Low-Flammability Hybrid Polymer Materials Based on Epoxy Oligomers and In Situ-Synthesized Zinc-Containing Microparticles
Abstract
1. Introduction
2. Materials and Methods
3. Results
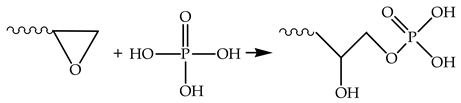

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PMC | polymer matrix composites |
| DGEBA | bisphenol A diglycidyl ether |
| TETA | triethylenetetramine |
| LOI | limiting oxygen index |
| SEM | scanning electron microscopy |
| THR | total heat release |
| meanHRR | mean heat release rate |
| PHRR | peak heat release rate |
| TSR | total smoke release |
| FIGRA | fire growth rate |
| Qef. | effective heat of combustion |
| initial first-stage temperature of thermal-oxidative degradation marking the beginning of mass loss intensity on the DTG curve | |
| final first-stage temperature of thermal-oxidative degradation based on the point of intersection of tangents drawn through the TG curve bends | |
| PMLR | peak mass loss rate |
| Tm | PMLR temperature |
| ΔmI | mass loss after the first stage of thermal-oxidative degradation |
References
- Askaralievich, M.A.A. Application of Composite Materials in Designs Unmanned Aerial Vehicles. Am. J. Technol. Adv. 2024, 1, 5–9. [Google Scholar]
- Slavin, A.V.; Donetskiy, K.I.; Khrulkov, A.V. Prospects for Use of Polymer Composite Materials in Aviation Structures in 2025–2035 (Overview). VIAM Work. 2022, 11, 81–92. [Google Scholar]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Ogabi, R.; Manescau, B.; Chetehouna, K.; Gascoin, N. A Study of Thermal Degradation and Fire Behaviour of Polymer Composites and Their Gaseous Emission Assessment. Energies 2021, 14, 7070. [Google Scholar] [CrossRef]
- Barbotko, S.L.; Bochenkov, M.M.; Volnyj, O.S.; Korobeinichev, O.; Shmakov, A. Evaluation of the effectiveness of the fire retardants, promising for the creation of new polymer composite materials intended for aviation techniques. Proc. VIAM 2021, 2, 20–29. [Google Scholar] [CrossRef]
- Antipov, V.V.; Konovalov, A.N.; Serebrennikova, N.Y. Influence of Structure on Fire Resistance and Fireproofing of SIAL Class Aluminum-Glass Fiber Reinforced Plastics and the Prospects for Use of These Materials in Aircraft Engineering. VIAM Work. 2019, 1, 40–46. [Google Scholar] [CrossRef]
- Gunyaeva, A.G.; Sidorina, A.I.; Klimenko, O.N. Polymer Composite Material Based on Hybrid Metal-Carbon Filler: Properties and Application. VIAM Work. 2020, 10, 30–39. [Google Scholar] [CrossRef]
- Zaripov, I.I.; Vikhareva, I.; Buylova, E.; Berestova, T.; Mazitova, A. Additives to Reduce the Flammability of Polymers. Nanotechnol. Constr. 2022, 14, 156–161. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. A Review of Current Flame Retardant Systems for Epoxy Resins. J. Fire Sci. 2004, 22, 25–40. [Google Scholar] [CrossRef]
- Hergenrother, P.M.; Thompson, C.M.; Smith, J.G.; Connell, J.W. Flammability of Epoxy Resins Containing Phosphorus; Report DOT/FAA/AR-TN05/44; FAA: Washington, DC, USA, 2005; 32p.
