Biodegradable Film Is Enriched with Pomegranate Seed Oil and Microalgae for Preservation of Cajarana (Spondias dulcis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Film Solutions
2.3. Experimental Design
2.4. Characterization of Films
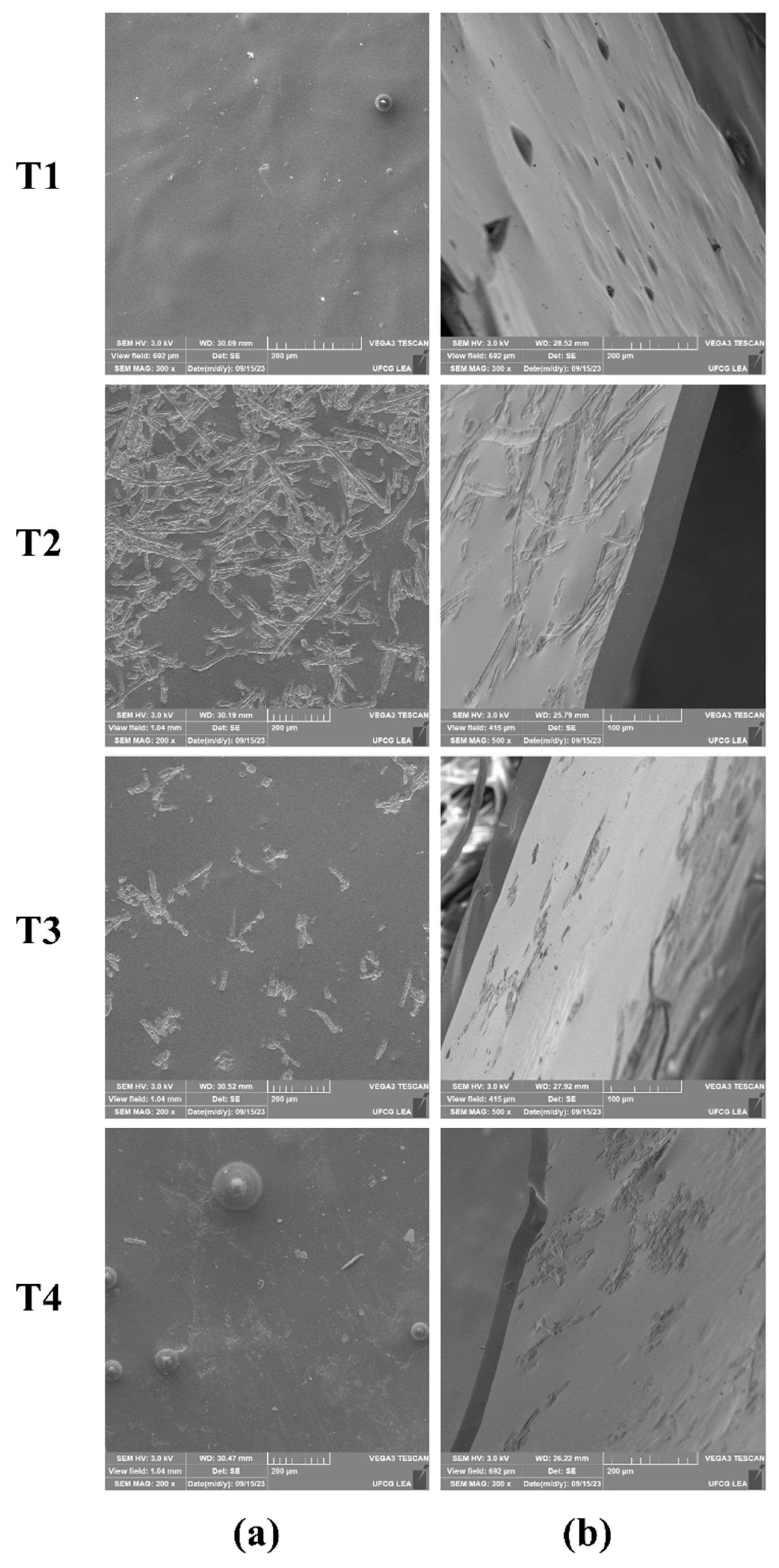
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Thickness
2.4.3. Solubility
2.4.4. Films Color Parameters
2.4.5. Water Vapor Permeability (WVP)
2.4.6. Mechanical Tests
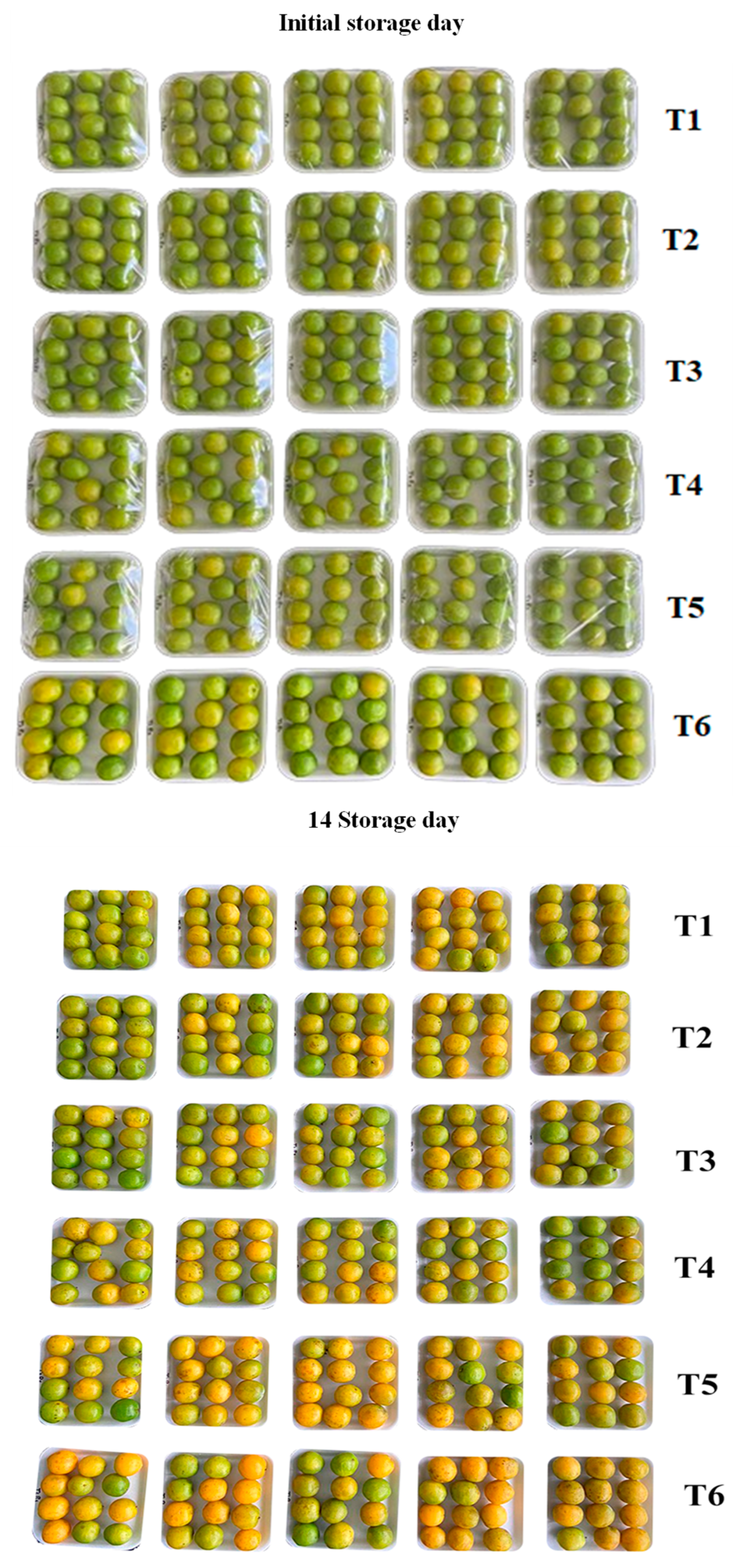
2.5. Treatments and Application of Films on Cajarana Fruits (Spondias dulcis)
2.5.1. External Appearance
2.5.2. Loss of Fresh Mass
2.5.3. Shell Coloration
2.5.4. Soluble Solids, Titratable Acidity, SS/TA Ratio, and pH
2.5.5. Vitamin C
2.5.6. Total Sugars
2.6. Statistical Analysis
3. Results and Discussion
3.1. Visual Appearance and Morphological Characteristics
3.2. Colorimetric Parameters of the Films
3.3. Thickness, Water Vapor Permeability and Solubility
3.4. Mechanical Tests
3.5. Application of Films on Cajarana Fruits (Spondias dulcis)
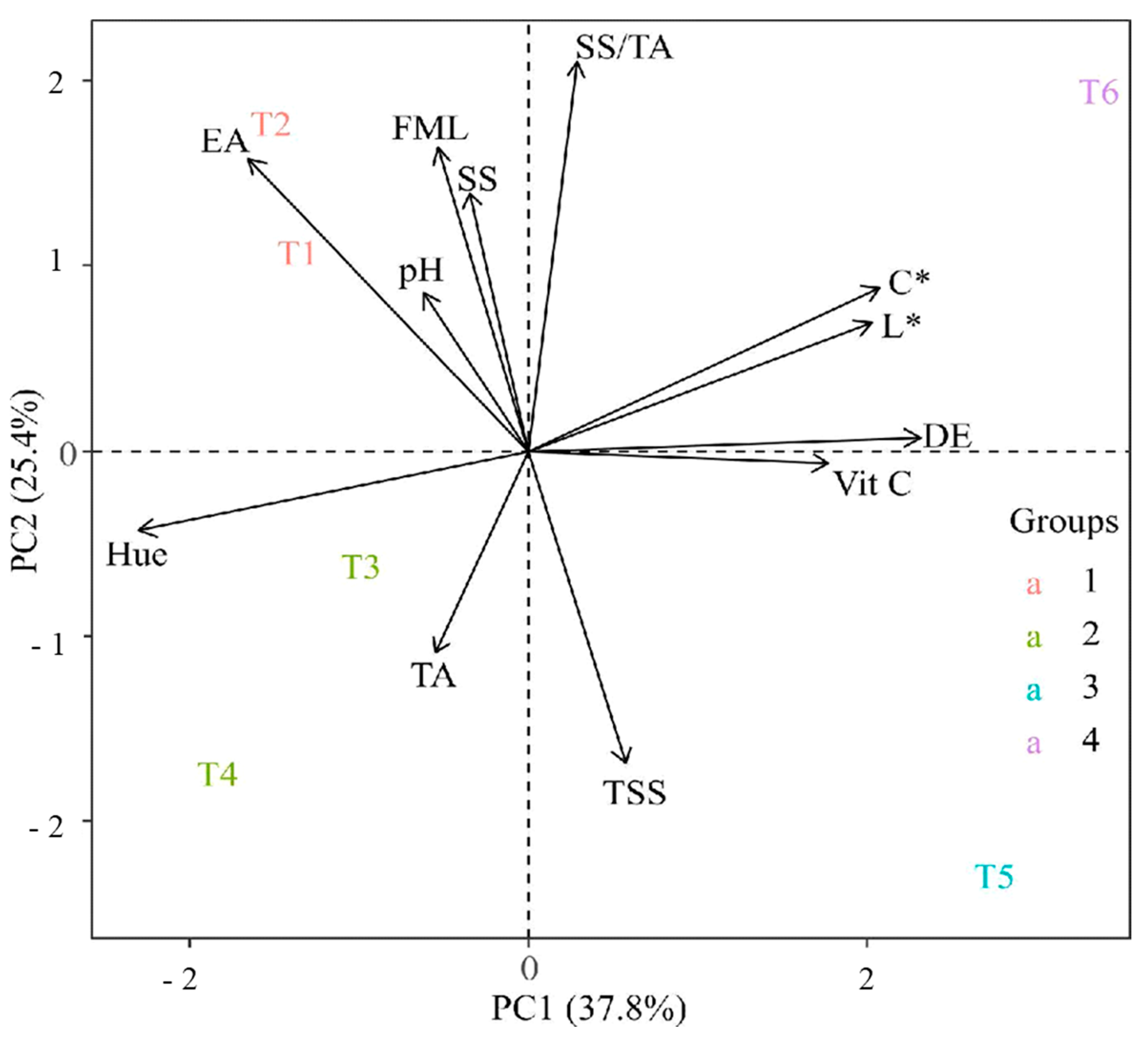
3.6. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseini, S.F.; Mousavi, Z.; McClements, D.J. Beeswax: A review on the recent progress in developing superhydrophobic films/coatings and their applications in fruit preservation. Food Chem. 2023, 424, 136404. [Google Scholar] [CrossRef] [PubMed]
- Thakwani, Y.; Karwa, A.; Bg, P.K.; Purkait, M.K.; Changmai, M. A composite starch-date seeds extract based biodegradable film for food packaging application. Food Biosci. 2023, 54, 102818. [Google Scholar] [CrossRef]
- Kupervaser, M.G.; Traffano-Schiffo, M.V.; Dellamea, M.L.; Flores, S.K.; Sosa, C.A. Trends in starch-based edible films and coatings enriched with tropical fruits extracts: A review. Food Hydrocolloids Health 2023, 4, 100138. [Google Scholar] [CrossRef]
- Pedreiro, S.; Figueirinha, A.; Silva, A.S.; Ramos, F. Bioactive edible films and coatings based on gums and starch: Phenolic enrichment and food application. Coatings 2021, 11, 1393. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Łopusiewicz, Ł.; Biswas, D.; Chandel, V.; Rhim, J.W. Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: A review. Int. J. Biolog. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Shabbir, M.A.; Khan, M.R.; Saeed, M.; Pasha, I.; Khalil, A.A.; Siraj, N. Punicic acid: A striking health substance to combat metabolic syndromes in humans. Lipids Health Disease 2017, 16, 99. [Google Scholar] [CrossRef]
