Exploring Metal Interactions with Released Polysaccharides from Cyanothece sp. CE4: A Chemical and Spectroscopic Study on Biosorption Mechanism
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Plan
2.2. RPS-Containing Supernatant Experiment
Analytical Procedures
2.3. Extracted RPS Experiment
3. Results and Discussion
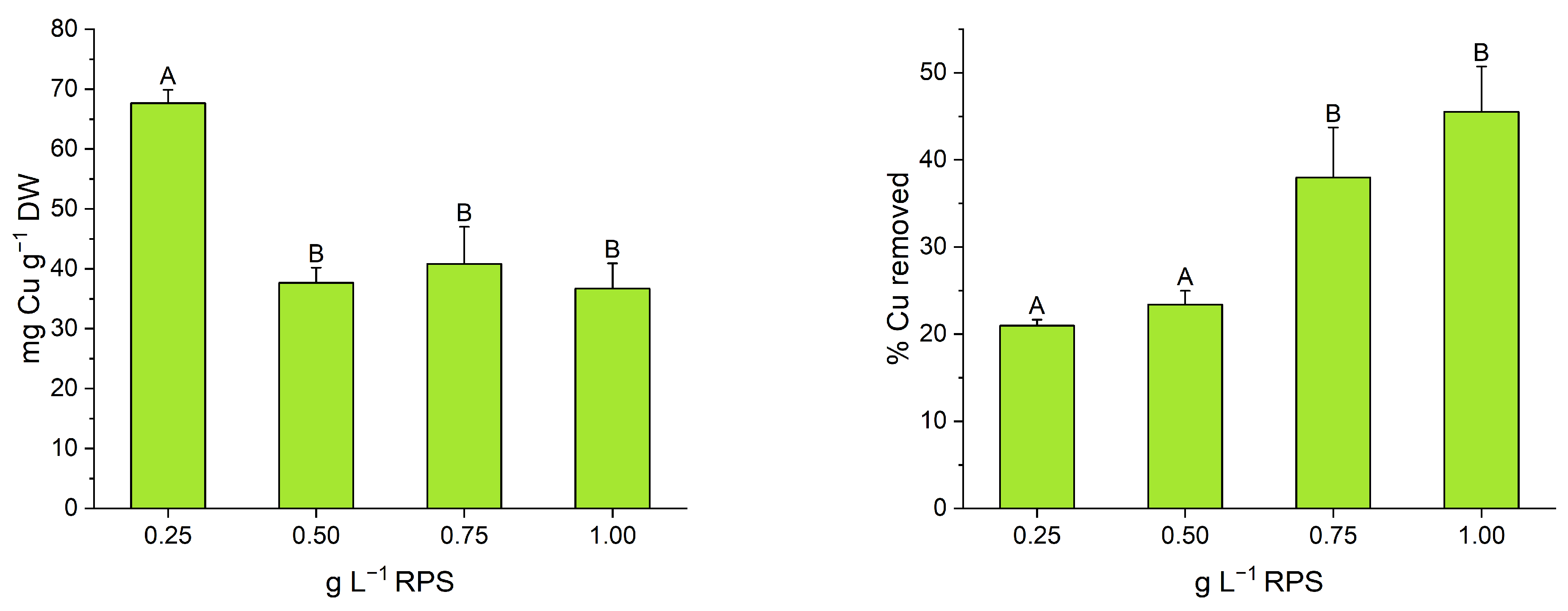
3.1. RPS-Containing Supernatant Experiment
3.2. Extracted RPS Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lourembam, J.; Haobam, B.; Singh, K.B.; Verma, S.; Rajan, J.P. The Molecular Insights of Cyanobacterial Bioremediations of Heavy Metals: The Current and the Future Challenges. Front. Microbiol. 2024, 15, 1450992. [Google Scholar] [CrossRef] [PubMed]
- Parsy, A.; Monlau, F.; Guyoneaud, R.; Sambusiti, C. Nutrient Recovery in Effluents from the Energy Sectors for Microalgae and Cyanobacteria Biomass Production: A Review. Renew. Sustain. Energy Rev. 2024, 191, 114207. [Google Scholar] [CrossRef]
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Mota, R.; Flores, C.; Tamagnini, P. Cyanobacterial Extracellular Polymeric Substances (EPS). In Polysaccharides of Microbial Origin; Oliveira, J.M., Radhouani, H., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 139–165. ISBN 978-3-030-42214-1. [Google Scholar]
- De Philippis, R.; Micheletti, E. Heavy Metal Removal with Exopolysaccharide-Producing Cyanobacteria. In Handbook of Advanced Industrial and Hazardous Wastes Management; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-11742-3. [Google Scholar]
- Mota, R.; Rossi, F.; Andrenelli, L.; Pereira, S.B.; De Philippis, R.; Tamagnini, P. Released Polysaccharides (RPS) from Cyanothece Sp. CCY 0110 as Biosorbent for Heavy Metals Bioremediation: Interactions between Metals and RPS Binding Sites. Appl. Microbiol. Biotechnol. 2016, 100, 7765–7775. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.; Vasconcelos, V.; Pierre, G.; Michaud, P.; Delattre, C. Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development. Appl. Sci. 2020, 10, 3763. [Google Scholar] [CrossRef]
- Pagliaccia, B.; Carretti, E.; Severi, M.; Berti, D.; Lubello, C.; Lotti, T. Heavy Metal Biosorption by Extracellular Polymeric Substances (EPS) Recovered from Anammox Granular Sludge. J. Hazard. Mater. 2022, 424, 126661. [Google Scholar] [CrossRef] [PubMed]