- Ciesielski, M.; Diederichs, J.; Döring, M.; Schäfer, A. Advanced Flame-Retardant Epoxy Resins for Composite Materials. In Fire and Polymers V; American Chemical Society: Washington, DC, USA, 2009; pp. 174–190. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Influence of Nano-Silica on the Flame Retardancy and Smoke Suppression Properties of Transparent Intumescent Fire-Retardant Coatings. Prog. Org. Coat. 2017, 112, 319–329. [Google Scholar] [CrossRef]
- Roenner, N.; Hutheesing, K.; Fergusson, A.; Rein, G. Simultaneous Improvements in Flammability and Mechanical Toughening of Epoxy Resins through Nano-Silica Addition. Fire Saf. J. 2017, 91, 200–207. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Q.; Liu, G.; Li, Z. Preparation of Zinc Ferrite Nanorods and Their Effect on the Flame Retardancy of Epoxy Resin. In Proceedings of the 2nd Asia-Oceania Symposium on Fire Safety Materials Science and Engineering, Shenzhen, China, 27–29 October 2017; p. 169. [Google Scholar]
- Rafique, I.; Kausar, A.; Anwar, Z.; Muhammad, B. Exploration of Epoxy Resins, Hardening Systems, and Epoxy/Carbon Nanotube Composite Designed for High Performance Materials: A Review. Polym. Plast. Technol. Eng. 2015, 55, 312–333. [Google Scholar] [CrossRef]
- Islam, M.E.; Mahdi, T.H.; Hosur, M.V.; Jeelani, S. Characterization of Carbon Fiber Reinforced Epoxy Composites Modified with Nanoclay and Carbon Nanotubes. Procedia Eng. 2015, 105, 821–828. [Google Scholar] [CrossRef]
- Hayati, D.; Ghasemi, S.; Lakouraj, M.M.; Mousavi, F. A smart epoxy coating based on Zn-imidazole metal-organic framework/graphene oxide nanocomposite for corrosion protection. Compos. Commun. 2025, 59, 102556. [Google Scholar] [CrossRef]
- Kuzub, L.I.; Gurieva, L.L.; Khodos, I.I.; Badamshina, E.R. Influence of Precursor Concentration on the Formation of Silver Nanoparticles with Oligostyrylmonocarboxylate Ligands in ED-20 Epoxy Oligomer. Polym. Sci. Ser. B 2020, 62, 222–229. [Google Scholar] [CrossRef]
- Kuzub, L.I.; Gurieva, L.L.; Khodos, I.I.; Grishchuk, A.A.; Estrin, Y.I.; Badamshina, E.R. Regularities of the Formation of Silver Nanoparticles with Oligostyrylmonocarboxylate Ligands in ED-20 Epoxy Oligomer. Polym. Sci. Ser. B 2017, 59, 348–354. [Google Scholar] [CrossRef]
- Kuzub, L.I.; Gurieva, L.L.; Grishchuk, A.A.; Estrina, G.A.; Estrin, Y.I.; Badamshina, E.R. Regularities of the Formation of Silver Nanoparticles with Oligostyrylcarboxylate Ligands. Polym. Sci. Ser. B 2015, 57, 608–615. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kashiwagi, Y.; Yamamoto, M. Synthesis and Size Regulation of Gold Nanoparticles by Controlled Thermolysis of Ammonium Gold(I) Thiolate in the Absence or Presence of Amines. Inorg. Chim. Acta 2005, 358, 4229–4236. [Google Scholar] [CrossRef]
- Charan, S.; Singh, N.; Khanna, P.K.; Patil, K.R. Direct Synthesis of Nanocrystalline Silver from the Reaction Between Silver Carboxylates and n-Trioctylphosphine. J. Nanosci. Nanotechnol. 2006, 6, 2095–2102. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Yamamoto, M.; Nakamoto, M. Facile Size-Regulated Synthesis of Silver Nanoparticles by Controlled Thermolysis of Silver Alkylcarboxylates in the Presence of Alkylamines with Different Chain Lengths. J. Colloid Interface Sci. 2006, 300, 169–175. [Google Scholar] [CrossRef]