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; Sahafi, S.M. Formulation and characterization of novel nanostructured lipid carriers made from beeswax, propolis wax and pomegranate seed oil. Food Chem. 2018, 244, 83–92. [Google Scholar] [CrossRef]
- Oliveira, A.M.F.; Araújo, R.H.C.R.; de Araújo Alves, K.; Onias, E.A.; Candeia, R.A.; Lopes, M.F.; de Lima, J.F.; Barbosa, L.S.; Dias, G.A. Composition of fatty acids and antioxidant activity of pomegranate seed oil cv. ‘Molar’. J. Exp. Agric. Int. 2019, 37, 1–9. [Google Scholar] [CrossRef]
- Teodosio, A.E.M.M.; Araújo, R.H.C.R.; Santos, B.G.F.L.; Linné, J.A.; da Silva Medeiros, M.L.; Onias, E.A.; de Lima, J.F. Effects of edible coatings of Chlorella sp. containing pomegranate seed oil on quality of Spondias tuberosa fruit during cold storage. Food Chem. 2021, 338, 127916. [Google Scholar] [CrossRef]
- Liu, N.; Ren, G.; Faiza, M.; Li, D.; Cui, J.; Zhang, K.; Zhao, M. Comparison of conventional and green extraction methods on oil yield, physicochemical properties, and lipid compositions of pomegranate seed oil. J. Food Comp. Analysis 2022, 114, 104747. [Google Scholar] [CrossRef]
- Teodosio, A.E.M.D.M.; Onias, E.A.; Oliveira, L.M.D.; Rodrigues, M.H.B.S.; Ribeiro, J.A.; Queiroga, T.B.; Santos, B.G.F.L. Influence of different coatings on quality and shelf-life of guava under different storage temperature. J. Exp. Agric. Int. 2018, 26, 1–10. [Google Scholar] [CrossRef]
- Teodosio, A.E.M.; Araujo, R.H.; de Lima, J.F.; Onias, E.A.; Ferreira, A.P.; Santos, B.G.; de Silva, K.G. Effect of the biodegradable coatings based on microalgae and oil of the seed of the pomegranate in the conservation post-harvest of the papaya ‘golden’. J. Agric. Sci. 2018, 10, 367–377. [Google Scholar] [CrossRef]
- Costa, A.M.M.; Silva, L.O.; Torres, A.G. Chemical composition of commercial cold-pressed pomegranate (Punica granatum) seed oil from Turkey and Israel, and the use of bioactive compounds for samples’ origin preliminary discrimination. J. Food Comp. Analysis 2019, 75, 8–16. [Google Scholar] [CrossRef]
- Sogut, E.; Balqis, A.I.; Hanani, Z.N.; Seydim, A.C. The properties of κ-carrageenan and whey protein isolate blended films containing pomegranate seed oil. Polym. Test. 2019, 77, 105886. [Google Scholar] [CrossRef]
