Study of Construction of Innovative Barite/Waterborne Polyurethane/Low-Density Polyethylene Composites for Enhanced X-Ray Shielding Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
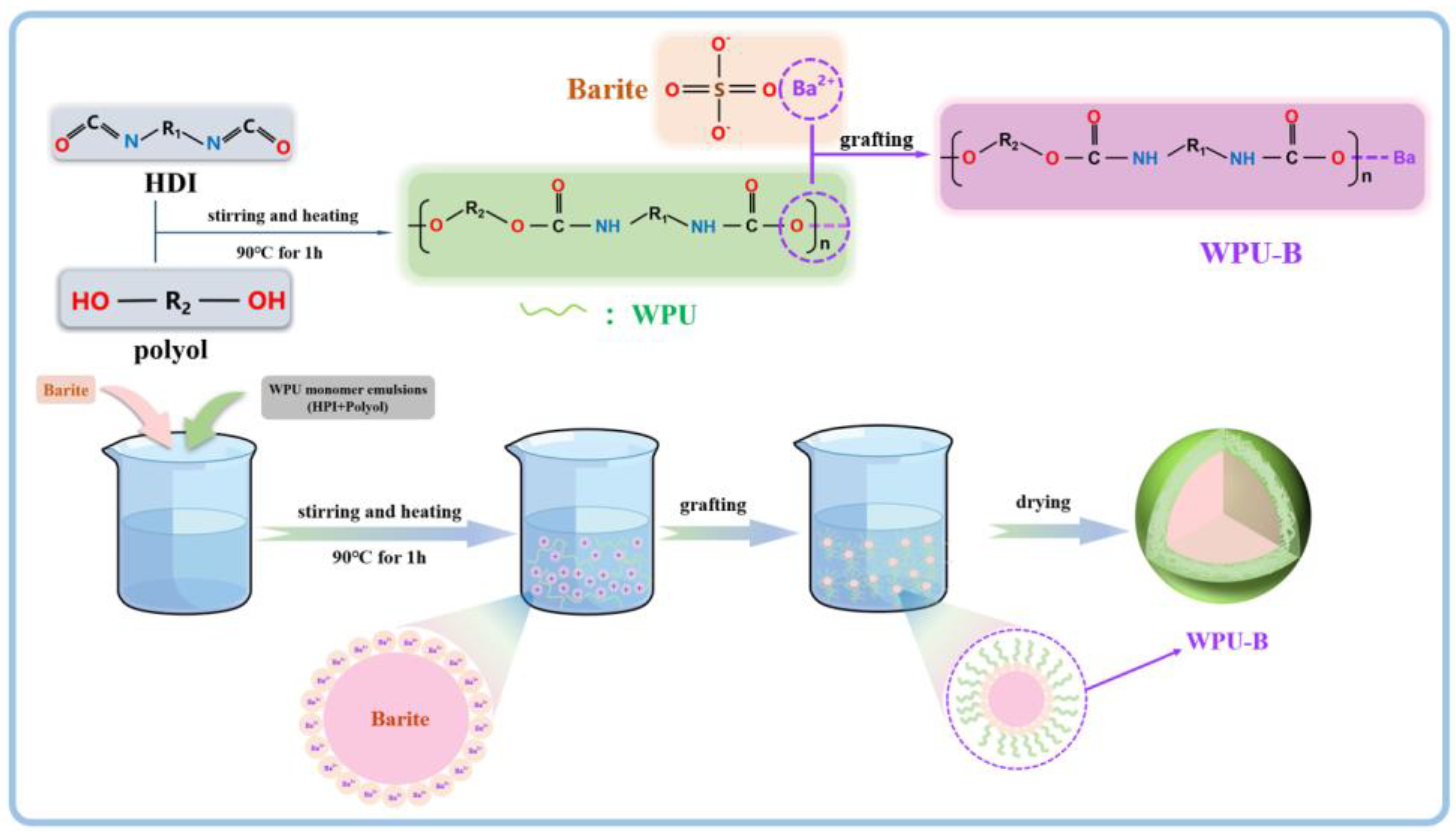
2.2. Experimental Procedure
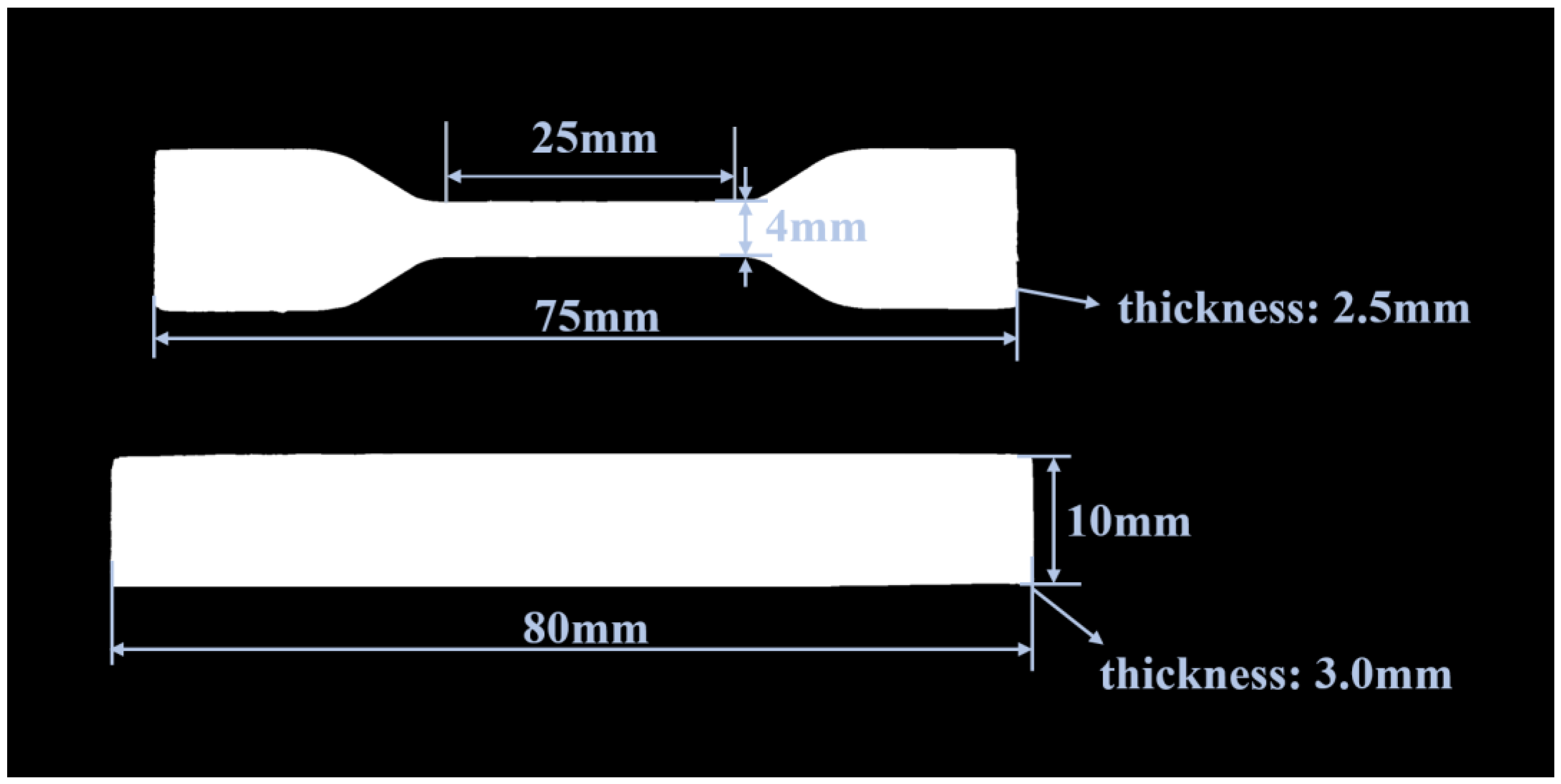
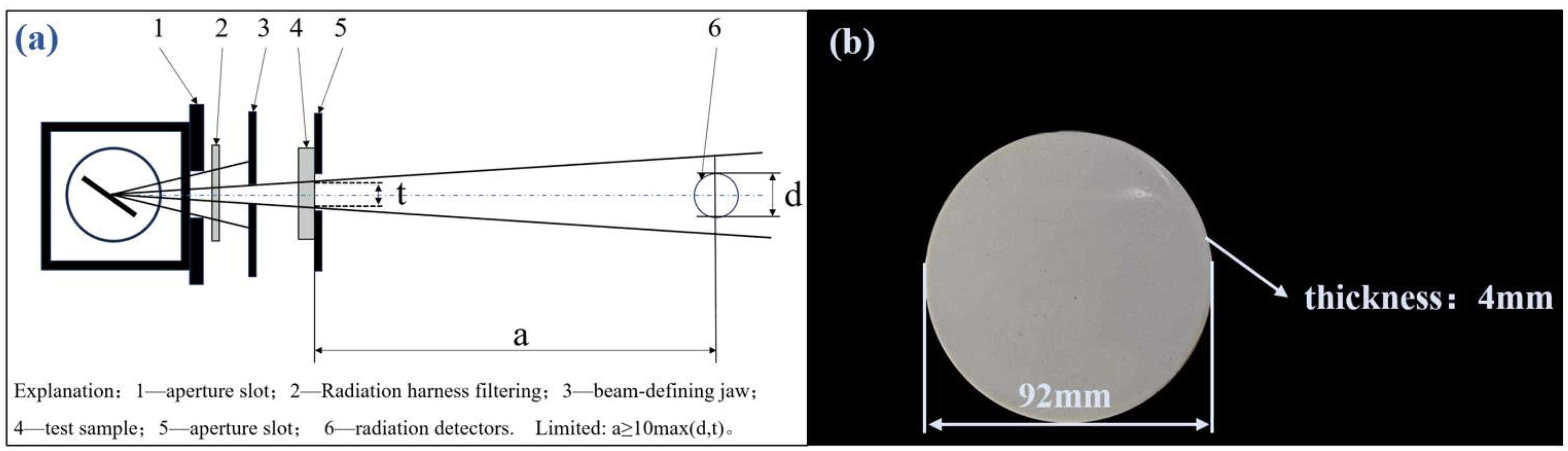
2.3. Testing and Analysis Methods
- (1)
- Attenuation efficiency (RPE): Attenuation efficiency refers to the ratio of the attenuated X-ray dose rate (I) after a certain dose of X-ray passes through the sample to the incident X-ray dose rate (I0). The unit is %, and the calculation formula is as follows:
- (2)
- Linear attenuation coefficient (LAC, μ): The linear attenuation coefficient is used to describe the X-ray attenuation effect per unit thickness of material and is calculated using the following formula:
- (3)
- Mass attenuation coefficient (MAC, μm): The percentage of reduction in X-ray intensity per gram per centimeter thick of the absorbing substance is the mass attenuation coefficient, with the unit of cm2‧g−1. The calculation formula is as follows:
- (4)
- Half-Value Layer (HVL): The half-value layer is the thickness of the material required to reduce the X-ray intensity by half, expressed in cm, calculated as follows:
- (5)
- Lead Equivalent: The lead equivalent is the thickness of lead that provides identical shielding effectiveness to that of the test material when both are exposed to the same X-ray source, expressed in mmPb. The calculation method of lead equivalent involves irradiating the material to be tested with X-rays, measuring its radiation dose, and comparing it to the radiation dose of a lead plate of known thickness under the same conditions, thereby calculating the lead equivalent of the material to be tested.
