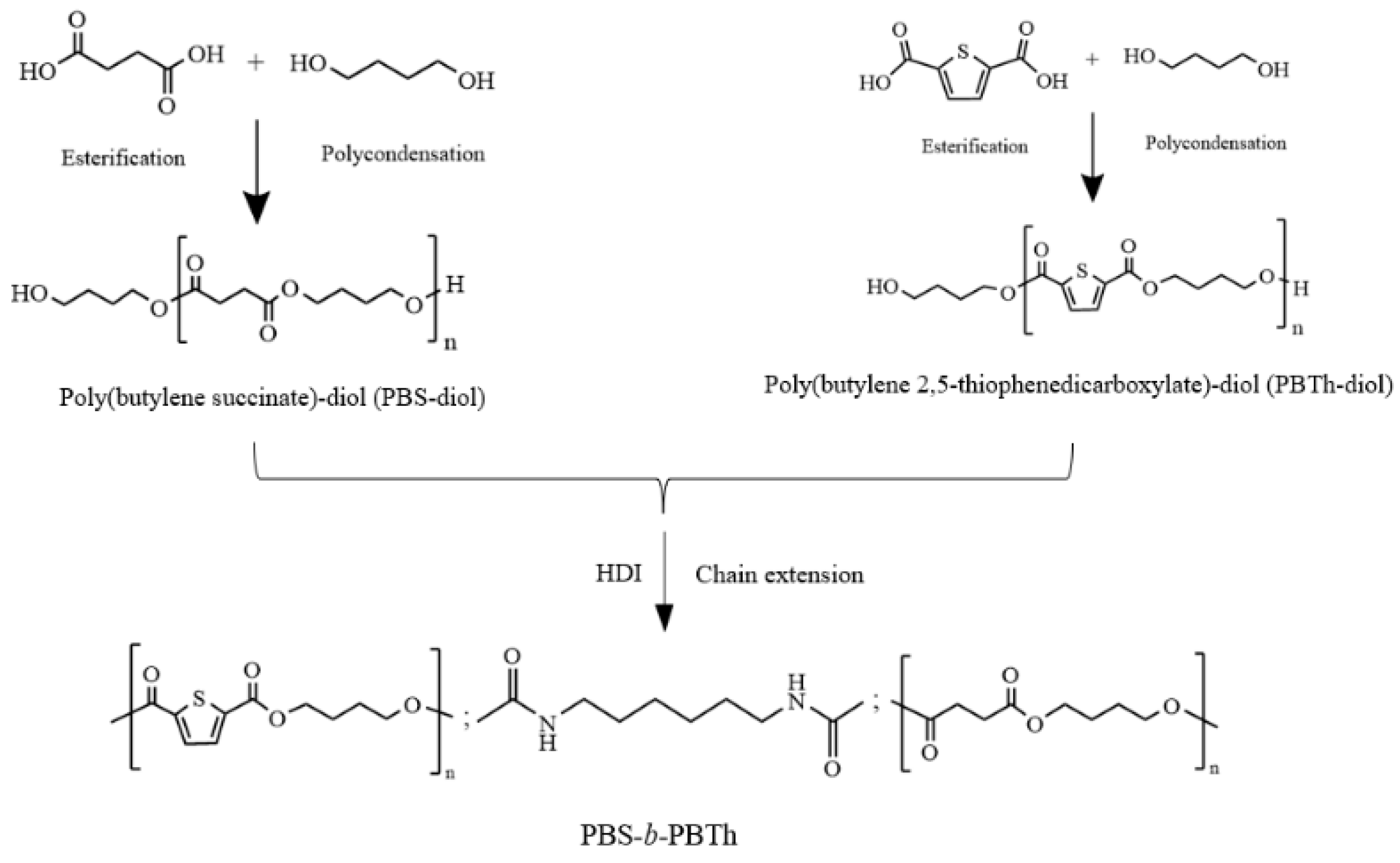
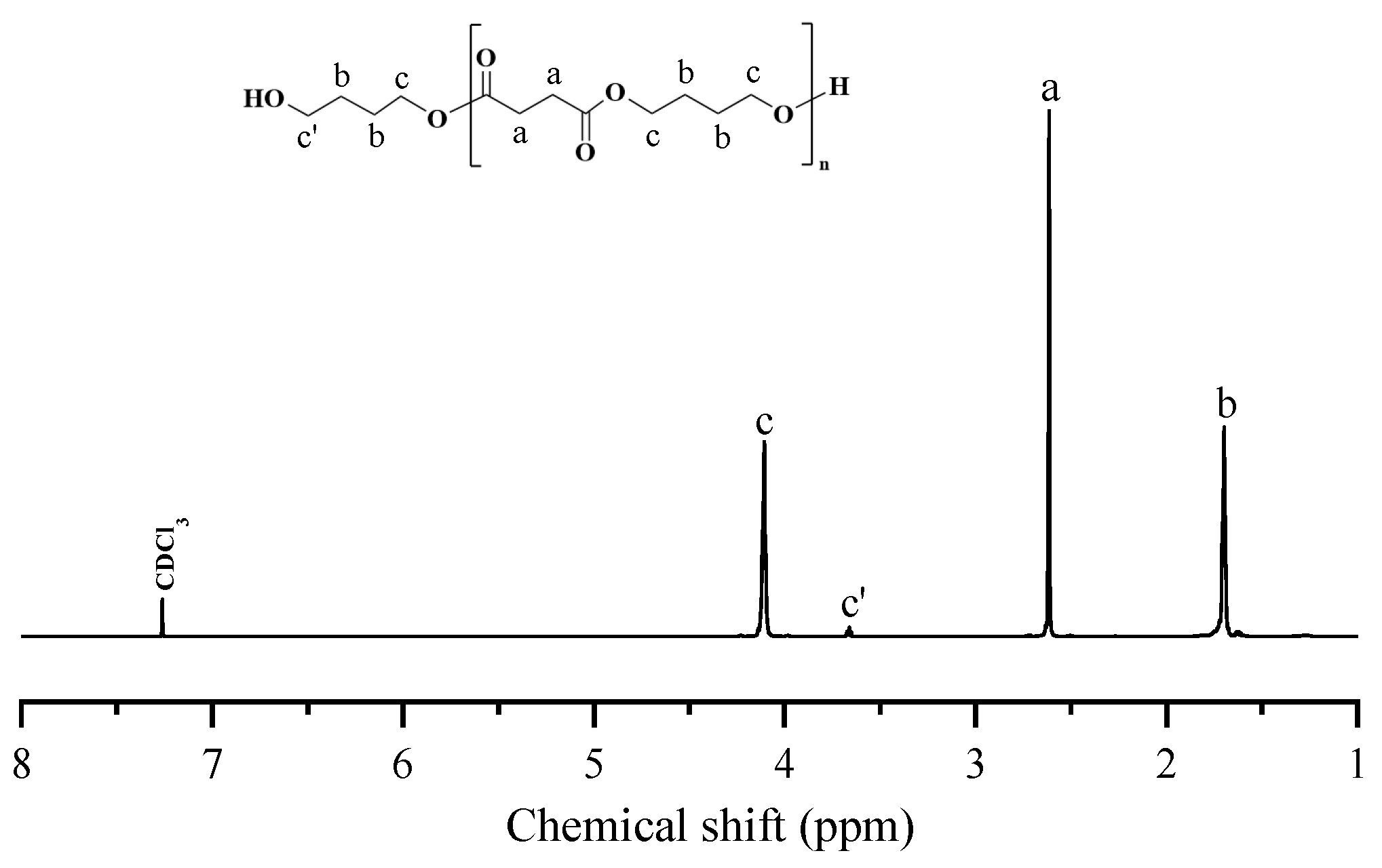
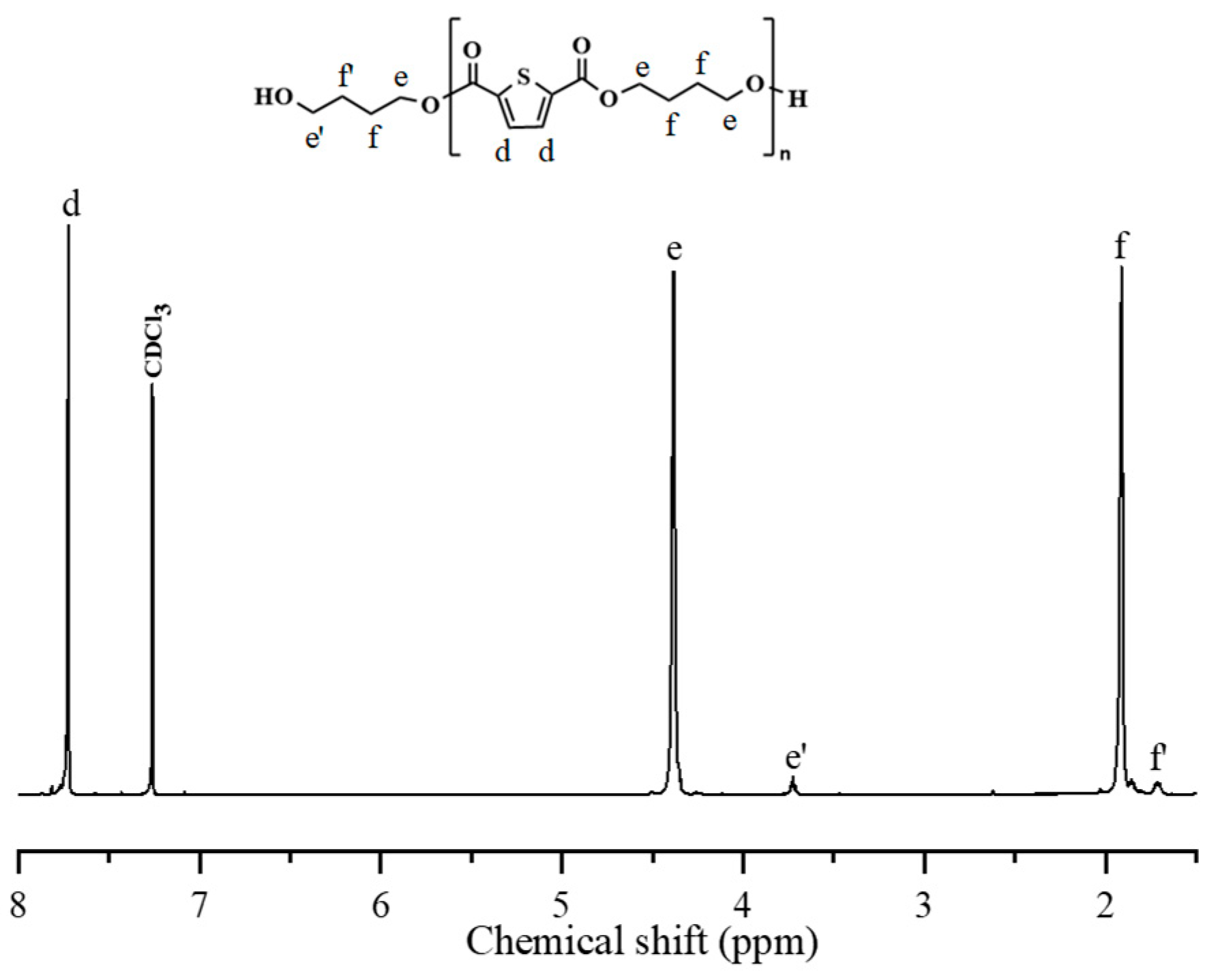
2.2. Synthesis of PBS-Diol and PBTh-Diol Prepolymers
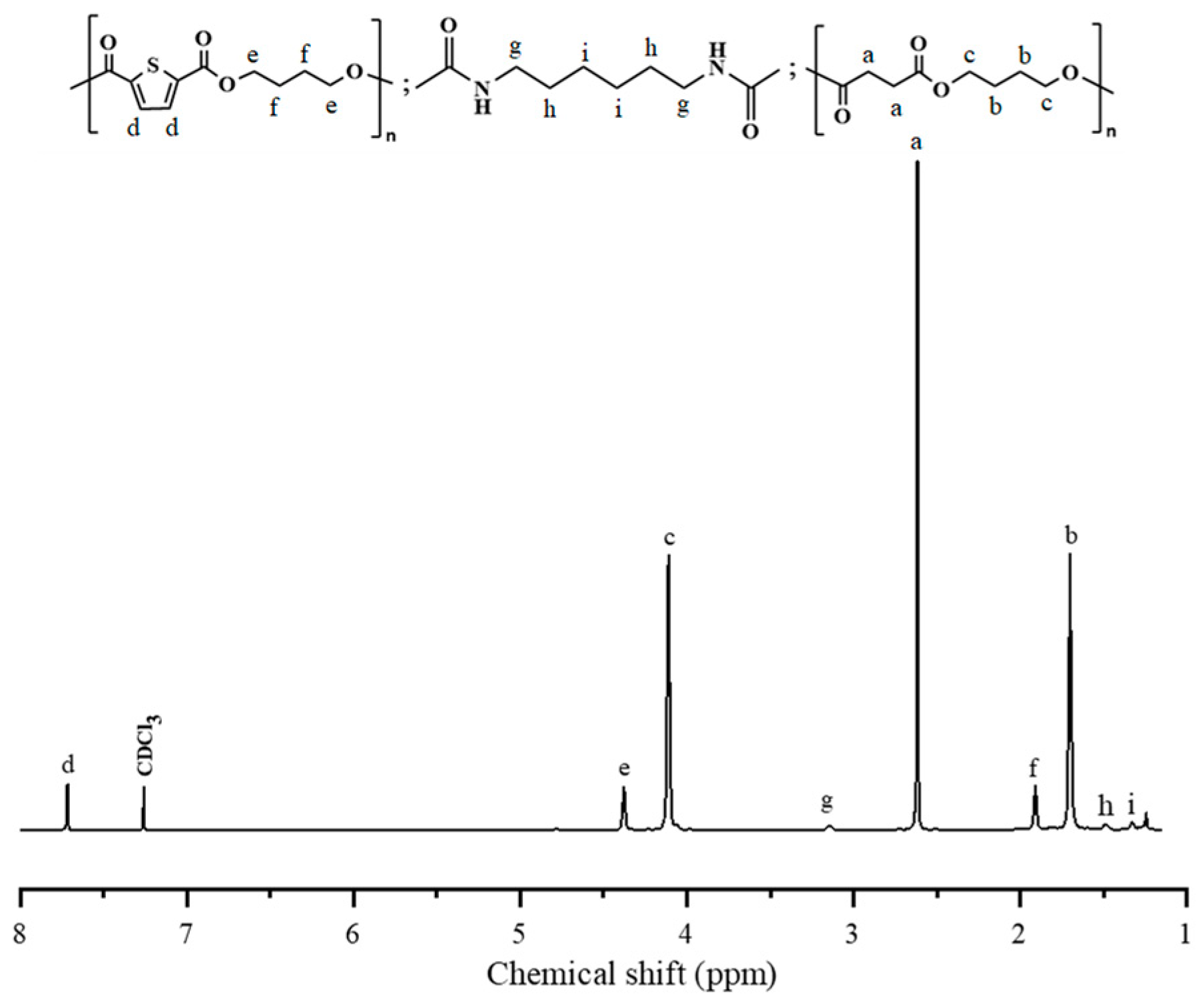
2.3. Synthesis of PBS-b-PBTh Multiblock Copolymers
2.4. Characterization
3. Results and Discussion
3.1. 1H NMR and Intrinsic Viscosity Studies
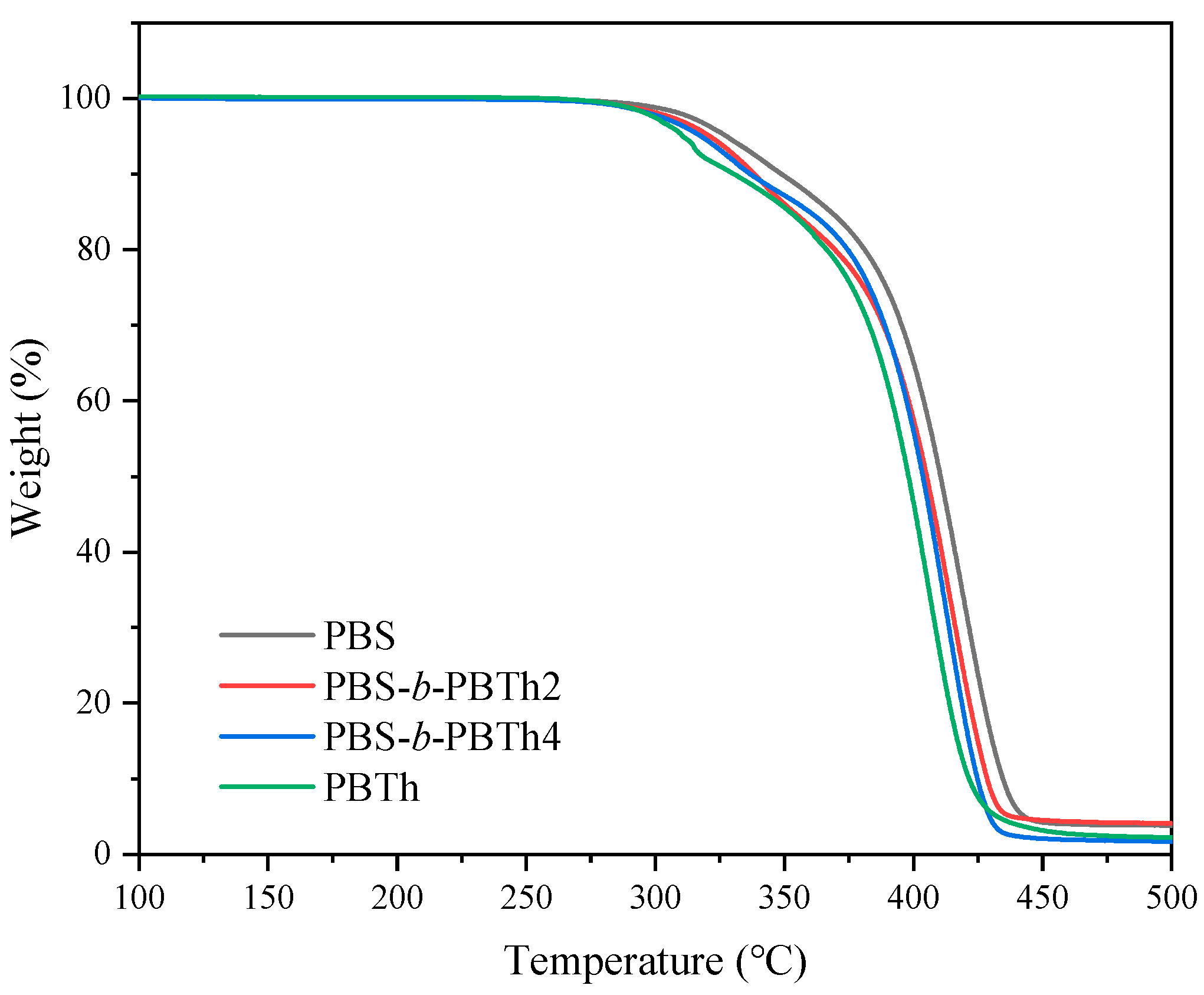
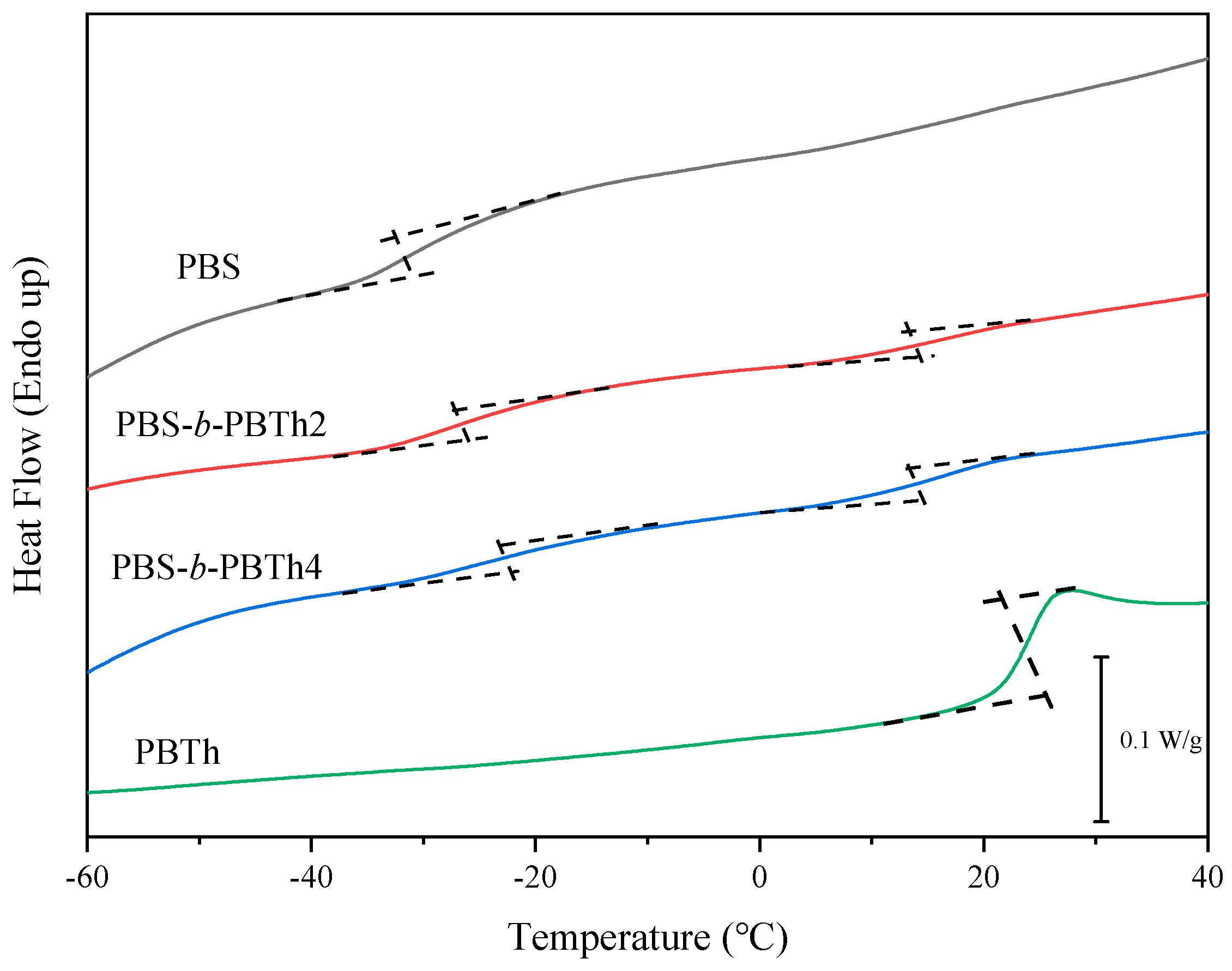
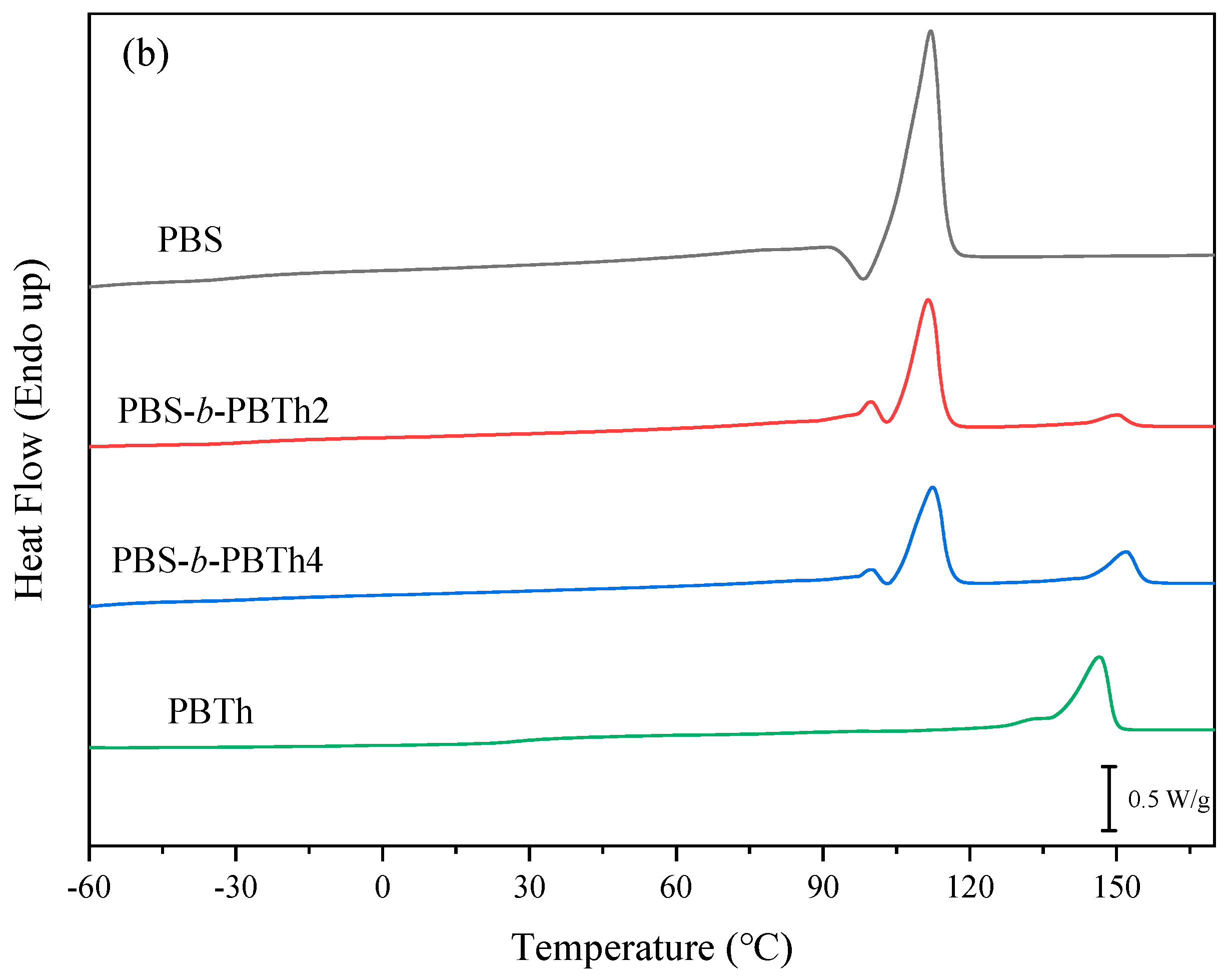
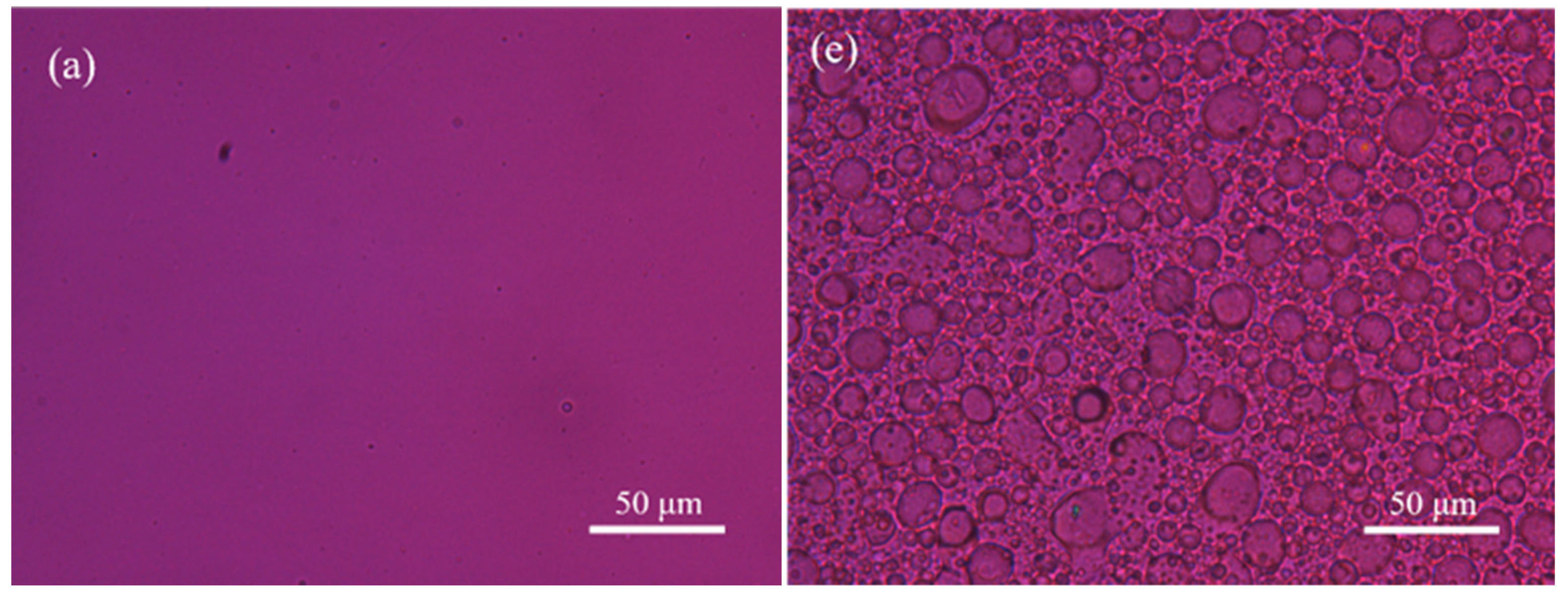
3.2. Thermal Properties and Crystalline Morphology Studies
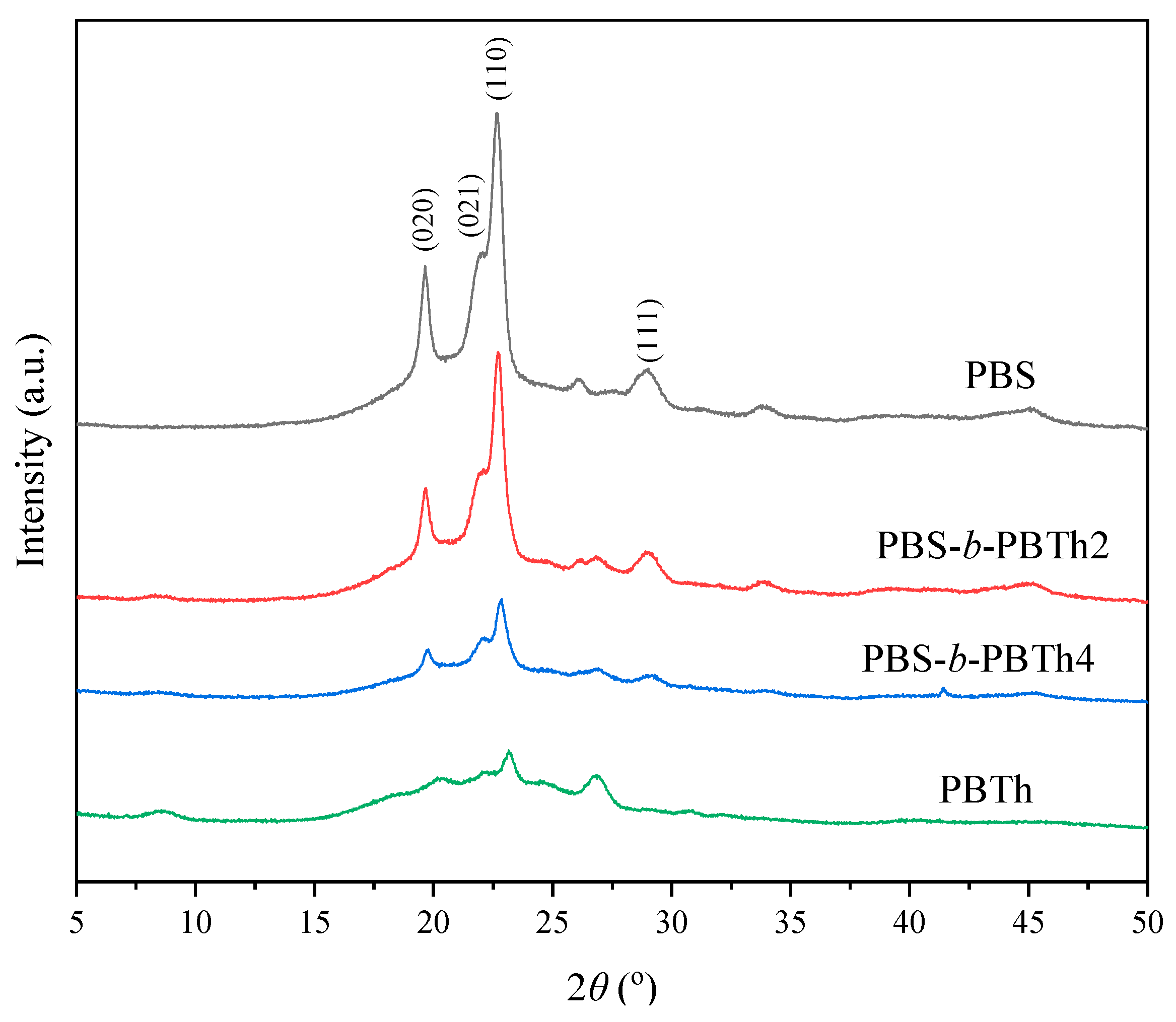
3.3. Crystal Structure Study
3.4. Mechanical Properties Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rafiqah, S.; Khalina, A.; Harmaen, A.; Tawakkal, I.; Zaman, K.; Asim, M.; Nurrazi, M.; Lee, C. A review on properties and application of bio-based poly(butylene succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(butylene succinate) (PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Savitha, K.; Paghadar, B.; Kumar, M.; Jagadish, R. Polybutylene succinate, a potential bio-degradable polymer: Synthesis, copolymerization and bio-degradation. Polym. Chem. 2022, 13, 3562–3612. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Achilias, D.; Bikiaris, D. Crystallization kinetics of biodegradable poly(butylene succinate) under isothermal and non-isothermal conditions. Macromol. Chem. Phys. 2007, 208, 1250–1264. [Google Scholar] [CrossRef]
- Sun, Z.; Jiang, Z.; Qiu, Z. Thermal, crystallization and mechanical properties of branched poly(butylene succinate) copolymers with 1,2-decanediol being the comonomer. Polymer 2021, 213, 123197. [Google Scholar] [CrossRef]