- Durmaz, Y.; Kilicli, M.; Toker, O.S.; Konar, N.; Palabiyik, I.; Tamtürk, F. Using spray-dried microalgae in ice cream formulation as a natural colorant: Effect on physicochemical and functional properties. Algal Res. 2020, 47, 101811. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Techno-functional properties of microalgae in food products. In Handbook of Food and Feed from Microalgae, 1st ed.; Jacob-Lopes, E., Queiroz, M.I., Maroneze, M.M., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 1, pp. 293–304. [Google Scholar] [CrossRef]
- Bernaerts, T.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Oliveira, L.M.; de Oliveira, Á.M.F.; Araújo, R.H.C.R.; Dias, G.A.; de Medeiros Teodósio, A.E.M.; de Lima, J.F.; Barbosa, L.S.; Guedes, W.A. Spirulina platensis coating for the conservation of pomegranate. AIMS Agric. Food 2020, 5, 76–85. [Google Scholar] [CrossRef]
- Teodosio, A.E.M.D.M.; Araujo, R.H.C.R.; Santos, B.G.F.L.; Linne, J.A.; Silva, K.G.D.; Gomes, F.A.L.; Souza, G.L.F.; Lima, J.F.D. Analysis of bioactive compounds in umbu (Spondias tuberosa) by application of edible coating based on Chlorella sp during storage. Food Sci. Technol. 2020, 40, 756–760. [Google Scholar] [CrossRef]
- Oliveira, A.M.F.; de Araújo, R.H.C.R.; da Silva, T.I.; da Cruz, M.C.M.; dos Santos, V.F.; Medeiros, E.A.A.; de Sousa, M.M.; Alves, K.A.; Soares, N.F.F.; de Lima, J.F.; et al. Characterization of individual microalgae subparticles and as edible coatings. Food Biophys. 2025, 20, 9. [Google Scholar] [CrossRef]
- Guimarães, A.R.D.; Leão, K.V.; Mapeli, A.M.; Schneider, L.C. Physical and chemical characterization of cajarana fruits (Spondias dulcis Parkinson). Braz. J. Develop. 2020, 6, 6693–6701. (In Portuguese) [Google Scholar] [CrossRef]
- Silva, F.C.D.; Santana, H.A.; Menezes, J.O.S.D.; Tavares, M.C.; Martins, R.D.; Siqueira, A.P.S. Use of indole-3-acetic acid (IAA) in postharvest cajá-manga (Spondias dulcis). Rev. Colombiana Ciências Hortícolas 2020, 14, 201–208. [Google Scholar] [CrossRef]
- Chaves Neto, J.R.C.; Dos Santos, L.F.; Dantas, R.L.; Dantas, A.L.; Schunemann, A.P.P.; Silva, S.D.M. Quality of fruit of caja-hospit accesses during maturation. Bol. Cent. Pesqui. E Process. Aliment. 2018, 36, 39–54. (In Portuguese) [Google Scholar] [CrossRef]
