Enhanced Sugar and Bioethanol Production from Broom Grass via NaOH-Autoclave Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.3. Pretreatment Procedure for Broom Grass Raw Material
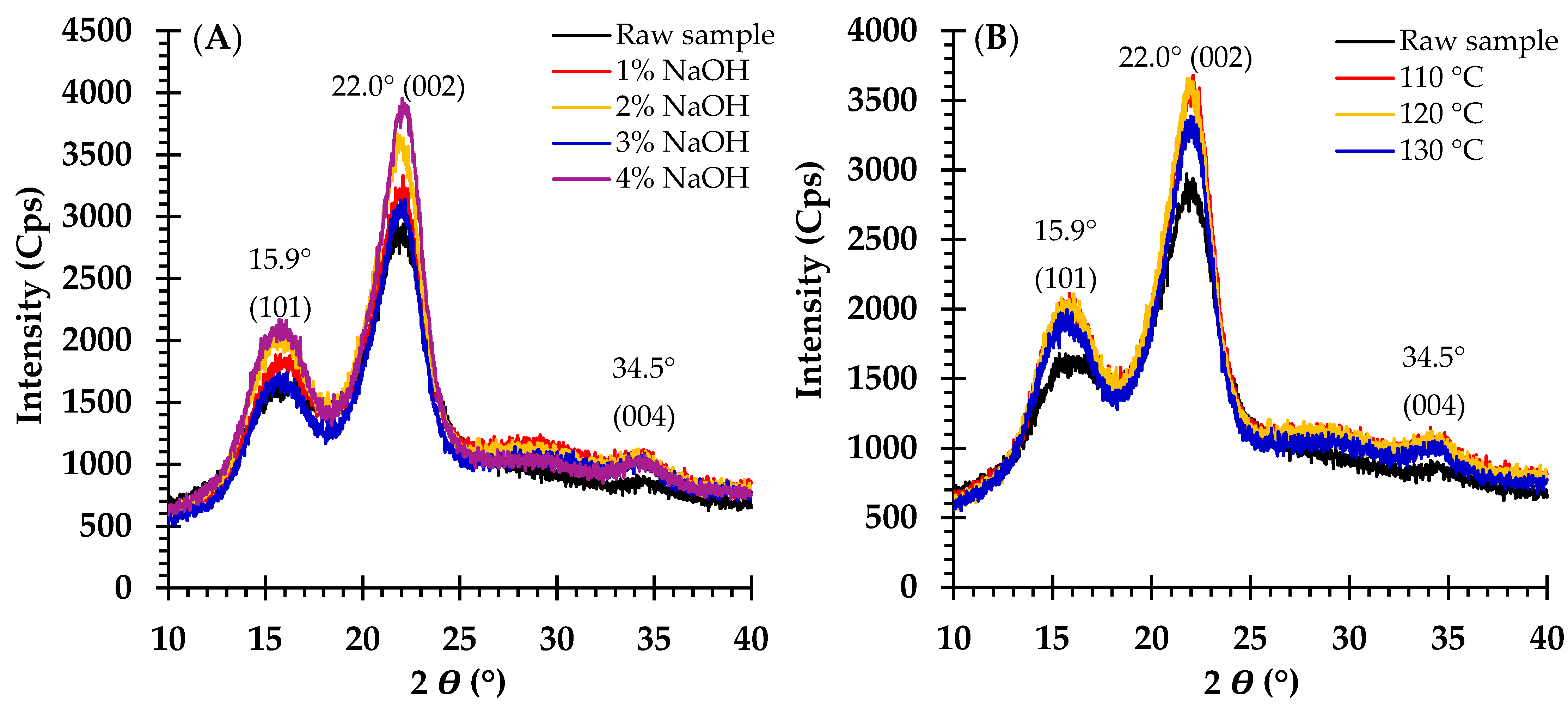
2.4. Crystallinity Analysis
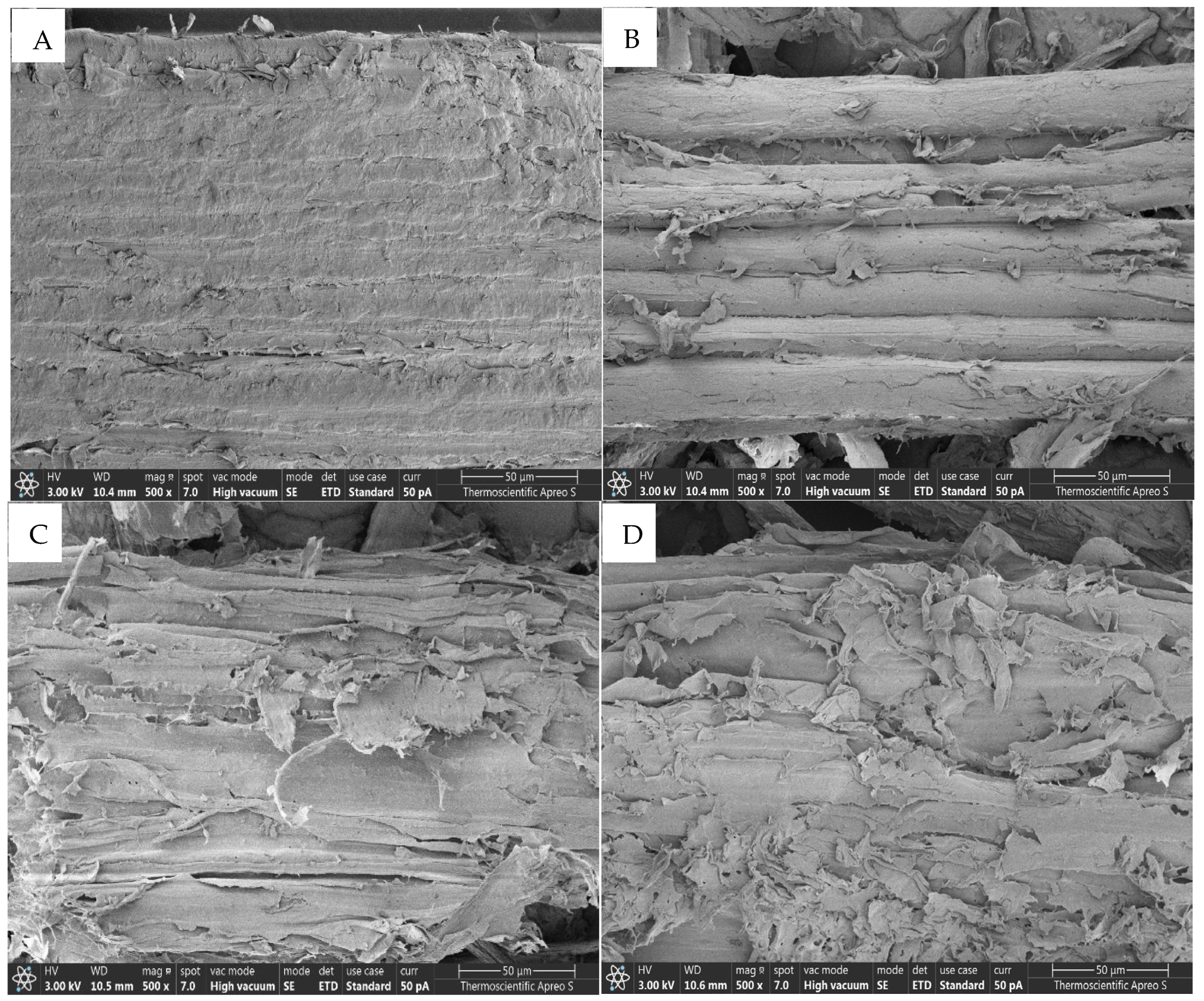
2.5. Morphology Analysis
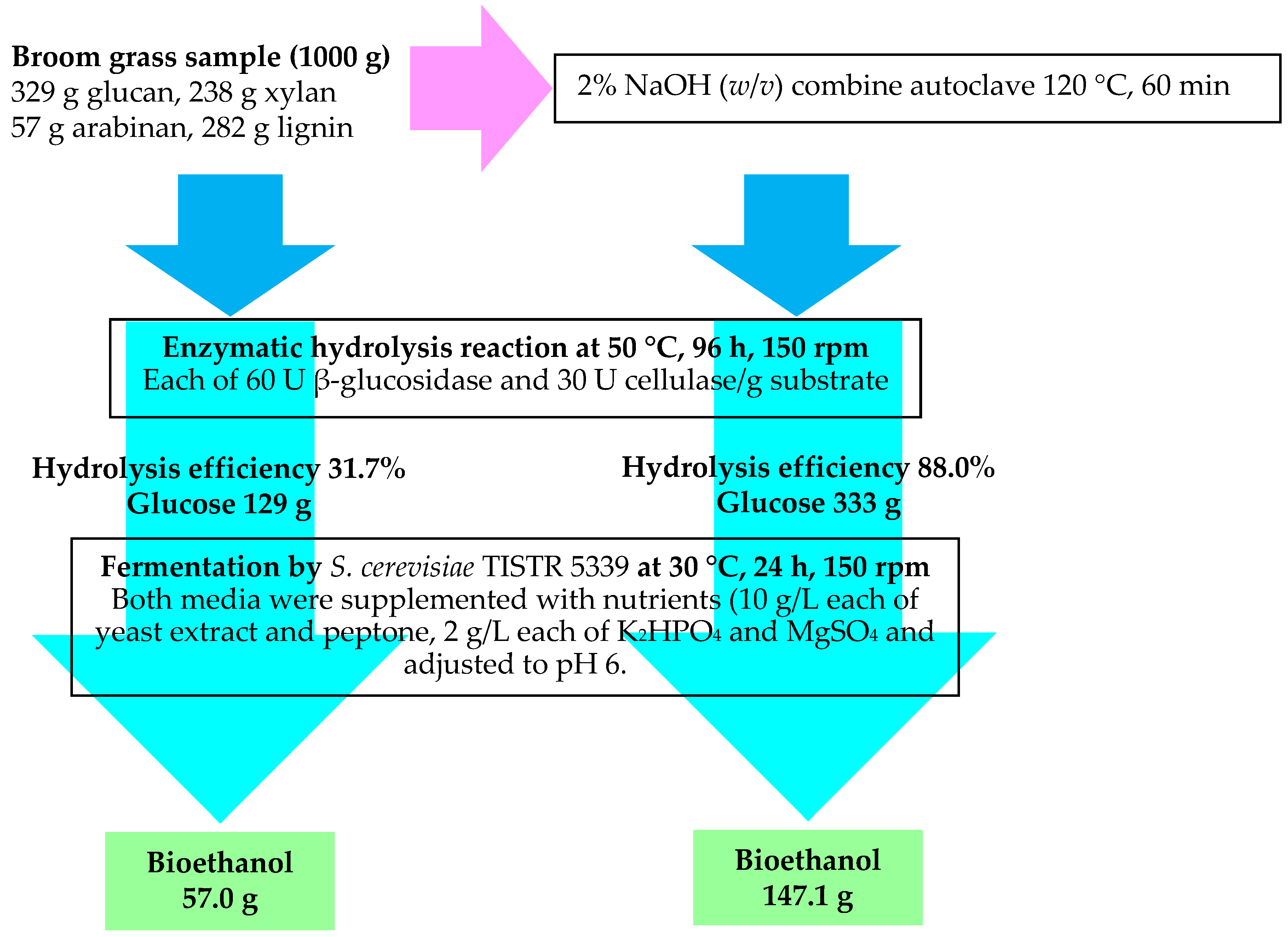