- Yao, W.; Pan, S.; Qiu, Z. Crystallization bhavior and mchanical poperty of bodegradable ply(butylene succinate-co-2-methyl succinate)/cellulose nanocrystals composites. Polymers 2024, 16, 1735. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, C.; Jiao, L.; Lu, X.; Wang, Y.; Wang, X. Synthesis and properties of biodegradable poly(butylene succinate-co-diethylene glycol succinate) copolymers. Ind. Eng. Chem. Res. 2012, 51, 12258–12265. [Google Scholar] [CrossRef]
- Ye, H.; Wang, R.; Liu, J.; Xu, J.; Guo, B. Isomorphism in poly(butylene succinate-co-butylene fumarate) and its application as polymeric nucleating agent for poly(butylene succinate). Macromolecules 2012, 45, 5667–5675. [Google Scholar] [CrossRef]
- Yao, W.; Chen, M.; Qiu, Z. Synthesis, crystallization behavior and mechanical property of biodegradable poly(butylene succinate-co-2-methyl succinate) copolyesters. Polymer 2024, 303, 127114. [Google Scholar] [CrossRef]
- Qiu, Z.; Komura, M.; Ikehara, T.; Nishi, T. DSC and TMDSC study of melting behaviour of poly(butylene succinate) and poly(ethylene succinate). Polymer 2003, 44, 7781–7785. [Google Scholar] [CrossRef]
- Wang, G.; Qiu, Z. Synthesis, crystallization kinetics, and morphology of novel biodegradable poly(butylene succinate-co-hexamethylene succinate) copolyesters. Ind. Eng. Chem. Res. 2012, 51, 16369–16376. [Google Scholar] [CrossRef]
- Gan, Z.; Abe, H.; Doi, Y. Crystallization, melting, and enzymatic degradation of biodegradable poly(butylene succinate-co-14 mol% ethylene succinate) copolyester. Biomacromolecules 2001, 2, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Mincheva, R.; Delangre, A.; Raquez, J.; Narayan, R.; Dubois, P. Biobased polyesters with composition-dependent thermomechanical properties: Synthesis and characterization of poly(butylene succinate-co-butylene azelate). Biomacromolecules 2013, 14, 890–899. [Google Scholar] [CrossRef]