- Sinan, K.I.; Zengin, G.; Zheleva-Dimitrova, D.; Gevrenova, R.; Picot-Allain, M.C.N.; Dall’Acqua, S.; Mahomoodally, M.F. Exploring the chemical profiles and biological values of two Spondias species (S. dulcis and S. mombin): Valuable sources of bioactive natural products. Antioxidants 2021, 10, 1771. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.P.; de Medeiros, E.S.; Fernandes, F.A.; da Silva, V.F.; de Morais, A.R. Adjustment of non-linear models to describe the cajá-manga fruit. Sigmae 2019, 8, 221–226. (In Portuguese) [Google Scholar]
- Zhou, Y.; Zhong, Y.; Li, L.; Jiang, K.; Gao, J.; Zhong, K.; Yan, B. A multifunctional chitosan-derived conformal coating for the preservation of passion fruit. LWT 2022, 163, 113584. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.Y.; Li, W. Preparation and characterization of multilayer films composed of chitosan, sodium alginate and carboxymethyl chitosan-ZnO nanoparticles. Food Chem. 2019, 283, 397–403. [Google Scholar] [CrossRef]
- Pace, B.; Cefola, M. Innovative preservation technology for fresh fruit and vegetables. Foods 2021, 10, 719. [Google Scholar] [CrossRef]
- Liu, W.; Kang, S.; Zhang, Q.; Chen, S.; Yang, Q.; Yan, B. Self-assembly fabrication of chitosan-tannic acid/MXene composite film with excellent antibacterial and antioxidant properties for fruit preservation. Food Chem. 2023, 410, 135405. [Google Scholar] [CrossRef]
- Lima, J.F.; Queiroz, A.J.D.M.; Maria, R.; De Figueirêdo, F.; Araújo, R.H.C.R.; De Souza, W.R.; Maciel, A.P. Enhancement of lipid accumulation in Chlorella sp. Farming culture held in open photobioreactors. Int. J. Eng. Sci. 2018, 7, 88–99. [Google Scholar]
- Espitia, P.J.P.; Soares, N.D.F.F.; Teófilo, R.F.; dos Reis Coimbra, J.S.; Vitor, D.M.; Batista, R.A.; Medeiros, E.A.A. Physical–mechanical and antimicrobial properties of nanocomposite films with pediocin and ZnO nanoparticles. Carbohydrate Polym. 2013, 94, 199–208. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Li, X.; Xu, Y.; Ma, X.; Shi, R.; Mu, C. Development of active rosmarinic acid-gelatin biodegradable films with antioxidant and long-term antibacterial activities. Food Hydrocolloids 2018, 83, 308–316. [Google Scholar] [CrossRef]
- ASTM E96-95; Standard Test Methods for Water Vapor Transmission of Materials. ASTM: West Conshohocken, PA, USA, 2004.
