3.1. Characterization of the Fabricated MnCo2O4 Samples
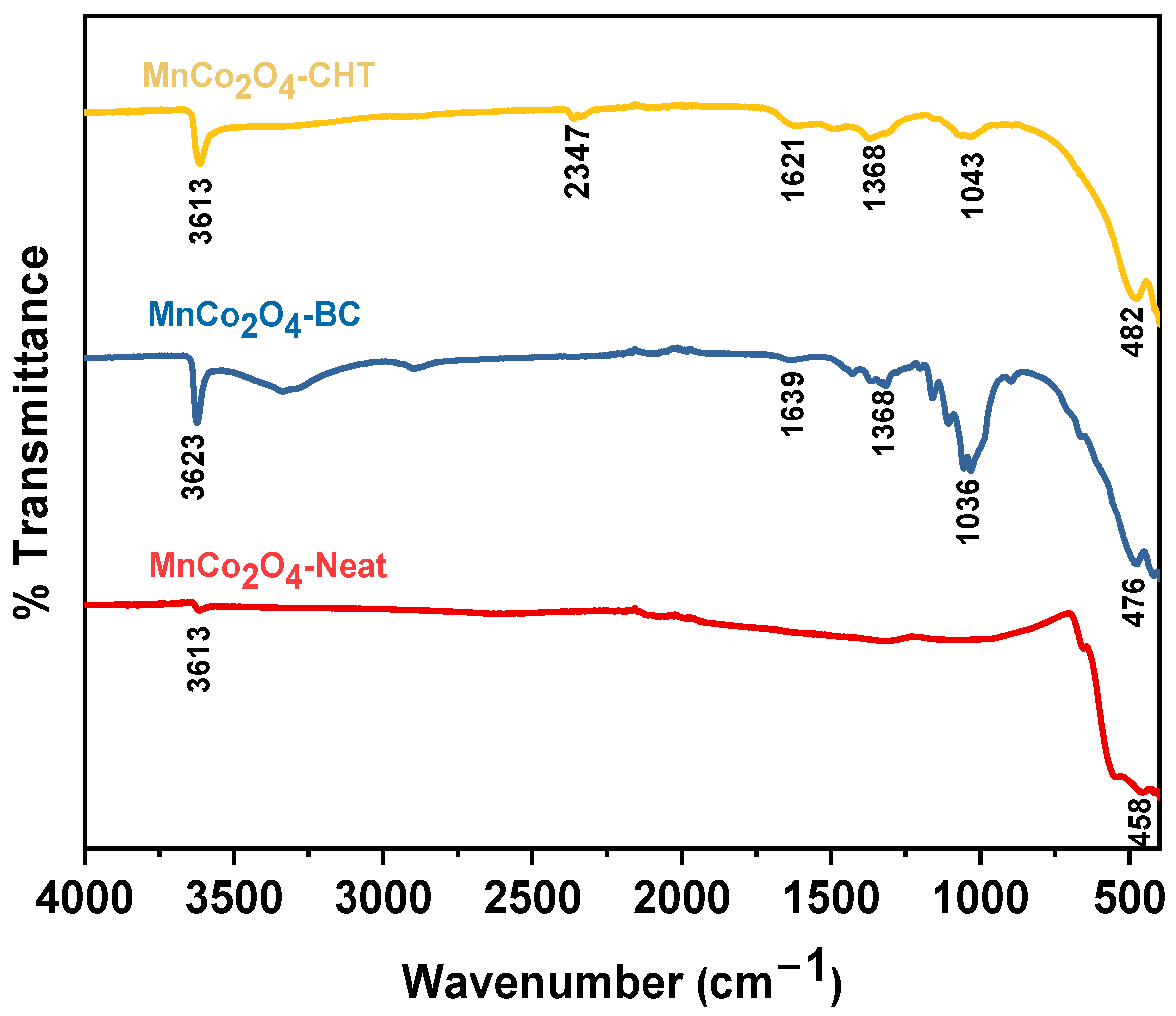
Figure 1 illustrates the FTIR spectra of MnCo
2O
4 synthesized with no stabilizer (MnCo
2O
4-Neat), with biomass-cellulose (MnCo
2O
4-BC), and with chitosan (MnCo
2O
4-CHT) as biodegradable stabilizers. The analysis highlights key functional groups, structural features, and the influence of stabilizers on the MnCo
2O
4 nanoparticles, emphasizing the role of surface modifications in enhancing their catalytic properties. All three samples exhibit broad absorption bands in the range of 3613–3623 cm
−1, attributed to the O–H stretching vibrations of adsorbed water molecules or surface hydroxyl groups. These hydrophilic groups are crucial for catalytic processes as they enhance interaction with polar dye molecules like methylene blue (MB). The distinct peaks observed at 458–482 cm
−1 and 1036–1043 cm
−1 correspond to Co–O and Mn–O stretching vibrations, respectively, confirming the successful formation of the MnCo
2O
4 spinel structure across all samples. The MnCo
2O
4-Neat sample, synthesized without any stabilizer, shows relatively weaker and less pronounced peaks compared to the stabilized samples. This indicates a simpler surface chemistry with fewer functional groups, which might limit its interaction with reactants and potentially reduce its catalytic efficiency. In contrast, MnCo
2O
4-BC, synthesized using biomass-cellulose, displays notable peaks at 1639 cm
−1 and 1368 cm
−1, corresponding to C=O and C–O stretching vibrations. These peaks signify the presence of cellulose residues on the nanoparticle surface, which likely enhance its adsorption capabilities by providing additional polar functional groups. This surface modification may improve the photocatalytic degradation of MB by increasing the interaction between the dye and the catalyst surface. Similarly, MnCo