- Concórdio-Reis, P.; Reis, M.A.M.; Freitas, F. Biosorption of Heavy Metals by the Bacterial Exopolysaccharide FucoPol. Appl. Sci. 2020, 10, 6708. [Google Scholar] [CrossRef]
- Freire-Nordi, C.S.; Vieira, A.A.H.; Nascimento, O.R. The Metal Binding Capacity of Anabaena Spiroides Extracellular Polysaccharide: An EPR Study. Process Biochem. 2005, 40, 2215–2224. [Google Scholar] [CrossRef]
- Ozturk, S.; Aslim, B.; Suludere, Z.; Tan, S. Metal Removal of Cyanobacterial Exopolysaccharides by Uronic Acid Content and Monosaccharide Composition. Carbohydr. Polym. 2014, 101, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Decorosi, F.; Ratti, C.; De Philippis, R.; Adessi, A. Semi-Continuous Cultivation of EPS-Producing Marine Cyanobacteria: A Green Biotechnology to Remove Dissolved Metals Obtaining Metal-Organic Materials. New Biotechnol. 2024, 82, 33–42. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Maneechote, W.; Srinuanpan, S.; Angelidaki, I. Microalgae as Tools for Bio-Circular-Green Economy: Zero-Waste Approaches for Sustainable Production and Biorefineries of Microalgal Biomass. Bioresour. Technol. 2023, 387, 129620. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Adessi, A. Cyanoremediation and Phyconanotechnology: Cyanobacteria for Metal Biosorption toward a Circular Economy. Front. Microbiol. 2023, 14, 1166612. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Prabha, S.; Vijay, A.K.; Paul, R.R.; George, B. Cyanobacterial Biorefinery: Towards Economic Feasibility through the Maximum Valorization of Biomass. Sci. Total Env. 2022, 814, 152795. [Google Scholar] [CrossRef] [PubMed]
- D’Acapito, F.; Lepore, G.O.; Puri, A.; Laloni, A.; La Manna, F.; Dettona, E.; De Luisa, A.; Martin, A. The LISA Beamline at ESRF. J. Synchrotron Rad. 2019, 26, 551–558. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-Ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Ravel, B. ATOMS: Crystallography for the X-Ray Absorption Spectroscopist. J. Synchrotron Rad. 2001, 8, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Ankudinov, A.L.; Ravel, B.; Rehr, J.J.; Conradson, S.D. Real-Space Multiple-Scattering Calculation and Interpretation of x-Ray-Absorption near-Edge Structure. Phys. Rev. B 1998, 58, 7565–7576. