Features of Selective Sorption of Neodymium and Praseodymium Ions by Interpolymer Systems Based on Industrial Sorbents KU-2-8 and AV-17-8
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Materials
2.3. Experiment
3. Results and Discussion
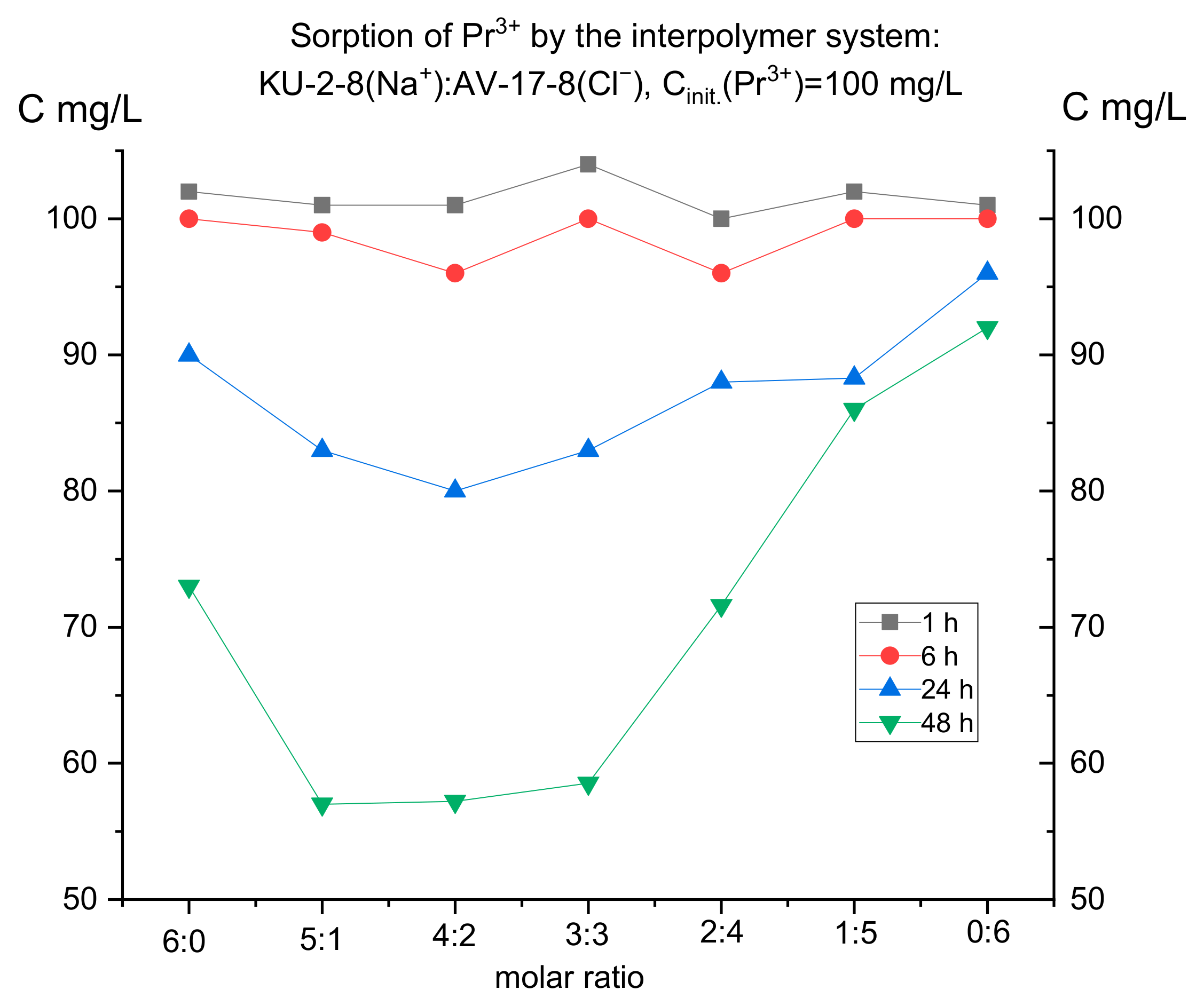
3.1. Study of Sorption of Target Metal Ions by Interpolymer Systems
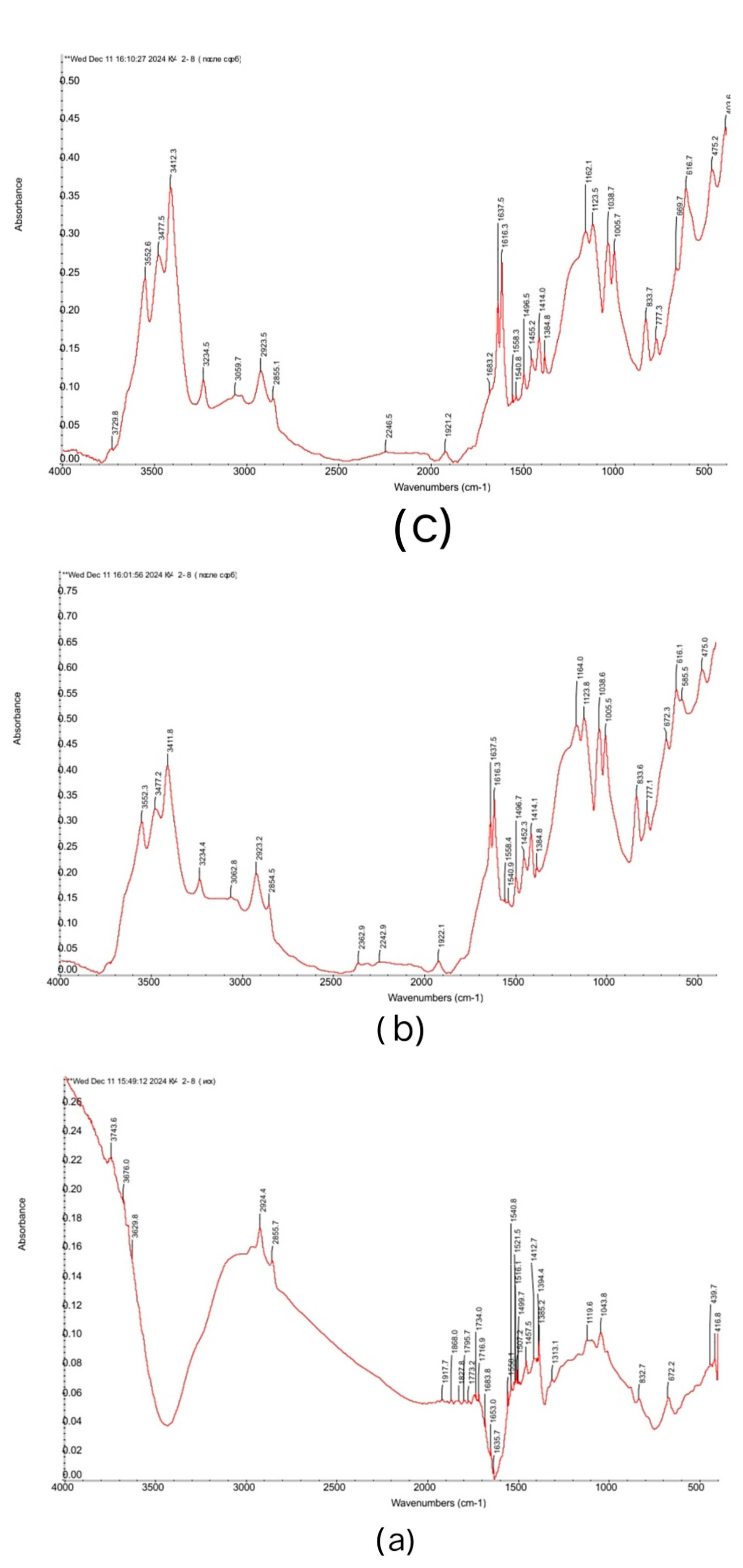
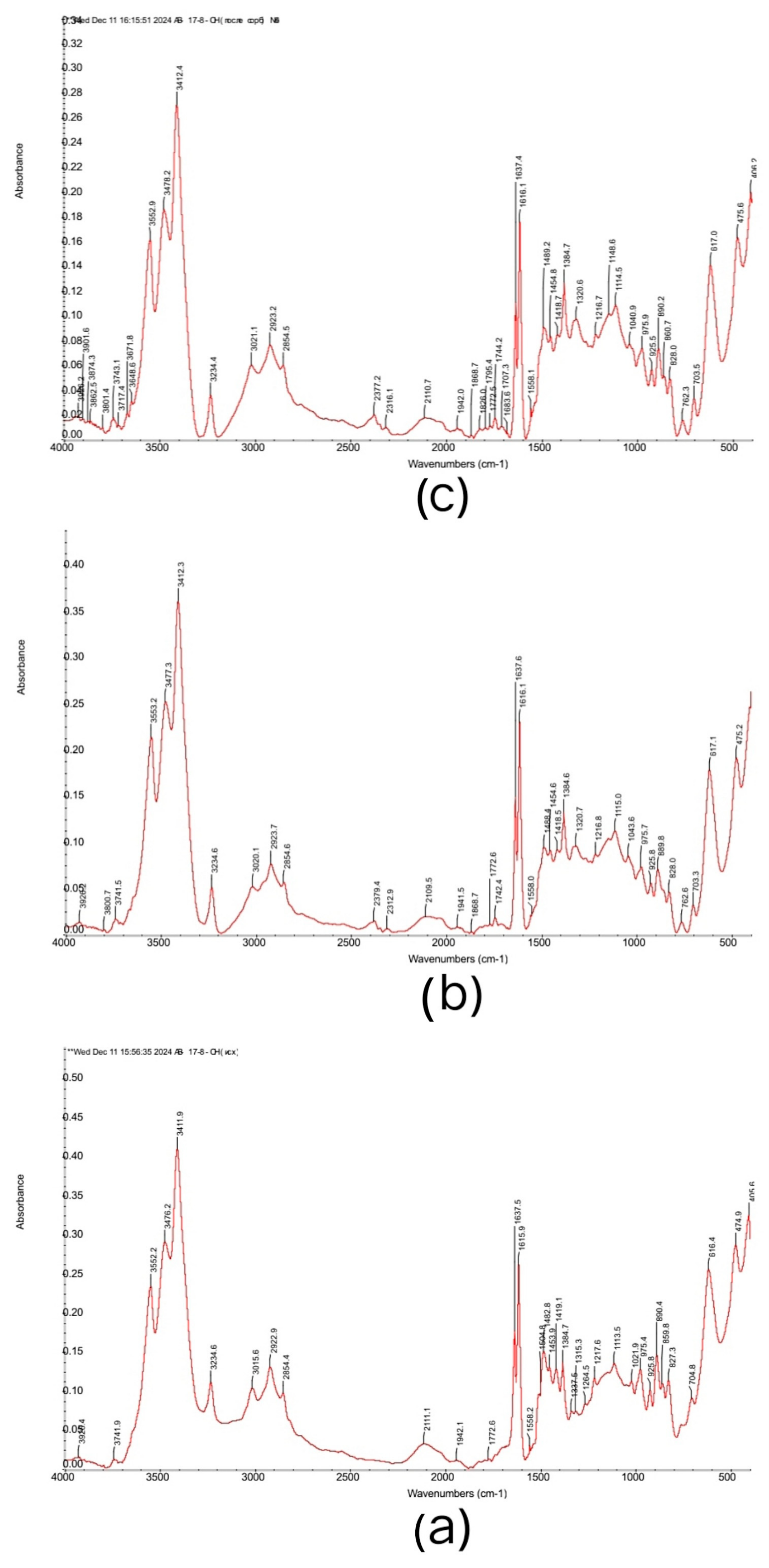
3.2. FTIR Spectra of the Initial Ion Exchangers and the Interpolymer Systems
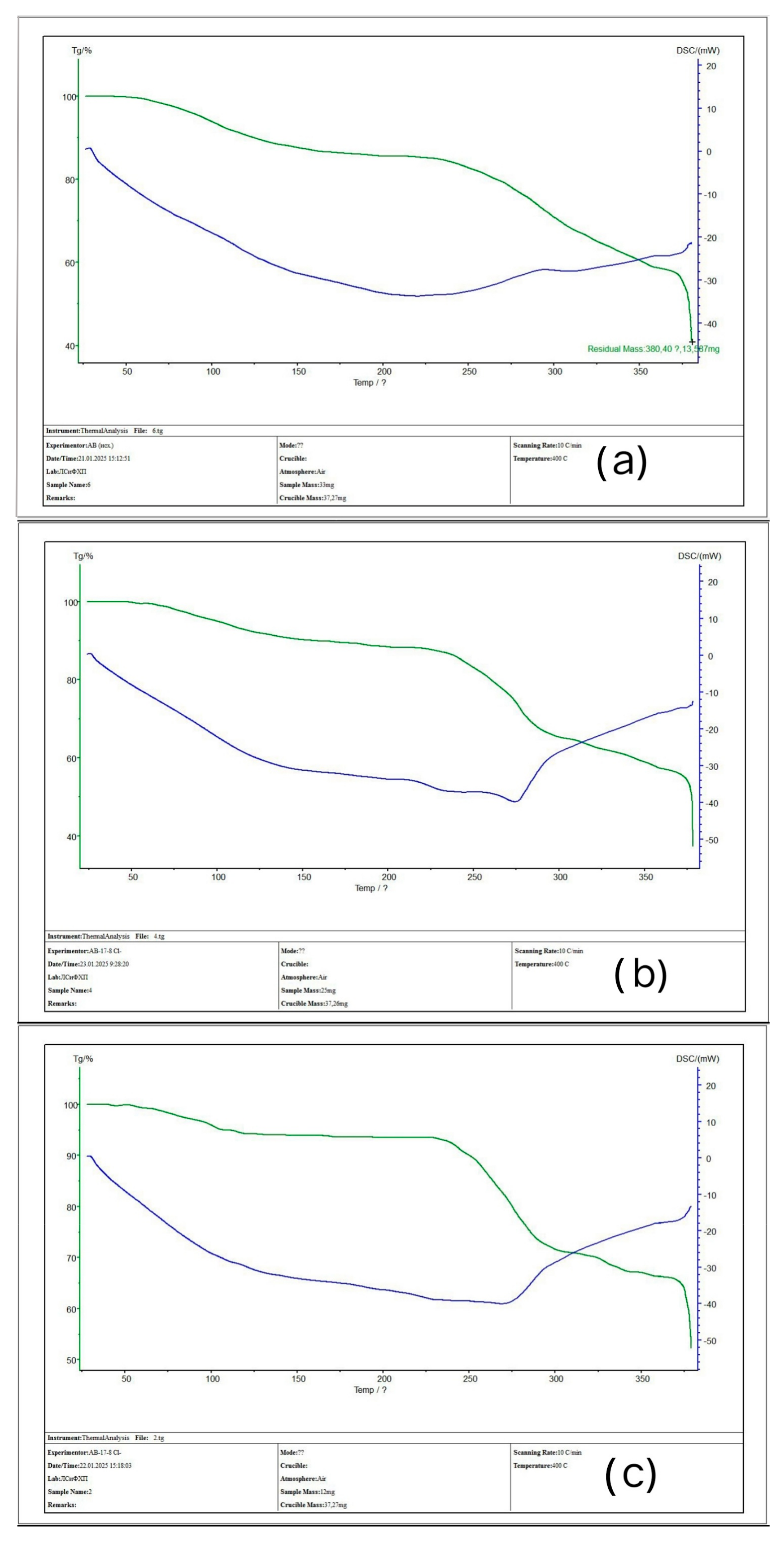
3.3. TGA Analysis of the Initial Ion-Exchangers and the Interpolymer Systems (4:2 and 3:3)
3.4. Study of Desorption of Target Metal Ions by Interpolymer Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Balaram, V. Potential future alternative resources for rare earth elements: Opportunities and challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Ivanik, S.A.; Ilyukhin, D.A. Flotation extraction of elemental sulfur from gold-bearing cakes. J. Min. Inst. 2020, 242, 202–208. [Google Scholar] [CrossRef]
- Cui, H.; Feng, X.; Sh, J.; Liu, W.; Yan, N.; Rao, G.; Wang, W. A facile process for enhanced rare earth elements separation from dilute solutions using N, N-di(2-ethylhexyl)-diglycolamide grafted polymer resin. Sep. Purif. Technol. 2020, 234, 116096. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Baulin, V.E.; Baulin, D.V.; Khvostikov, V.A. Extraction of Rare-Earth Elements (III) from Nitric Acid Solutions with Diethyl 2-[(Diphenylphosphoryl)methoxy]-5-ethylphenylphosphonate. Russ. J. Inorg. Chem. 2019, 64, 1297–1303. [Google Scholar] [CrossRef]