2O
4-CHT, synthesized with chitosan as the stabilizer, exhibits strong peaks at 1621 cm
−1 and 1368 cm
−1, corresponding to amide I (C=O stretching) and amide III (C–N stretching and N–H bending) vibrations. The presence of these functional groups suggests a robust interaction between chitosan and the MnCo
2O
4 nanoparticles, leading to a modified surface with enhanced adsorption sites. The sharper peaks indicate a stronger impact of chitosan in tailoring the surface chemistry compared to cellulose, which may contribute to improved dispersion and reactivity in photocatalytic applications. The comparison across the three samples highlights the critical role of stabilizers in modifying the surface properties of MnCo
2O
4 nanoparticles. While MnCo
2O
4-Neat has a simpler structure, the incorporation of biomass-cellulose and chitosan introduces additional functional groups, enhancing the nanoparticles’ ability to adsorb and degrade MB. These modifications are expected to improve the photocatalytic activity of MnCo
2O
4 by facilitating better interaction with MB and enhancing the degradation process. In conclusion, the FTIR spectra confirm the successful synthesis of MnCo
2O
4 spinel nanoparticles and underline the significant influence of biodegradable stabilizers on their surface chemistry [
30,
31,
32,
33]. The results demonstrate that the choice of stabilizer, whether biomass-cellulose or chitosan, plays a crucial role in tailoring the nanoparticles’ properties for environmental remediation applications, such as the photocatalytic degradation of MB. This work underscores the potential of bio-degradable stabilizers to enhance the performance of MnCo
2O
4 in catalytic processes, paving the way for more efficient and sustainable solutions in water treatment.