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Cryst. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Marmiroli, M.; Lepore, G.O.; Pagano, L.; D’Acapito, F.; Gianoncelli, A.; Villani, M.; Lazzarini, L.; White, J.C.; Marmiroli, N. The Fate of CdS Quantum Dots in Plants as Revealed by Extended X-Ray Absorption Fine Structure (EXAFS) Analysis. Environ. Sci. Nano 2020, 7, 1150–1162. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-Valence Parameters for Solids. Acta Cryst. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive Derivation of Bond-Valence Parameters for Ion Pairs Involving Oxygen. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 562–578. [Google Scholar] [CrossRef]
- Liu, W.; Thorp, H.H. Bond Valence Sum Analysis of Metal-Ligand Bond Lengths in Metalloenzymes and Model Complexes. 2. Refined Distances and Other Enzymes. Inorg. Chem. 1993, 32, 4102–4105. [Google Scholar] [CrossRef]
- Moia, I.C.; Pereira, S.B.; Domizio, P.; De Philippis, R.; Adessi, A. Phormidium Ambiguum and Leptolyngbya Ohadii Exopolysaccharides under Low Water Availability. Polymers 2023, 15, 1889. [Google Scholar] [CrossRef]
- Dodgson, K.S. Determination of Inorganic Sulphate in Studies on the Enzymic and Non-Enzymic Hydrolysis of Carbohydrate and Other Sulphate Esters. Biochem. J. 1961, 78, 312–319. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Wu, J.; Yang, X.; Yang, S.; Zhu, W.; Liu, Y.; Tang, W.; Nie, S.; Hassouna, A.; et al. Fucoidan Extracted From Sporophyll of Undaria pinnatifida Grown in Weihai, China—Chemical Composition and Comparison of Antioxidant Activity of Different Molecular Weight Fractions. Front. Nutr. 2021, 8, 636930. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Gola, D.; Dey, P.; Malik, A. Synergistic and Antagonistic Effects on Metal Bioremediation with Increasing Metal Complexity in a Hexa-Metal Environment by Aspergillus fumigatus. Int. J. Environ. Res. 2020, 14, 761–770. [Google Scholar] [CrossRef]