- Maria, L.; Cruz, A.; Carretas, J.M.; Monteiro, B.; Galinha, C.; Gomes, S.S.; Araújo, M.F.; Paiva, I.; Marçalo, J.; Leal, J.P. Improving the selective extraction of lanthanides by using functionalised ionic liquid. Sep. Purif. Technol. 2020, 237, 116354. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S.; Acharya, M.R. Hydrometallurgical route for recovery and separation of samarium (III) and cobalt (II) from simulated waste solution using tri-n-octyl phosphine oxide—A novel pathway for synthesis of samarium and cobalt oxides nanoparticles. J. Alloys Compd. 2020, 815, 152423. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Filho, W.L.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding rare earth elements as critical raw materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y. Polymeric Materials for Rare Earth Elements Recovery. Gels 2023, 9, 775. [Google Scholar] [CrossRef]
- Artiushenko, O.; da Silva, R.F.; Zaitsev, V. Recent advances in functional materials for rare earth recovery: A review. Sustain. Mater. Technol. 2023, 37, e00681. [Google Scholar] [CrossRef]
- Charalampides, G.; Vatalis, K.; Karayannis, V.; Baklavaridis, A. Environmental Defects and Economic Impact on Global Market of Rare Earth Metals. IOP Conf. Ser. Mater. Sci. Eng. 2016, 161, 012069. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H. Recent Advances in Selective Separation Technologies of Rare Earth Elements: A Review. J. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar] [CrossRef]