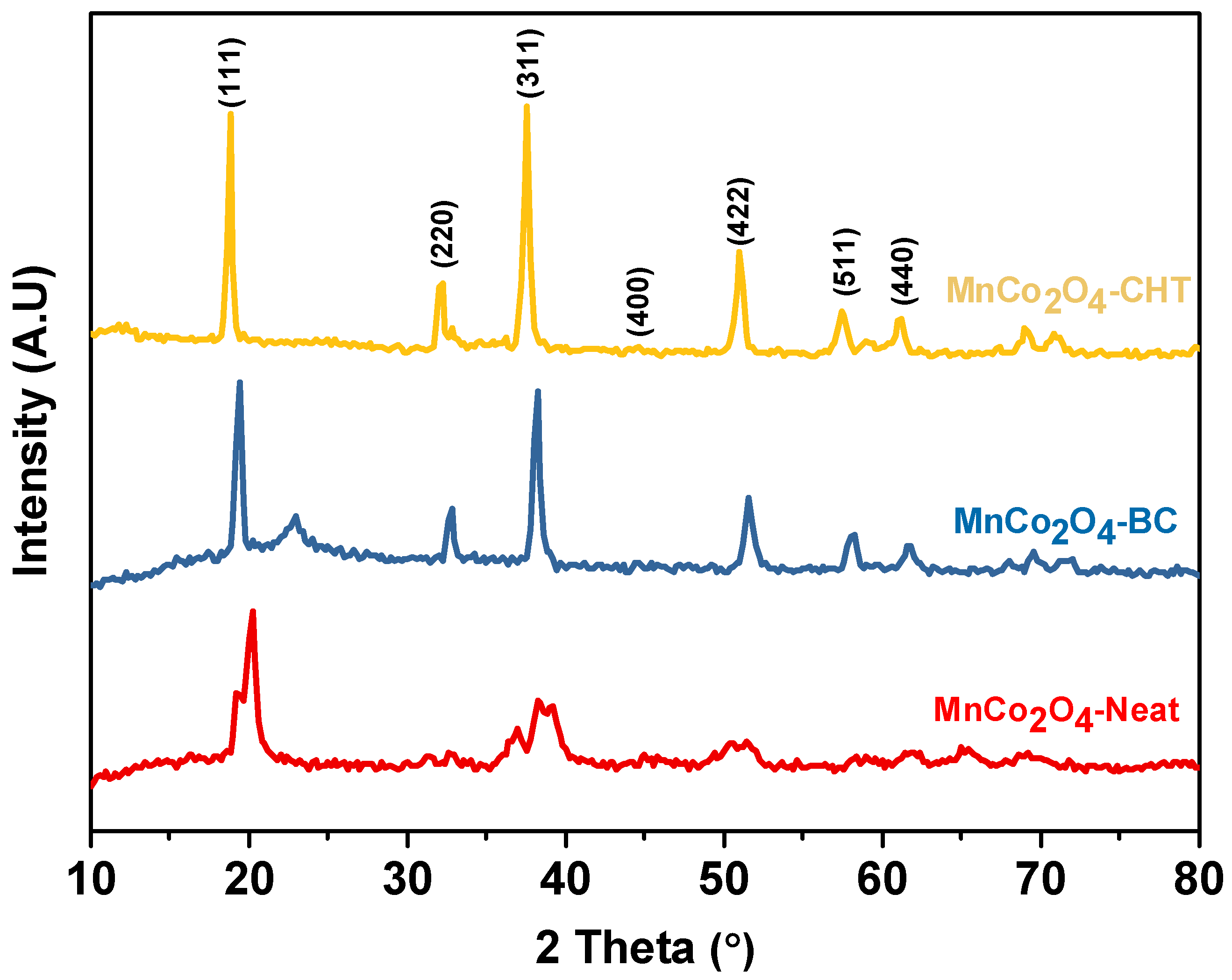
Figure 2 displays the X-ray diffraction (XRD) patterns of MnCo
2O
4 synthesized with and without stabilizers: MnCo
2O
4-Neat (without a stabilizer), MnCo
2O
4-BC (using biomass-cellulose as a stabilizer), and MnCo
2O
4-CHT (using chitosan as a stabilizer). The diffraction peaks at the 2θ values of 18.71, 32.11, 37.6, 44.4, 50.9, 57.56, and 61.2°, which are correspond to the characteristic planes of the MnCo
2O
4 spinel structure, including (111), (220), (311), (400), (511), and (440). These patterns confirm the successful formation of the spinel MnCo
2O
4 crystalline phase while demonstrating the significant impact of the stabilizers on the material’s crystallinity and structural integrity. The XRD patterns of MnCo
2O
4 closely align with the standard JCPDS card no. 023-1237, confirming the successful formation of the expected spinel structure. However, notable differences in the sharpness, intensity, and width of the peaks highlight the influence of stabilizers on the crystallinity and grain size of the nanoparticles. The absence of any additional peaks indicates the phase purity of the synthesized MnCo
2O
4 compound. The XRD pattern of MnCo
2O
4-Neat shows broader peaks with relatively low intensity, indicating lower crystallinity and smaller grain size. The absence of a stabilizing agent likely led to uncontrolled nucleation and growth, resulting in less uniform particles and higher structural disorder. These characteristics could negatively impact the material’s performance, as lower crystallinity may hinder charge carrier mobility in photocatalytic applications. The XRD pattern of MnCo
2O
4-BC reveals sharper and more intense peaks compared to MnCo
2O
4-Neat, reflecting improved crystallinity. Biomass-cellulose acts as a stabilizing scaffold during synthesis, facilitating controlled nucleation and growth of the crystalline phase. The cellulose matrix likely ensures a more uniform dispersion of the metal precursors, preventing agglomeration and promoting the formation of well-ordered particles. This enhancement in crystallinity contributes to improved material properties, such as better structural stability and potential photocatalytic activity. Additionally, the MnCo
2O
4-CHT sample exhibits the sharpest and most intense diffraction peaks, indicating the highest degree of crystallinity among the three samples. Chitosan’s chelating ability, attributed to its functional groups (e.g., –NH