- Micheletti, E.; Colica, G.; Viti, C.; Tamagnini, P.; De Philippis, R. Selectivity in the Heavy Metal Removal by Exopolysaccharide-Producing Cyanobacteria. J. Appl. Microbiol. 2008, 105, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Deschatre, M.; Ghillebaert, F.; Guezennec, J.; Colin, C.S. Sorption of Copper(II) and Silver(I) by Four Bacterial Exopolysaccharides. Appl. Biochem. Biotechnol. 2013, 171, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, J.; Shen, B.; Hou, W.; Zhang, Y. Biosorption of Copper(II) and Cadmium(II) by a Novel Exopolysaccharide Secreted from Deep-Sea Mesophilic Bacterium. Colloids Surf. B Biointerfaces 2009, 72, 295–302. [Google Scholar] [CrossRef]
- Fertu, D.I.; Dragoi, E.N.; Bulgariu, L.; Curteanu, S.; Gavrilescu, M. Modeling the Biosorption Process of Heavy Metal Ions on Soybean-Based Low-Cost Biosorbents Using Artificial Neural Networks. Processes 2022, 10, 603. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Biosorption of Heavy Metal Ions by Green Alga Neochloris oleoabundans: Effects of Metal Ion Properties and Cell Wall Structure. J. Hazard. Mater. 2021, 418, 126336. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Lan, C.Q. Effects of Culture pH on Cell Surface Properties and Biosorption of Pb(II), Cd(II), Zn(II) of Green Alga Neochloris oleoabundans. Chem. Eng. J. 2023, 468, 143579. [Google Scholar] [CrossRef]
- Rahman, M.S.; Sathasivam, K.V. Heavy Metal Biosorption Potential of a Malaysian Rhodophyte (Eucheuma denticulatum) from Aqueous Solutions. Int. J. Environ. Sci. Technol. 2016, 13, 1973–1988. [Google Scholar] [CrossRef]
- Pagnucco, G.; Overfield, D.; Chamlee, Y.; Shuler, C.; Kassem, A.; Opara, S.; Najaf, H.; Abbas, L.; Coutinho, O.; Fortuna, A.; et al. Metal Tolerance and Biosorption Capacities of Bacterial Strains Isolated from an Urban Watershed. Front. Microbiol. 2023, 14, 1278886. [Google Scholar] [CrossRef]
- Caichiolo, M.; Zampieri, R.M.; Adessi, A.; Ciani, M.; Caldara, F.; Dalla Valle, L.; La Rocca, N. Microbial Polysaccharides Extracted from Different Mature Muds of the Euganean Thermal District Show Similar Anti-Inflammatory Activity In Vivo. Int. J. Mol. Sci. 2024, 25, 4999. [Google Scholar] [CrossRef] [PubMed]
- Comte, S.; Guibaud, G.; Baudu, M. Relations between extraction protocols for activated sludge extracellular polymeric substances (EPS) and EPS complexation properties: Part I. Comparison of the efficiency of eight EPS extraction methods. Enzym. Microb. Technol. 2006, 38, 237–245. [Google Scholar] [CrossRef]
