High-Quality Agar Polysaccharide from Unexplored Gelidium micropterum Kützing Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of Native Agar
2.3. Preparation of Alkali-Treated (AT) Agar
2.4. Testing Physical Properties
2.5. Sulphate Content Analysis
2.6. FTIR Characterisation
2.7. 1H and 13C-NMR Characterisation
2.8. Molecular Weight Determination
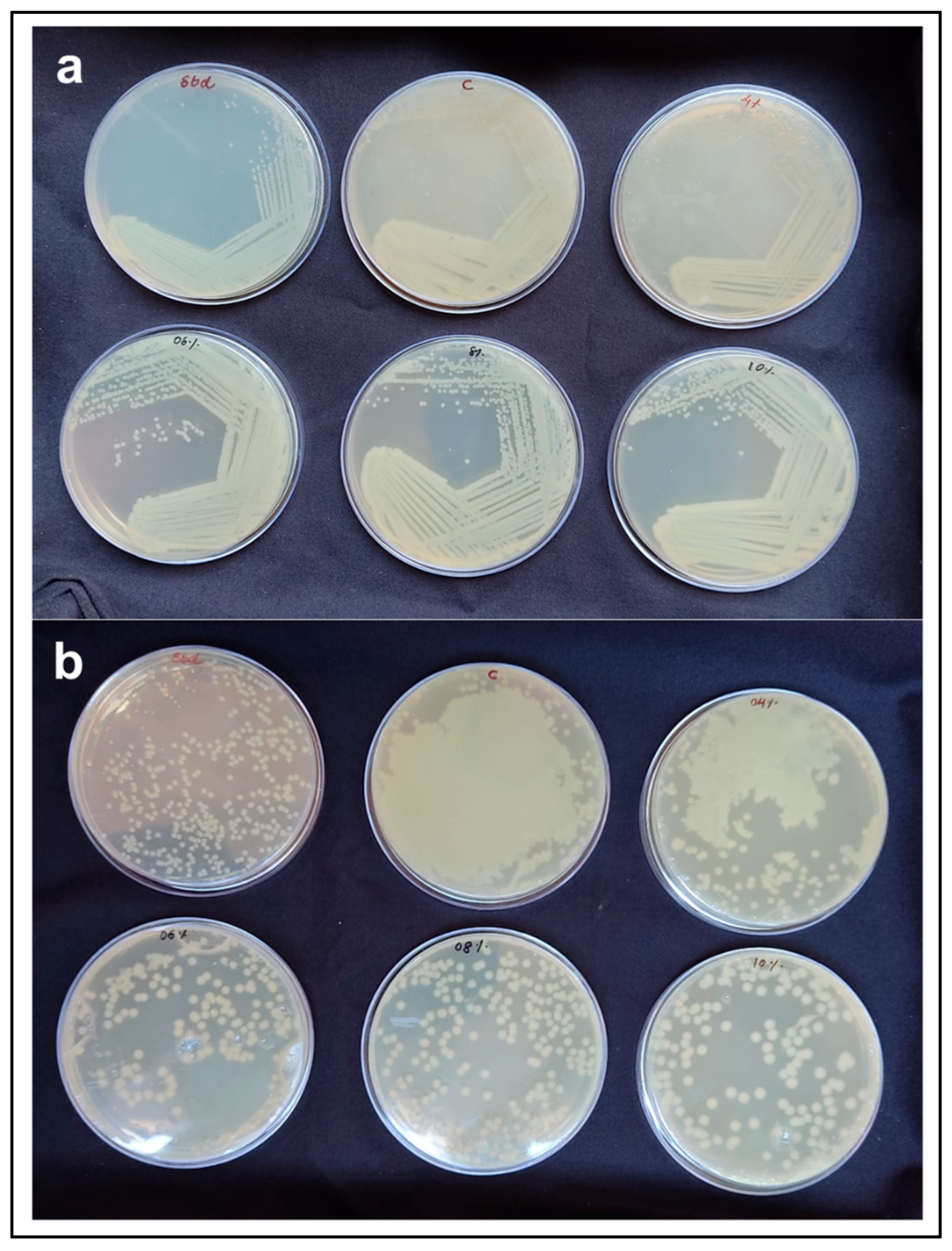
2.9. Testing Suitability of Agars for Bacteriological Application
3. Results and Discussion
3.1. Agar Yields
3.2. Agar Physical Properties
3.3. Sulphate Content
3.4. FT-IR Analysis
3.5. 1H and 13C-NMR Analysis
3.6. Molecular Weights
3.7. Suitability of Agars for Bacteriological Application
3.8. Applicability of Extracted Agar for Food Industry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT | Alkali-treated |
| ATA | Alkali-treated Agar |
| DW | Dry weight |
| FTIR | Fourier transform infrared spectroscopy |
| NMR | Nuclear Magnetic Resonance |
References
- Zhang, L.; Ye, S.; Chen, F.; Xiao, Q.; Weng, H.; Xiao, A. Super absorbent glutaric anhydride-modified agar: Structure, properties, and application in biomaterial delivery. Int. J. Biol. Macromol. 2023, 231, 123524. [Google Scholar] [CrossRef]
- Baghel, R.S.; Sharma, A.A.K.; Sandhya, S.V. Preparation and characterization of agar polysaccharide from red alga Phycocalidia vietnamensis (Bangiales). Waste Biomass Valoriz. 2024, 15, 6243–6249. [Google Scholar] [CrossRef]
- Polat, T.G.; Duman, O.; Tunç, S. Agar/κ-carrageenan/montmorillonite nanocomposite hydrogels for wound dressing applications. Int. J. Biol. Macromol. 2020, 164, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Mantri, V.A.; Shah, Y.; Thiruppathi, S. Feasibility of farming the agarose-yielding red alga Gracilaria dura using tube-net cultivation in the open sea along the Gujarat coast of NW India. Appl. Phycol. 2020, 1, 12–19. [Google Scholar] [CrossRef]
- Baghel, R.S.; Reddy, C.R.K.; Jha, B. Characterization of agarophytic seaweeds from the biorefinery context. Bioresour. Technol. 2014, 159, 280–285. [Google Scholar] [CrossRef]
- Reddy, C.R.K.; Rao, P.S.; Ganesan, M.; Eswaran, K.; Zaidi, S.H.; Mantri, V.A. The seaweed resources of India. In World Seaweed Resources; ACritchely, T., Ohno, M., Largo, D.B., Eds.; Eti Information Services Ltd.: Wokingham, UK, 2006; p. 25. [Google Scholar]
- Qin, Y. (Ed.) Seaweed bioresources. In Bioactive Seaweeds for Food Applications; Academic Press: Cambridge, MA, USA, 2018; pp. 3–24. [Google Scholar]