- Souza, C.O.; Veiga-Santos, P.; Druzian, J.I. Natural ingredients as additive for active antioxidant food packaging. In Food Quality, Safety and Technology, 1st ed.; Lima, G., Vianello, F., Eds.; Springer: Vienna, Austria, 2013; pp. 179–188. [Google Scholar] [CrossRef]
- ASTM D882-12; ASTM International. Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2012.
- ASTM F1306-90; ASTM—American Society for Testing and Materials. Standard Test Method for Slow Rate Penetration Resistance of Flexible Barrier Films and Laminates. ASTM: West Conshohocken, PA, USA, 1994.
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2006. [Google Scholar]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. B. J. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- The R Core Team. R: A Language and Environment for Statistical Computing, Version 4.4.2; The R Core Team: Vienna, Austria, 2022; Available online: https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf (accessed on 18 December 2024).
- Kawabata, K.; Totani, M.; Kawaguchi, D.; Matsuno, H.; Tanaka, K. Two-dimensional cellular patterning on a polymer film based on interfacial stiffness. Langmuir 2022, 37, 14911–14919. [Google Scholar] [CrossRef]
- Parlak, M.E.; Uzuner, K.; Kirac, F.T.; Ozdemir, S.; Dundar, A.N.; Sahin, O.I.; Saricaoglu, F.T. Production and characterization of biodegradable bi-layer films from poly(lactic) acid and zein. Int. J. Biolog. Macromol. 2023, 227, 1027–1037. [Google Scholar] [CrossRef]
- Silva, V.D.M.; Macedo, M.C.C.; Rodrigues, C.G.; dos Santos, A.N.; Loyola, A.C.D.F.; Fante, C.A. Biodegradable edible films of ripe banana peel and starch enriched with extract of Eriobotrya japonica leaves. Food Biosci. 2020, 38, 100750. [Google Scholar] [CrossRef]
- Han Lyn, F.; Tan, C.P.; Zawawi, R.M.; Nur Hanani, Z.A. Enhancing the mechanical and barrier properties of chitosan/graphene oxide composite films using trisodium citrate and sodium tripolyphosphate crosslinkers. J. Appl. Polym. Sci. 2021, 138, 50618. [Google Scholar] [CrossRef]
- Mehboob, S.; Ali, T.M.; Sheikh, M.; Hasnain, A. Effects of cross-linking and/or acetylation on sorghum starch and film characteristics. Int. J. Biolog. Macromol. 2020, 155, 786–794. [Google Scholar] [CrossRef]
- Jaderi, Z.; Tabatabaee Yazdi, F.; Mortazavi, S.A.; Koocheki, A. Effects of glycerol and sorbitol on a novel biodegradable edible film based on Malva sylvestris flower gum. Food Sci. Nutrition 2023, 11, 991–1000. [Google Scholar] [CrossRef]
- Akhila, V.; Badwaik, L.S. Recent advancement in improvement of properties of polysaccharides and proteins based packaging film with added nanoparticles: A review. Int. J. Biolog. Macromol. 2022, 203, 515–525. [Google Scholar] [CrossRef]
- Tong, W.Y.; Rafiee, A.R.A.; Leong, C.R.; Tan, W.N.; Dailin, D.J.; Almarhoon, Z.M.; Chuah, L.F. Development of sodium alginate-pectin biodegradable active food packaging film containing cinnamic acid. Chemosphere 2023, 336, 139212. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.D.S.; Flôres, S.H.; Brandelli, A.; Vargas, C.G.; Ritter, A.C.; da Cruz Rodrigues, A.M.; da Silva, L.H.M. Development and properties of biodegradable film from peach palm (Bactris gasipaes). Food Res. Int. 2023, 173, 113172. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.N.T.; Nguyen, H.T.; Le, N.L.; Khoi, T.T.; Richel, A. Biodegradable films from dragon fruit (Hylocereus polyrhizus) peel pectin and potato starches cross-linked with glutaraldehyde. Food Packag. Shelf Life 2023, 37, 101084. [Google Scholar] [CrossRef]