- Flores, C.; Lima, R.T.; Adessi, A.; Sousa, A.; Pereira, S.B.; Granja, P.L.; De Philippis, R.; Soares, P.; Tamagnini, P. Characterization and Antitumor Activity of the Extracellular Carbohydrate Polymer from the Cyanobacterium Synechocystis ΔsigF Mutant. Int. J. Biol. Macromol. 2019, 136, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Gee, C.W.; Andersen-Ranberg, J.; Boynton, E.; Rosen, R.Z.; Jorgens, D.; Grob, P.; Holman, H.-Y.N.; Niyogi, K.K. Implicating the Red Body of Nannochloropsis in Forming the Recalcitrant Cell Wall Polymer Algaenan. Nat. Commun. 2024, 15, 5456. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Su, L.; Chen, S.; Zhao, L.; Wang, H.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical Characterization and Functional Analysis of the Polysaccharide from the Edible Microalga Nostoc sphaeroides. Molecules 2018, 23, 508. [Google Scholar] [CrossRef]
- Parikh, A.; Madamwar, D. Partial Characterization of Extracellular Polysaccharides from Cyanobacteria. Bioresour. Technol. 2006, 97, 1822–1827. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Miao, C.; McDonald, A.; Chen, S. Concomitant Extraction of Bio-Oil and Value Added Polysaccharides from Chlorella sorokiniana Using a Unique Sequential Hydrothermal Extraction Technology. Fuel 2012, 95, 63–70. [Google Scholar] [CrossRef]
- Carr, C.E.; Foster, A.W.; Maroney, M.J. An XAS Investigation of the Nickel Site Structure in the Transcriptional Regulator InrS. J. Inorg. Biochem. 2017, 177, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhou, C.; Cai, P.; Chen, W.; Rong, X.; Dai, K.; Liang, W.; Gu, J.-D.; Huang, Q. Binding Characteristics of Copper and Cadmium by Cyanobacterium Spirulina platensis. J. Hazard. Mater. 2011, 190, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, O.S.; Pokrovski, G.S.; Shirokova, L.S.; Gonzalez, A.G.; Emnova, E.E.; Feurtet-Mazel, A. Chemical and Structural Status of Copper Associated with Oxygenic and Anoxygenic Phototrophs and Heterotrophs: Possible Evolutionary Consequences. Geobiology 2012, 10, 130–149. [Google Scholar] [CrossRef]