- Meena, R.; Prasad, K.; Ganesan, M.; Siddhanta, A.K. Superior quality agar from Gracilaria species (Gracilariales, Rhodophyta) collected from the Gulf of Mannar, India. J. Appl. Phycol. 2008, 20, 397–402. [Google Scholar] [CrossRef]
- Jha, B.; Reddy, C.R.K.; Thakur, M.C.; Rao, M.U. Seaweeds of India: The Diversity and Distribution of Seaweeds of Gujarat Coast; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Baghel, R.S.; Sharma, A.A.K.; Vas, A.D.; Reddy, C.R.K. Production of quality biomass of Gelidium micropterum Kützing through optimization of nutrients and salinity for sustainable land-based cultivation. J. Appl. Phycol. 2024, 36, 3537–3547. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Talha, M.A.; Baten, M.A.; Newaz, A.W.; Choi, J.S. Yield optimization, physicochemical characterizations, and antioxidant properties of food grade agar from Gracilaria tenuistipitata of Cox’s Bazar coast, Bangladesh. Food Sci. Nutr. 2023, 11, 2852–2863. [Google Scholar] [CrossRef]
- Belattmania, Z.; Bentiss, F.; Jama, C.; Nadri, A.; Reani, A.; Sabour, B. Spectroscopic characterization and gel properties of agar from two Gelidium species from the atlantic coast of morocco. Biointerface Res. Appl. Chem. 2021, 11, 12642–12652. [Google Scholar] [CrossRef]
- Veeragurunathan, V.; Vadodariya, N.; Chaudhary, J.P.; Gogda, A.; Saminathan, K.R.; Meena, R. Experimental cultivation of Gelidium pusillum in open sea along the south east Indian coast. Indian J. Geo-Mar. Sci. 2018, 47, 336–345. [Google Scholar]
- Vuai, S.A. Characterization of agar extracted from Gracilaria species collected along Tanzanian coast. Heliyon 2022, 8, e09002. [Google Scholar] [CrossRef]
- Zambuto, S.G.; Kolluru, S.S.; Ferchichi, E.; Rudewick, H.F. Evaluation of gelatin bloom strength on gelatin methacryloyl hydrogel properties. J. Mech. Behav. Biomed. Mater. 2024, 154, 106509. [Google Scholar] [CrossRef]
- Shukla, M.K.; Kumar, M.; Prasad, K.; Reddy, C.R.K.; Jha, B. Partial characterization of sulfohydrolase from Gracilaria dura and evaluation of its potential application in improvement of the agar quality. Carbohydr. Polym. 2011, 85, 157–163. [Google Scholar] [CrossRef]
- Murano, E.; Toffanin, R.; Zanetti, F.; Knutsen, S.H.; Paoletti, S.; Rizzo, R. Chemical and macromolecular characterisation of agar polymers from Gracilaria dura (C. Agardh) J. Agardh (Gracilariaceae, Rhodophyta). Carbohydr. Polym. 1992, 18, 171–178. [Google Scholar] [CrossRef]
- Torres, P.B.; Nagai, A.; Jara, C.E.P.; Santos, J.P.; Chow, F.; Santos, D.Y.A.C.D. Determination of sulfate in algal polysaccharide samples: A step-by-step protocol using microplate reader. Ocean Coast. Res. 2021, 69, e21021. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef]