- Paudel, S.; Regmi, S.; Janaswamy, S. Effect of glycerol and sorbitol on cellulose-based biodegradable films. Food Packag. Shelf Life 2023, 37, 101090. [Google Scholar] [CrossRef]
- Hiremani, V.D.; Khanapure, S.; Gasti, T.; Goudar, N.; Vootla, S.K.; Masti, S.P.; Chougale, R.B. Preparation and physicochemical assessment of bioactive films based on chitosan and starchy powder of white turmeric rhizomes (Curcuma zedoaria) for green packaging applications. Int. J. Biolog. Macromol. 2021, 193, 2192–2201. [Google Scholar] [CrossRef]
- Schaefer, E.W.; Pavoni, J.M.F.; Luchese, C.L.; Faccin, D.J.L.; Tessaro, I.C. Influence of turmeric incorporation on physicochemical, antimicrobial and mechanical properties of the cornstarch and chitosan films. Int. J. Biolog. Macromol. 2020, 148, 342–350. [Google Scholar] [CrossRef]
- Khan, M.R.; Vapenka, L.; Sadiq, M.B.; Torrieri, E.; Rajchl, A. Comparative influence of active PLA and PP films on the quality of minimally processed cherry tomatoes. Food Packag. Shelf Life 2024, 44, 101313. [Google Scholar] [CrossRef]
- Salazar, A.S.S.; Cavazos, P.A.S.; Paz, H.M.; Fragoso, A.V. External factors and nanoparticles effect on water vapor permeability of pectin-based films. J. Food Eng. 2019, 245, 73–79. [Google Scholar] [CrossRef]
- Shaikh, M.; Haider, S.; Ali, T.M.; Hasnain, A. Physical, thermal, mechanical and barrier properties of pearl millet starch films as affected by levels of acetylation and hydroxypropylation. Int. J. Biolog. Macromol. 2019, 124, 209–219. [Google Scholar] [CrossRef]
- Turan, D. Water vapor transport properties of polyurethane films for packaging of respiring foods. Food Eng. Rev. 2021, 13, 54–65. [Google Scholar] [CrossRef]
- González Sandoval, D.C.; Luna Sosa, B.; Martínez-Ávila, G.C.G.; Rodríguez Fuentes, H.; Avendaño Abarca, V.H.; Rojas, R. Formulation and Characterization of Edible Films Based on Organic Mucilage from Mexican Opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef]
- Lin, X.; Chen, S.; Wang, R.; Li, C.; Wang, L. Fabrication, characterization and biological properties of pectin and/or chitosan-based films incorporated with noni (Morinda citrifolia) fruit extract. Food Hydrocoll. 2023, 134, 108025. [Google Scholar] [CrossRef]
- Luna-Sosa, B.; Martínez-Ávila, G.C.; Rodríguez-Fuentes, H.; Azevedo, A.G.; Pastrana, L.M.; Rojas, R.; Cerqueira, M.A. Pectin-based films loaded with hydroponic nopal mucilages: Development and physicochemical characterization. Coatings 2020, 10, 467. [Google Scholar] [CrossRef]
- Carissimi, M.; Flôres, S.H.; Rech, R. Effect of microalgae addition on active biodegradable starch film. Algal Res. 2018, 32, 201–209. [Google Scholar] [CrossRef]
- Pirsa, S.; Mohtarami, F. Biodegradable film based on barley sprout powder/pectin modified with quercetin and V2O5 nanoparticles: Investigation of physicochemical and structural properties. Heliyon 2024, 10, e2024. [Google Scholar]
- Gonçalves, S.M.; Dos Santos, D.C.; Motta, J.F.G.; Dos Santos, R.R.; Chávez, D.W.H.; de Melo, N.R. Structure and functional properties of cellulose acetate films incorporated with glycerol. Carbohydr. Polym. 2019, 209, 190–197. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current advancements in pectin: Extraction, properties and multifunctional applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Jayarathna, P.L.; Jayawardena, J.A.E.; Vanniarachchy, M.P.G. Identification of physical, chemical properties and flavor profile of Spondias dulcis in three maturity stages. Int. Res. J. Adv. Eng. Sci. 2020, 5, 208–211. [Google Scholar]