2.6. Biomass Enzyme Saccharification of Broom Grass
2.7. Biomass Hydrolysate Preparation
2.8. Ethanol Fermentation Medium
2.9. Culture Preparation
2.10. Statistical Analysis
3. Results
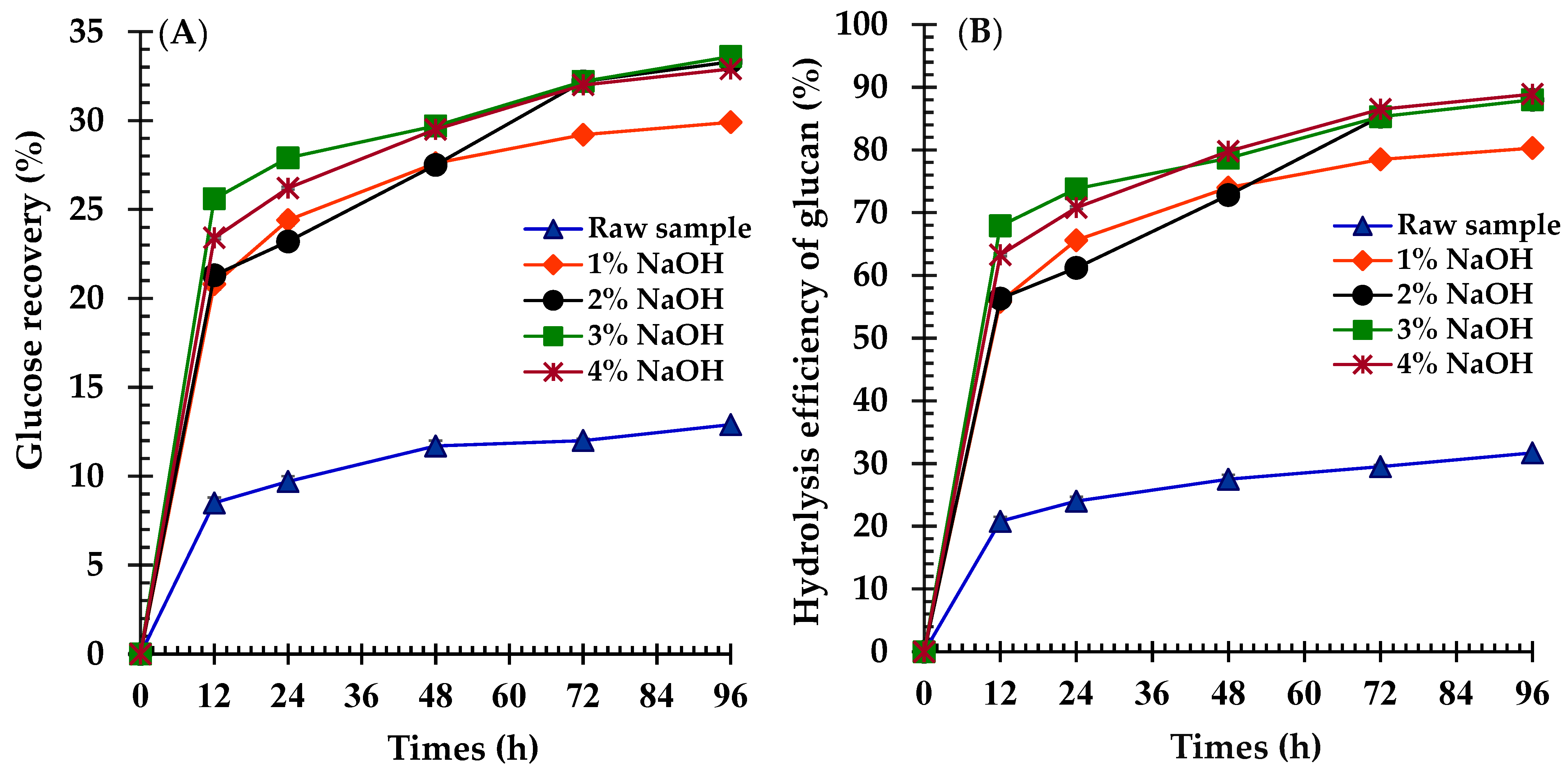
3.1. Effects of NaOH Concentration Pretreatment on Biomass Fractionation
3.2. Effects of Temperature on Biomass Fractionation