- Sekkal, M.; Huvenne, J.P.; Legrand, P.; Sombret, B.; Mollet, J.C.; Mouradi-Givernaud, A.; Verdus, M.C. Direct structural identification of polysaccharides from red algae by FTIR microspectrometry I: Localization of agar in Gracilaria verrucosa sections. Mikrochim. Acta 1993, 112, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Z.; Mu, H.; Lin, Y.; Zhang, J.; Jiang, X. Impact of alkali pretreatment on yield, physico-chemical and gelling properties of high quality agar from Gracilaria tenuistipitata. Food Hydrocoll. 2017, 70, 356–362. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Lemus, A.; Bird, K.; Kapraun, D.F.; Koehn, F. Agar yield, quality and standing crop biomass of Gelidium serrulatum, Gelidium floridanum and Pterocladia capillacea in Venezuela. J. Appl. Phycol. 1991, 3, 469–479. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, X.; Zhang, J.; Zhang, Y.; Chen, J.; Chen, F.; Xiao, A. Pretreatment techniques and green extraction technologies for agar from Gracilaria lemaneiformis. Mar. Drugs 2021, 19, 617. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Shin, H.-J. Introduction of alkali soaking and microwave drying processes to improve agar quality of Gracilaria verrucosa. Korean J. Chem. Eng. 2017, 34, 3163–3169. [Google Scholar] [CrossRef]
- Veeragurunathan, V.; Prasad, K.; Singh, N.; Malarvizhi, J.; Mandal, S.K.; Mantri, V.A. Growth and biochemical characterization of green and red strains of the tropical agarophytes Gracilaria debilis and Gracilaria edulis (Gracilariaceae, Rhodophyta). J. Appl. Phycol. 2016, 28, 3479–3489. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Alves, V.D.; Morais, S.; Delerue-Matos, C.; Gonçalves, M.P. Agar extraction from integrated multitrophic aquacultured Gracilaria vermiculophylla: Evaluation of a microwave-assisted process using response surface methodology. Bioresour. Technol. 2010, 101, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Rodarte, M.A.; Hernández-Carmona, G.; Rodríguez-Montesinos, Y.E.; Arvizu-Higuera, D.L.; Riosmena-Rodríguez, R.; Murillo-Álvarez, J.I. Seasonal variation of agar from Gracilaria vermiculophylla, effect of alkali treatment time, and stability of its Colagar. J. Appl. Phycol. 2010, 22, 753–759. [Google Scholar] [CrossRef]
- Villanueva, R.D.; Sousa, A.M.M.; Gonçalves, M.P.; Nilsson, M.; Hilliou, L. Production and properties of agar from the invasive marine alga, Gracilaria vermiculophylla (Gracilariales, Rhodophyta). J. Appl. Phycol. 2010, 22, 211–220. [Google Scholar] [CrossRef]