- Khanna, P.K.; Kulkarni, D.; Beri, R.K. Synthesis and Characterization of Myristic Acid Capped Silver Nanoparticles. J. Nanopart. Res. 2008, 10, 1059–1062. [Google Scholar] [CrossRef]
- Moazzen, K.; Rossegger, E.; Alabiso, W.; Shaukat, U.; Schlögl, S. Organic Phosphates and Phosphonates in Catalyzing Dynamic Exchange Reactions in Thiol-Click Vitrimers. Macromol. Chem. Phys. 2021, 222, 2100072. [Google Scholar] [CrossRef]
- Chen, F.; Cheng, Q.; Gao, F.; Zhong, J.; Shen, L.; Lin, C.; Lin, Y. The Effect of Latent Plasticity on the Shape Recovery of a Shape Memory Vitrimer. Eur. Polym. J. 2021, 147, 110304. [Google Scholar] [CrossRef]
- Dertnig, C.; Guedes de la Cruz, G.; Neshchadin, D.; Schlögl, S.; Griesser, T. Blocked Phosphates as Photolatent Catalysts for Dynamic Photopolymer Networks. Angew. Chem. Int. Ed. 2023, 62, e202215525. [Google Scholar] [CrossRef]
- Bergoglio, M.; Reisinger, D.; Schlögl, S.; Griesser, T.; Sangermano, M. Sustainable Bio-Based UV-Cured Epoxy Vitrimer from Castor Oil. Polymers 2023, 15, 1024. [Google Scholar] [CrossRef]
- Feng, X.; Li, G. Versatile Phosphate Diester-Based Flame Retardant Vitrimers via Catalyst-Free Mixed Transesterification. ACS Appl. Mater. Interfaces 2020, 12, 55217–55224. [Google Scholar] [CrossRef] [PubMed]
- Wink, R.; Majumdar, S.; van Benthem, R.A.T.M.; Heuts, J.P.A.; Sijbesma, R.P. RNA-Inspired Phosphate Diester Dynamic Covalent Networks. Polym. Chem. 2023, 14, 4294–4302. [Google Scholar] [CrossRef]
- Pavlenko, E.V.; Borisov, S.V.; Vaniev, M.A. Modification of Epoxy Resin ED-20 with Aluminum Phosphate. VSTU Bull. 2021, 259, 99–103. [Google Scholar] [CrossRef]
- Lyubibogov, A.A.; Khamzina, D.A.; Borisov, S.V.; Stenina, I.A.; Evtushenko, Y.M.; Vaniev, M.A.; Novakov, I.A. Modification of Epoxy Resin ED-20 with Copper and Ammonium Phosphates. Polym. Sci. Ser. D 2025, 18, 567–573. [Google Scholar] [CrossRef]
- Lyubibogov, A.A.; Khamzina, D.A.; Borisov, S.V.; Rogozhkina, M.A.; Vaniev, M.A.; Novakov, I.A. Research of Combustion Products of Epoxy Polymers Modified with Copper and Ammonium Phosphates. VSTU Bull. 2024, 295, 144–153. [Google Scholar] [CrossRef]
- GOST 4648–2014; Plastic. Methods for Determining Flexural Properties. Rosstandart: Moscow, Russia, 2014.
- GOST R 57941–2017; Polymer Composites. Infrared Spectroscopy. Qualitative Analysis. Rosstandart: Moscow, Russia, 2017.
- Malysheva, Z.N.; Novakov, I.A. Theoretical and Practical Guide to the Discipline “Surface Phenomena and Dispersed Systems”; VolgGTU Publishing: Volgograd, Russia, 2011; 347p. (In Russian) [Google Scholar]
- GOST 21793–76; Plastics. Method for Determination of the Oxygen Index. Rosstandart: Moscow, Russia, 1976.
- GOST 12423-2013 (ISO 291:2008); Plastics. Standard Atmospheres for Conditioning and Testing. Rosstandart: Moscow, Russia, 2013.