3.3. Effects of NaOH and Temperature Pretreatment on Morphological Structure
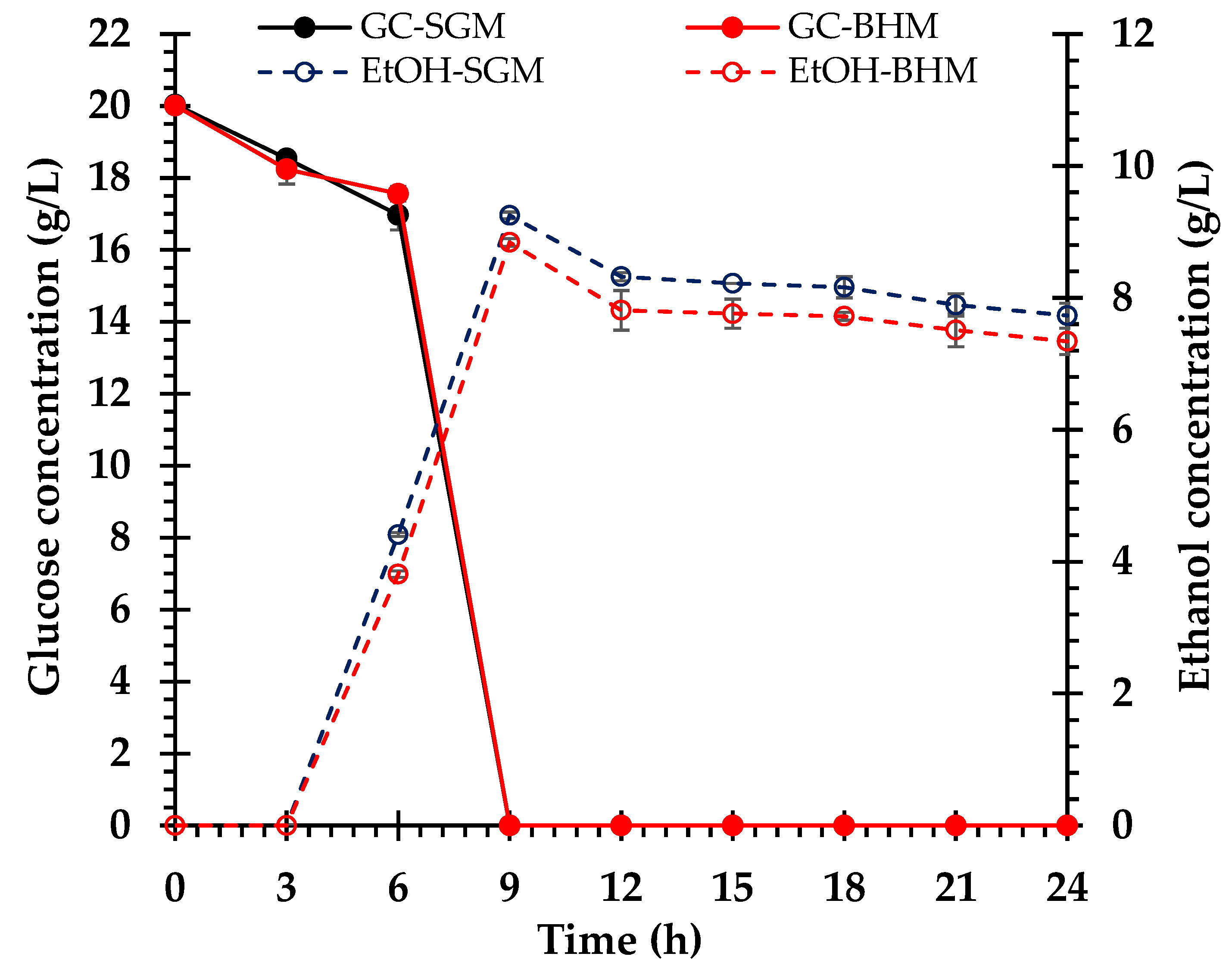
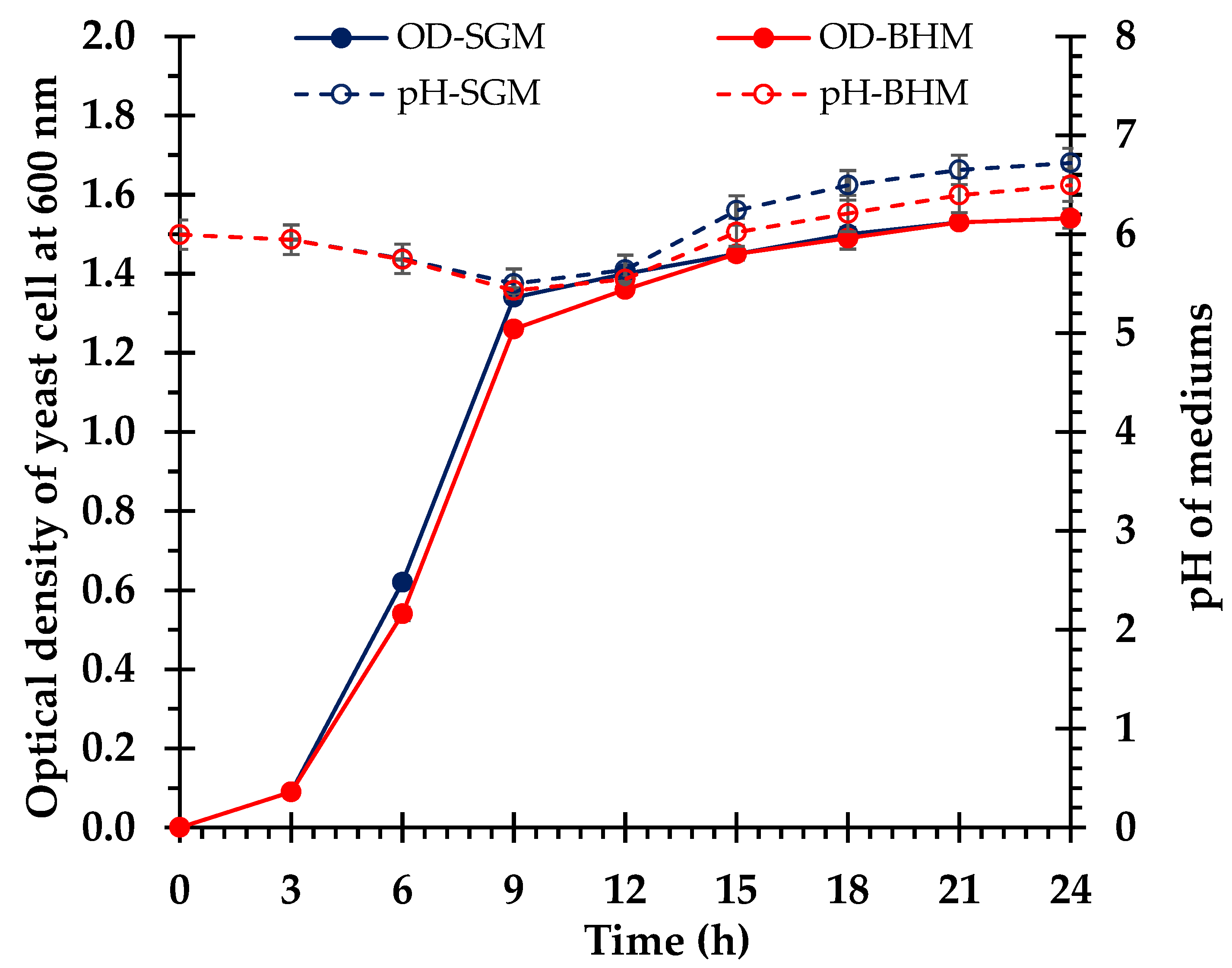
3.4. Ethanol Fermentation
4. Discussion
4.1. Effects of NaOH Concentration and Temperature on Biomass Fractionation
4.2. Effects of NaOH Concentration and Temperature on Crystallinity
4.3. Effects of the NaOH Concentration and the Temperature on the Morphological Structure
4.4. Effects of NaOH Concentration and Temperature on Glucose Recovery Yields
4.5. Comparative Fermentation Efficiency of S. cerevisiae in the Control and Biomass Hydrolysate Media
5. Conclusions
- −
- Production costs at various scales.