- González-Leija, J.A.; Hernández-Garibay, E.; Pacheco-Ruíz, I.; Guardado-Puentes, J.; Espinoza-Avalos, J.; López-Vivas, J.M.; Bautista-Alcantar, J. Optimization of the yield and quality of agar from Gracilariopsis lemaneiformis (Gracilariales) from the Gulf of California using an alkaline treatment. J. Appl. Phycol. 2009, 21, 321–326. [Google Scholar] [CrossRef]
- Meena, R.; Prasad, K.; Siddhanta, A.K. Preparation of superior quality products from two Indian agarophytes. J. Appl. Phycol. 2011, 23, 183–189. [Google Scholar] [CrossRef]
- Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Murillo-Álvarez, J.I.; Muñoz-Ochoa, M.; Hernández-Carmona, G. Effect of alkali treatment time and extraction time on agar from Gracilaria vermiculophylla. J. Appl. Phycol. 2008, 20, 515–519. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Murano, E. Agars from three species of Gracilaria (Rhodophyta) from Yucatán Peninsula. Bioresour. Technol. 2005, 96, 295–302. [Google Scholar] [CrossRef]
- Rath, J.; Adhikary, S.P. Effect of alkali treatment on the yield and quality of agar from red alga Gracilaria verrucosa (Rhodophyta, Gracilariales) occurring at different salinity gradient of Chilika lake. Indian J. Mar. Sci. 2004, 33, 202–205. [Google Scholar]
- Freile-Pelegrín, Y.; Robledo, D. Influence of alkali treatment on agar from Gracilaria cornea from Yucatan, mexico. J. Appl. Phycol. 1997, 9, 533–539. [Google Scholar] [CrossRef]
- Sasuga, K.; Yamanashi, T.; Nakayama, S.; Ono, S.; Mikami, K. Optimization of yield and quality of agar polysaccharide isolated from the marine red macroalga Pyropia yezoensis. Algal Res. 2017, 26, 123–130. [Google Scholar] [CrossRef]
- Zhao, P.; Niu, J.; Huan, L.; Gu, W.; Wu, M.; Wang, G. Agar extraction from Pyropia haitanensis residue after the removal and purification of phycobiliproteins. J. Appl. Phycol. 2019, 31, 2497–2505. [Google Scholar] [CrossRef]
- Zhang, C.; An, D.; Xiao, Q.; Weng, H.; Zhang, Y.; Yang, Q.; Xiao, A. Preparation, characterization, and modification mechanism of agar treated with hydrogen peroxide at different temperatures. Food Hydrocoll. 2020, 101, 105527. [Google Scholar] [CrossRef]
