Enhancing Cassava Starch Bioplastics with Vismia guianensis Alcoholic Extract: Characterization with Potential Applications
Abstract
:1. Introduction
2. Materials and Methods
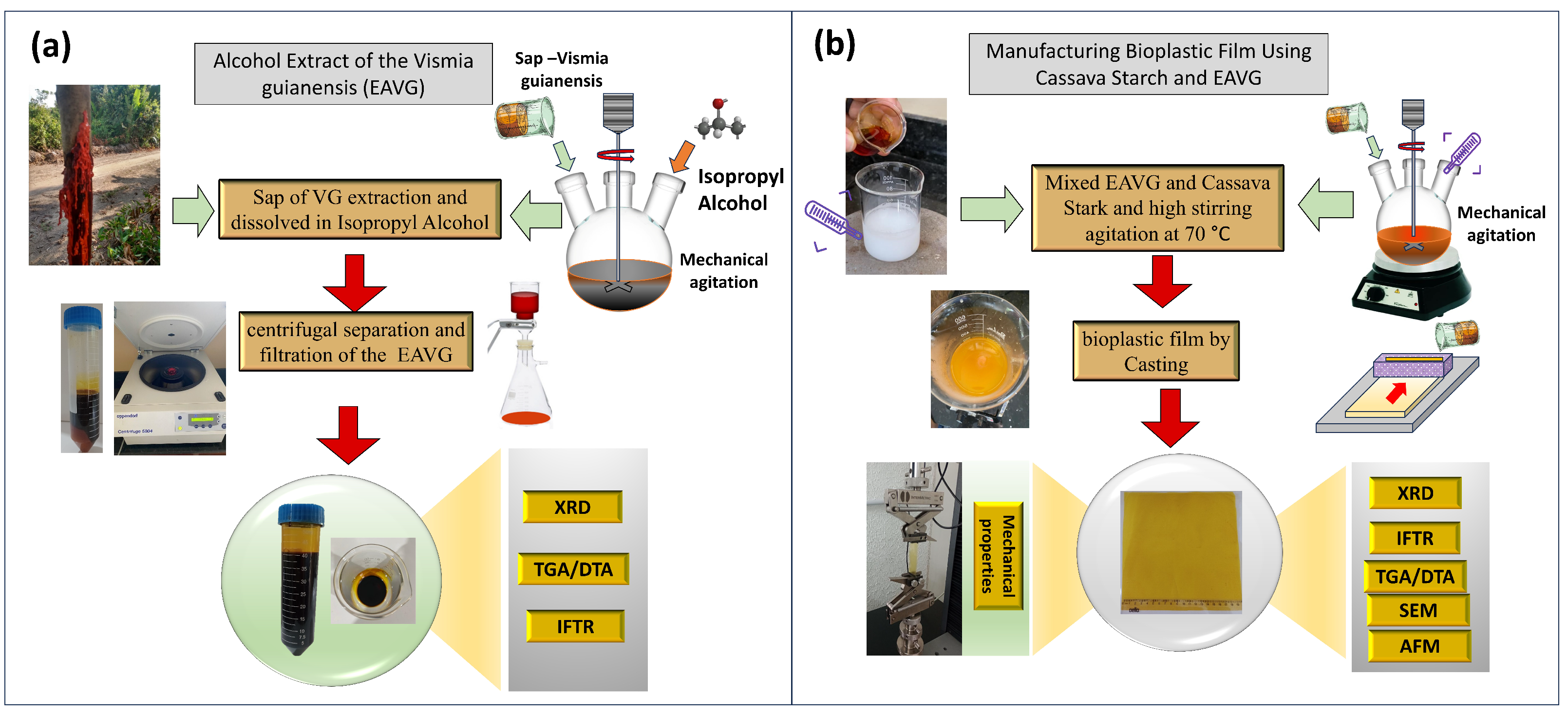
2.1. The Extract of Vismia guianensis in Isopropyl Alcohol (EAVG)
2.2. Bioplastic Film Manufacturing by Casting Method
3. Results
3.1. EAVG Characterization
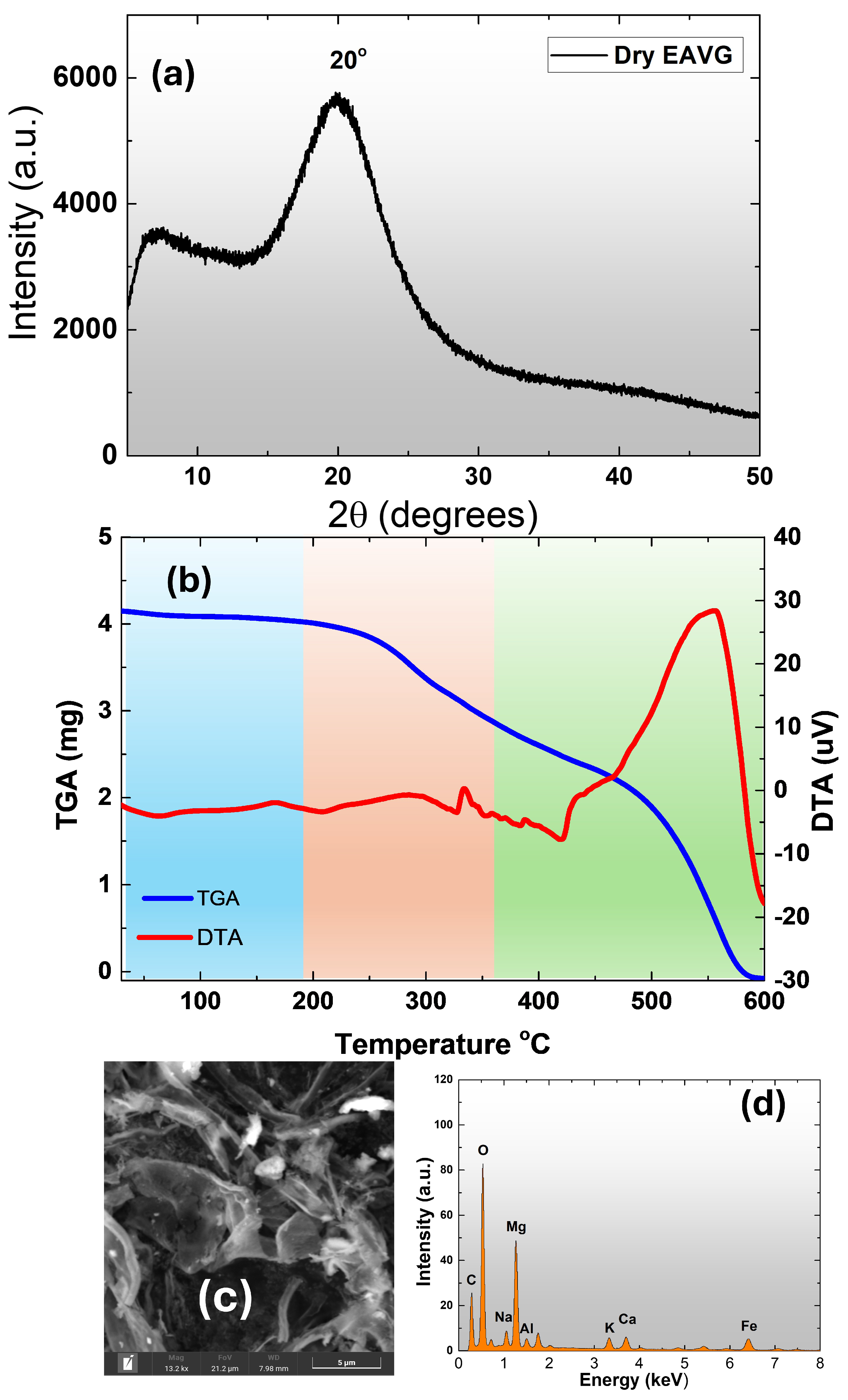
3.1.1. X-Ray Diffraction, TGA-DTA, and SEM-EDS
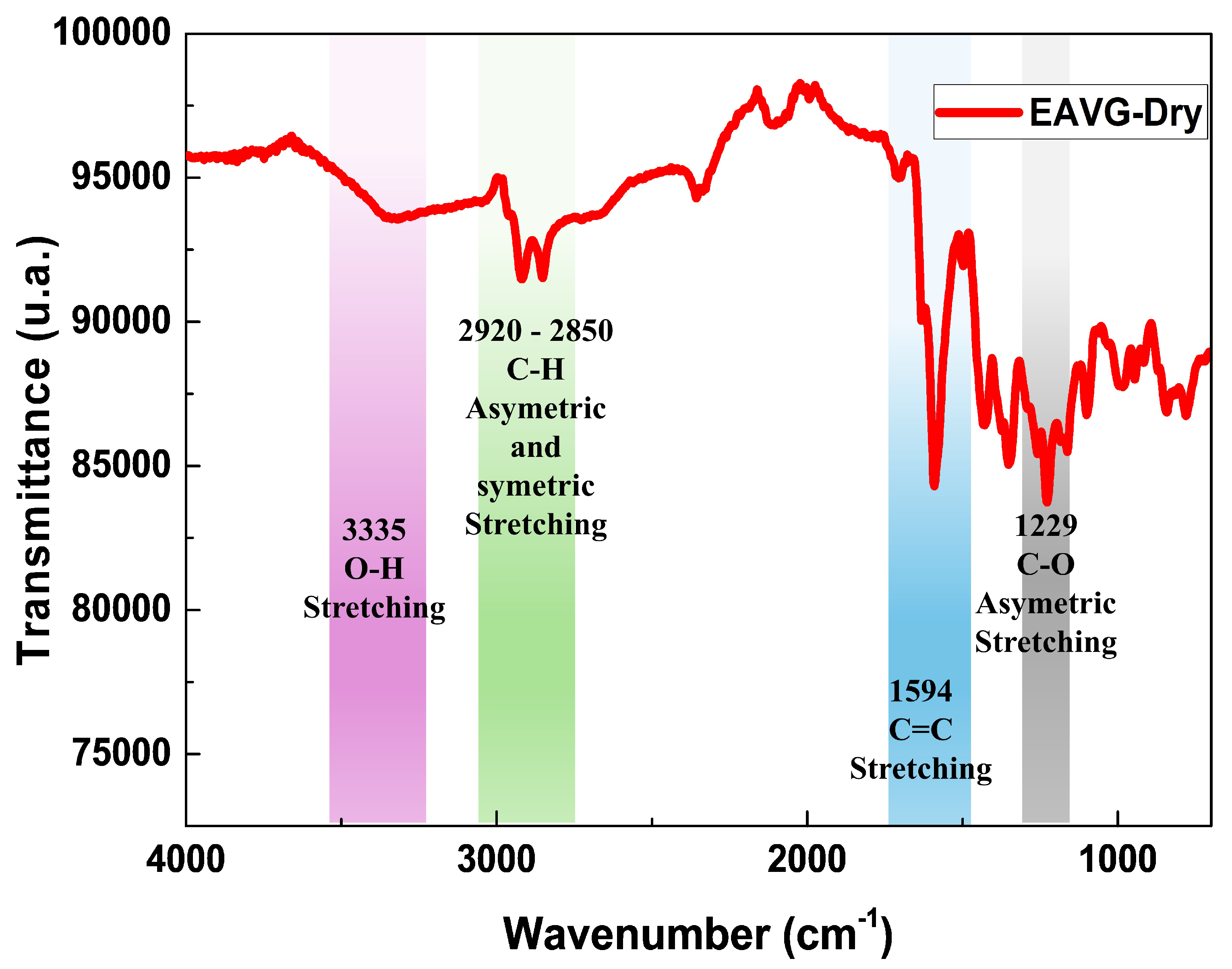
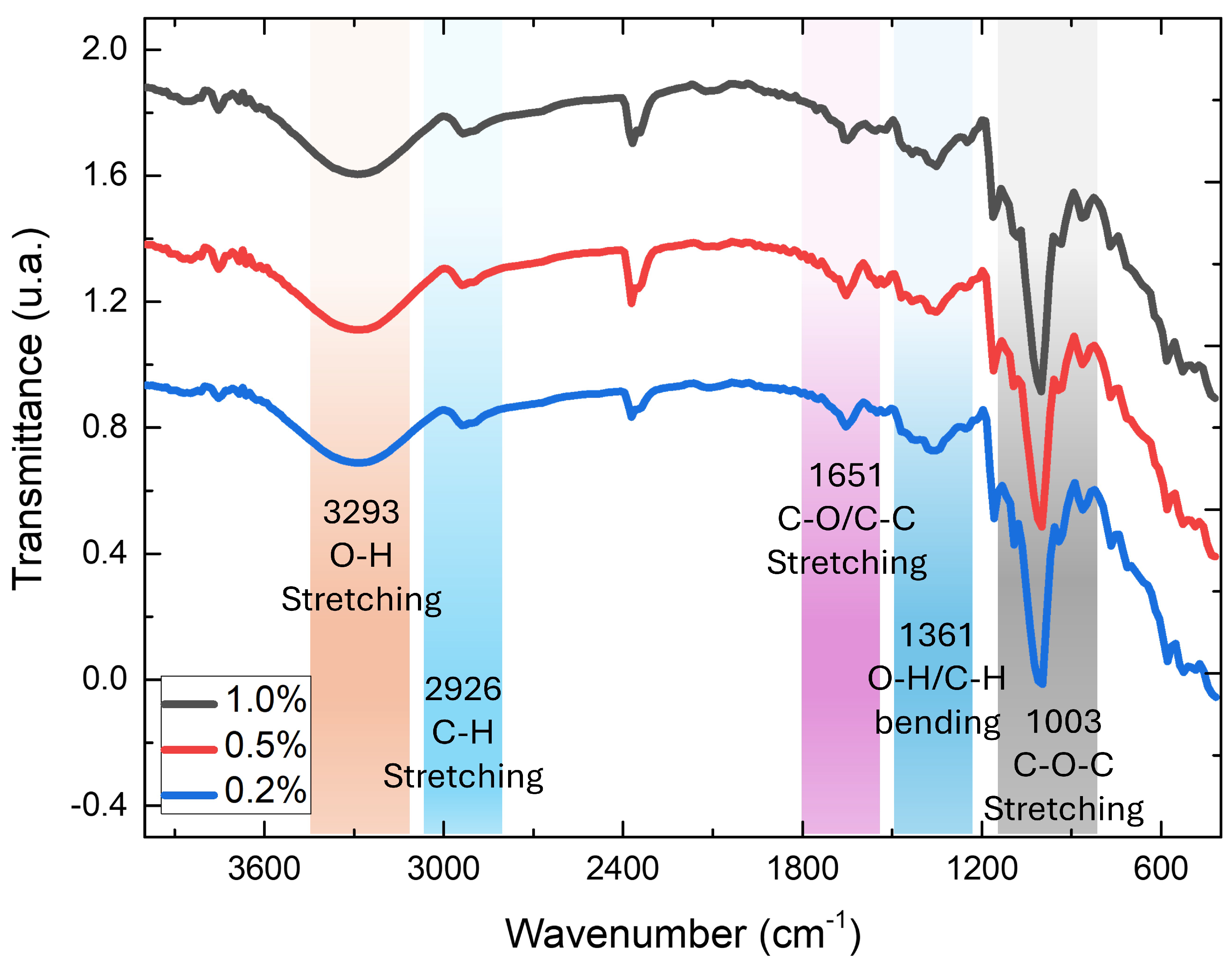
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Bioplastics Characterization
3.2.1. Scanning Electron Microscopy/Energy Dispersive Spectroscopy (SEM/EDS)
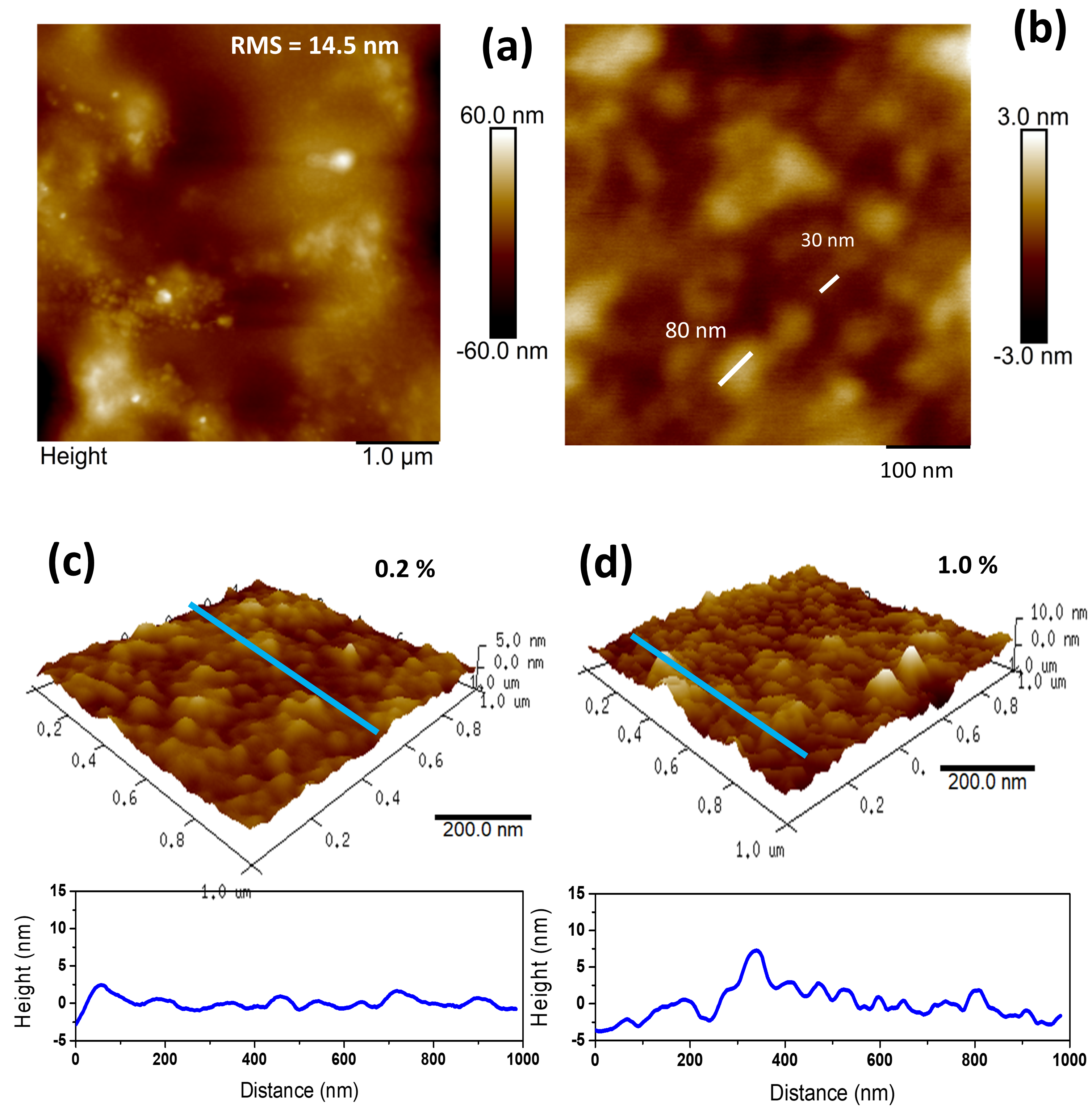
3.2.2. Atomic Force Microscopy (AFM): Surface Roughness (SR) and Particle Size (PS)
3.2.3. FTIR of Bioplastic Film
3.2.4. TGA and DTA Thermal Analysis
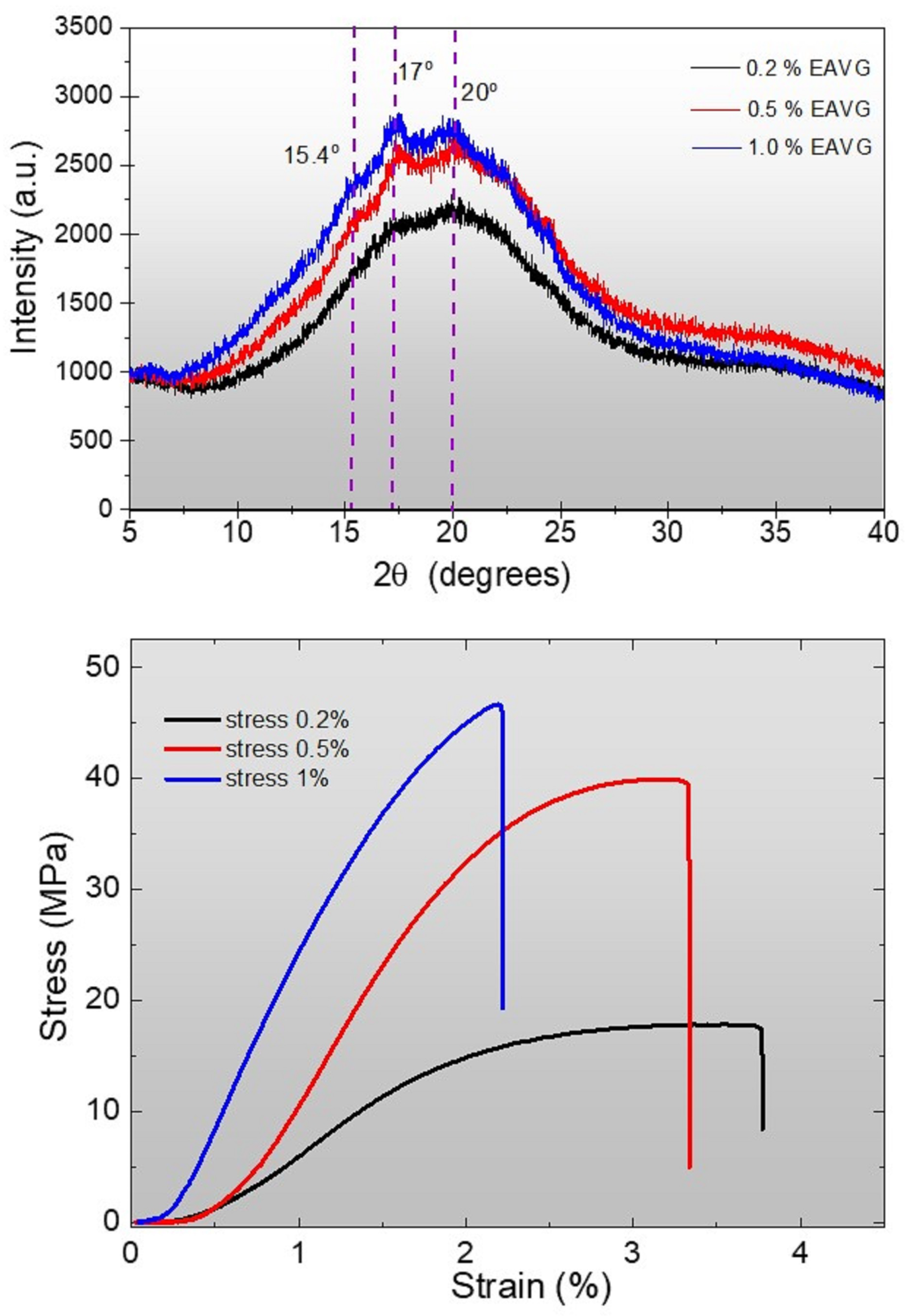
3.2.5. X-Ray Diffraction (XRD) and Stress–Strain Curves Analysis of Cassava Starch Bioplastic Films Doped with EAVG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| ATR | Attenuated total reflectance |
| DTA | Differential thermal analysis |
| EDS | Energy dispersive spectroscopy |
| EAVG | Vismia guianensis alcoholic extract |
| FTIR | Fourier transform infrared spectroscopy |
| PAC | Perturbed angular correlations |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| XRD | X-ray diffraction |
References
- The multifaceted challenges of bioplastics. Nat. Rev. Bioeng. 2024, 2, 279. [CrossRef]
- Feria-Reyes, R.; Ramírez-Cruz, S.; Ruiz-Aquino, F.; Robledo-Taboada, L.; Sánchez-Medina, M.; Mijangos-Ricárdez, O.; Gabriel-Parra, R.; Suárez-Mota, M.; Puc-Kauil, R.; Porcallo-Vargas, J. Pine bark as a potential source of condensed tannin: Analysis through Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), and Energy Dispersive X-ray (EDX). Forests 2023, 14, 1433. [Google Scholar] [CrossRef]