- −
- Market value assessment of the main products and by-products.
- −
- Waste management costs and strategies.
- −
- Potential revenue streams from lignin and hemicellulose utilization.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIL | Acid-insoluble lignin |
| ASL | Acid-soluble lignin |
| BHM | Biomass hydrolysate medium |
| CrI | Crystallinity index |
| DMRT | Duncan’s multiple-range test |
| GR | Glucose recovery |
| HEG | Hydrolysis efficiency of glucose |
| HPLC | High-performance liquid chromatography system |
| SEM | Scanning electron microscopy |
| SGM | Synthetic glucose medium |
| UV | Ultraviolet |
| XRD | X-ray diffraction |
| OD | Optical density |
References
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Khattab, S.M.R.; Okano, H.; Kimura, C.; Fujita, T.; Watanabe, T. Efficient integrated production of bioethanol and antiviral glycerolysis lignin from sugarcane trash. Biotechnol. Biofuels Bioprod. 2023, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tsai, M.-L.; Sharma, V.; Sun, P.-P.; Nargotra, P.; Bajaj, B.K.; Chen, C.-W.; Dong, C.-D. Environment friendly pretreatment approaches for the bioconversion of lignocellulosic biomass into biofuels and value-added products. Environments 2023, 10, 6. [Google Scholar] [CrossRef]
- Kaur, S.; Gaurav, K. Lignocellulosic hydrolysates for the production of bioethanol: A comprehensive analysis. Sugar Tech. 2024, 26, 1068–1077. [Google Scholar] [CrossRef]
- Johannes, L.P.; Xuan, T.D. Comparative analysis of acidic and alkaline pretreatment techniques for bioethanol production from perennial grasses. Energies 2024, 17, 1048. [Google Scholar] [CrossRef]
- Manandhar, A.; Sha, A. Feedstock Logistics for Agricultural Residues and Energy Crops: Moving Biomass from the Field to Biorefinery Gate. Agriculture and Natural Resources. 2018. Available online: https://ohioline.osu.edu/factsheet/fabe-6604 (accessed on 10 January 2025).
- Cheah, W.Y.; Sankaran, R.; Show, P.L.; Ibrahim, T.N.B.T.; Chew, K.W.; Culaba, A.; Chang, J.-S. Pretreatment methods for lignocellulosic biofuels production: Current advances, challenges and future prospects. Biofuel Res. J. 2020, 7, 1115–1127. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X.; Zalesny, R.S. Pretreatment of woody biomass for biofuel production: Energy efficiency, technologies, and recalcitrance. Appl. Microbiol. Biotechnol. 2010, 87, 847–857. [Google Scholar] [CrossRef]
- El Hage, M.; Louka, N.; Rezzoug, S.-A.; Maugard, T.; Sablé, S.; Koubaa, M.; Debs, E.; Maache-Rezzoug, Z. Bioethanol Production from Woody biomass: Recent advances on the effect of pretreatments on the bioconversion process and energy yield aspects. Energies 2023, 16, 5052. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B. Bioethanol production from renewable raw materials and its separation and purification: A review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mishra, S.S.; Bhalla, P.; Thatoi, H. Engineering grass biomass for sustainable and enhanced bioethanol production. Planta 2019, 250, 395–412. [Google Scholar] [CrossRef]
- Premjet, S.; Doungporn, P.; Yoo, H.Y.; Kim, S.W. Improvement of sugar recovery from Sida acuta (Thailand Weed) by NaOH pretreatment and application to bioethanol production. Korean J. Chem. Eng. 2018, 35, 2413–2420. [Google Scholar] [CrossRef]
- Obeng, A.K.; Premjet, D.; Premjet, S. Combining autoclaving with mild alkaline solution as a pretreatment technique to enhance glucose recovery from the invasive weed Chloris barbata. Biomolecules 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Wongleang, S.; Premjet, D.; Premjet, S. Cellulosic ethanol production from weed biomass hydrolysate of Vietnamosasa pusilla. Polymers 2023, 15, 1103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Wongleang, S.; Premjet, D.; Premjet, S. investigating the potential of grass biomass (Thysanolaena latifolia) as an alternative feedstock for sugar platforms and bioethanol production. Energies 2024, 17, 4017. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Shukla, R.P.; Lynser, M.B.; Tynsong, H. Growth pattern, production, and marketing of Thysanolaena maxima (Roxb.) Kuntze: An important non-timber forest product of Meghalaya, India. For. Trees Livelihoods 2012, 21, 176–187. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. biofuels bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.F.; Zhang, C.C.; Nan, J.; Ren, N.Q.; Lee, D.J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef]
- Hute, T.; Sanusi, I.A.; Kana, E.B.G.; Meyer, E.L.; Sewsynker-Sukai, Y. Comparative assessment of autoclave- and microwave-facilitated seawater pretreatments for the enhancement of sugar recovery from banana pseudostem. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]