- Gadelha, T.M.; do Nascimento, A.M.; da Silva, T.I.; da Silva, J.L.; Sales, G.N.B.; Ribeiro, W.S.; Queiroga, R.C.F.; Almeida, F.A.; Souza, P.A.; Ribeiro, J.E.S.; et al. Comprehensive analysis of quality and bioactive compounds in umbu-cajá fruit during different ripening stages. Sci. Hortic. 2024, 327, 112886. [Google Scholar] [CrossRef]
- Freitas, R.V.D.S.; Souza, P.A.D.; Coelho, E.L.; Souza, F.X.D.; Beserra, H.N.B.R. Storage of mombin fruits coated with cassava starch and PVC film. Rev. Caatinga 2017, 30, 244–249. [Google Scholar] [CrossRef]
- Koubala, B.B.; Kansci, G.; Ralet, M.C. Ambarella. In Exotic Fruits; Academic Press: Cambridge, MA, USA, 2018; pp. 15–22. [Google Scholar]
- Oliveira, V.R.L.; Santos, F.K.G.; Leite, R.H.L.; Aroucha, E.M.M.; Silva, K.N.O. Use of biopolymeric coating hydrophobized with beeswax in post-harvest conservation of guavas. Food Chem. 2018, 259, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Guhathakurta, D.; Mathur, M.; Yadav, R.; Gupta, M.; Kumaraguru, P. Tweetboost: Influence of social media on NFT valuation. In Proceedings of the WWW ’22: Companion Proceedings of the Web Conference, 2022, Lyon, France, 25–29 April 2022; pp. 621–629. [Google Scholar] [CrossRef]
- Reges, B.M.; Batista, E.M.; do Nascimento Almeida, É.J.; dos Reis Lemos, L.M.; da Silva, E.F.; de Souza, P.A. Post-harvest of sugar aplle (Annona squamosa L.) covered with PVC. Rev. Verde Agroecol. Desenvol. Sustent. 2018, 13, 445–451. (In Portuguese) [Google Scholar] [CrossRef]
- Gomes, F.R.; Silva, D.F.P.D.; Costa, G.S.; Souza, P.H.M.D.; Silveira-Neto, A.N.D.; Cruz, S.C.S. Calcium carbide in anticipation and standardization of ripening in Cajá-manga fruits. Rev. Bras. Fruticult. 2023, 45, e-139. (In Portuguese) [Google Scholar] [CrossRef]
- Kaynarca, G.B.; Kamer, D.D.A.; Yucel, E.; Yılmaz, O.S.; Henden, Y.; Kaymaz, E.; Gumus, T. The potential of pectin-based films enriched with bioactive components for strawberry preservation: A sustainable and innovative coating. Sci. Horticult. 2024, 334, 113294. [Google Scholar] [CrossRef]
- Alves, T.P.; da Silva, R.A.C.; Santos, N.S.; de Sales Silva, J.C.; Dantas, F.A.L. Post-harvest quality of umbuzeiro fruits (Spondias tuberosa Arruda) stored under modified atmosphere. Diversitas J. 2020, 5, 1523–1535. (In Portuguese) [Google Scholar] [CrossRef]
| Parameters | T1 | T2 | T3 | T4 | CV (%) |
|---|---|---|---|---|---|
| L* | 90.46 ± 0.33 ab | 90.10 ± 0.25 b | 90.68 ± 0.18 ab | 90.72 ± 0.10 a | 0.25 |
| C* | 2.80 ± 0.30 a | 1.66 ± 0.11 b | 2.85 ± 0.12 a | 2.94 ± 0.20 a | 7.73 |
| °h | 129.52 ± 5.60 a | 125.40 ± 2.56 a | 122.63 ± 0.68 a | 122.20 ± 0.57 a | 2.49 |
| Thickness (mm) | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a | - |
| WVP (g·mm/m2·h·kPa) | 0.779 ± 0.001 a | 0.779 ± 0.001 a | 0.782 ± 0.006 a | 0.778 ± 0.002 a | 0.42 |
| Solubility (%) | 92.88 ± 10.50 a | 85.31 ± 13.14 ab | 91.98 ± 3.78 a | 63.17 ± 12.30 b | 12.70 |
| Parameters | T1 | T2 | T3 | T4 | T5 | CV (%) |
|---|---|---|---|---|---|---|
| TS (MPa) | 0.068 ± 0.009 a | 0.062 ± 0.007 a | 0.042 ± 0.004 b | 0.040 ± 0.004 b | 0.0170 ± 0.010 c | 16.72 |
| D (%) | 11.46 ± 0.80 b | 12.45 ± 2.56 b | 9.77 ± 1.10 b | 9.99 ± 2.16 b | 19.23 ± 3.06 a | 16.84 |
| T (MPa) | 0.0059 ± 0.001 ab | 0.0066 ± 0.002 a | 0.0023 ± 0.0008 c | 0.0025 ± 0.0012 c | 0.0028 ± 0.0009 bc | 38.07 |
| ME (MPa) | 1.10 ± 0.11 a | 0.90 ± 0.18 ab | 0.65 ± 0.13 bc | 0.59 ± 0.07 cd | 0.35 ± 0.10 d | 17.46 |
| MTF (N) | 8.82 ± 1.13 a | 8.05 ± 0.86 a | 5.37 ± 0.98 b | 5.22 ± 0.55 b | 2.87 ± 0.22 c | 13.48 |