- Iroegbu, A.O.C.; Ray, S.S.; Mbarane, V.; Carlos Bordado, J.; Sardinha, J.P. Plastic pollution: A perspective on matters arising: Challenges and opportunities. ACS Omega 2021, 6, 19343–19355. [Google Scholar] [CrossRef]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Ptential health impact of microplastics: A review of environmental distribution, human exposure, and toxic effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Kong, U.; Rawi, N.F.M.; Tay, G.S. The potential applications of reinforced bioplastics in various industries: A review. Polymers 2023, 15, 2399. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in applications and prospects of bioplastics and biopolymers: A review. Environ. Chem. Lett. 2021, 20, 379–395. [Google Scholar] [CrossRef]
- Kim, M.S.; Chang, H.; Zheng, L.; Yan, Q.; Pfleger, B.F.; Klier, J.; Nelson, K.; Majumder, E.L.W.; Huber, G.W. A review of biodegradable plastics: Chemistry, applications, properties, and future research needs. Chem. Rev. 2023, 123, 9915–9939. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Papchenko, K.; Esposti, M.D.; Tondi, G.; Angelis, M.G.D.; Morselli, D.; Fabbri, P. Fully biobased polyhydroxyalkanoate/tannin films as multifunctional materials for smart food packaging applications. ACS Appl. Mater. interf. 2023, 15, 28594–28605. [Google Scholar] [CrossRef] [PubMed]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-based smart materials for food packaging and sensors—A review. Front. Mater. 2020, 7, 82. [Google Scholar] [CrossRef]
- Bozó, É.; Ervasti, H.; Halonen, N.; Shokouh, S.H.H.; Tolvanen, J.; Pitkänen, O.; Järvinen, T.; Pálvölgyi, P.S.; Szamosvölgyi, Á.; Sápi, A.; et al. Bioplastics and carbon-based sustainable materials, components, and devices: Toward green electronics. ACS Appl. Mater. interf. 2021, 13, 49301–49312. [Google Scholar] [CrossRef]