- González, A.G.; Jimenez-Villacorta, F.; Beike, A.K.; Reski, R.; Adamo, P.; Pokrovsky, O.S. Chemical and Structural Characterization of Copper Adsorbed on Mosses (Bryophyta). J. Hazard. Mater. 2016, 308, 343–354. [Google Scholar] [CrossRef]
- Belfiore, L.A.; McCurdie, M.P.; Das, P.K. Macromolecule–Metal Complexes: Ligand Field Stabilization and Thermophysical Property Modification. Polymer 2001, 42, 09995–10006. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Biological Inorganic Chemistry of Zinc Ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef]
- Mogany, T.; Swalaha, F.M.; Allam, M.; Mtshali, P.S.; Ismail, A.; Kumari, S.; Bux, F. Phenotypic and Genotypic Characterisation of an Unique Indigenous Hypersaline Unicellular Cyanobacterium, Euhalothece sp.nov. Microbiol. Res. 2018, 211, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.M.; Javed, M.A.; Aly Hassan, A.; Sahle-Demessie, E.; Harmon, S. Biodesalination Using Halophytic Cyanobacterium Phormidium Keutzingianum from Brackish to the Hypersaline Water. Chemosphere 2022, 307, 136082. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Paperi, R.; Sili, C. Heavy Metal Sorption by Released Polysaccharides and Whole Cultures of Two Exopolysaccharide-Producing Cyanobacteria. Biodegradation 2007, 18, 181–187. [Google Scholar] [CrossRef]

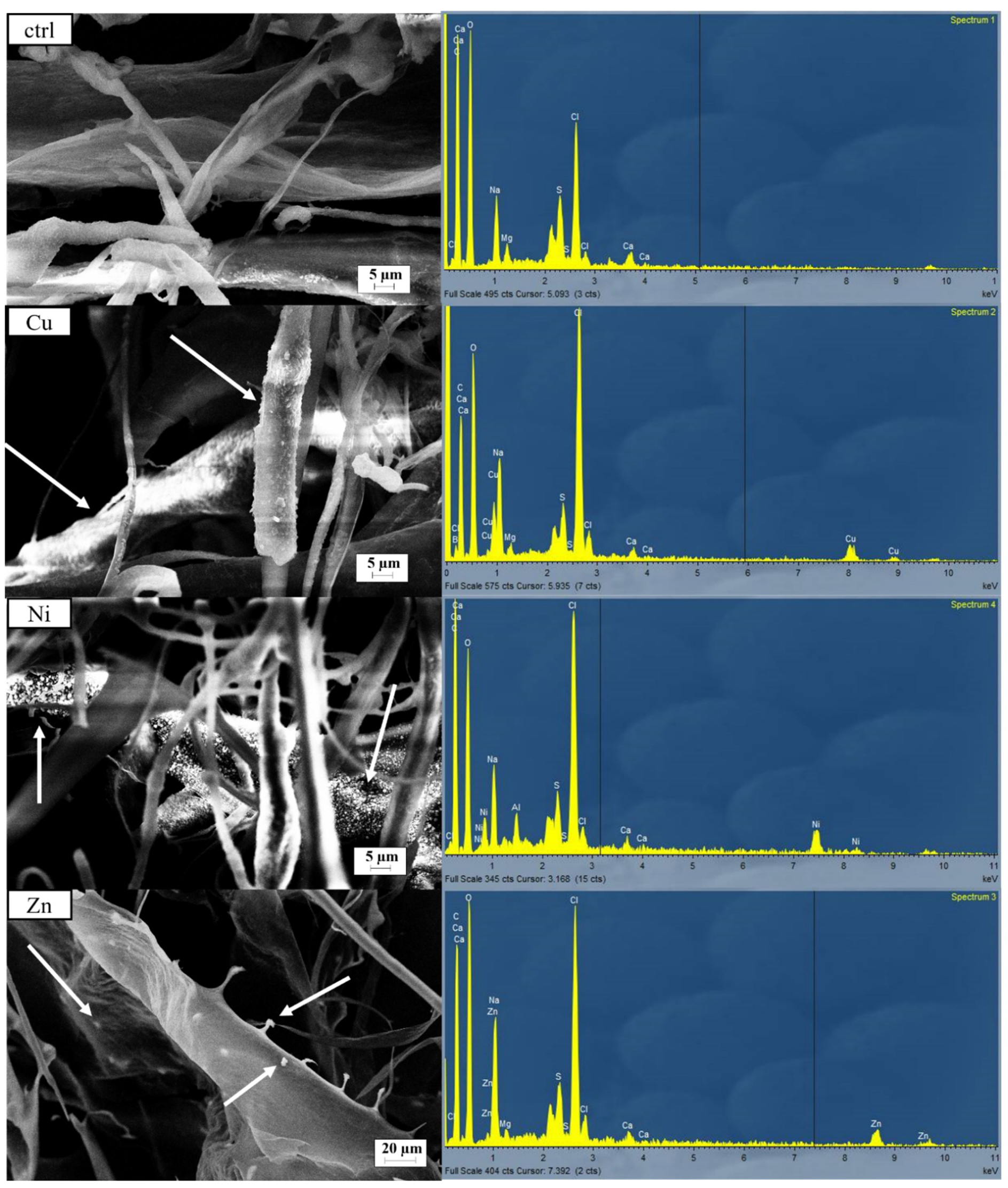
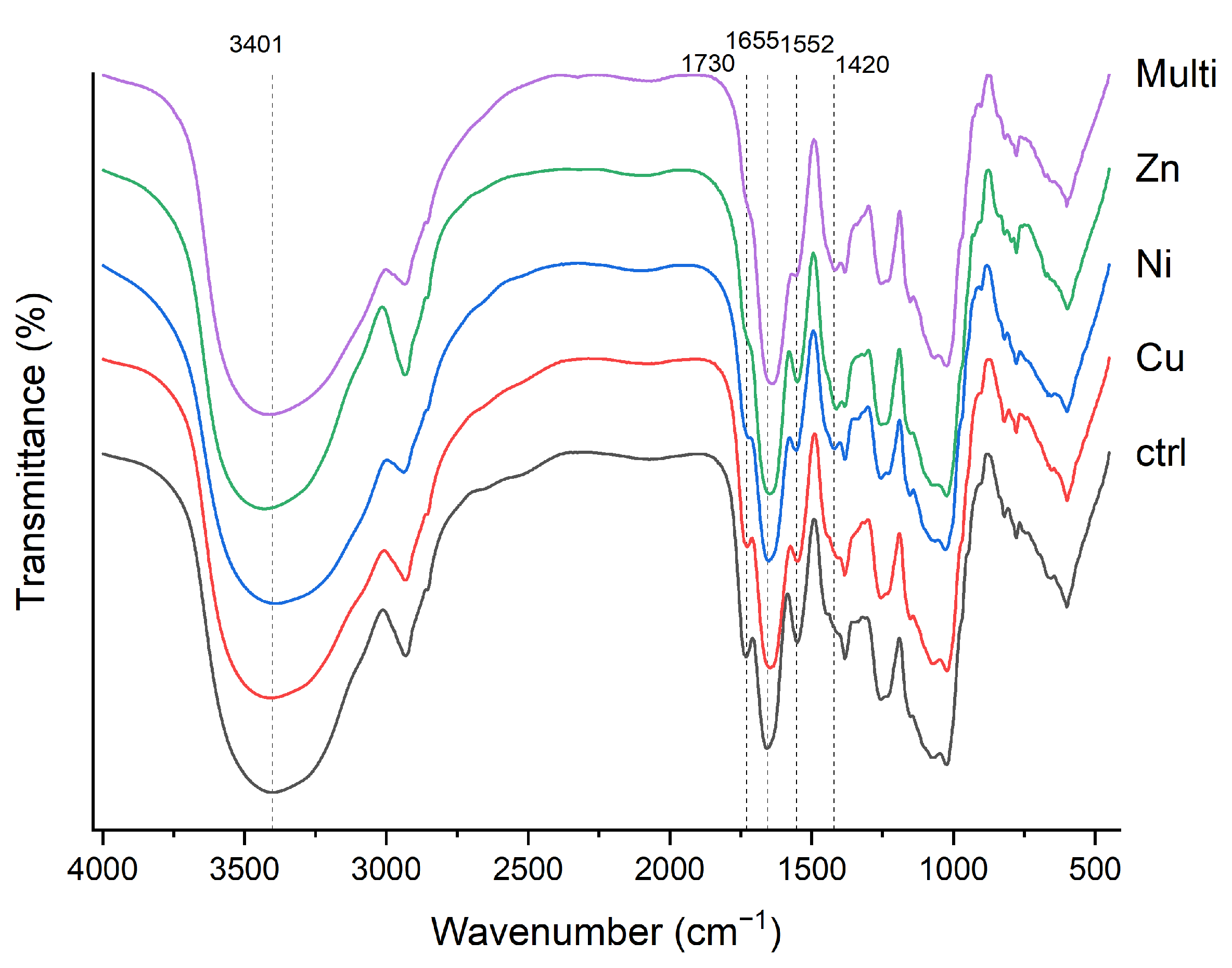
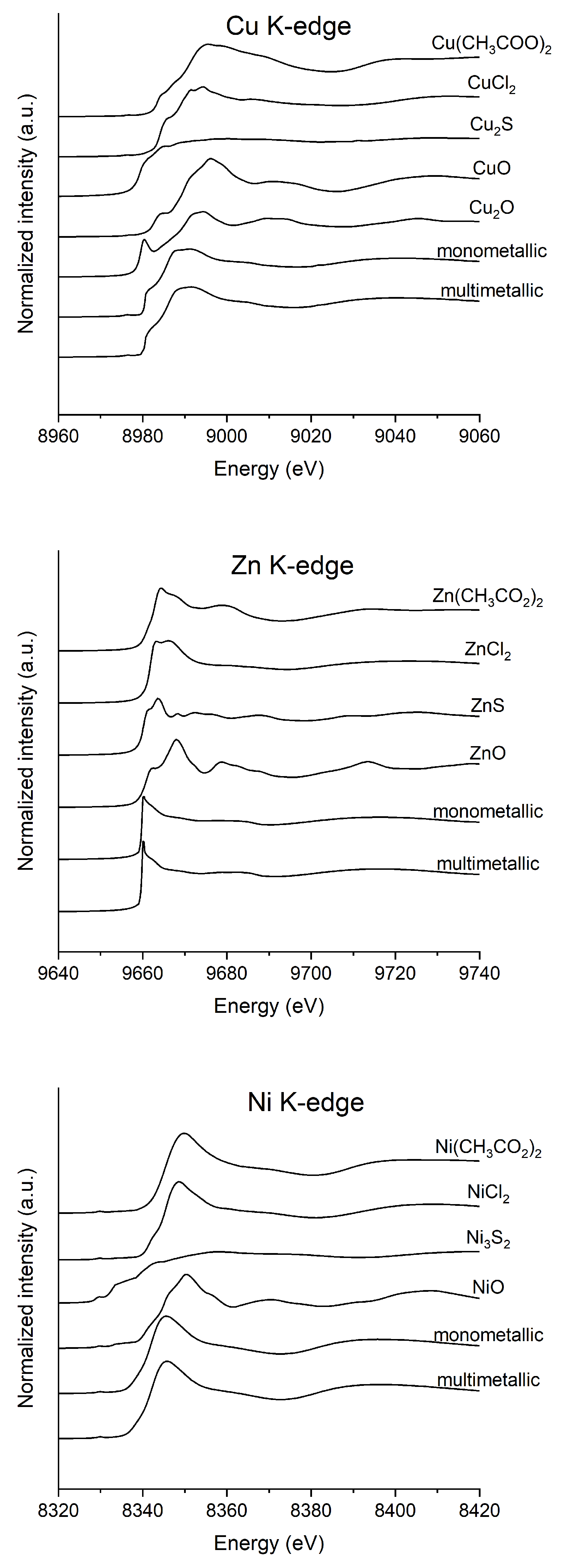
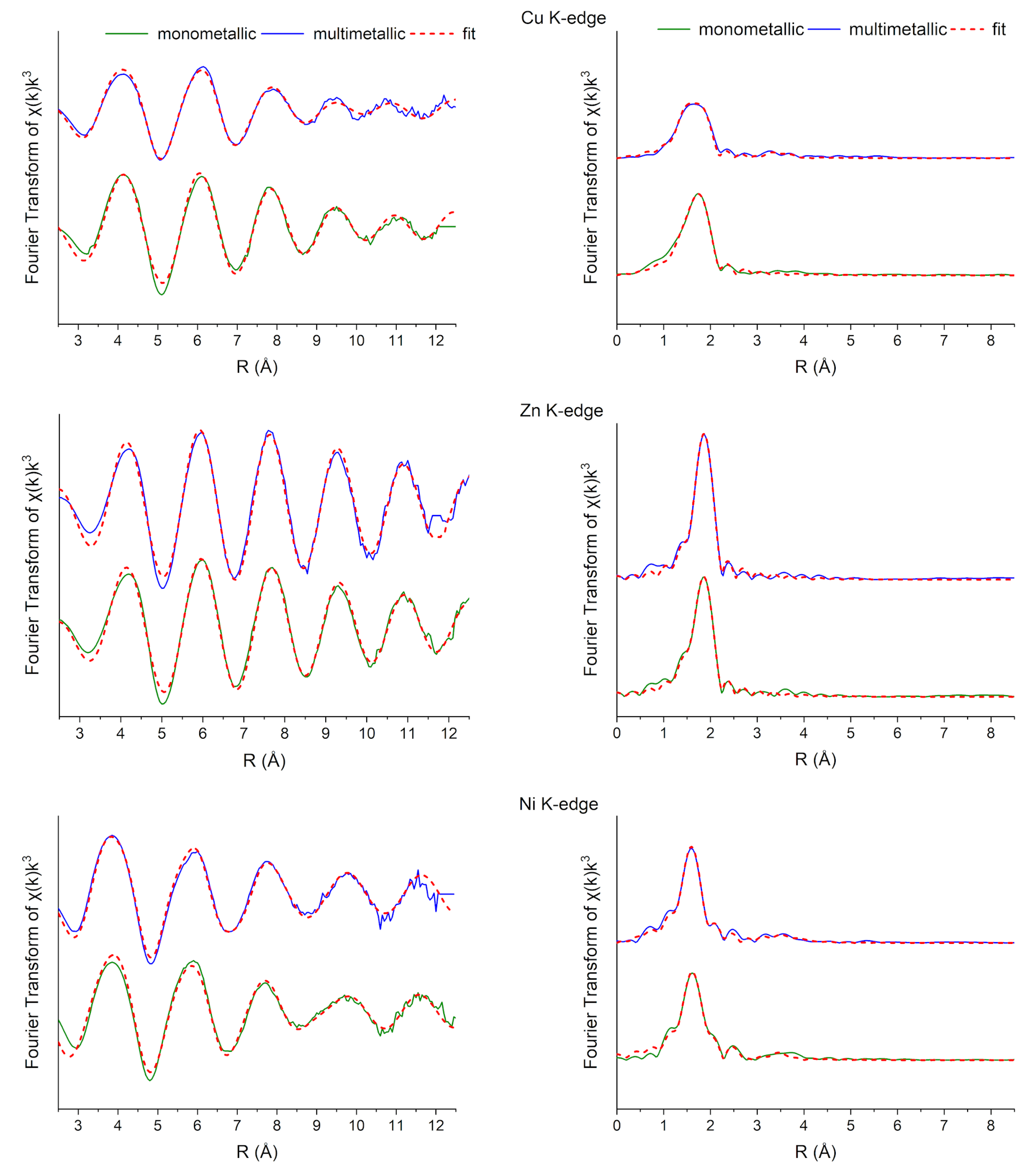
| Cu (%) | Ni (%) | Zn (%) | ||||
|---|---|---|---|---|---|---|
| Average | St. Dev. | Average | St. Dev. | Average | St. Dev. | |
| Ctrl | 0.05 | 0.07 | 0.00 | 0.01 | 0.09 | 0.12 |
| Multi | 5.14 | 0.75 | 3.73 | 0.08 | 3.72 | 0.03 |
| Ni | 0.04 | 0.00 | 7.91 | 1.60 | 0.10 | 0.02 |
| Cu | 9.28 | 0.52 | 0.06 | 0.01 | 0.11 | 0.04 |
| Zn | 0.04 | 0.00 | 0.00 | 0.00 | 5.42 | 0.23 |
| Wave Number (cm−1) | ||||||
|---|---|---|---|---|---|---|
| Ctrl | Cu | Ni | Zn | Multi | Assignment | References |
| 3401 | 3406 | 3871 | 3429 | 3401 | O-H | [37,38] |
| 2929 | 2933 | 2938 | 2933 | 2938 | C-H | [39,40,41] |
| 1730 | 1726 | nd | nd | nd | C=O | [41] |
| 1655 | 1646 | 1651 | 1646 | 1642 | C=O. C-N | [34,38,41] |
| 1552 | 1548 | 1552 | 1552 | 1557 | N-H. C-N | [6,38,44] |