2 and –OH), plays a critical role in coordinating metal ions during synthesis. This interaction promotes a highly ordered arrangement of atoms in the spinel structure. Additionally, the polymeric network of chitosan provides a template that ensures uniform nucleation and growth, resulting in enhanced structural properties. The degree of crystallinity has direct implications for the photocatalytic performance of MnCo
2O
4. Higher crystallinity, as observed in MnCo
2O
4-BC and MnCo
2O
4-CHT, reduces defect sites and improves charge carrier mobility, essential for efficient photocatalysis. Additionally, the functional groups introduced by the stabilizers, such as hydroxyl and amino groups, enhance surface adsorption of methylene blue, further improving the degradation efficiency. The superior crystallinity and surface properties of MnCo
2O
4-CHT suggest that it may exhibit the best photocatalytic performance among the three samples. The XRD analysis underscores the critical role of stabilizers in influencing the structural properties of MnCo
2O
4 nanoparticles. While the MnCo
2O
4-Neat sample demonstrates lower crystallinity, the use of biomass-cellulose and chitosan significantly enhances the crystallinity and structural order. Among the stabilizers, chitosan proves to be the most effective, yielding the sharpest peaks and the highest crystallinity. These structural improvements, facilitated by the stabilizers, are expected to enhance the photocatalytic degradation efficiency of methylene blue, demonstrating the importance of stabilizer selection in optimizing material properties for environmental remediation applications. The crystallite size (t) was estimated using Scherer’s formula [
33]:
where, t is the average crystallites size, λ is the wavelength of the X-ray (Cu Kα1, 1.5418 Å), β is the full-width half maximum, and θ is the diffraction angle. The calculated crystallite sizes are 19.1 nm for MnCo
2O
4-Neat, 16.4 nm for MnCo
2O
4-BC, and 13.3 nm for MnCo
2O
4-CHT. The differences in crystallite size are attributed to the influence of the specific additives used during the synthesis of each sample. The average lattice parameter (
a) for all samples was calculated using the equation
a = d
, based on the (311) plane. The obtained lattice parameters were 8.463 Å for MnCo
2O
4-Neat, 8.475 Å for MnCo
2O
4-BC, and 8.482 Å for MnCo
2O
4-CHT, all of which are close to the theoretical value of 8.49 Å [
34,
35].