- GOST R 56721–2015; Plastics. Thermogravimetry of Polymers. Part 1. General Principles. Rosstandart: Moscow, Russia, 2015.
- GOST 4650–2014; Plastic. Methods for Determining Water Absorption. Rosstandart: Moscow, Russia, 2014.
- GOST R 56782–2015; Polymer Composites. Prepregs. Determination of Prepreg Component Content by Soxhlet Extraction. Rosstandart: Moscow, Russia, 2015.
- GOST 25271-93; Plastics. Liquid Resins, Emulsions or Dispersions. Determination of Apparent Viscosity by the Brookfield Test Method. Rosstandart: Moscow, Russia, 1993.
- Lidin, R.A.; Molochko, V.A.; Andreeva, L.L. Reactivity of Inorganic Substances: Handbook; Lidin, R.A., Ed.; Drofa: Moscow, Russia, 2007; 637p. [Google Scholar]
- Zinc Granular, 30-100 Mesh, 99 7440-66-6. Available online: https://www.sigmaaldrich.com/RU/en/product/aldrich/565148 (accessed on 12 November 2025).
- Zinc Sulfate Puriss. p.a., ACS Reagent, Reag. ISO, Reag. Ph. Eur., ≥99.5 7446-20-0. Available online: https://www.sigmaaldrich.com/RU/en/product/sigald/31665 (accessed on 12 November 2025).
- Wang, K.; Wang, L.; Wu, J.; Chen, L.; He, C. Preparation of highly exfoliated epoxy/clay nanocomposites by “slurry compounding”: Process and mechanisms. Macromolecules 2005, 38, 788–800. [Google Scholar] [CrossRef]
- Wang, X.; Jin, J.; Song, M. An Investigation of the Mechanism of Graphene Toughening Epoxy. Carbon 2013, 65, 324–333. [Google Scholar] [CrossRef]
- Advani, S.G.; Hsiao, K.-T. Manufacturing Techniques for Polymer Matrix Composites (PMCs); Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Verrey, J.; Michaud, V.; Månson, J.-A.E. Dynamic Capillary Effects in Liquid Composite Moulding with Non-Crimp Fabrics. Compos. Part A Appl. Sci. Manuf. 2006, 37, 92–102. [Google Scholar] [CrossRef]
- Triethylenetetramine (TETA). Nouryon. 2021. Available online: https://www.nouryon.com/content-search/?q=Triethylenetetramine%20 (accessed on 30 April 2025).
- Tarasevich, B.N. IR Spectra of the Main Classes of Organic Compounds: Reference Materials; LMSU: Moscow, Russia, 2012; 54p. [Google Scholar]
- Pechkovsky, V.V.; Melnikova, R.Y.; Dzyuba, E.D.; Barannikova, T.I.; Nikanovich, M.V. Atlas of Infrared Spectra of Phosphates. Orthophosphates; Nauka: Moscow, Russia, 1981; 248p. [Google Scholar]
- Schartel, B.; Hull, T.R. Development of Fire-Retarded Materials—Interpretation of Cone Calorimeter Data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Sundström, B. The Development of a European Fire Classification System for Building Products—Test Methods and Mathematical Modelling. Ph.D. Thesis, Lund University, Lund, Sweden, 2007. [Google Scholar]
- Hopkin, C.; Spearpoint, M.; Hopkin, D. A Review of Design Values Adopted for Heat Release Rate Per Unit Area. Fire Technol. 2019, 55, 1599–1618. [Google Scholar] [CrossRef]
- Shcherbakov, N.A.; Dvoenko, O.V.; Kruglov, E.Y.; Aseeva, R.M. Heat Release Characteristics during Flame Combustion of Wood Fiber Board. Technol. Technosphere Saf. 2024, 105, 130–144. [Google Scholar] [CrossRef]
- EN 13501-1:2007; Fire Classification of Construction Products and Building Elements. Part 1: Classification Using Data from Reaction to Fire Tests. European Committee for Standardization (CEN): Brussels, Belgium, 2007.