- Huang, M.; Dong, X.; Wang, L.; Zheng, L.; Liu, G.; Gao, X.; Li, C.; Müller, A.; Wang, D. Reversible lamellar periodic structures induced by sequential crystallization/melting in PBS-co-PCL multiblock copolymer. Biomacromolecules 2018, 51, 1100–1109. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Bikiaris, D. Synthesis, cocrystallization, and enzymatic degradation of novel poly(butylene-co-propylene succinate) copolymers. Biomacromolecules 2007, 8, 2437–2449. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, H.; Qiu, Z. Thermal properties and crystallization behavior of novel biodegradable poly(hexamethylene succinate-co-6 mol% butylene succinate) and poly(hexamethylene succinate). J. Polym. Environ. 2018, 26, 1320–1327. [Google Scholar] [CrossRef]
- Qin, P.; Wu, L.; Li, B.; Li, N.; Pan, X.; Dai, J. Superior gas barrier properties of biodegradable PBST vs. PBAT copolyesters: A comparative study. Polymers 2021, 13, 3449. [Google Scholar] [CrossRef] [PubMed]
- Gang, M.; Wang, Y.; Zhang, Y.; Liu, L.; Shi, Y. The relationship between microstructure and mechanical properties of PBST two-component crystalline random copolymers with different BT contents. Polymers 2023, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Luo, F.; Zheng, L.; Niu, R.; Liu, Y.; Xie, Q.; Song, D.; Zhang, Y.; Zhou, T.; Zhu, S. Biodegradable poly(butylene succinate-co-butylene furandicarboxylate): Effect of butylene furandicarboxylate unit on thermal, mechanical, and ultraviolet shielding properties, and biodegradability. J. Appl. Polym. Sci. 2022, 139, e53122. [Google Scholar] [CrossRef]
- Zhi, W.; Hu, Y.; Liang, M.; Liu, Y.; Li, J.; Yin, J.; Shi, Y. Solid–liquid equilibrium and thermodynamic of 2,5-thiophenedicarboxylic acid in different organic solvents. Fluid Phase Equilib. 2014, 375, 110–114. [Google Scholar] [CrossRef]