3. Results and Discussion
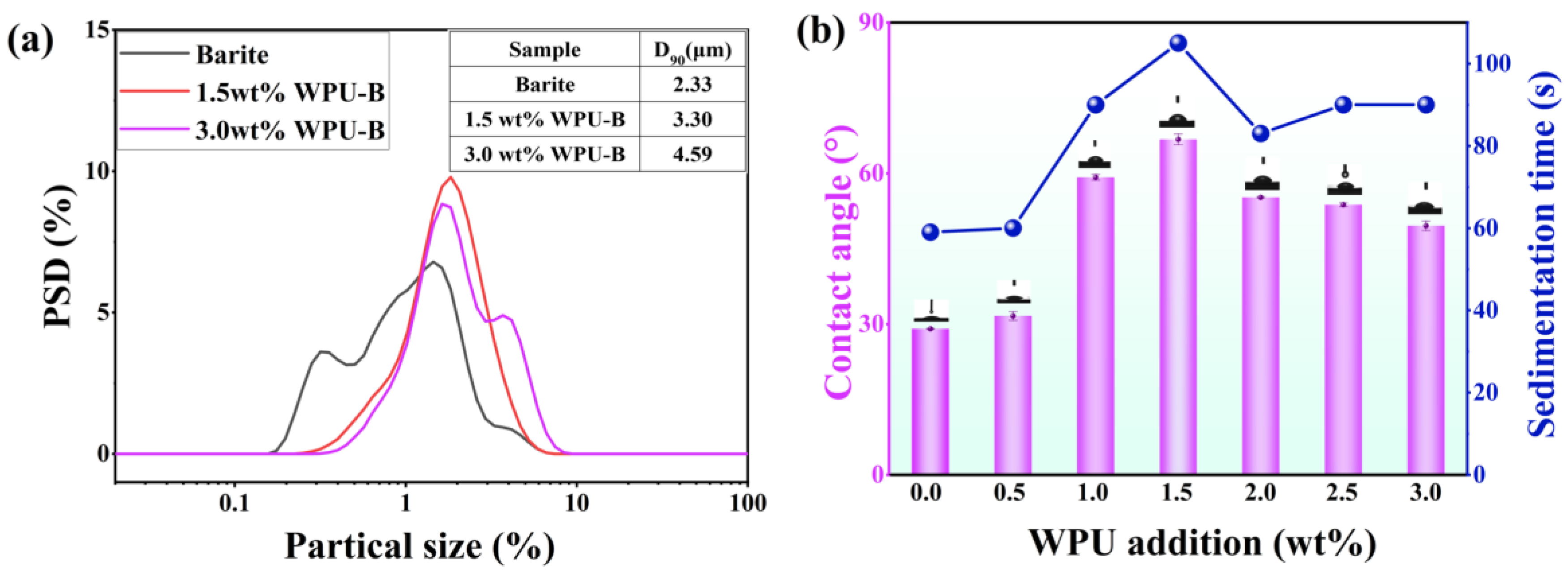
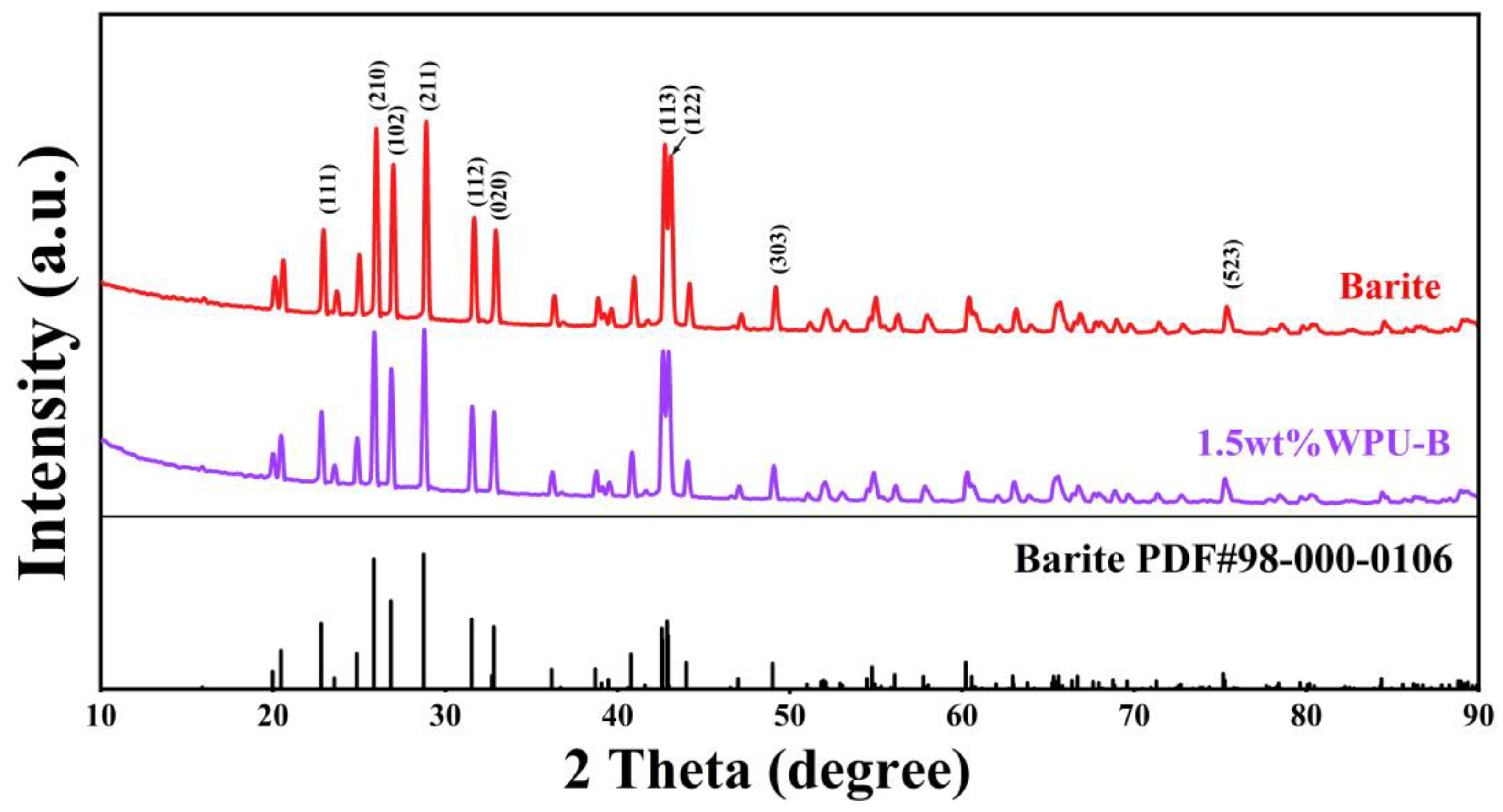
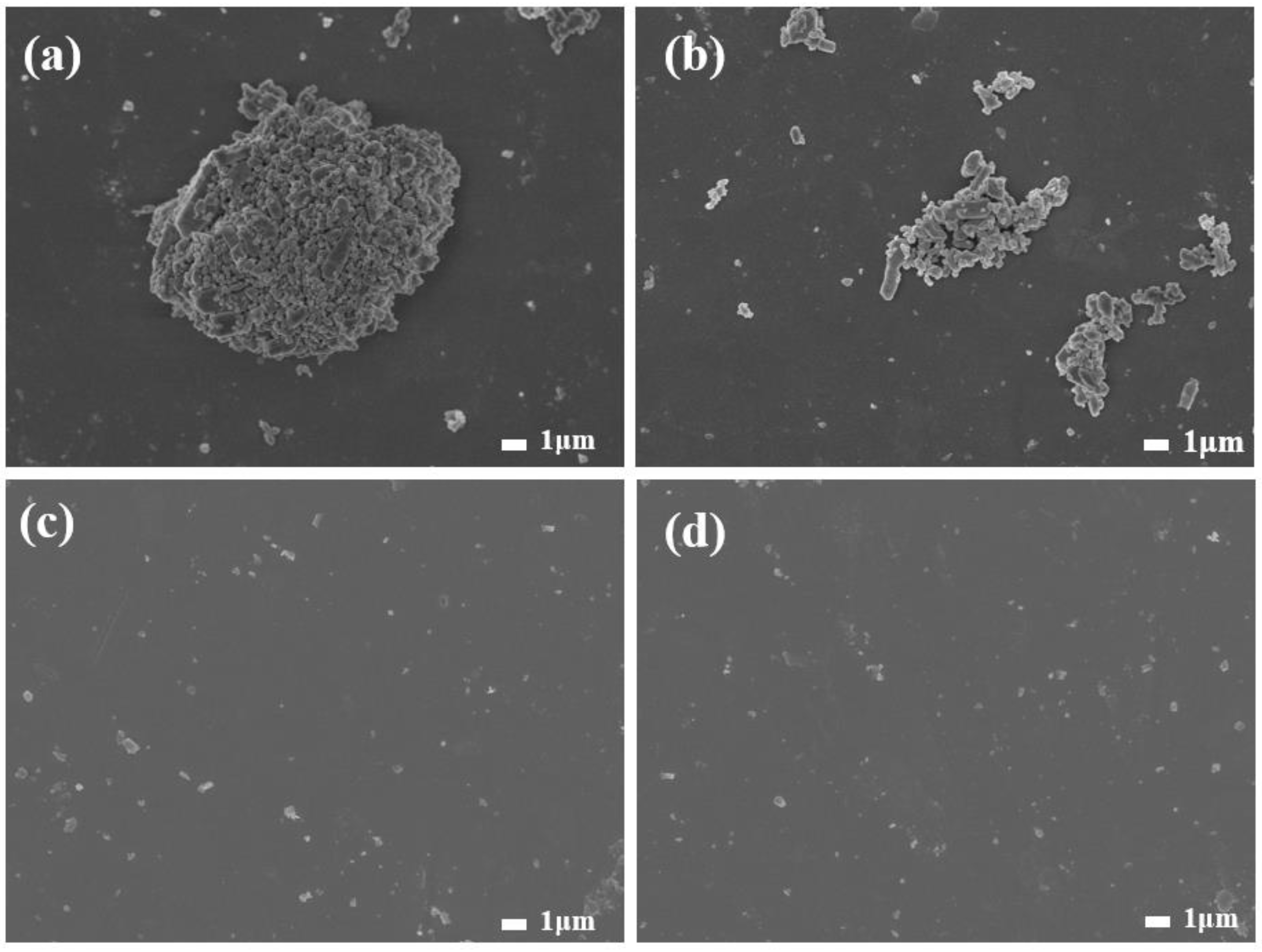
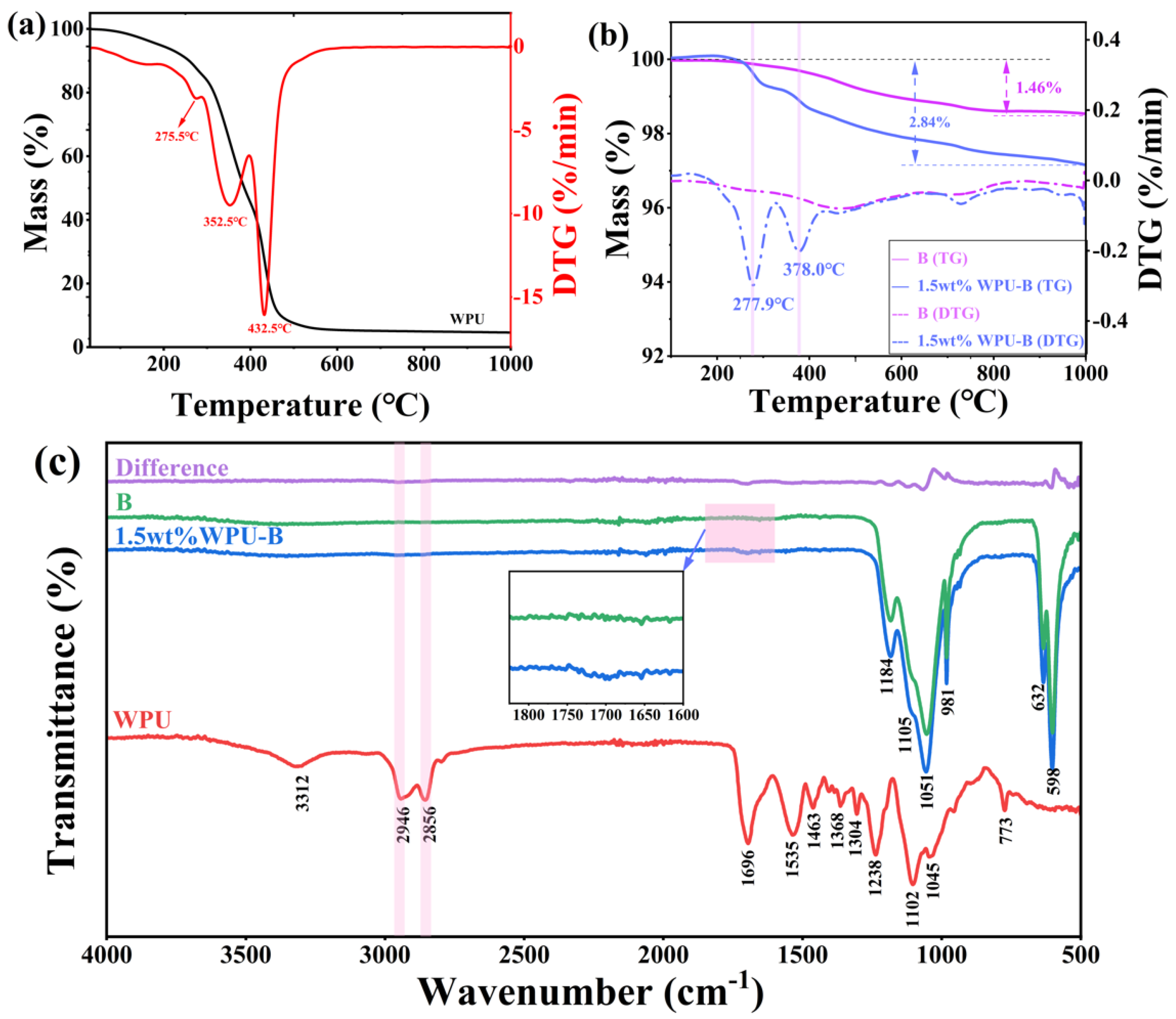
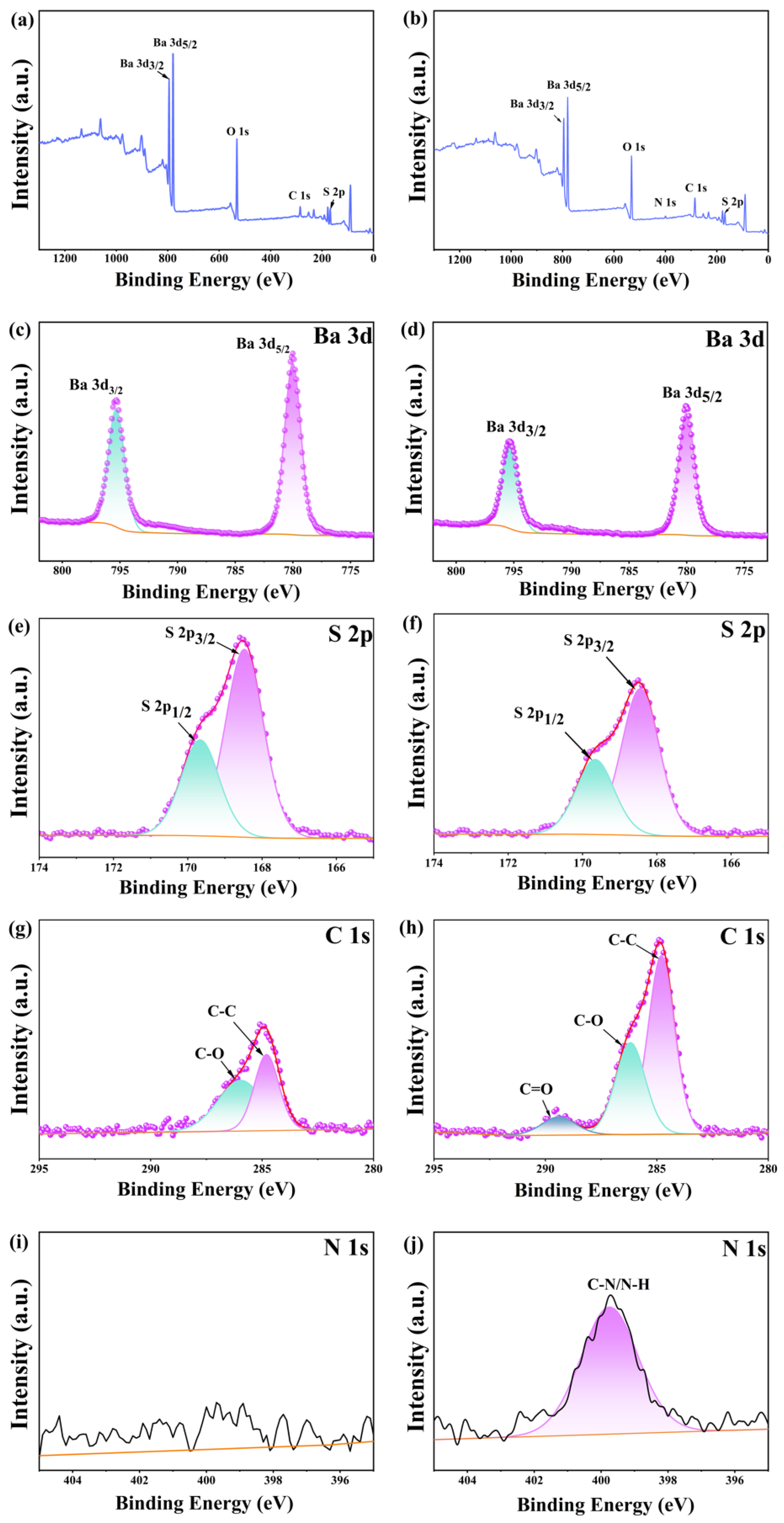
3.1. Discussion of Modification Effect
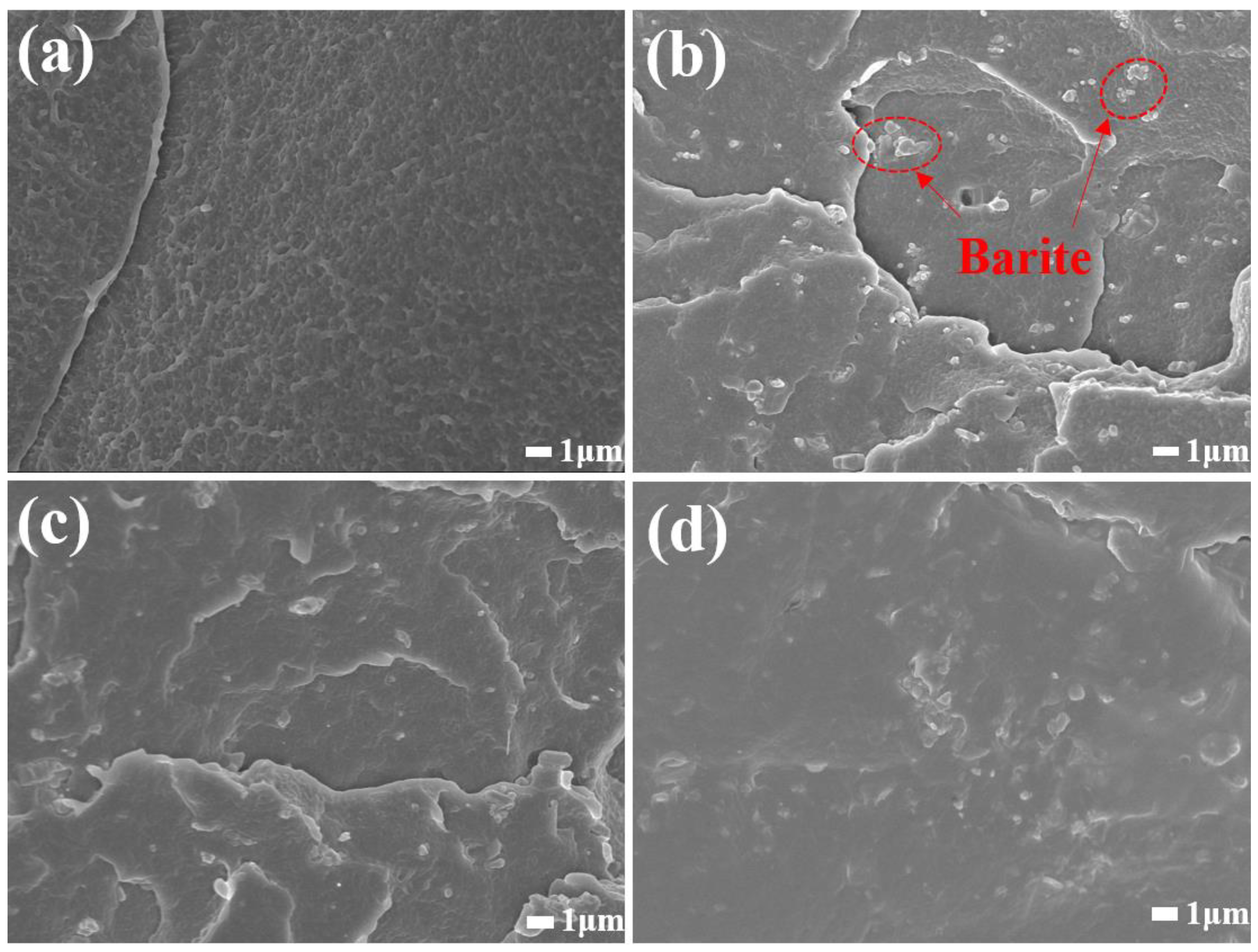
3.2. Analysis of Modification Mechanism
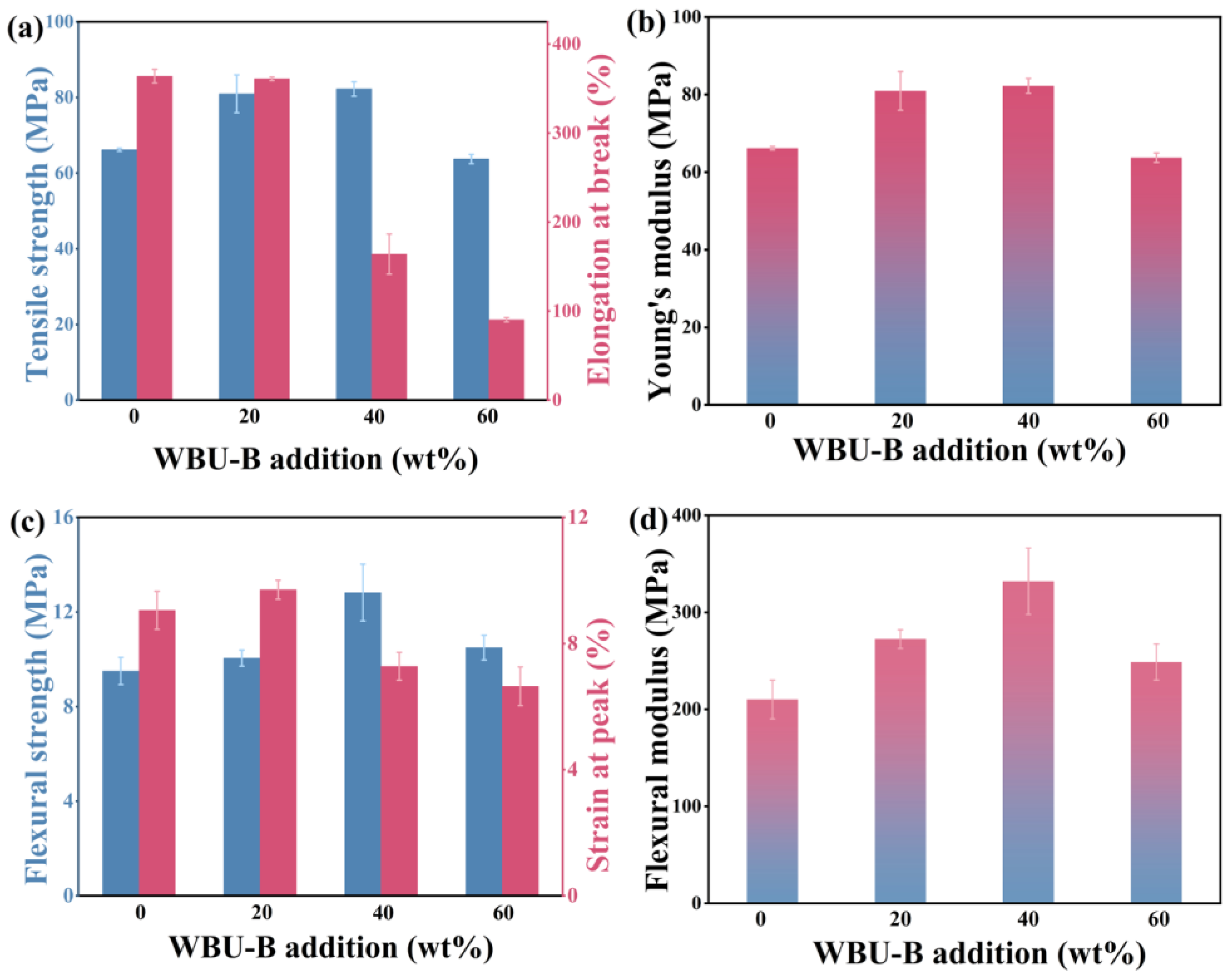
3.3. Performance Analysis of LDPE/WPU-B Composites
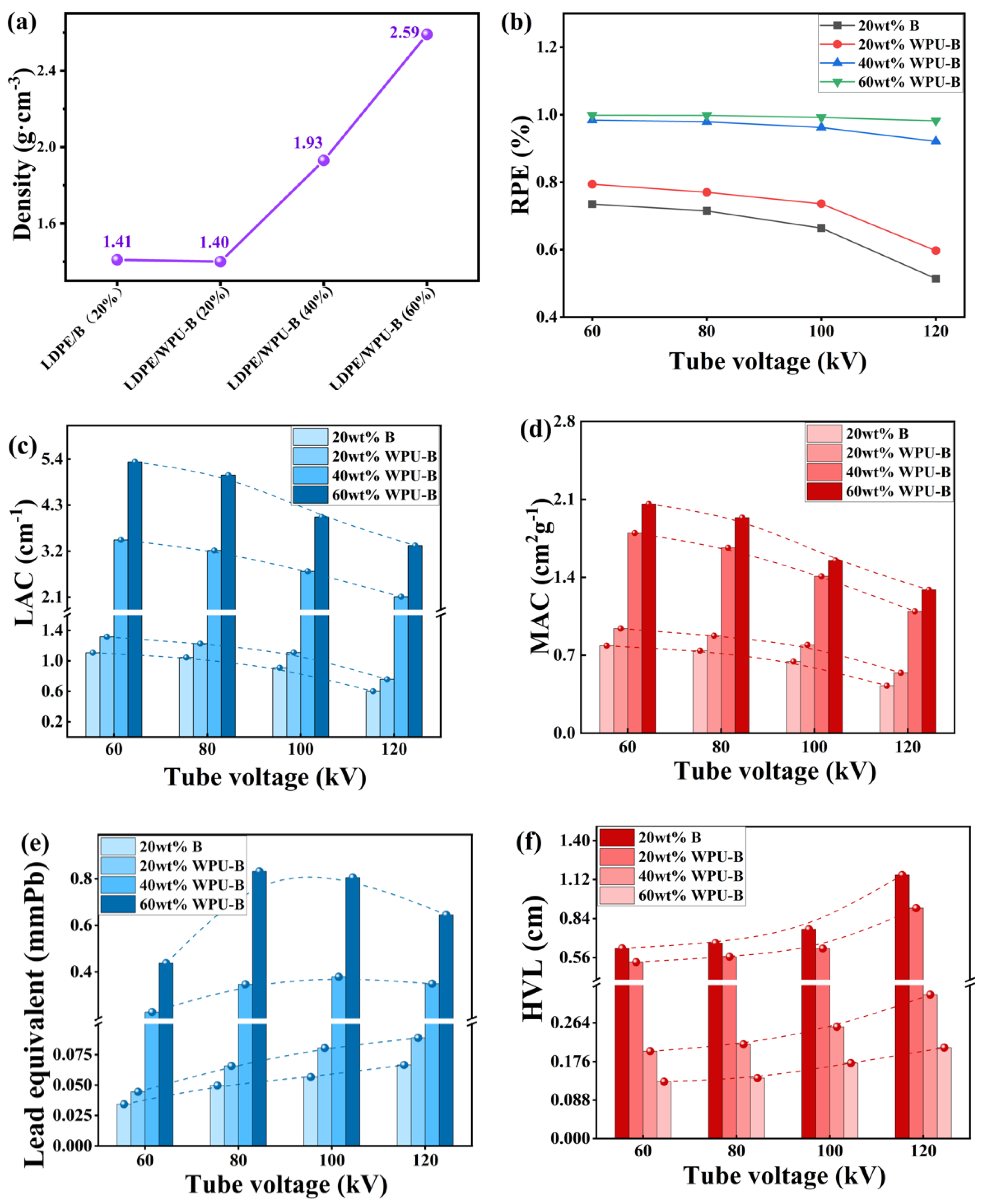
3.4. Radiation Resistance of LDPE/WPU-B Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.F.; Song, J.B.; Chen, X.Y.; Yang, H.H. X-ray-activated nanosystems for theranostic applications. Chem. Soc. Rev. 2019, 48, 3073–3101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, G.; Tao, Y.; Zhai, P.; Chen, H.; He, H.; Cai, T. Multi-task contrastive learning for automatic CT and X-ray diagnosis of COVID-19. Pattern Recognit. 2021, 114, 107848. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Nishimura, S.; Hashimoto, R.; Ohnuma, M.; Yamada, A. Mechanism of Sodium Storage in Hard Carbon: An X-Ray Scattering Analysis. Adv. Energy Mater. 2020, 10, 9. [Google Scholar] [CrossRef]