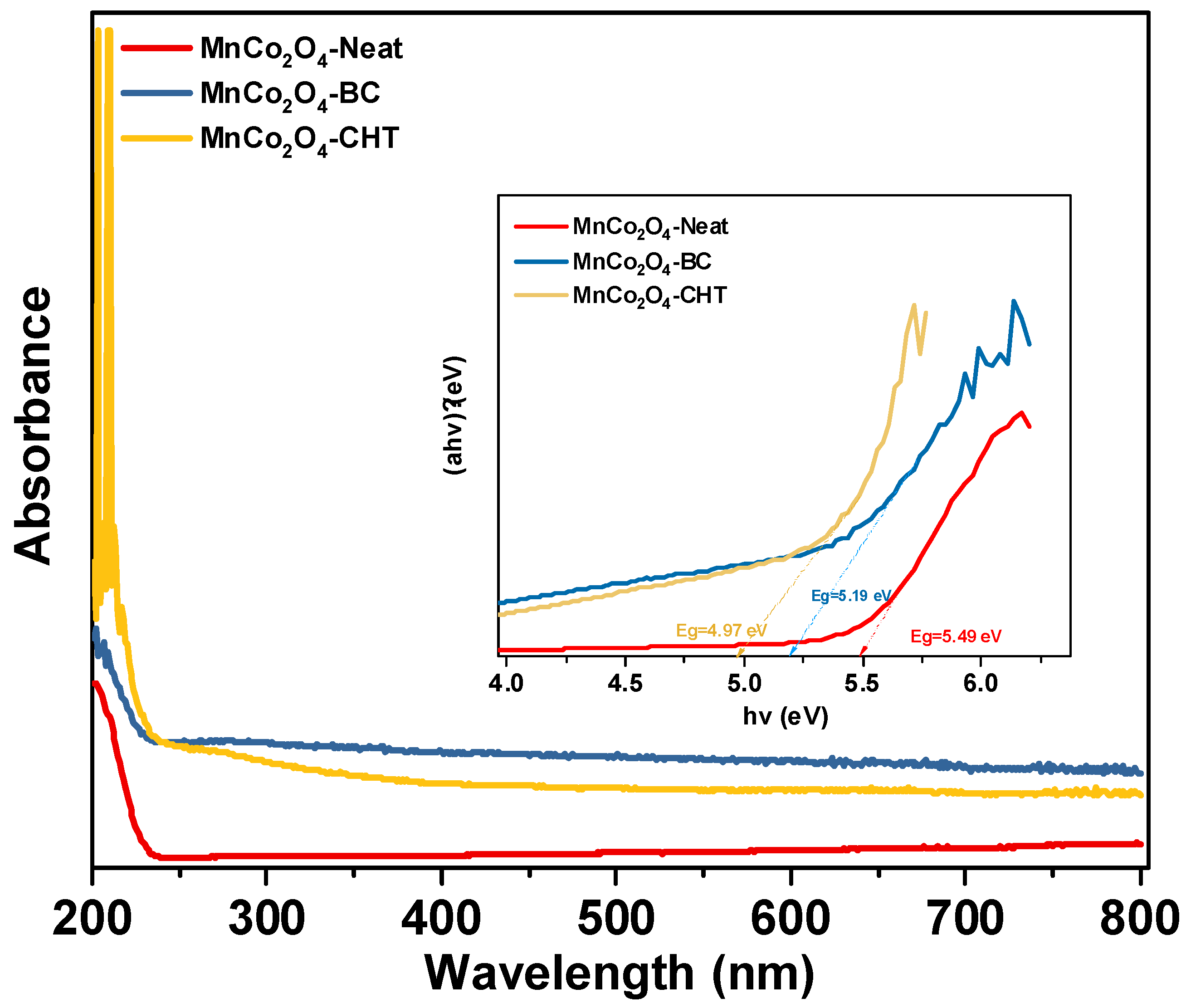
The UV–Vis absorption spectra and corresponding Tauc plots (inset) (
Figure 3) of MnCo
2O
4-Neat, MnCo
2O
4-CHT, and MnCo
2O
4-BC provide valuable insights into the optical properties of these materials and the effect of biodegradable stabilizers on their electronic structure. All samples exhibit significant absorption in the UV and visible regions, indicative of their ability to absorb light across a broad wavelength range, which is beneficial for photocatalytic applications. The absorption onset in the visible region corresponds to the charge transfer transitions between the Co
3+/Mn
3+ d-orbitals and the O
2− p-orbitals, a characteristic of the MnCo
2O
4 spinel structure. The MnCo
2O
4-Neat sample shows a lower absorption intensity in the visible region compared to MnCo
2O
4-BC and MnCo
2O
4-CHT, indicating that the absence of stabilizers results in less efficient light-harvesting capability. The MnCo
2O
4-BC sample, synthesized with biomass-cellulose, demonstrates enhanced light absorption in the visible region, likely due to the improved crystallinity and better dispersion of particles facilitated by the stabilizer. MnCo
2O
4-CHT, synthesized using chitosan as a stabilizer, exhibits the highest absorption intensity, which can be attributed to the strong interaction between chitosan’s functional groups and the metal ions during synthesis, leading to improved particle homogeneity and surface modification. The Tauc plots (
inset) were used to estimate the optical band gaps of the samples. The calculated band gap energies are approximately 5.49 eV, 5.19 V, and 4.97 eV for MnCo
2O
4-Neat, MnCo
2O
4-BC, and MnCo
2O
4-CHT, respectively. The slight reduction in the band gap for MnCo
2O
4-BC and MnCo
2O
4-CHT compared to MnCo
2O
4-Neat suggests that the stabilizers introduce surface states or modify the electronic structure, enhancing visible light absorption. Chitosan, in particular, appears to lower the band gap the most, potentially due to its chelating effects and the incorporation of functional groups that alter the band structure. The results demonstrate that the choice of stabilizer significantly affects the optical properties of MnCo
2O
4. The improved light absorption and reduced band gap observed in MnCo
2O
4-BC and MnCo
2O
4-CHT highlight the importance of biodegradable stabilizers in tailoring the material’s electronic and optical properties, making them more suitable for photocatalytic applications such as the degradation of methylene blue. These findings underscore the critical role of stabilizer selection in optimizing MnCo
2O
4 for enhanced photocatalytic performance [
30,
32,
34,
36].