- Kharmalova, K.I.; Selezneva, L.D.; Simonov-Emelyanov, I.D. Optimization of the Particle Size and Structure Parameters to Receive Dispersed Filled Polymer Composites with Maximum Strength Characteristics. Plast. Massy 2020, 9–10, 13–18. [Google Scholar] [CrossRef]
- Huynh, T.; Poiroux, K.; O’Brien, R.; West, K.; Davis, J.; West, C.W. Fusion and Thermal Degradation Behavior of Symmetric Sulfur-Containing Quaternary Ammonium Bromides. J. Phys. Chem. B 2016, 120, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, A.R.; Price, D. Fire Retardant Materials; Woodhead Publishing: Cambridge, UK, 2001; 448p. [Google Scholar]
- Tian, C.M.; Xie, J.X.; Guo, H.Z.; Xu, J.Z. The Effect of Metal Ions on Thermal Oxidative Degradation of Cotton Cellulose Ammonium Phosphate. J. Therm. Anal. Calorim. 2003, 73, 827–834. [Google Scholar] [CrossRef]





 —0.03,
—0.03,  —0.07,
—0.07,  —0.13,
—0.13,  —0.22,
—0.22,  —0.35.
—0.35.
 —0.03,
—0.03,  —0.07,
—0.07,  —0.13,
—0.13,  —0.22,
—0.22,  —0.35.
—0.35.




 ) unmodified reference sample; (B–F) samples modified with H3PO4 (1.98 wt%) containing different amounts of ZnSO4·7H2O: (
) unmodified reference sample; (B–F) samples modified with H3PO4 (1.98 wt%) containing different amounts of ZnSO4·7H2O: ( ) 0.03 wt%, (
) 0.03 wt%, ( ) 0.07 wt%, (
) 0.07 wt%, ( ) 0.13 wt%, (
) 0.13 wt%, ( ) 0.22 wt%.
) 0.22 wt%.
 ) unmodified reference sample; (B–F) samples modified with H3PO4 (1.98 wt%) containing different amounts of ZnSO4·7H2O: (
) unmodified reference sample; (B–F) samples modified with H3PO4 (1.98 wt%) containing different amounts of ZnSO4·7H2O: ( ) 0.03 wt%, (
) 0.03 wt%, ( ) 0.07 wt%, (
) 0.07 wt%, ( ) 0.13 wt%, (
) 0.13 wt%, ( ) 0.22 wt%.
) 0.22 wt%.


| Formulation Number | DGEBA, wt% | TETA, wt% | Modifying Additive, wt% | |
|---|---|---|---|---|
| ZnSO4·7H2O | H3PO4 | |||
| 0 | 90.90 | 9.10 | - | - |
| 1 | 89.77 | 8.98 | 0.0075 | 1.2425 |
| 2 | 90.45 | 9.05 | 0.4925 | |
| 3 | 90.71 | 9.07 | 0.2075 | |
| 4 | 90.80 | 9.08 | 0.1175 | |
| 5 | 88.64 | 8.86 | 0.0150 | 2.4850 |
| 6 | 90.00 | 9.00 | 0.9850 | |
| 7 | 90.52 | 9.05 | 0.4130 | |
| 8 | 90.68 | 9.07 | 0.2350 | |
| 9 | 87.51 | 8.75 | 0.0224 | 3.7176 |
| 10 | 89.55 | 8.95 | 1.4775 | |
| 11 | 90.33 | 9.03 | 0.6176 | |
| 12 | 90.57 | 9.06 | 0.3516 | |
| 13 | 88.64 | 8.86 | 0.0390 | 2.11 |
| 14 | 88.55 | 8.85 | 2.5610 | |
| 15 | 89.90 | 8.99 | 1.0760 | |