- Mosai, A.K.; Tutu, H. Simultaneous Sorption of Rare Earth Elements (Including Scandium and Yttrium) from Aqueous Solutions Using Zeolite Clinoptilolite: A Column and Speciation Study. Miner. Eng. 2021, 161, 106740. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, P.; Zou, Y.; Gao, Q.; Xiong, S.; Liang, B.; Xiao, B. Overview of Functionalized Porous Materials for Rare-Earth Element Separation and Recovery. Molecules 2024, 29, 2824. [Google Scholar] [CrossRef]
- Jumadilov, T.K.; Ismailova, S.A. Intergel interactions of three-dimensional structures based on polyacrylic acid and polyethyleneimine. Bull. KazNU Ser. Chem. 2005, 4, 60–64. [Google Scholar]
- Ismailova, S.A.; Jumadilov, T.K.; Bekturov, E.A. Interaction of rare-crosslinked polyacrylic acid with nitrogen-containing polymers. Bull. KazNTU 2004, 3, 186–189. [Google Scholar]
- Schneider, S.; Linse, P. Swelling of cross-linked polyelectrolyte gels. Eur. Phys. J. E 2002, 8, 457–460. [Google Scholar] [CrossRef]
- Ismailova, S.A. Features of Interaction of Three-Dimensional Structures Based on Polycarboxylic Acids and Nitrogen-Containing Polymers: Dissertation for the Degree of Candidate of Chemical Sciences; ICN MES RK: Almaty, Kazakhstan, 2006; 109p. [Google Scholar]
- Kokufuta, E.; Ogawa, K.; Miyake, M. Polyelectrolyte complex formation between anionic and cationic nanogels in salt-free aqueous solution. In Proceedings of the Abstr. 6th Int. Symp. Polyelectrolytes, Dresden, Germany, August 2006; pp. 1324–1326. [Google Scholar]
- Karpushkin, E.A.; Kechekyan, A.S.; Zezin, A.B. Interpolyelectrolyte reaction between particles of oppositely charged microgels. High-Mol. Compd. Ser. B 2006, 48, 2053–2057. [Google Scholar] [CrossRef]