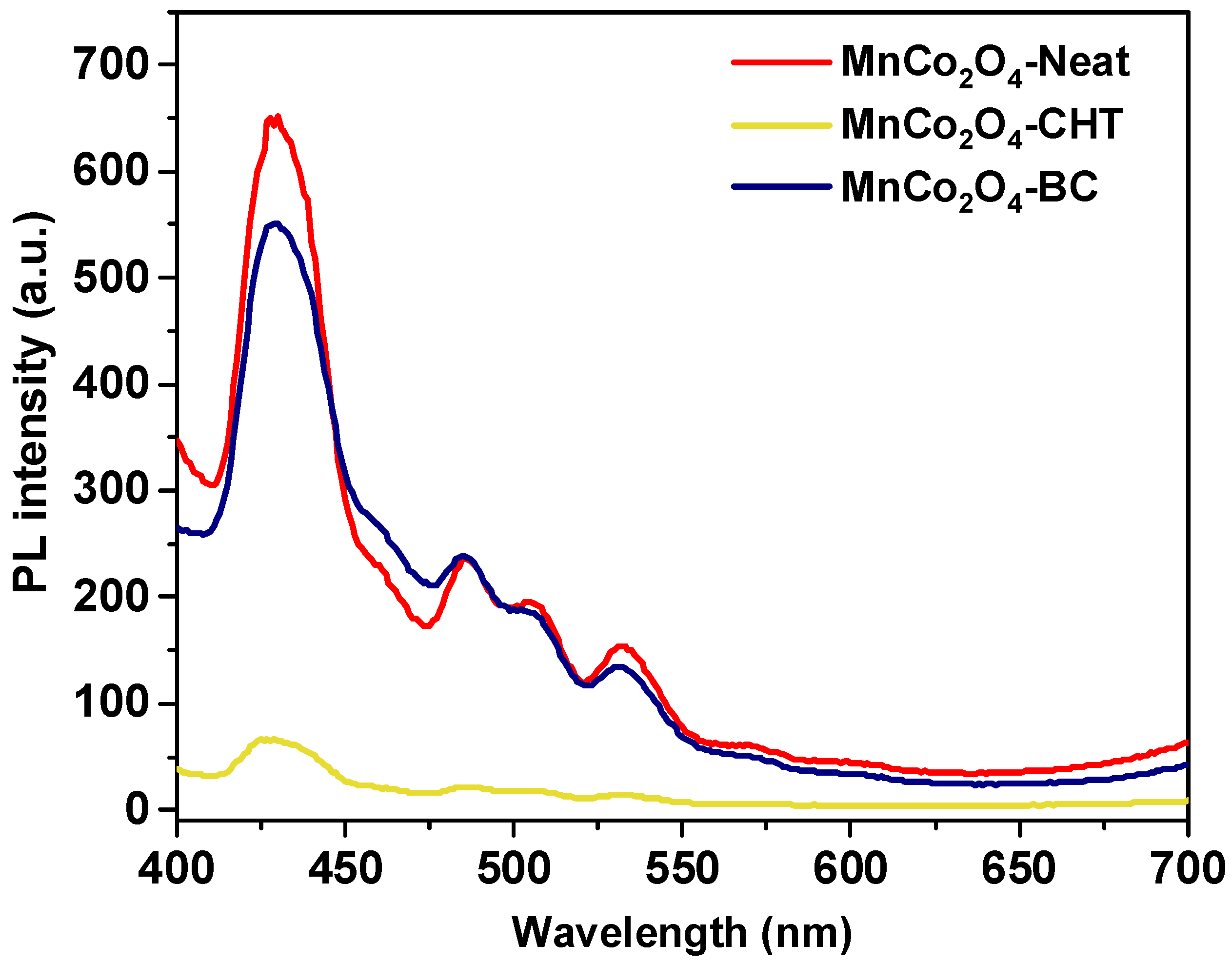
The photoluminescence (PL) spectra in
Figure 4 illustrate the recombination rates of photogenerated electron-hole pairs for MnCo
2O
4-Neat, MnCo
2O
4-BC, and MnCo
2O
4-CHT, synthesized with and without biodegradable stabilizers. PL spectra are a critical tool to evaluate charge recombination processes in photocatalysts, as lower PL intensity indicates more effective separation of charge carriers, enhancing photocatalytic performance. The MnCo
2O
4-Neat sample displays the highest PL intensity, indicating a higher recombination rate of electron-hole pairs. The absence of stabilizers results in minimal surface modifications or structural optimization, contributing to inefficient charge separation and limiting photocatalytic efficiency. For MnCo
2O
4-BC, synthesized with biomass-cellulose, the PL intensity is significantly reduced compared to MnCo
2O
4-Neat. This reduction can be attributed to the stabilizing effect of cellulose, which enhances the crystalline quality and introduces surface modifications. These changes lower the recombination rate of charge carriers, improving photocatalytic efficiency. On the other hand, MnCo
2O
4-CHT, synthesized using chitosan, exhibits the lowest PL intensity among the samples, suggesting the most effective suppression of charge recombination. The strong chelating and templating effects of chitosan during synthesis likely improve structural homogeneity and introduce functional groups that act as charge trapping sites, further enhancing charge separation and transport. The observed differences in PL intensity correlate directly with the electronic properties of the materials. The HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) levels of MnCo
2O
4 are critical for charge transfer processes. The incorporation of biodegradable stabilizers appears to influence these levels: (i): A higher recombination rate of MnCo
2O