| 16 | 90.32 | 9.03 | 0.6110 | |
| 17 | 90.00 | 9.00 | 0.006 | 0.994 |
| 18 | 90.00 | 9.00 | 0.015 | 0.985 |
| 19 | 89.97 | 9.00 | 0.036 | 0.994 |
| 20 | 89.95 | 9.00 | 0.058 | 0.992 |
| 21 | 89.09 | 8.90 | 0.03 | 1.98 |
| 22 | 89.05 | 8.90 | 0.07 | 1.98 |
| 23 | 88.99 | 8.90 | 0.13 | 1.98 |
| 24 | 88.95 | 8.85 | 0.22 | 1.98 |
| 25 | 88.84 | 8.83 | 0.35 | 1.98 |
| 26 | 88.64 | 8.86 | 0.015 | 2.485 |
| 27 | 88.55 | 8.85 | 0.039 | 2.561 |
| 28 | 88.64 | 8.86 | 0.087 | 2.413 |
| 29 | 88.50 | 8.85 | 0.159 | 2.491 |
| 30 | 88.18 | 8.82 | 0.018 | 2.982 |
| 31 | 88.18 | 8.82 | 0.045 | 2.955 |
| 32 | 88.05 | 8.80 | 0.189 | 2.961 |
| Component A | Component B | Compatibility Assessment |
|---|---|---|
| Orthophosphoric acid (H3PO4) | Metallic zinc (Zn) | Dissolves (5 wt% or more) at 20–25 °C within 24 h. Releases gas during dissolution. |
| Zinc oxide (ZnO) | Dissolves (up to 2 wt%) only after 12 h’ exposure to 80 °C, but forms a scale deposit on the vessel walls 72 h after dissolution. | |
| Zinc phosphate (Zn3(PO4)2) | Dissolves (up to 2 wt%) only after 12 h’ exposure to 80 °C, but precipitates within 7 days as a white sediment apparent due to opalescence. | |
| Zinc metasilicate (ZnSiO3) | Insoluble (both at room temperature and upon heating) | |
| Zinc sulfate 7-hydrate (ZnSO4·7H2O) | Dissolves (up to 20 wt%) at 20–25 °C within 24 h. Remains stable during storage. |
| Element | Theoretical Content | Average Element Content, wt% | |
|---|---|---|---|
| Polymer Matrix | Particle | ||
| C | 72.8 | 82.0 | 73.9 |
| O | 17.8 | 14.1 | 14.3 |
| N | 3.39 | 3.6 | 4.0 |
| P | 0.46 | 0.3 | 7.1 |
| S | 0.03 | 0 | 0.3 |
| Zn | 0.06 | 0 | 0.4 |
| H 1 | 5.46 | - | - |
| Medium/Formulation | Distilled Water | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| pH | 6.02 | 6.50 | 6.60 | 6.80 | 7.00 | 7.40 |
| Content of ZnSO4· 7H2O, wt% | Content (Theor.) of P, wt% | Content (Theor.) of Zn, 103 wt% | LOI, vol% | THR, MJ/m2 | meanHRR, kW/m2 | PHRR, kW/m2 | FIGRA, kJ/m2c2 | Qef., kJ/g | TSR m2/m2 | Char Residue from Cone Calorimeter Test, wt% |
|---|---|---|---|---|---|---|---|---|---|---|
| - | - | - | 19.0 | 117.4 ± 1.3 | 361.3 ± 14.5 | 1254.6 ± 107.6 | 8.5 ± 0.2 | 21.4 ± 1.4 | 2242 ± 91 | 8.7 ± 0.5 |
| 0.03 | 0.46 | 6.8 | 23.1 | 81.9 ± 14.8 | 168.0 ± 14.0 | 517.5 ± 8.0 | 3.1 ± 0.3 | 16.7 ± 2.4 | 1920 ± 65 | 14.9 ± 0.2 |