- Valles, A.; Capilla, M.; Álvarez-Hornos, F.J.; García-Puchol, M.; San-Valero, P.; Gabaldón, C. Optimization of alkali pretreatment to enhance rice straw conversion to butanol. Biomass Bioenergy 2021, 150, 106131. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. Thermochemical pretreatment enhanced bioconversion of elephant grass (Pennisetum purpureum): Insight on the production of sugars and lignin. Biomass Convers. Biorefin. 2022, 12, 1125–1138. [Google Scholar] [CrossRef]
- Sawisit, A.; Jampatesh, S.; Jantama, S.S.; Jantama, K. Optimization of sodium hydroxide pretreatment and enzyme loading for efficient hydrolysis of rice straw to improve succinate production by metabolically engineered Escherichia coli KJ122 under simultaneous saccharification and fermentation. Bioresour. Technol. 2018, 260, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Papathoti, N.K.; Laemchiab, K.; Megavath, V.S.; Keshav, P.K.; Numparditsub, P.; Le Thanh, T.; Buensanteai, N. Augmented ethanol production from alkali-assisted hydrothermal pretreated cassava peel waste. Energy Source Part A Recovery Util. Environ. Eff. 2021, 1–11. [Google Scholar] [CrossRef]
- Kontogianni, N.; Barampouti, E.M.; Mai, S.; Malamis, D.; Loizidou, M. Effect of alkaline pretreatments on the enzymatic hydrolysis of wheat straw. Environ. Sci. Pollut. Res. 2019, 26, 35648–35656. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report TP-510-42618; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2012; pp. 1–15. [Google Scholar]
- Premjet, S.; Dana, S.; Obeng, A.K.; Premjet, D. Enzymatic response to structural and chemical transformations in Hibiscus sabdariffa var. altissima bark and core during phosphoric acid pretreatment. BioResources 2018, 13, 6778–6789. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin Jr, A.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Saratale, G.D.; Oh, M.-K. Improving alkaline pretreatment method for preparation of whole rice waste biomass feedstock and bioethanol production. RSC Adv. 2015, 5, 97171–97179. [Google Scholar] [CrossRef]
- Bala, R.; Mondal, M.K. Exhaustive characterization on chemical and thermal treatment of sawdust for improved biogas production. Biomass Convers. Biorefin. 2018, 8, 991–1003. [Google Scholar] [CrossRef]
- Obeng, A.K.; Premjet, D.; Premjet, S. Improved glucose recovery from durian peel by alkaline-catalyzed steam pretreatment. PeerJ 2021, 9, e12026. [Google Scholar] [CrossRef]
- An, H.-E.; Lee, K.H.; Jang, Y.W.; Kim, C.-B.; Yoo, H.Y. improved glucose recovery from Sicyos angulatus by NaOH pretreatment and application to bioethanol production. Processes 2021, 9, 245. [Google Scholar] [CrossRef]
- Wongleang, S.; Premjet, D.; Premjet, S. Physicochemical pretreatment of Vietnamosasa pusilla for bioethanol and xylitol production. Polymers 2023, 15, 3990. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, F.; Zhang, P.; Jin, S.; Tao, X.; Tang, X.; Ye, J.; Nabi, M.; Wang, H. Ultrasound assisted alkaline pretreatment to enhance enzymatic saccharification of grass clipping. Energy Convers. Manag. 2017, 149, 409–415. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, J.J.; Sharma-Shivappa, R.R.; Burns, J.C. Sodium hydroxide pretreatment of switchgrass for ethanol production. Energy Fuels 2010, 24, 2113–2119. [Google Scholar] [CrossRef]
- Wunna, K.; Nakasaki, K.; Auresenia, J.L.; Abella, L.C.; Gaspillo, P.-a.D. Effect of alkali pretreatment on removal of lignin from sugarcane bagasse. Chem. Eng. Trans. 2017, 56, 1831–1836. [Google Scholar] [CrossRef]
- Shah, T.A.; Khalid, S.; Nafidi, H.-A.; Salamatullah, A.M.; Bourhia, M. Sodium hydroxide hydrothermal extraction of lignin from rice straw residue and fermentation to biomethane. Sustainability 2023, 15, 8755. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S. Effects of sodium hydroxide pretreatment on structural components of biomass. Trans. ASABE 2014, 57, 1187–1198. [Google Scholar]
- Nath, P.; Maibam, P.D.; Singh, S.; Rajulapati, V.; Goyal, A. Sequential pretreatment of sugarcane bagasse by alkali and organosolv for improved delignification and cellulose saccharification by chimera and cellobiohydrolase for bioethanol production. 3 Biotech 2021, 11, 59. [Google Scholar] [CrossRef]
- Matsushita, Y.; Kakehi, A.; Miyawaki, S.; Yasuda, S. Formation and chemical structures of acid-soluble lignin II: Reaction of aromatic nuclei model compounds with xylan in the presence of a counterpart for condensation, and behavior of lignin model compounds with guaiacyl and syringyl nuclei in 72% sulfuric acid. J. Wood Sci. 2004, 50, 136–141. [Google Scholar] [CrossRef]
- Xu, H.; Li, B.; Mu, X. review of alkali-based pretreatment to enhance enzymatic saccharification for lignocellulosic biomass conversion. Ind. Eng. Chem. Res. 2016, 55, 8691–8705. [Google Scholar] [CrossRef]
- Hoşgün, E.Z.; Bozan, B. Effect of different types of thermochemical pretreatment on the enzymatic hydrolysis and the composition of Hazelnut Shells. Waste Biomass Valori. 2020, 11, 3739–3748. [Google Scholar] [CrossRef]