- Lomelí-Ramírez, M.G.; Kestur, S.G.; Manríquez-González, R.; Iwakiri, S.; de Muniz, G.B.; Flores-Sahagun, T.S. Bio-composites of cassava starch-green coconut fiber: Part II—Structure and properties. Carbohydr. Polym. 2014, 102, 576–583. [Google Scholar] [CrossRef]
- Surendren, A.; Mohanty, A.; Liu, Q.; Misra, M. A review of biodegradable thermoplastic starches, their blends and composites: Recent developments and opportunities for single-use plastic packaging alternatives. Green Chem. 2022, 24, 8606–8636. [Google Scholar] [CrossRef]
- Lenzi, L.; Esposti, M.D.; Braccini, S.; Siracusa, C.; Quartinello, F.; Guebitz, G.M.; Puppi, D.; Morselli, D.; Fabbri, P. Further Step in the Transition from Conventional Plasticizers to Versatile Bioplasticizers Obtained by the Valorization of Levulinic Acid and Glycerol. ACS Sustain. Chem. Eng. 2023, 11, 9455–9469. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers derived from biomass resources: A short review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef]
- Seo, H.J.; Seo, Y.H.; Park, S.U.; Lee, H.J.; Lee, M.R.; Park, J.H.; Cho, W.Y.; Lee, P.C.; Lee, B.Y. Glycerol-derived organic carbonates: Environmentally friendly plasticizers for PLA. RSC Adv. 2024, 14, 4702–4716. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, J.; Wu, M.; Wu, Q.; Liu, J.; Zhang, J. Biobased plasticizers from tartaric acid: Synthesis and effect of alkyl chain length on the properties of poly(vinyl chloride). ACS Omega 2021, 6, 13161–13169. [Google Scholar] [CrossRef] [PubMed]
- Navasingh, R.J.H.; Gurunathan, M.K.; Nikolova, M.P.; Królczyk, J.B. Sustainable bioplastics for food packaging produced from renewable natural sources. Polymers 2023, 15, 3760. [Google Scholar] [CrossRef]