- Tian, S.; Du, Y.; Wang, P.; Chen, T.; Xu, J.; Yu, H.; Guo, B. Effects of different isomers of thiophenedicarboxylic acids on the synthesis and properties of thiophene-based sustainable polyesters. ACS Sustain. Chem. Eng. 2023, 11, 6652–6664. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Shen, A.; Zhu, J.; Song, P.; Wang, H.; Liu, X. Synthesis and properties investigation of thiophene-aromatic polyesters: Potential alternatives for the 2,5-furandicarboxylic acid-based ones. Chin. J. Polym. Sci. 2020, 38, 1082–1091. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Gazzano, M.; Siracusa, V.; Lotti, N. Poly(alkylene 2,5-thiophenedicarboxylate) polyesters: A new class of bio-based high-performance polymers for sustainable packaging. Polymers 2021, 13, 2460. [Google Scholar] [CrossRef]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Poly(butylene 2,5-thiophenedicarboxylate): An added value to the class of high gas barrier biopolyesters. Polymers 2018, 10, 167. [Google Scholar] [CrossRef]
- Chen, C.; Pan, S.; Qiu, Z. Crystallization kinetics and melting behavior of novel biobased poly(butylene 2,5-thiophenedicarboxylate). ChemistrySelect 2024, 9, e202401725. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. poly(propylene 2,5-thiophenedicarboxylate) vs. poly(propylene 2,5-furandicarboxylate): Two examples of high gas barrier bio-based polyesters. Polymers 2018, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Gigli, M.; Quartinellob, F.; Soccioc, M.; Pellisb, A.; Lottic, N.; Guebitzb, G.; Licocciaa, S.; Munaric, A. Enzymatic hydrolysis of poly(1,4-butylene 2,5-thiophenedicarboxylate) (PBTF) and poly(1,4-butylene 2,5-furandicarboxylate) (PBF) films: A comparison of mechanisms. Environ. Int. 2019, 130, 104852. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Salatelli, E.; Gazzano, M.; Siracusa, V.; Munari, A. Tailoring poly(butylene 2,5-thiophenedicarboxylate) features by the introduction of adipic acid co-units: Biobased and biodegradable aliphatic/aromatic polyesters. Polymer 2018, 145, 11–20. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Wang, J.; Hu, H.; Dong, Y.; O’Young, D.; Hu, D.; Zhang, X.; Wei, D.; Zhu, J. Biobased biodegradable copolyesters from 2,5-thiophenedicarboxylic acid: Effect of aliphatic diols on barrier properties and degradation. Biomacromolecules 2023, 24, 5884–5897. [Google Scholar] [CrossRef]
- Djouonkep, L.; Tamo, C.; Simo, B.; Issah, N.; Tchouagtie, M.; Selabi, N.; Doench, I.; Tamo, A.; Xie, B.; Osorio-Madrazo, A. Synthesis by melt-polymerization of a novel series of bio-based and biodegradable thiophene-containing copolyesters with promising gas barrier and high thermomechanical properties. Molecules 2023, 28, 1825. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dong, Y.; Hao, X.; Zhang, L.; Sun, R. Bio-based poly(butylene furandicarboxylate-co-butylene 2,5-thiophenedicarboxylate): Synthesis, thermal properties, crystallization properties and mechanical properties. Polym. Bull. 2023, 80, 5373–5395. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Y.; Jiang, M.; Wang, R.; Wang, H.; Liang, Y.; Zhou, G. Fully bio-based polyesters poly(ethylene-co-1,5-pentylene 2,5-thiophenedicarboxylate)s (PEPTs) with high toughness: Synthesis, characterization and thermo-mechanical properties. Polymer 2020, 204, 122800. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Wang, Q.; Dong, Y.; Zhu, J. New biodegradable polyesters synthesized from 2,5-thiophenedicarboxylic acid with excellent gas barrier properties. Polym. Degrad. Stab. 2022, 205, 110148. [Google Scholar] [CrossRef]
- Wang, G.; Hao, X.; Dong, Y.; Zhang, L.; Sun, R. Fully bio-based poly(butylene succinate-co-butylene 2,5-thiophenedicarboxylate) with derived from 2,5-thiophenedicarboxylic acid. Express Polym. Lett. 2022, 16, 772–784. [Google Scholar] [CrossRef]