- Jumadilov, T.; Khimersen, K.; Malimbayeva, Z.; Kondaurov, R. Effective sorption of europium ions by interpolymer system based on industrial ion-exchanger resins amberlite IR120 and AB-17-8. Materials 2021, 14, 3837. [Google Scholar] [CrossRef]
- Jumadilov, T.; Yskak, L.; Imangazy, A.; Suberlyak, O. Ion exchange dynamics in cerium nitrate solution regulated by remotely activated industrial ion exchangers. Materials 2021, 14, 3491. [Google Scholar] [CrossRef] [PubMed]
- Pismenskaya, N.; Sarapulova, V.; Klevtsova, A.; Mikhaylin, S.; Bazinet, L. Adsorption of anthocyanins by cation and anion exchange resins with aromatic and aliphatic polymer matrices. Int. J. Mol. Sci. 2020, 21, 7874. [Google Scholar] [CrossRef] [PubMed]
- Grebeniyk, V.D.; Mazo, A.A. Water Demineralization with Ion Exchangers; Chemistry: Moscow, Russia, 1982. (In Russian) [Google Scholar]
- Kravchenko, T.A.; Sakardina, E.A.; Kalinichev, A.I.; Zolotukhina, E.V. Stabilization of copper nanoparticles with volume- and surface-distribution inside ion-exchange matrices. Russ. J. Phys. Chem. A 2015, 89, 1648–1654. [Google Scholar] [CrossRef]
- Petrukhin, O.M. Apractitioner of the Physico-Mathematical Method of Analysis; Chemistry: Moscow, Russia, 1987; pp. 77–80. [Google Scholar]
- Saha, D.; Akkoyunlu, S.D.; Thorpe, R.; Hensley, D.K.; Chen, J. Adsorptive Recovery of Neodymium and Dysprosium in Phosphorous Functionalized Nanoporous Carbon. J. Environ. Chem. Eng. 2017, 5, 4684–4692. [Google Scholar] [CrossRef]