- Tan, S.X.; Andriyana, A.; Ong, H.C.; Lim, S.; Ngoh, Y.L.P.G.C. A comprehensive review on the emerging roles of nanofillers and plasticizers towards sustainable starch-based bioplastic fabrication. Polymers 2022, 10, 664. [Google Scholar] [CrossRef]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and disadvantages of bioplastics production from starch and lignocellulosic components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, G.M.; Mohanty, A.K.; Misra, M. Green approaches to engineer tough biobased epoxies: A review. ACS Sustain. Chem. Eng. 2017, 11, 9528–9541. [Google Scholar] [CrossRef]
- Motta, E.P.; Farias, J.R.; da Costa, A.A.C.; da Silva, A.F.; Lopes, A.J.O.; do Socorro Sousa Cartágenes, M.; Nicolete, R.; Abreu, A.G.; Fernandes, E.S.; Nascimento, F.R.F. The Anti-Virulence Effect of Vismia guianensis against Candida albicans and Candida glabrata. Antibiotics 2022, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.H.; de Oliveira, G.G.; Neto, F.C.; Portuondo, D.F.; Batista-Duharte, A.; Carlos, I.Z. Anti-inflammatory activity of Vismia guianensis (Aubl.) Pers. extracts and antifungal activity against Sporothrix schenckii. J. Ethnopharmacol. 2017, 195, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.C.; Alves, P.P.; Chaves, F.C.M.; Kluczkovski, A.M.; Cortez, A.C.A.; Souza, J.V.B.; Souza, T.P. Investigation of antifungal activity from Vismia guianensis (Aubl.) standardized extract. Concilium 2024, 24, 565–578. [Google Scholar] [CrossRef]
- Deng, Y.; Chin, Y.W.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Anthraquinones with Quinone Reductase-Inducing Activity and Benzophenones from Morinda citrifolia (Noni) Roots. J. Nat. Prod. 2007, 70, 2049–2052. [Google Scholar] [CrossRef] [PubMed]
- Handique, J.G.; Baruah, J.B. Polyphenolic compounds: An overview. React. Funct. Polym. 2002, 52, 163–188. [Google Scholar] [CrossRef]
- Trung, N.Q.; Thong, N.M.; Cuong, D.H.; Manh, T.D.; Hoang, L.P.; Hien, N.K.; Nam, P.C.; Quang, D.T.; Mechler, A.; Vo, Q.V. Radical Scavenging Activity of Natural Anthraquinones: A Theoretical Insight. ACS Omega 2021, 6, 13391–13397. [Google Scholar] [CrossRef] [PubMed]