- Zheng, L.; Li, C.; Zhang, D.; Guan, G.; Xiao, Y.; Wang, D. Synthesis, characterization and properties of novel biodegradable multiblock copolymers comprising poly(butylene succinate) and poly(1,2-propylene terephthalate) with hexamethylene diisocyanate as a chain extender. Polym. Int. 2011, 60, 666–675. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, Q.; Lu, X.; He, Y.; Wang, Y. From miscible to partially miscible biodegradable double crystalline poly(ethylene succinate)-b-poly(butylene succinate) multiblock copolymers. Polym. Chem. 2012, 3, 399–408. [Google Scholar] [CrossRef]
- Shang, Y.; Jiang, Z.; Qiu, Z. Synthesis, thermal and mechanical properties of novel biobased, biodegradable and double crystalline poly(butylene succinate)-b-poly(butylene sebacate) multiblock copolymers. Polymer 2021, 214, 23248. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, K.; Jiang, Z.; Qiu, Z. Synthesis and characterization of novel poly(butylene succinate)-b-poly(diethylene glycol terephthalate) multiblock copolyesters with high melting point and significantly improved mechanical property. Polymer 2021, 232, 124151. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Z.; Wu, S.; Li, C.; Zhang, D.; Xiao, Y. Novel poly(butylene fumarate) and poly(butylene succinate) multiblock copolymers bearing reactive carbon−carbon double bonds: Synthesis, characterization, cocrystallization, and properties. Ind. Eng. Chem. Res. 2013, 52, 6147–6155. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Y.; Zhu, Q.; Yang, K.; Wang, X.; Wang, Y. A novel biodegradable multiblock poly(ester urethane) containing poly(l-lactic acid) and poly(butylene succinate) blocks. Polymer 2009, 50, 1178–1186. [Google Scholar] [CrossRef]