- Babu, C.M.; Binnemans, K.; Roosen, J. Ethylenediaminetriacetic Acid-Functionalized Activated Carbon for the Adsorption of Rare Earths from Aqueous Solutions. Ind. Eng. Chem. Res. 2018, 57, 1487–1497. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G.; Alevizos, G. Adsorption of scandium and neodymium on biochar derived after low-temperature pyrolysis of sawdust. Minerals 2017, 7, 200. [Google Scholar] [CrossRef]
- Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Praseodymium (III) removal from aqueous solutions using living and non-living Arthrospira platensis biomass. Water 2023, 15, 2064. [Google Scholar] [CrossRef]
- Stashkiv, O.; Vasylechko, V.; Gryshchouk, G.; Patsay, I. Solid phase extraction of trace amounts of praseodymium using transcarpathian clinoptilolite. Colloids Interfaces 2019, 3, 27. [Google Scholar] [CrossRef]
- Masry, B.A.; Abu Elgoud, E.M.; Rizk, S.E. Modeling and equilibrium studies on the recovery of praseodymium (III), dysprosium (III) and yttrium (III) using acidic cation exchange resin. BMC Chem. 2022, 16, 37. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 86. [Google Scholar]
- Ulusoy Erol, H.B.; Hestekin, C.N.; Hestekin, J.A. Effects of resin chemistries on the selective removal of industrially relevant metal ions using wafer-enhanced electrodeionization. Membranes 2021, 11, 45. [Google Scholar] [CrossRef]
- Ghosh, S.; Dhole, K.; Tripathy, M.K.; Kumar, R.; Sharma, R.S. FTIR spectroscopy in the characterization of the mixture of nuclear grade cation and anion exchange resins. J. Radioanal. Nucl. Chem. 2015, 304, 917–923. [Google Scholar] [CrossRef]