- Stepczyńska, M.; Rytlewski, P.; Moraczewski, K.; Pawłowska, A.; Karasiewicz, T. Novel Biocomposite of Starch and Flax Fiber Modified with Tannic Acid with Biocidal Properties. Polymers 2024, 16, 1108. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, P.; Chen, X.D. Mechanistic insights into the influence of flavonoids from dandelion on physicochemical properties and in vitro digestibility of cooked potato starch. Food Hydrocoll. 2022, 130, 107714. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Kupervaser, M.G.; Traffano-Schiffo, M.V.; Dellamea, M.L.; Flores, S.K.; Sosa, C.A. Trends in starch-based edible films and coatings enriched with tropical fruits extracts: A review. Food Hydrocoll. Health 2023, 4, 100138. [Google Scholar] [CrossRef]
- Xie, F. Natural polymer starch-based materials for flexible electronic sensor development: A review of recent progress. Carbohydr. Polym. 2024, 337, 122116. [Google Scholar] [CrossRef] [PubMed]
- Diel, K.A.P.; Filho, P.C.S.; Silveira, P.P.; Ribeiro, R.L.; Teixeira, P.C.; Júnior, L.C.R.; Marinho, L.C.; Romao, P.R.T.; von Poser, G.L. Antiprotozoal potential of Vismia species (Hypericaceae), medicinal plants used to fight cutaneous leishmaniasis. J. Ethnopharmacol. 2024, 328, 118028. [Google Scholar] [CrossRef] [PubMed]
- Bajer, D. Eco-Friendly, Biodegradable Starch-Based Packaging Materials with Antioxidant Features. Polymers 2024, 16, 958. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.; Tiwari, B.; O’Donnell, C. 6—Improved thermal processing for food texture modification. In Modifying Food Texture; Chen, J., Rosenthal, A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2015; pp. 115–131. [Google Scholar] [CrossRef]