- Li, C.; Fan, M.; Xie, J.; Zhang, H. Effect of NaOH-catalyzed organosolv pretreatment on the co-production of ethanol and xylose from poplar. Ind. Crops. Prod. 2023, 200, 116774. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Ishiguro, M.; Endo, T. Addition of alkali to the hydrothermal–mechanochemical treatment of Eucalyptus enhances its enzymatic saccharification. Bioresour. Technol. 2014, 153, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.L.; Gonçalves, E.; Suarez, C.A.G.; de Sousa Rodrigues, D.; Montano, I.C. Effect of reaction time and sodium hydroxide concentration on delignification and enzymatic hydrolysis of brewer’s spent grain from two brazilian brewers. Cellulose Chem. Technol. 2021, 55, 101–112. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review. Sustain. Energy Fuels 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Rajulapati, V.; Goyal, A. Evaluation of pre-treatment methods for Lantana camara stem for enhanced enzymatic saccharification. 3 Biotech 2020, 10, 37. [Google Scholar] [CrossRef]
- Debiagi, F.; Madeira, T.B.; Nixdorf, S.L.; Mali, S. Pretreatment efficiency using autoclave high-pressure steam and ultrasonication in sugar production from liquid hydrolysates and access to the residual solid fractions of wheat bran and oat hulls. Appl. Biochem. Biotechnol. 2020, 190, 166–181. [Google Scholar] [CrossRef]
- Gao, F.; Wang, H.; Li, F.; De, Y.; Ma, Y.; Jiang, H.; Jing, Y.; Qu, H.; Li, P. Evaluation of lignocellulose degradation and ethanol production via dilute acid and alkali pretreatment of hybrid Pennisetum. BioRes. 2022, 17, 4517–4531. [Google Scholar] [CrossRef]
- Xu, F.; Shi, Y.-C.; Wang, D. X-ray scattering studies of lignocellulosic biomass: A review. Carbohydr. Polym. 2013, 94, 904–917. [Google Scholar] [CrossRef]
- Wang, X.; Le, H.; Guo, Y.; Zhao, Y.; Deng, X.; Zhang, J.; Zhang, L. Preparation of cellulose nanocrystals from jujube cores by fractional purification. Molecules 2022, 27, 3236. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, H.; Qian, J. Pretreatment with a combination of steam explosion and NaOH increases butanol production of enzymatically hydrolyzed corn stover. Renew. Energy 2023, 203, 301–311. [Google Scholar] [CrossRef]
- Manokhoon, P.; Rangseesuriyachai, T. Effect of two-stage sodium hydroxide pretreatment on the composition and structure of Napier grass (Pakchong 1) (Pennisetum purpureum). Int. J. Green Energy 2020, 17, 864–871. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Fan, C.; Zheng, X.; Zhang, W.; Wu, D.; Wang, X.; Kong, H. Optimisation of enzymatic saccharification of wheat straw pre-treated with sodium hydroxide. Sci. Rep. 2021, 11, 23234. [Google Scholar] [CrossRef]
- Wu, W.; Li, P.; Huang, L.; Wei, Y.; Li, J.; Zhang, L.; Jin, Y. The Role of Lignin Structure on Cellulase Adsorption and Enzymatic Hydrolysis. Biomass 2023, 3, 96–107. [Google Scholar] [CrossRef]
- Junior, C.S.; Milagres, A.M.F.; Ferraz, A.; Carvalho, W. The effects of lignin removal and drying on the porosity and enzymatic hydrolysis of sugarcane bagasse. Cellulose 2013, 20, 3165–3177. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Chen, C.; Wang, S.; Min, D. Lignin interaction with cellulase during enzymatic hydrolysis. Pap. Biomater. 2019, 4, 15–30. [Google Scholar] [CrossRef]
- Cui, P.; Ye, Z.; Chai, M.; Yuan, J.; Xiong, Y.; Yang, H.; Yao, L. Effective fractionation of lignocellulose components and lignin valorization by combination of deep eutectic solvent with ethanol. Front. Bioeng. Biotechnol. 2022, 10, 1115469. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Lee, K.H.; Lee, S.K.; Chun, Y.; Kim, S.W.; Park, C.; Yoo, H.Y. Improvement of bioethanol production from waste chestnut shells via evaluation of mass balance-based pretreatment and glucose recovery process. Environ. Technol. Innov. 2022, 28, 102955. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, C.; Lin, W.; Bian, B.; Lai, C.; Ling, Z.; Yong, Q. A structure–activity understanding of the interaction between lignin and various cellulase domains. Bioresour. Technol. 2022, 351, 127042. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, G.; Li, M.; Jiang, H.; Wang, S.; Min, D. Impact of bagasse lignin-carbohydrate complexes structural changes on cellulase adsorption behavior. Int. J. Biol. Macromol. 2020, 162, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Moysés, D.N.; Reis, V.C.; Almeida, J.R.; Moraes, L.M.; Torres, F.A. Xylose fermentation by Saccharomyces cerevisiae: Challenges and prospects. Int. J. Mol. Sci. 2016, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Endalur Gopinarayanan, V.; Nair, N.U. Pentose metabolism in Saccharomyces cerevisiae: The need to engineer global regulatory systems. Biotechnol. J. 2019 14, e1800364. [CrossRef]