| Parameters | Cajaranas (Spondias dulcis) |
|---|---|
| L* | 52.38 ± 2.31 |
| C* | 34.14 ± 1.49 |
| h° | 114.90 ± 2.06 |
| External appearance | 4.00 ± 0.00 |
| Weight (g) | 23.88 ± 1.20 |
| Longitudinal diameter (mm) | 33.76 ± 1.11 |
| Cross diameter (mm) | 36.90 ± 1.04 |
| Treatable acidity (TA) (% citric acid) | 1.57 ± 0.21 |
| pH | 1.99 ± 0.11 |
| Total soluble solids (SS) (°Brix) | 12.73 ± 0.24 |
| SS/TA ratio | 8.10 ± 1.18 |
| Ascorbic acid (mg·100 mL−1) | 12.14 ± 1.38 |
| Total sugars (g·100 g−1) | 10.74 ± 1.24 |
| Parameters | T1 | T2 | T3 | T4 | T5 | T6 | CV (%) |
|---|---|---|---|---|---|---|---|
| pH | 2.41 ± 0.08 a | 2.36 ± 0.12 a | 2.34 ± 0.09 a | 2.34 ± 0.05 a | 2.35 ± 0.04 a | 2.34 ± 0.15 a | 3.97 |
| SS | 14.34 ± 0.52 a | 13.20 ± 0.37 cd | 13.42 ± 0.52 bcd | 13.58 ± 0.25 bc | 12.74 ± 0.18 d | 14.04 ± 0.13 ab | 2.69 |
| TA | 1.66 ± 0.090 a | 1.40 ± 0.073 c | 1.53 ± 0.096 abc | 1.60 ± 0.045 ab | 1.56 ± 0.045 ab | 1.47 ± 0.070 bc | 4.73 |
| SS/TA | 8.65 ± 0.63 bc | 9.44 ± 0.57 ab | 8.76 ± 0.35 bc | 8.51 ± 0.28 c | 8.16 ± 0.24 c | 9.58 ± 0.42 a | 4.96 |
| TS | 10.10 ± 0.51 a | 9.28 ± 0.33 ab | 10.63 ± 0.55 ab | 11.05 ± 1.24 b | 10.67 ± 0.85 ab | 10.59 ± 0.58 ab | 7.0 |
| LFM | 7.24 ± 0.31 b | 6.99 ± 0.20 b | 7.22 ± 0.37 b | 7.32 ± 0.42 b | 2.67 ± 0.08 c | 9.19 ± 0.97 a | 7.14 |
| AA | 8.29 ± 0.35 b | 8.11 ± 0.66 b | 8.17 ± 0.36 b | 8.67 ± 0.40 b | 8.63 ± 0.51 ab | 9.20 ± 0.39 a | 5.39 |
| °h | 103.38 ± 2.53 bc | 106.12 ± 3.71 ab | 108.13 ± 1.96 ab | 109.14 ± 1.69 a | 97.85 ± 4.23 cd | 95.87 ± 2.22 d | 2.78 |
| C* | 31.40 ± 0.56 ab | 31.07 ± 1.20 ab | 31.14 ± 0.56 ab | 29.65 ± 0.82 b | 32.05 ± 0.37 a | 32.67 ± 1.49 a | 3.41 |
| L* | 51.62 ± 1.11 ab | 51.22 ± 1.05 ab | 51.19 ± 0.67 ab | 49.82 ± 1.19 b | 52.35 ± 1.18 a | 52.43 ± 1.08 a | 2.12 |
| CD | 7.20 ± 1.33 b | 6.31 ± 1.29 b | 5.05 ± 0.78 b | 6.06 ± 0.85 b | 11.06 ± 1.27 a | 11.54 ± 1.10 a | 14.28 |
| EA | 3.40 ± 0.28 a | 3.28 ± 0.23 ab | 2.68 ± 0.30 abc | 2.80 ± 0.42 bc | 2.20 ± 0.40 c | 2.60 ± 0.45 bc | 12.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, K.A.; Araújo, R.H.C.R.; Silva, A.S.; Almeida, E.S.; Oliveira, Á.M.F.; Rocha, N.S.; Araújo, M.C.; Gusmão, T.A.S.; Lima, J.F.; Delgado, J.M.P.Q.; et al. Biodegradable Film Is Enriched with Pomegranate Seed Oil and Microalgae for Preservation of Cajarana (Spondias dulcis). Polymers 2025, 17, 367. https://doi.org/10.3390/polym17030367
Alves KA, Araújo RHCR, Silva AS, Almeida ES, Oliveira ÁMF, Rocha NS, Araújo MC, Gusmão TAS, Lima JF, Delgado JMPQ, et al. Biodegradable Film Is Enriched with Pomegranate Seed Oil and Microalgae for Preservation of Cajarana (Spondias dulcis). Polymers. 2025; 17(3):367. https://doi.org/10.3390/polym17030367
Chicago/Turabian StyleAlves, Kalinny A., Railene H. C. R. Araújo, Adriano S. Silva, Evanilson S. Almeida, Ágda M. F. Oliveira, Nayara S. Rocha, Max C. Araújo, Thaisa A. S. Gusmão, José F. Lima, João M. P. Q. Delgado, and et al. 2025. "Biodegradable Film Is Enriched with Pomegranate Seed Oil and Microalgae for Preservation of Cajarana (Spondias dulcis)" Polymers 17, no. 3: 367. https://doi.org/10.3390/polym17030367
APA StyleAlves, K. A., Araújo, R. H. C. R., Silva, A. S., Almeida, E. S., Oliveira, Á. M. F., Rocha, N. S., Araújo, M. C., Gusmão, T. A. S., Lima, J. F., Delgado, J. M. P. Q., Pereira, J. F., Santos, R. S., & Lima, A. G. B. (2025). Biodegradable Film Is Enriched with Pomegranate Seed Oil and Microalgae for Preservation of Cajarana (Spondias dulcis). Polymers, 17(3), 367. https://doi.org/10.3390/polym17030367