- Abhilash, V.; Rajender, N.; Suresh, K. Chapter 14—X-ray diffraction spectroscopy of polymer nanocomposites. In Spectroscopy of Polymer Nanocomposites; Thomas, S., Rouxel, D., Ponnamma, D., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 410–451. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, B.K.; Bhattacharjee, S.; Seal, T. Nutritional composition, mineral content, antioxidant activity and quantitative estimation of water soluble vitamins and phenolics by RP-HPLC in some lesser used wild edible plants. Heliyon 2019, 5, e01431. [Google Scholar] [CrossRef] [PubMed]
- Kainat, S.; Gilani, S.R.; Asad, F.; Khalid, M.Z.; Khalid, W.; Ranjha, M.M.A.N.; Bangar, S.P.; Lorenzo, J.M. Determination and comparison of phytochemicals, phenolics, and flavonoids in Solanum lycopersicum using FTIR spectroscopy. Food Anal. Methods 2022, 15, 2931–2939. [Google Scholar] [CrossRef]
- Hussain, H.; Hussain, J.; Al-Harrasi, A.; Saleem, M.; Green, I.; van Ree, T.; Ghulam, A. Chemistry and biology of genus Vismia. Pharm. Biol. 2012, 11, 1448–1462. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Sanogo, R.; Ndjoko, K.; Guilet, D.; Wolfender, J.L.; Hostettmann, K.; Morelli, I. HPLC-UV/PAD and HPLC-MS analyses of leaf and root extracts of Vismia guineensis and isolation and identification of two new bianthrones. Phytochem. Anal. 2004, 15, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, C.; Jiang, S.; Xiong, L.; Sun, Q. Characterization of starch nanoparticles prepared by nanoprecipitation: Influence of amylose content and starch type. Ind. Crops Prod. 2016, 87, 182–190. [Google Scholar] [CrossRef]
- Herrera, M.P.; Vasanthan, T.; Hoover, R.; Izydorczyk, M. Molecular size distribution and amylase resistance of maize starch nanoparticles prepared by acid hydrolysis. Cereal Chem. 2017, 94, 262–269. [Google Scholar] [CrossRef]
- Bel Haaj, S.; Magnin, A.; Pétrier, C.; Boufi, S. Starch nanoparticles formation via high power ultrasonication. Carbohydr. Polym. 2012, 92, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, C.N.; Kling, I.C.S.; Ferreira, W.H.; Andrade, C.T.; Simao, R.A. Starch films containing starch nanoparticles as produced in a single step green route. Ind. Crops Prod. 2022, 177, 114481. [Google Scholar] [CrossRef]