- Pandit, B.; Johansen, M.; Andersen, B.P.; Martínez-Cisneros, C.S.; Levenfeld, B.; Ravnsbæk, D.B.; Varez, A. All-solid-state sodium-ion batteries operating at room temperature based on NASICON-type NaTi2(PO4)3 cathode and ceramic NASICON solid electrolyte: A complete in situ synchrotron X-ray study. Chem. Eng. J. 2023, 472, 144509. [Google Scholar] [CrossRef]
- Lu, L.; Sun, M.Z.; Lu, Q.Y.; Wu, T.; Huang, B.L. High energy X-ray radiation sensitive scintillating materials for medical imaging, cancer diagnosis and therapy. Nano Energy 2021, 79, 21. [Google Scholar] [CrossRef]
- Lusic, H.; Grinstaff, M.W. X-ray-Computed Tomography Contrast Agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, H.K.; Kim, M.S.; Rhim, J.D.; Park, Y.J. Development of a new nanocrystalline alloy for X-ray shielding. Radiat. Eff. Defects Solids 2018, 173, 643–656. [Google Scholar] [CrossRef]
- Lakhwani, O.P.; Dalal, V.; Jindal, M.; Nagala, A. Radiation protection and standardization. J. Clin. Orthop. Trauma 2019, 10, 738–743. [Google Scholar] [CrossRef]
- Yu, L.; Pereira, A.L.C.; Tran, D.N.H.; Santos, A.M.C.; Losic, D. Bismuth Oxide Films for X-ray shielding: Effects of particle size and structural morphology. Mater. Chem. Phys. 2021, 260, 124084. [Google Scholar] [CrossRef]
- Yu, L.; Yap, P.L.; Santos, A.M.C.; Tran, D.N.H.; Losic, D. Lightweight polyester fabric with elastomeric bismuth titanate composite for high-performing lead-free X-ray shielding. Radiat. Phys. Chem. 2023, 205, 9. [Google Scholar] [CrossRef]
- Xu, L.; Huang, L.; Yu, J.; Si, Y.; Ding, B. Ultralight and Superelastic Gd2O3/Bi2O3 Nanofibrous Aerogels with Nacre-Mimetic Brick-Mortar Structure for Superior X-ray Shielding. Nano Lett. 2022, 22, 8711–8718. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, M.; Alberto, G.; Cantamessa, S.; Cantino, G.; Conterosito, E.; Palin, L.; Milanesio, M. Light Weight, Easy Formable and Non-Toxic Polymer-Based Composites