| nd | nd | 1422 | 1412 | 1417 | C-H | [6,42] |
| 1384 | 1384 | 1384 | 1382 | 1382 | C-H. S=O | [6,41,42,43] |
| 1253 | 1256 | 1255 | 1255 | 1256 | C-O | [41,42,43] |
| 1155 | 1152 | 1153 | 1153 | 1153 | C-O-C | [39] |
| 1075 | 1075 | 1075 | 1075 | 1071 | C-O | [42] |
| 1025 | 1025 | 1026 | 1025 | 1026 | C-O-S. C-O-C. C-O. C-C | [41,42,43,44] |
| 821 | 820 | 819 | 818 | 816 | C-O-S. C-O-C. C-O. C-C | [41,42,43,44] |
| 780 | 779 | 778 | 778 | 778 | C-O-S | [41,42,43] |
| 599 | 599 | 599 | 599 | 599 | C-H | [38] |
| Solution | k Range (Å−1) | Path | R (Å) | N | vi | (O, N):(Cl, S) | σ2 (Å−2) | R-Factor |
|---|---|---|---|---|---|---|---|---|
| Cu | 3.0–12.6 | Cu-O | 1.91 (5) | 3.73 | 0.54 | 0.16 (24) | 0.001 (8) | 0.013 |
| Cu-Cl | 2.22 (2) | 3.63 | 0.55 | 0.003 (4) | ||||
| Cu-O | 1.98 (5) | 4.52 | 0.44 | 0.69 (23) | 0.009 (0) | 0.018 | ||
| Cu-S | 2.26 (2) | 5.94 | 0.34 | 0.004 (3) | ||||
| Cu-N | 2.01 (5) | 5.82 | 0.34 | 0.71 (25) | 0.009 (0) | 0.026 | ||
| Cu-S | 2.27 (3) | 6.02 | 0.33 | 0.004 (4) | ||||
| Cu-N | 1.91 (2) | 4.52 | 0.44 | 0.10 (29) | 0.008 (3) | 0.023 | ||
| Cu-Cl | 2.22 (4) | 3.39 | 0.58 | 0.008 (3) | ||||
| Cu (multi) | 2.9–12.3 | Cu-O | 1.93 (3) | 3.93 | 0.51 | 0.34 (24) | 0.003 (4) | 0.008 |
| Cu-Cl | 2.23 (2) | 3.75 | 0.53 | 0.008 (2) | ||||
| Cu-O | 1.96 (2) | 4.34 | 0.46 | 0.71 (40) | 0.008 (3) | 0.016 | ||
| Cu-S | 2.26 (4) | 5.96 | 0.34 | 0.006 (7) | ||||
| Cu-N | 1.91 (4) | 4.60 | 0.43 | 0.19 (29) | 0.001 (7) | 0.017 | ||
| Cu-S | 2.22 (2) | 5.35 | 0.37 | 0.011 (2) | ||||
| Cu-N | 1.94 (2) | 4.85 | 0.41 | 0.26 (17) | 0.001 (3) | 0.009 | ||
| Cu-Cl | 2.22 (2) | 2.67 | 0.54 | 0.008 (1) | ||||
| Zn | 3.2–12.8 | Zn-O | 2.06 (7) | 5.29 | 0.38 | 0.39 (23) | 0.010 (2) | 0.008 |
| Zn-Cl | 2.27 (2) | 4.08 | 0.49 | 0.004 (1) | ||||
| Zn-O | 2.09 (4) | 5.73 | 0.35 | 0.47 (19) | 0.006 (2) | 0.013 | ||
| Zn-S | 2.31 (1) | 3.58 | 0.56 | 0.003 (1) | ||||
| Zn-N | 2.08 (4) | 4.63 | 0.43 | 0.36 (26) | 0.002 (3) | 0.015 | ||
| Zn-S | 2.29 (2) | 3.45 | 0.58 | 0.002 (1) | ||||
| Zn-N | 1.98 (12) | 3.57 | 0.56 | 0.12 (32) | 0.001 (11) | 0.010 | ||
| Zn-Cl | 2.26 (1) | 3.88 | 0.52 | 0.005 (2) | ||||
| Zn (multi) | 3.2–12.8 | Zn-Cl | 2.27 (0) | 3.97 | 0.49 | 0.00 | 0.005 (0) | 0.002 |