- Fazeli, M.; Lipponen, J. Developing self-assembled starch nanoparticles in starch nanocomposite films. ACS Omega 2022, 49, 44962–44971. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Ghozali, M.; Meliana, Y.; Chalid, M. Novel in situ modification for thermoplastic starch preparation based on arenga pinnata palm starch. Polymers 2022, 14, 4813. [Google Scholar] [CrossRef] [PubMed]
- Chaffa, T.Y.; Meshesha, B.T.; Mohammed, S.A.; Jabasingh, S.A. Production, characterization, and optimization of starch-based biodegradable bioplastic from waste potato (Solanum tuberosum) peel with the reinforcement of false banana (Ensete ventricosum) fiber. Biomass Conv. Bioref. 2022, 14, 27365–27377. [Google Scholar] [CrossRef]
- Shi, M.; Jing, Y.; Yang, L.; Huang, X.; Wang, H.; Yan, Y.; Liu, Y. Structure and Physicochemical Properties of Malate Starches from Corn, Potato, and Wrinkled Pea Starches. Polymers 2019, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Castillo, L.E.; Leite, M.A.; Tisnado, V.J.A.; nad Paulo José do Amaral Sobral, C.D.; Moraes, I.C.F. Cassava starch films containing quinoa starch nanocrystals: Physical and surface properties. Foods 2023, 12, 576. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, C.M.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Mikus, P.Y.; Alix, S.; Soulestin, J.; Lacrampe, M.; Krawczak, P.; Coqueret, X.; Dole, P. Deformation mechanisms of plasticized starch materials. Carbohydr. Polym. 2014, 114, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.Q.; Li, F.Y.; Li, J.Y.; Li, J.F.; Zhang, C.W.; Chen, S.; Sun, X.; Cui, J.F. Optimisation of compatibility for improving elongation at break of chitosan/starch films. RSC Adv. 2019, 9, 24451–24459. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. 23—Plasticizers in edible films and coatings. In Innovations in Food Packaging; Han, J.H., Ed.; Food Science and Technology; Academic Press: London, UK, 2005; pp. 403–433. [Google Scholar] [CrossRef]