for Hard X-ray Shielding: A Theoretical and Experimental Study. Int. J. Mol. Sci. 2020, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, M.; Palin, L.; Alberto, G.; Cantamessa, S.; Milanesio, M. Epoxy resins composites for X-ray shielding materials additivated by coated barium sulfate with improved dispersibility. Mater. Today Commun. 2021, 26, 12. [Google Scholar] [CrossRef]
- Qu, L.J.; Tian, M.W.; Zhang, X.S.; Guo, X.Q.; Zhu, S.F.; Han, G.T.; Li, C.L. Barium sulfate/regenerated cellulose composite fiber with X-ray radiation resistance. J. Ind. Text. 2015, 45, 352–367. [Google Scholar] [CrossRef]
- Pulford, S.; Fergusson, M. A textile platform for non-lead radiation shielding apparel. J. Text. Inst. 2016, 107, 1610–1616. [Google Scholar] [CrossRef]
- Kinnunen, P.; Pelto, J.; Viitanen, P.; Olin, M.; Nieminen, M. Valorisation of baryte tailings for radiation shielding in plastics and nuclear waste disposal. Heliyon 2024, 10, 8. [Google Scholar] [CrossRef]
- Messele, A.G.; Penev, K.I.; Mequanint, K.; Mekonnen, T.H. Lead-free single and dual-filler loaded polychloroprene X-ray shielding nanocomposites. Appl. Mater. Today 2025, 42, 102558. [Google Scholar] [CrossRef]
- Nakamura, K.; Kubo, K.; Hirata, M.; Sakai, Y.; Nakamura, Y.; Kosaka, H.; Monzen, H. Evaluation of the shielding effectiveness of a non-toxic, double-layered BaSO4/W composite against diagnostic X-rays. Radiat. Phys. Chem. 2024, 219, 111684. [Google Scholar] [CrossRef]
- Mungpayaban, H.; Rindhatayathon, P.; Ninlaphruk, S.; Rueanngoen, A.; Ekgasit, S.; Pengprecha, S. X-ray protective materials from barium sulfate/amorphous cellulose/natural rubber composites. Radiat. Phys. Chem. 2022, 194, 110011. [Google Scholar] [CrossRef]
- Jackson, D.F.; Hawkes, D.J. X-ray attenuation coefficients of elements and mixtures. Phys. Rep. 1981, 70, 169–233. [Google Scholar] [CrossRef]
- YY/T 0292.1-2020; Medical Diagnostic X-ray Radiation Protection Equipment Part 1: Determination of Material Attenuation Performance. National Medical Products Administration (NMPA): Beijing, China, 2020.