| Zn-S | 2.28 (0) | 4.40 | 0.60 | 0.00 | 0.005 (0) | 0.003 | ||
| Ni | 2.8–12.0 | Ni-O | 2.07 (2) | 6.01 | 0.33 | 0.93 (10) | 0.007 (2) | 0.007 |
| Ni-Cl | 2.40 (3) | 5.54 | 0.36 | 0.001 (8) | ||||
| Ni-O | 2.07 (2) | 5.98 | 0.33 | 0.94 (9) | 0.007 (1) | 0.007 | ||
| Ni-S | 2.40 (3) | 7.10 | 0.28 | 0.001 (9) | ||||
| Ni-N | 2.06 (2) | 6.30 | 0.32 | 0.57 (19) | 0.003 (2) | 0.008 | ||
| Ni-Cl | 2.32 (2) | 4.47 | 0.45 | 0.007 (3) | ||||
| Ni-N | 2.06 (1) | 6.28 | 0.32 | 0.55 (20) | 0.003 (1) | 0.008 | ||
| Ni-S | 2.33 (2) | 5.76 | 0.35 | 0.009 (4) | ||||
| Ni (multi) | 2.8–12.0 | Ni-O | 2.06 (2) | 5.87 | 0.34 | 0.96 (9) | 0.007 (1) | 0.005 |
| Ni-Cl | 2.41 (3) | 5.77 | 0.35 | 0.000 (9) | ||||
| Ni-O | 2.07 (2) | 5.97 | 0.34 | 0.99 (8) | 0.008 (1) | 0.006 | ||
| Ni-S | 2.43 (3) | 7.54 | 0.27 | 0.018 (11) | ||||
| Ni-N | 2.06 (1) | 6.14 | 0.33 | 0.69 (14) | 0.003 (2) | 0.004 | ||
| Ni-Cl | 2.33 (2) | 4.57 | 0.44 | 0.007 (3) | ||||
| Ni-N | 2.06 (1) | 6.13 | 0.33 | 0.68 (17) | 0.003 (5) | 0.004 | ||
| Ni-S | 2.34 (4) | 5.91 | 0.34 | 0.008 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciani, M.; Lepore, G.O.; Puri, A.; Facchetti, G.; Adessi, A. Exploring Metal Interactions with Released Polysaccharides from Cyanothece sp. CE4: A Chemical and Spectroscopic Study on Biosorption Mechanism. Polymers 2025, 17, 371. https://doi.org/10.3390/polym17030371
Ciani M, Lepore GO, Puri A, Facchetti G, Adessi A. Exploring Metal Interactions with Released Polysaccharides from Cyanothece sp. CE4: A Chemical and Spectroscopic Study on Biosorption Mechanism. Polymers. 2025; 17(3):371. https://doi.org/10.3390/polym17030371
Chicago/Turabian StyleCiani, Matilde, Giovanni Orazio Lepore, Alessandro Puri, Giorgio Facchetti, and Alessandra Adessi. 2025. "Exploring Metal Interactions with Released Polysaccharides from Cyanothece sp. CE4: A Chemical and Spectroscopic Study on Biosorption Mechanism" Polymers 17, no. 3: 371. https://doi.org/10.3390/polym17030371
APA StyleCiani, M., Lepore, G. O., Puri, A., Facchetti, G., & Adessi, A. (2025). Exploring Metal Interactions with Released Polysaccharides from Cyanothece sp. CE4: A Chemical and Spectroscopic Study on Biosorption Mechanism. Polymers, 17(3), 371. https://doi.org/10.3390/polym17030371