- Topaloğlu, A.; Esen, Ö.; Turanlı-Yıldız, B.; Arslan, M.; Çakar, Z.P. From Saccharomyces cerevisiae to ethanol: Unlocking the power of evolutionary engineering in metabolic engineering applications. J. Fungi 2023, 9, 984. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Zhang, J.; Geng, A.; Yao, C.; Lu, Y.; Li, Q. Effects of lignin-derived phenolic compounds on xylitol production and key enzyme activities by a xylose utilizing yeast Candida athensensis SB18. Bioresour. Technol. 2012, 121, 369–378. [Google Scholar] [CrossRef]
- Rage, G.; Atasi, O.; Wilhelmus, M.M.; Hernández-Sánchez, J.F.; Haut, B.; Scheid, B.; Legendre, D.; Zenit, R. Bubbles determine the amount of alcohol in Mezcal. Sci. Rep. 2020, 10, 11014. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Li, C.; Sakakibara, K.; Tanaka, S.; Kong, H. Factors affecting ethanol fermentation using Saccharomyces cerevisiae BY4742. Biomass Bioenergy 2012, 47, 395–401. [Google Scholar] [CrossRef]
- Stanley, D.; Fraser, S.; Chambers, P.J.; Rogers, P.; Stanley, G.A. Generation and characterisation of stable ethanol-tolerant mutants of Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2010, 37, 139–149. [Google Scholar] [CrossRef]


| Composition | NaOH | ||||
|---|---|---|---|---|---|
| Untreated | 1% | 2% | 3% | 4% | |
| Glucan | 32.9 ± 0.5 e | 48.3 ± 0.2 d | 54.6 ± 0.2 c | 57.3 ± 0.3 b | 61.3 ± 0.3 a |
| Xylan | 23.8 ± 0.2 a | 19.8 ± 0.3 b | 19.5 ± 0.1 b | 18.2 ± 0.3 c | 15.4 ± 0.2 d |
| Arabinan | 5.7 ± 0.0 a | 4.9 ± 0.2 b | 4.6 ± 0.1 c | 4.3 ± 0.2 d | 3.8 ± 0.0 e |
| AIL | 22.2 ± 0.5 a | 13.3 ± 0.4 b | 10.1 ± 0.1 c | 9.2 ± 0.2 d | 8.6 ± 0.1 e |
| ASL | 5.9 ± 0.1 a | 2.8 ± 0.1 b | 2.6 ± 0.1 c | 2.4 ± 0.0 d | 2.4 ± 0.0 d |
| Total lignin | 28.2 ± 0.4 a | 16.1 ± 0.4 b | 12.7 ± 0.1 c | 11.6 ± 0.2 d | 10.9 ± 0.1 e |
| CrI | 54.1% | 57.8% | 60.9% | 62.6% | 65.1% |
| Solid recovery | 100 a | 62.5 ± 0.5 b | 56.2 ± 0.4 c | 53.4 ± 0.1 d | 48.9 ± 0.5 e |
| Glucan recovery | 100 a | 91.8 ± 0.4 c | 93.2 ± 0.3 b | 93.1 ± 0.5 b | 91.1 ± 0.5 c |
| Xylan recovery | 100 a | 52.0 ± 0.7 b | 46.0 ± 0.3 c | 40.7 ± 0.6 d | 31.7 ± 0.5 e |
| Arabinan recovery | 100 | 53.9 ± 2.3 b | 45.4 ± 1.0 c | 40.1 ± 2.2 d | 32.1 ± 0.3 e |
| AIL recovery | 100 a | 37.5 ± 1.1 b | 25.5 ± 0.3 c | 22.1 ± 0.4 d | 18.9 ± 0.2 e |
| ASL recovery | 100 a | 29.6 ± 0.7 b | 24.4 ± 0.7 c | 21.6 ± 0.2 d | 19.4 ± 0.3 e |
| Total lignin recovery | 100 a | 35.8 ± 1.0 b | 25.3 ± 0.1 c | 22.0 ± 0.3 d | 19.0 ± 0.1 e |
| Lignin removal | 0.0 | 64.2 ± 1.0 d | 74.7 ± 0.1 c | 78.0 ± 0.3 b | 81.0 ± 0.1 a |
| Composition (%Dw) | Temperature | |||
|---|---|---|---|---|
| Untreated | 110 °C | 120 °C | 130 °C | |
| Glucan | 32.9 ± 0.5 d | 49.7 ± 0.6 c | 54.6 ± 0.2 b | 57.1 ± 0.2 a |
| Xylan | 23.8 ± 0.2 a | 20.8 ± 0.4 b | 19.5 ± 0.1 c | 18.7 ± 0.1 c |
| Arabinan | 5.7 ± 0.0 a | 4.8 ± 0.1 b | 4.6 ± 0.1 c | 4.5 ± 0.0 c |
| AIL | 22.2 ± 0.5 a | 14.9 ± 0.3 b | 10.1 ± 0.1 c | 8.5 ± 0.4 d |
| ASL | 5.9 ± 0.1 a | 2.9 ± 0.1 b | 2.6 ± 0.1 c | 2.4 ± 0.1 c |
| Total lignin | 28.2 ± 0.4 a | 17.9 ± 0.3 b | 12.7 ± 0.1 c | 10.9 ± 0.4 d |
| CrI | 54.1% | 60.7% | 60.9% | 62.2% |
| Solid recovery | 100 a | 58.2 ± 0.5 b | 56.2 ± 0.4 c | 54.2 ± 0.6 d |
| Glucan recovery | 100 a | 87.9 ± 1.0 c | 93.2 ± 0.4 b | 94.0 ± 0.2 b |
| Xylan recovery | 100 a | 50.8 ± 0.9 b | 46.0 ± 0.3 c | 42.6 ± 0.2 d |
| Arabinan recovery | 100 a | 48.4 ± 0.7 b | 45.5 ± 1.0 c | 42.7 ± 0.3 d |
| AIL recovery | 100 a | 29.1 ± 0.7 b | 25.5 ± 0.3 c | 20.7 ± 1.1 d |
| ASL recovery | 100 a | 28.8 ± 0.9 b | 24.4 ± 0.7 c | 21.9 ± 0.6 d |
| Total lignin recovery | 100 a | 36.9 ± 0.6 b | 25.3 ± 0.1 c | 20.9 ± 0.8 d |
| Lignin removal | 0.0 d | 63.1 ± 0.3 c | 74.7 ± 0.1 b | 79.1 ± 0.8 a |
| Feedstocks | Pretreatment Conditions | Lignin Removal (%) | Glucose Recovery (%) | Reference |
|---|---|---|---|---|
| Chloris barbata | 2% NaOH, 110 °C, 60 min | 71% | 30.7% | [13] |
| Durian peel (Cultivar Monthong) | 2% NaOH, 110 °C, 60 min | 77% | 36.1% | [31] |
| Durian peel (Cultivar Chanee) | 2% NaOH, 110 °C, 60 min | 74% | 34.9% | [31] |
| Sicyos angulatus | 2% NaOH, 110 °C, 10 min | - | 8.5% | [32] |
| Vietnamosasa pusilla | 2% NaOH, 120 °C, 60 min | 50.5% | 42.4% | [33] |
| Broom grass | 2% NaOH, 120 °C, 60 min | 74.7% | 33.3% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Premjet, D.; Premjet, S. Enhanced Sugar and Bioethanol Production from Broom Grass via NaOH-Autoclave Pretreatment. Polymers 2025, 17, 266. https://doi.org/10.3390/polym17030266
Premjet D, Premjet S. Enhanced Sugar and Bioethanol Production from Broom Grass via NaOH-Autoclave Pretreatment. Polymers. 2025; 17(3):266. https://doi.org/10.3390/polym17030266
Chicago/Turabian StylePremjet, Duangporn, and Siripong Premjet. 2025. "Enhanced Sugar and Bioethanol Production from Broom Grass via NaOH-Autoclave Pretreatment" Polymers 17, no. 3: 266. https://doi.org/10.3390/polym17030266
APA StylePremjet, D., & Premjet, S. (2025). Enhanced Sugar and Bioethanol Production from Broom Grass via NaOH-Autoclave Pretreatment. Polymers, 17(3), 266. https://doi.org/10.3390/polym17030266