- Paluch, M.; Ostrowska, J.; Tyński, P.; Sadurski, W.; Konkol, M. Structural and thermal properties of starch plasticized with glycerol/urea mixture. J. Polym. Environ. 2022, 30, 728–740. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.H. Crystallization of High-Amylose Starch by the Addition of Plasticizers at Low and Intermediate Concentrations. J. Food Sci. 2010, 75, N8–N16. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.F.; Silva, C.W.C.; Silva, B.P.G.; Britto-Costa, P.H.; Costa, C.S.; Otubo, L.; Carbonari, A.W.; Cabrera-Pasca, G.A. Enhancing Cassava Starch Bioplastics with Vismia guianensis Alcoholic Extract: Characterization with Potential Applications. Polymers 2025, 17, 419. https://doi.org/10.3390/polym17030419
Santos JF, Silva CWC, Silva BPG, Britto-Costa PH, Costa CS, Otubo L, Carbonari AW, Cabrera-Pasca GA. Enhancing Cassava Starch Bioplastics with Vismia guianensis Alcoholic Extract: Characterization with Potential Applications. Polymers. 2025; 17(3):419. https://doi.org/10.3390/polym17030419
Chicago/Turabian StyleSantos, Josiel F., Crystian Willian C. Silva, Barbara P. G. Silva, Pedro H. Britto-Costa, Cleidilane S. Costa, Larissa Otubo, Artur W. Carbonari, and Gabriel A. Cabrera-Pasca. 2025. "Enhancing Cassava Starch Bioplastics with Vismia guianensis Alcoholic Extract: Characterization with Potential Applications" Polymers 17, no. 3: 419. https://doi.org/10.3390/polym17030419
APA StyleSantos, J. F., Silva, C. W. C., Silva, B. P. G., Britto-Costa, P. H., Costa, C. S., Otubo, L., Carbonari, A. W., & Cabrera-Pasca, G. A. (2025). Enhancing Cassava Starch Bioplastics with Vismia guianensis Alcoholic Extract: Characterization with Potential Applications. Polymers, 17(3), 419. https://doi.org/10.3390/polym17030419