- Kim, J.; Park, C.W.; Lee, K.W.; Lee, T.S. Adsorption of ethylenediaminetetraacetic acid on a gel-type ion-exchange resin for purification of liquid waste containing Cs ions. Polymers 2019, 11, 297. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
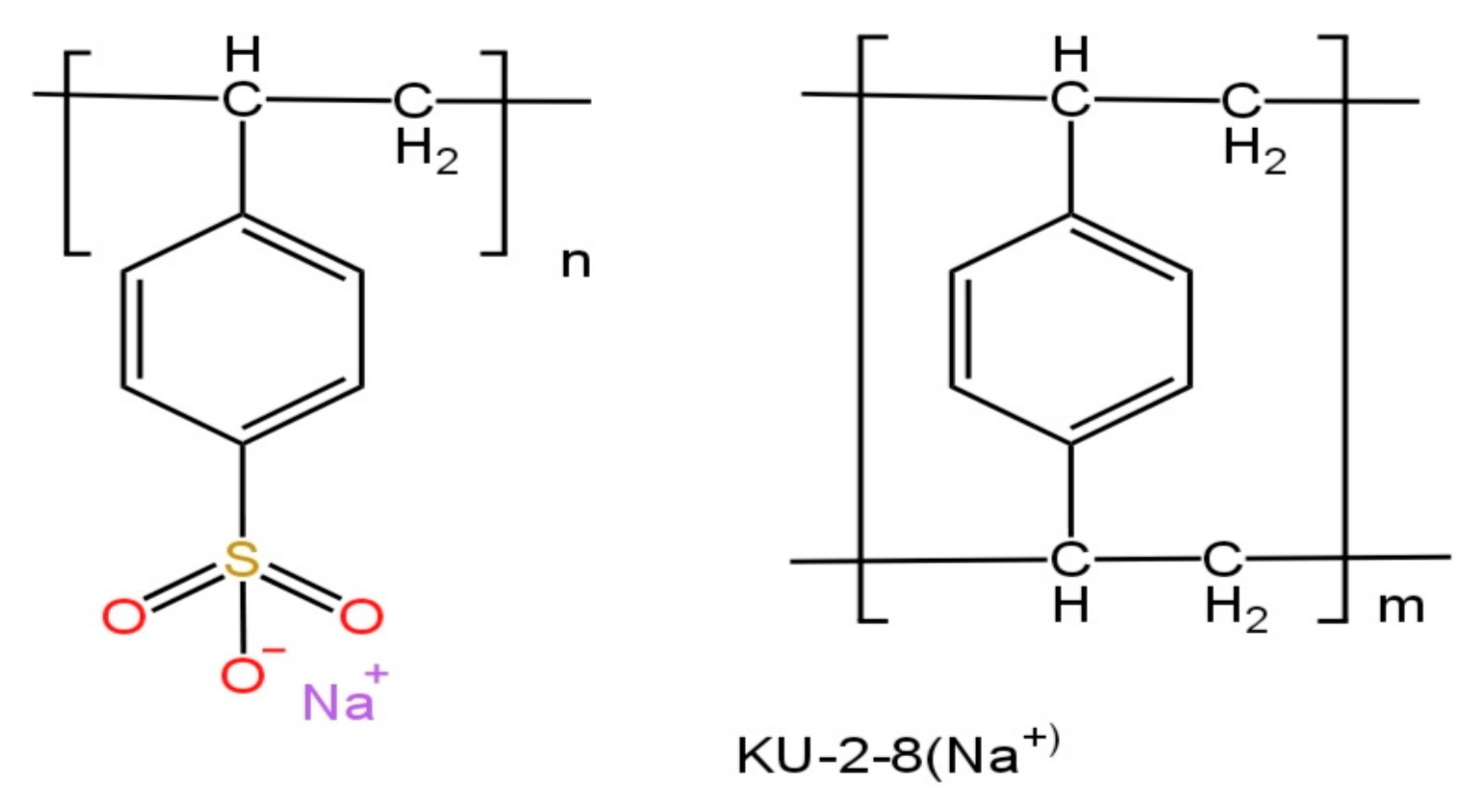



| IERs | Fixed Groups | Polymer Matrix | True Density, g/cm3 | Total Ion-Exchange Capacity, mmol cm−3 |
|---|---|---|---|---|
| KU-2-8 | -N+ (CH3)3 | DVB8% + PS | 1.13 [25] | 1.12 ± 0.02 [26] |
| AV-17-8 | -SO3− | DVB8% + PS | 1.25 [25] | 1.80 ± 0.01 [26] |
| PE Ratios and Sorption Mode | Nd (mg/L) | Pr (mg/L) |
|---|---|---|
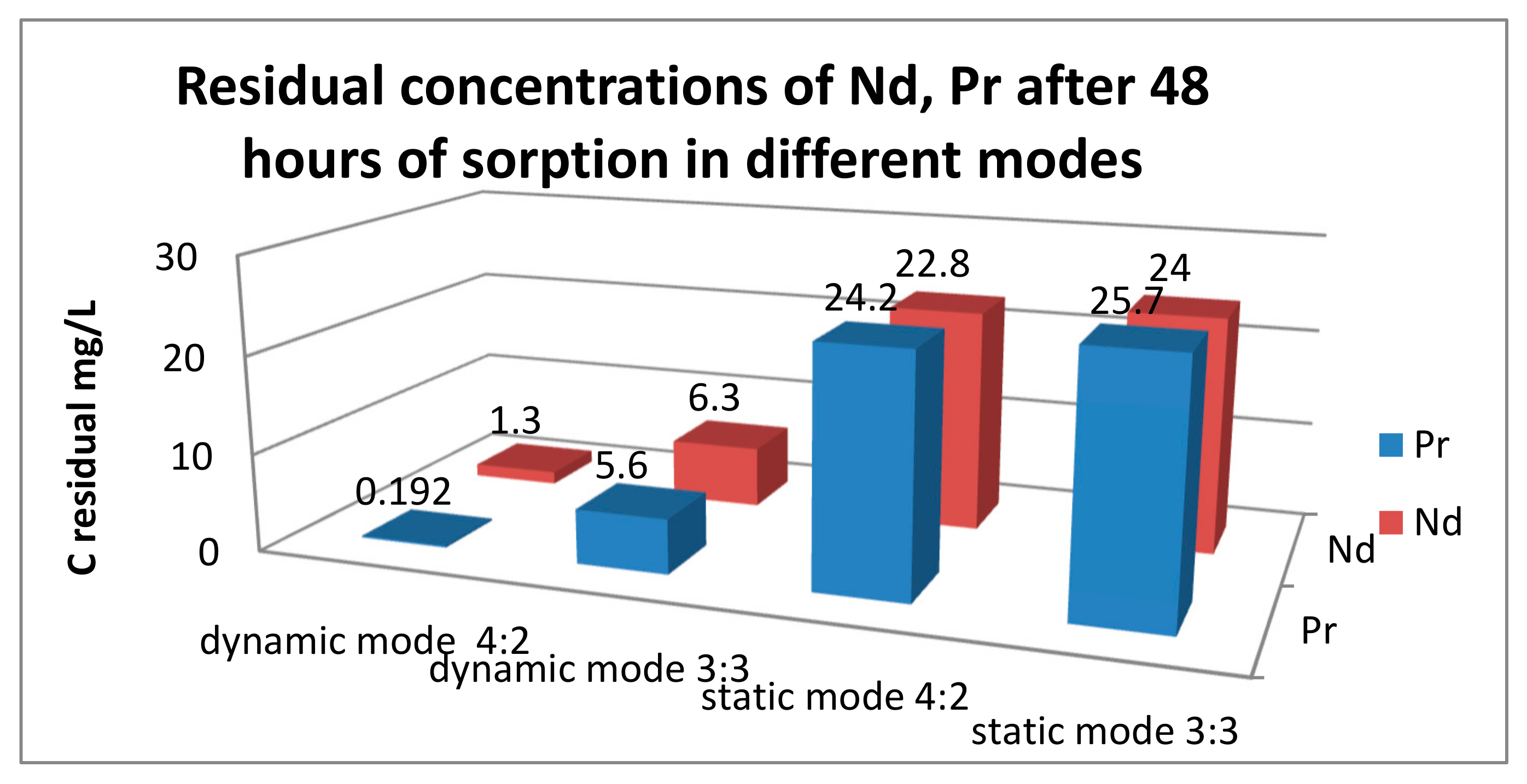
| KU-2-8(Na):AV-17-8(Cl) 4:2 dynamic mode | 1.3 | 0.192 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 dynamic mode | 6.3 | 5.6 |
| KU-2-8(Na):AV-17-8(Cl) 4:2 static mode | 22.8 | 24.2 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 static mode | 24.0 | 25.7 |
| PE Ratios and Sorption Mode | q (Nd) mg/g | q (Pr) mg/g |
|---|---|---|
| KU-2-8(Na):AV-17-8(Cl) 4:2 dynamic mode | 72.65 | 75.46 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 dynamic mode | 80.3 | 82.71 |
| KU-2-8(Na):AV-17-8(Cl) 4:2 static mode | 18.22 | 14.68 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 static mode | 20.33 | 14.57 |
| PE Ratios | pH (15 min) | pH (120 min) | pH (360 min) |
|---|---|---|---|
| KU-2-8(Na):AV-17-8(Cl) 4:2 dynamic mode | 5.06 | 5.27 | 5.14 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 dynamic mode | 4.97 | 5.19 | 4.98 |