- Solomon, O.; Ciuta, I. Détermination de La Viscosité Intrinsèque de Solutions de Polymères Par Une Simple Détermination de La Viscosité. J. Appl. Polym. Sci. 1962, 6, 683–686. [Google Scholar] [CrossRef]
- Simon, J.; Barla, F.; Kelemen-Haller, A.; Farkas, F.; Kraxner, M. Thermal stability of polyurethanes. Chromatographia 1988, 25, 99–106. [Google Scholar] [CrossRef]
- Mitra, M.; Muthukumar, M. Theory of spinodal decomposition assisted crystallization in binary mixtures. J. Chem. Phys. 2010, 132, 184908. [Google Scholar] [CrossRef]
- Shi, W.; Chen, F.; Zhang, Y.; Han, C. Viscoelastic phase separation and interface assisted crystallization in a highly immiscible iPP/PMMA blend. ACS Macro Lett. 2012, 1, 1086–1089. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, Z.; Qiu, Z. Biobased poly(ethylene succinate)-b-poly(triethylene terephthalate) multiblock copolyesters with high melting temperature and improved crystallization rate and mechanical property. Polymer 2022, 254, 125061. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Li, L. Multiple melting behavior of poly(butylene succinate). Eur. Polym. J. 2007, 43, 3163–3170. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Z. Crystallization kinetics and morphology of biodegradable poly(butylene succinate-co-ethylene succinate) copolyesters: Effects of comonomer composition and crystallization temperature. CrystEngComm 2011, 13, 2408. [Google Scholar] [CrossRef]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Ordered structures of poly(butylene 2,5-thiophenedicarboxylate) and their impact on material functional properties. Eur. Polym. J. 2018, 106, 284–290. [Google Scholar] [CrossRef]



| Samples | PBS | PBTh | HDI | [η] (dL/g) | |||
|---|---|---|---|---|---|---|---|
| fPBS (wt%) | FPBS (wt%) | fPBTh (wt%) | FPBTh (wt%) | fHDI (wt%) | FHDI (wt%) | ||
| PBS | 96.6 | 97.3 | / | / | 3.4 | 2.7 | 1.44 |
| PBS-b-PBTh2 | 77.4 | 78.9 | 19.4 | 18.6 | 3.2 | 2.5 | 1.38 |