- Yang, X.R.; Shuai, H.; Du, G.X.; Wang, J.; Shen, J. Study on the mechanism of waterborne polyurethane modified ultrafine ground calcium carbonate and its properties in polypropylene plastics. Polym. Compos. 2024, 13. [Google Scholar] [CrossRef]
- Xin, H.; Xu, Y.Y.; Chen, Y.; Peng, Q.; Li, X.Q.; Gao, B. Development of fluorosilicon bivalve isocyanate microcapsules for multifunctional coatings with excellent self-repairing properties, enhanced hydrophobicity, and corrosion protection properties. Prog. Org. Coat. 2024, 191, 12. [Google Scholar] [CrossRef]
- Li, R.; Shan, Z. Research on structural features and thermal conductivity of waterborne polyurethane. Prog. Org. Coat. 2017, 104, 271–279. [Google Scholar] [CrossRef]
- Chen, S.A.; Chan, W.C. Polyurethane cationomers. 2. Phase inversion and its effect on physical-properties. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 1515–1532. [Google Scholar] [CrossRef]
- Qiang, T.T.; Han, M.; Wang, X. Waterborne polyurethane/carbon quantum dot nanocomposite as a surface coating material exhibiting outstanding luminescent performance. Prog. Org. Coat. 2020, 138, 9. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hosseini, D.; Isfahani, A.P.; Dehghani, Z.; Shams, E. Waterborne polyurethane nanocomposite incorporated with phytic acid intercalated layered double hydroxides: A highly stable aqueous dispersion with desired corrosion protection capability. Polym. Adv. Technol. 2021, 32, 4014–4028. [Google Scholar] [CrossRef]
- Sivakumar, S.; Soundhirarajan, P.; Venkatesan, A.; Khatiwada, C.P. Synthesis, characterization and anti-bacterial activities of pure and Co-doped BaSO4 nanoparticles via chemical precipitation route. Spectroc. Acta Part A Mol. Biomol. Spectr. 2015, 137, 137–147. [Google Scholar] [CrossRef]
- Duan, H.; Liu, W.G.; Zhao, L.; Wang, X.Y. Flotation performance and selective adsorption mechanism of novel hydroxamic acid on the separation of fluorite from barite. Miner. Eng. 2021, 171, 10. [Google Scholar] [CrossRef]
- Yang, S.Y.; Xu, Y.L.; Liu, C.; Huang, L.Y.; Huang, Z.Q.; Li, H.Q. The anionic flotation of fluorite from barite using gelatinized starch as the depressant. Colloid Surf. A Physicochem. Eng. Asp. 2020, 597, 6. [Google Scholar] [CrossRef]
- Wen, J.; Sun, Z.; Xiang, J.; Fan, H.; Chen, Y.; Yan, J. Preparation and characteristics of waterborne polyurethane with various lengths of fluorinated side chains. Appl. Surf. Sci. 2019, 494, 610–618. [Google Scholar] [CrossRef]
- Xu, W.Q.; Lv, Y.D.; Kong, M.Q.; Huang, Y.J.; Yang, Q.; Li, G.X. In-situ polymerization of eco-friendly waterborne polyurethane/polydopamine-coated graphene oxide composites towards enhanced mechanical properties and UV resistance. J. Clean. Prod. 2022, 373, 11. [Google Scholar] [CrossRef]
- Alsaab, A.H.; Zeghib, S. Analysis of X-ray and gamma ray shielding performance of prepared polymer micro-composites. J. Radiat. Res. Appl. Sci. 2023, 16, 14. [Google Scholar] [CrossRef]
- Bhattacharya, A. Radiation and industrial polymers. Prog. Polym. Sci. 2000, 25, 371–401. [Google Scholar] [CrossRef]
- Sobczak, J.; Zyla, G. Nano and microcomposites as gamma and X-ray ionizing radiation shielding materials—A review. Energy 2024, 290, 28. [Google Scholar] [CrossRef]


| Abbreviation | Explanation |
|---|---|
| B | Barite |
| WPU-B | Barite powder modified with waterborne polyurethane |
| LDPE | Low-density polyethylene |
| LDPE/B | Composite material reinforced with barite and matrix composed of low-density polyethylene |
| LDPE/WPU-B | Composite material reinforced with WPU-B and matrix composed of low-density polyethylene |
| PSD | Particle size distribution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Shi, S.; Yang, X.; Shuai, H.; Du, G.; Wang, J. Study of Construction of Innovative Barite/Waterborne Polyurethane/Low-Density Polyethylene Composites for Enhanced X-Ray Shielding Performance. Polymers 2025, 17, 451. https://doi.org/10.3390/polym17040451
Xu X, Shi S, Yang X, Shuai H, Du G, Wang J. Study of Construction of Innovative Barite/Waterborne Polyurethane/Low-Density Polyethylene Composites for Enhanced X-Ray Shielding Performance. Polymers. 2025; 17(4):451. https://doi.org/10.3390/polym17040451
Chicago/Turabian StyleXu, Xi, Shujin Shi, Xianrong Yang, Huan Shuai, Gaoxiang Du, and Jiao Wang. 2025. "Study of Construction of Innovative Barite/Waterborne Polyurethane/Low-Density Polyethylene Composites for Enhanced X-Ray Shielding Performance" Polymers 17, no. 4: 451. https://doi.org/10.3390/polym17040451
APA StyleXu, X., Shi, S., Yang, X., Shuai, H., Du, G., & Wang, J. (2025). Study of Construction of Innovative Barite/Waterborne Polyurethane/Low-Density Polyethylene Composites for Enhanced X-Ray Shielding Performance. Polymers, 17(4), 451. https://doi.org/10.3390/polym17040451