| KU-2-8(Na):AV-17-8(Cl) 4:2 static mode | 5.04 | 4.94 | 5.09 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 static mode | 5.05 | 5.16 | 5.30 |
| PE Ratios and Sorption Mode | Nd Degree of Extraction % | Nd Degree of Extraction Per 1 mol in % | Pr Degree of Extraction % | Nd Degree of Extraction Per 1 mol in % |
|---|---|---|---|---|
| KU-2-8(Na):AV-17-8(Cl) 4:2 dynamic mode | 95.67 | 23.91 | 99.36 | 24.81 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 dynamic mode | 79 | 26.33 | 81.33 | 27.11 |
| KU-2-8(Na):AV-17-8(Cl) 4:2 static mode | 24.0 | 6 | 19.3 | 4.83 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 static mode | 20.0 | 6.67 | 14.33 | 4.77 |
| PE Ratios and Sorption Mode | (Nd) % | (Pr) % |
|---|---|---|
| KU-2-8(Na):AV-17-8(Cl) 4:2 dynamic mode | 49.75 | 52.88 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 dynamic mode | 54.78 | 57.72 |
| KU-2-8(Na):AV-17-8(Cl) 4:2 static mode | 12.48 | 10.29 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 static mode | 13.87 | 10.17 |
| PE Ratios and Sorption Mode | Q (Nd) mol/g | Q (Pr) mol/g |
|---|---|---|
| KU-2-8(Na):AV-17-8(Cl) 4:2 dynamic mode | 0.0025 | 0.0026 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 dynamic mode | 0.0027 | 0.0029 |
| KU-2-8(Na):AV-17-8(Cl) 4:2 static mode | 0.0006 | 0.0005 |
| KU-2-8(Na):AV-17-8(Cl) 3:3 static mode | 0.0007 | 0.0005 |
| PE Ratios and Sorption Mode | Nd (mg/L) | Pr (mg/L) |
|---|---|---|
| KU-2-8(Na) 4:2 dynamic mode | 15.8 | 15.4 |
| KU-2-8(Na) 3:3 dynamic mode | 14.6 | 14.2 |
| AV-17-8(Cl) 4:2 dynamic mode | 1.25 | 0.095 |
| AV-17-8(Cl) 3:3 dynamic mode | 1.52 | 0.33 |