| 0.07 | 0.46 | 15.9 | 23.2 | 86.3 ± 5.3 | 174.5 ± 8.5 | 512.4 ± 2.9 | 2.9 ± 0.1 | 17.9 ± 0.2 | 1849 ± 113 | 18.7 ± 2.1 |
| 0.13 | 0.46 | 29.6 | 23.3 | 77.7 ± 6.5 | 172.5 ± 1.5 | 534.7 ± 11.1 | 3.1 ± 0.1 | 16.6 ± 1.9 | 1570 ± 164 | 19.5 ± 0.2 |
| 0.22 | 0.46 | 50.0 | 23.5 | 73.0 ± 1.4 | 174.0 ± 13.0 | 610.9 ± 33.9 | 3.5 ± 0.5 | 15.3 ± 0.1 | 1610 ± 193 | 15.1 ± 0.5 |
| 0.35 | 0.46 | 79.6 | 23.9 | 71.1 ± 9.1 | 183.5 ± 3.5 | 504.0 ± 42.6 | 2.8 ± 0.3 | 15.3 ± 1.7 | 1575 ± 155 | 18.9 ± 3.2 |
| Content of ZnSO4·7H2O, wt% | Content (Theor.) of P, wt% | Content (Theor.) of Zn, 103 wt% | , °C | , °C | PMLR, %/min | Tm, °C | ΔmI, % |
|---|---|---|---|---|---|---|---|
| - | - | - | 294 | 401 | 20.6 | 360 | 40.4 |
| 0.03 | 0.46 | 6.8 | 276 | 384 | 17.5 | 364 | 46.5 |
| 0.07 | 0.46 | 15.9 | 295 | 385 | 19.8 | 364 | 51.5 |
| 0.13 | 0.46 | 29.6 | 298 | 385 | 21.6 | 363 | 47.3 |
| 0.22 | 0.46 | 50.0 | 281 | 382 | 16.0 | 361 | 44.3 |
| 0.35 | 0.46 | 79.6 | 289 | 382 | 23.1 | 363 | 45.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisov, S.V.; Buravov, B.A.; Kudryavtseva, D.A.; Kharlamov, V.O.; Kobelev, A.A.; Trubachev, S.A.; Vaniev, M.A.; Novakov, I.A. Low-Flammability Hybrid Polymer Materials Based on Epoxy Oligomers and In Situ-Synthesized Zinc-Containing Microparticles. Polymers 2025, 17, 3291. https://doi.org/10.3390/polym17243291
Borisov SV, Buravov BA, Kudryavtseva DA, Kharlamov VO, Kobelev AA, Trubachev SA, Vaniev MA, Novakov IA. Low-Flammability Hybrid Polymer Materials Based on Epoxy Oligomers and In Situ-Synthesized Zinc-Containing Microparticles. Polymers. 2025; 17(24):3291. https://doi.org/10.3390/polym17243291
Chicago/Turabian StyleBorisov, Sergey Vladimirovich, Boris Andreevich Buravov, Daria Andreevna Kudryavtseva, Valentin Olegovich Kharlamov, Artem Aleksandrovich Kobelev, Stanislav Albertovich Trubachev, Marat Abdurakhmanovich Vaniev, and Ivan Aleksandrovich Novakov. 2025. "Low-Flammability Hybrid Polymer Materials Based on Epoxy Oligomers and In Situ-Synthesized Zinc-Containing Microparticles" Polymers 17, no. 24: 3291. https://doi.org/10.3390/polym17243291
APA StyleBorisov, S. V., Buravov, B. A., Kudryavtseva, D. A., Kharlamov, V. O., Kobelev, A. A., Trubachev, S. A., Vaniev, M. A., & Novakov, I. A. (2025). Low-Flammability Hybrid Polymer Materials Based on Epoxy Oligomers and In Situ-Synthesized Zinc-Containing Microparticles. Polymers, 17(24), 3291. https://doi.org/10.3390/polym17243291