| PBS-b-PBTh4 | 58.2 | 55.9 | 38.8 | 40.1 | 3.0 | 4.0 | 0.94 |
| PBTh | / | / | 97.3 | 95.9 | 2.7 | 4.1 | 0.96 |
| Samples | Tg1 (°C) | Tg2 (°C) | Tcc1 (°C) | ΔHcc1 (J/g) | Tcc2 (°C) | ΔHcc2 (J/g) | Tm1 (°C) | Tm2 (°C) |
|---|---|---|---|---|---|---|---|---|
| PBS | −31.4 | / | 72.1 | 76.1 | / | / | 112.1 | / |
| PBS-b-PBTh2 | −27.2 | 17.9 | 78.5 | 44.1 | 92.7 | 2.9 | 111.5 | 150.5 |
| PBS-b-PBTh4 | −21.9 | 17.1 | 78.8 | 33.8 | 97.4 | 8.7 | 112.4 | 152.1 |
| PBTh | / | 24.5 | / | / | 88.1 | 31.1 | / | 146.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Feng, S.; Qiu, Z. Novel Biobased Double Crystalline Poly(butylene succinate)-b-poly(butylene 2,5-thiophenedicarboxylate) Multiblock Copolymers with Excellent Thermal and Mechanical Properties and Enhanced Crystallization Behavior. Polymers 2025, 17, 450. https://doi.org/10.3390/polym17040450
Yang H, Feng S, Qiu Z. Novel Biobased Double Crystalline Poly(butylene succinate)-b-poly(butylene 2,5-thiophenedicarboxylate) Multiblock Copolymers with Excellent Thermal and Mechanical Properties and Enhanced Crystallization Behavior. Polymers. 2025; 17(4):450. https://doi.org/10.3390/polym17040450
Chicago/Turabian StyleYang, Haidong, Shiwei Feng, and Zhaobin Qiu. 2025. "Novel Biobased Double Crystalline Poly(butylene succinate)-b-poly(butylene 2,5-thiophenedicarboxylate) Multiblock Copolymers with Excellent Thermal and Mechanical Properties and Enhanced Crystallization Behavior" Polymers 17, no. 4: 450. https://doi.org/10.3390/polym17040450
APA StyleYang, H., Feng, S., & Qiu, Z. (2025). Novel Biobased Double Crystalline Poly(butylene succinate)-b-poly(butylene 2,5-thiophenedicarboxylate) Multiblock Copolymers with Excellent Thermal and Mechanical Properties and Enhanced Crystallization Behavior. Polymers, 17(4), 450. https://doi.org/10.3390/polym17040450