| KU-2-8(Na) 4:2 static mode | 2.25 | 1.11 |
| KU-2-8(Na) 3:3 static mode | 2.24 | 1.09 |
| AV-17-8(Cl) 4:2 static mode | 1.32 | 0.17 |
| AV-17-8(Cl) 3:3 static mode | 1.33 | 0.18 |
| PE Ratios and Desorption Mode | (Nd) % | (Pr) % |
|---|---|---|
| KU-2-8(Na) 4:2 dynamic mode | 55 | 51.66 |
| KU-2-8(Na) 3:3 dynamic mode | 61.6 | 58.19 |
| KU-2-8(Na) static mode | 31.25 | 19.13 |
| KU-2-8(Na) static mode | 37.33 | 25.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talkybek, J.; Kabzhalelov, K.; Malimbayeva, Z.; Korganbayeva, Z. Features of Selective Sorption of Neodymium and Praseodymium Ions by Interpolymer Systems Based on Industrial Sorbents KU-2-8 and AV-17-8. Polymers 2025, 17, 440. https://doi.org/10.3390/polym17040440
Talkybek J, Kabzhalelov K, Malimbayeva Z, Korganbayeva Z. Features of Selective Sorption of Neodymium and Praseodymium Ions by Interpolymer Systems Based on Industrial Sorbents KU-2-8 and AV-17-8. Polymers. 2025; 17(4):440. https://doi.org/10.3390/polym17040440
Chicago/Turabian StyleTalkybek, Jumadilov, Kamil Kabzhalelov, Zamira Malimbayeva, and Zhanar Korganbayeva. 2025. "Features of Selective Sorption of Neodymium and Praseodymium Ions by Interpolymer Systems Based on Industrial Sorbents KU-2-8 and AV-17-8" Polymers 17, no. 4: 440. https://doi.org/10.3390/polym17040440
APA StyleTalkybek, J., Kabzhalelov, K., Malimbayeva, Z., & Korganbayeva, Z. (2025). Features of Selective Sorption of Neodymium and Praseodymium Ions by Interpolymer Systems Based on Industrial Sorbents KU-2-8 and AV-17-8. Polymers, 17(4), 440. https://doi.org/10.3390/polym17040440