- Nozid, N.A.C.; Ibrahim, N.H.; Yoshida, A.; Huang, Y.; Hirose, T.; Hirasaka, K. Valorization of agar extracted from Gelidium elegans via ultrasound-assisted extraction as potential film packaging by optimizing the incorporation of chitosan and curcumin. Int. J. Biol. Macromol. 2025, 327, 147323. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K. Structural analysis of agar-type polysaccharides by NMR spectroscopy. BBA Gen. Subj. 1973, 320, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, T.J.; Kumar, A. Efficient extraction of agarose from red algae using ionic liquids. Green Sustai. Chem. 2014, 4, 190–201. [Google Scholar] [CrossRef]
- Guerrero, P.; Etxabide, A.; Leceta, I.; Peñalba, M.; De la Caba, K. Extraction of agar from Gelidium sesquipedale (Rod-hopyta) and surface characterization of agar based films. Carbohydr. Polym. 2014, 99, 491–498. [Google Scholar] [CrossRef]
- Usov, A.I.; Ivanova, E.G.; Shashkov, A.S. Polysaccharides of algae XXXIII: Isolation and 13C-NMR spectral study of some new gel-forming polysaccharides from Japan sea red seaweeds. Bot. Mar. 1983, 26, 285–294. [Google Scholar] [CrossRef]
- Thandavamoorthy, R.; Devarajan, Y.; Thanappan, S. Analysis of the characterization of NaOH-treated natural cellulose fibre extracted from banyan aerial roots. Sci. Rep. 2023, 13, 12579. [Google Scholar] [CrossRef]
- Beruto, M.; Beruto, D.; Debergh, P. Influence of agar on in vitro cultures: I. Physicochemical properties of agar and agar gelled media. Vitr. Cell Dev. Biol. Plant 1999, 35, 86–93. [Google Scholar] [CrossRef]
- Reddy, C.R.; Baghel, R.S.; Trivedi, N.; Kumari, P.; Gupta, V.; Prasad, K.; Meena, R. An Integrated Process to Recover a Spectrum of Bioproducts from Fresh Seaweeds. U.S. Patent No. 10,000,579, 19 June 2018. [Google Scholar]
- Abbott, I.A.; Chapman, F.A. Evaluation of kappa carrageenan as a substitute for agar in microbiological media. Arch. Microbiol. 1981, 128, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Burey, P.; Bhandari, B.R.; Rutgers, R.P.G.; Halley, P.J.; Torley, P.J. Confectionery gels: A review on formulation, rheological and structural aspects. Int. J. Food Prop. 2009, 12, 176–210. [Google Scholar] [CrossRef]
- Liao, Y.C.; Chang, C.C.; Nagarajan, D.; Chen, C.Y.; Chang, J.S. Algae-derived hydrocolloids in foods: Applications and health-related issues. Bioengineered 2021, 12, 3787–3801. [Google Scholar] [CrossRef]



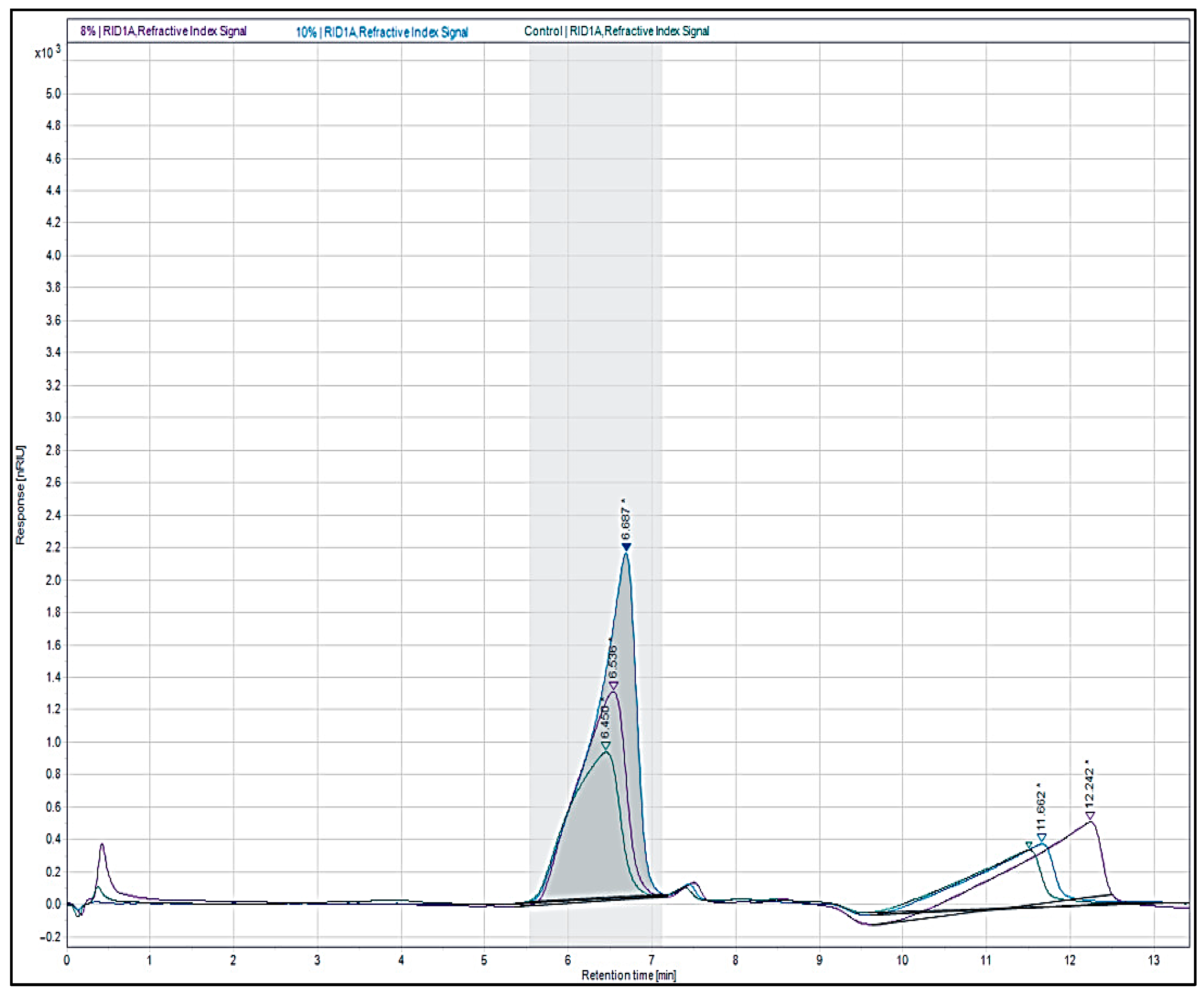
| NaOH Conc. % (w/v) | Agar Yields (% DW) | Gel Strength (g/cm2) | Gelling Temp. (°C) | Melting Temp. (°C) | Viscosity (mPa·S) |
|---|---|---|---|---|---|
| Native agar (0) | 26.03 ± 1.86 | 855 ± 51 | 39.8 ± 0.25 | 90.33 ± 0.57 | 18.34 ± 2.81 |
| 4 | 24.1 ± 0.8 | 1110 ± 34 | 40.6 ± 0.15 | 91.5 ± 0.5 | 26.15 ± 6.41 |
| 6 | 20.5. ± 1.5 | 1762 ± 37 | 41 ± 0.25 | 94 ± 0.5 | 30.32 ± 5.87 |
| 8 | 18.06 ± 1.3 | 1940 ± 12 | 41.6 ± 0.2 | 94.83 ± 0.28 | 62.54 ± 13.61 |
| 10 | 16.97 ± 0.78 | 2078 ± 55 | 42 ± 0.55 | 96.5 ± 0.5 | 63.03 ± 10.66 |
| S. No. | Species | Alkali Pre-Treatment Conc. % (Agar Yields % and Gel Strength g/cm2) | References |
|---|---|---|---|
| 1 | Gelidium micropterum | 0 (26 and 855), 4 (24 and 1110), 6 (20.5 and 1762), 8 (18 and 1940) 10 (16.97 and 2078) | Present study |
| 2 | Gelidium corneum | 0 (16 and 341), 10 (6 and 529) | [12] |
| 3 | Gelidium microdon | 0 (12 and 350), 10 (15 and 489) | |
| 4 | Gelidium sesquipedale | 0 (12 and 245), 10 (3 and 979) | [22] |
| 5 | Gelidium pusillum | 0 (13 and 350), 5 (11 and 1550), 8 (10 and 1800), 10 (9.5 and 2100), 15 (8 and 2100) | [13] |
| 6 | Gelidiella acerosa | 0 (28 and 800), 4 (24 and 1025), 6 (18 and 1400), 8 (13 and 1850), 10 (12 and 2000), 15 (11 and 2000) | [31] |
| 7 | Gelidium pusillum | 0 (19 and 740), 4 (18 and 1000), 6 (17 and 1200), 8 (12 and 1400), 10 (12 and 1500), 15 (10 and 1500) | |
| 8 | Pterocladia capillacea | 0 (32.1 and 753.3), 4 (27.6 and 1470) | [23] |
| 9 | Gelidium floridanum | 0 (31.7 and 1030), 4 (24.2 and 730) | |
| 10 | Gelidium serrulatum | 0 (33 and 380), 4 (15.4 and 687) |
| SI No. | Agar Type (Used Conc. %) | Total Bacterial Count (CFU mL−1) |
|---|---|---|
| 1 | Native agar (1.93) | Mat growth |
| 2 | 4% ATA (1.47) | Mat growth |
| 3 | 6% ATA (0.94) | 1.89 × 108 |
| 4 | 8% ATA (0.85) | 2.32 × 108 |
| 5 | 10% ATA (0.79) | 1.4 × 108 |
| 6 | Commercial standard agar | 4.28 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.A.K.; Baghel, R.S.; Sandhya, S.V.; Kaushik, R.; Jagtap, A.S.; Vaishnavi, B. High-Quality Agar Polysaccharide from Unexplored Gelidium micropterum Kützing Biomass. Polymers 2025, 17, 3278. https://doi.org/10.3390/polym17243278
Sharma AAK, Baghel RS, Sandhya SV, Kaushik R, Jagtap AS, Vaishnavi B. High-Quality Agar Polysaccharide from Unexplored Gelidium micropterum Kützing Biomass. Polymers. 2025; 17(24):3278. https://doi.org/10.3390/polym17243278
Chicago/Turabian StyleSharma, Anurag A. K., Ravi S. Baghel, S. V. Sandhya, Rahul Kaushik, Ashok S. Jagtap, and Balaji Vaishnavi. 2025. "High-Quality Agar Polysaccharide from Unexplored Gelidium micropterum Kützing Biomass" Polymers 17, no. 24: 3278. https://doi.org/10.3390/polym17243278
APA StyleSharma, A. A. K., Baghel, R. S., Sandhya, S. V., Kaushik, R., Jagtap, A. S., & Vaishnavi, B. (2025). High-Quality Agar Polysaccharide from Unexplored Gelidium micropterum Kützing Biomass. Polymers, 17(24), 3278. https://doi.org/10.3390/polym17243278