4-Neat, suggests less effective overlap or mismatch between HOMO and LUMO, leading to rapid electron-hole recombination. (ii) The cellulose stabilizer of MnCo
2O
4-BC improves HOMO-LUMO alignment by modifying the surface energy states, facilitating better charge transfer. (iii) Chitosan introduces functional groups that lower the recombination rate by optimizing HOMO-LUMO interactions of MnCo
2O
4-CHT and creating effective pathways for charge separation. The results of this study are supported by previous research highlighting the role of surface modifications and stabilizers in enhancing charge separation and suppressing recombination in photocatalysts. For example, the Ag
2CO
3/AgX heterojunctions synthesized by Dong et al. (2014) demonstrated improved photocatalytic activity due to efficient charge carrier separation and suppressed recombination at the interface, which aligns with the reduced PL intensity observed for MnCo
2O
4-BC and MnCo
2O
4-CHT [
37]. Similarly, Yadav et al. (2017) reported that tungsten-doped TiO
2/rGO composites exhibited reduced PL intensity due to the presence of tungsten as an electron trap, a phenomenon comparable to the charge trapping and improved HOMO-LUMO interactions introduced by chitosan in MnCo
2O
4-CHT [
38]. Moreover, Fakhravar et al. (2020) showed that the incorporation of Cu
2S and Ag
2S in a ternary heterostructure significantly enhanced charge carrier mobility and reduced recombination, similar to the role of biomass-cellulose and chitosan in optimizing MnCo
2O
4’s surface and electronic properties [
39]. Finally, Du et al. (2021) [
40,
41] highlighted the role of functional groups in reducing charge recombination in a g-C
3N
4/MnO
2/GO heterojunction, corroborating our findings that chitosan enhances surface functionality and reduces recombination in MnCo
2O
4-CHT. These studies collectively support our results, emphasizing the effectiveness of biodegradable stabilizers in improving the photocatalytic performance of MnCo
2O
4.
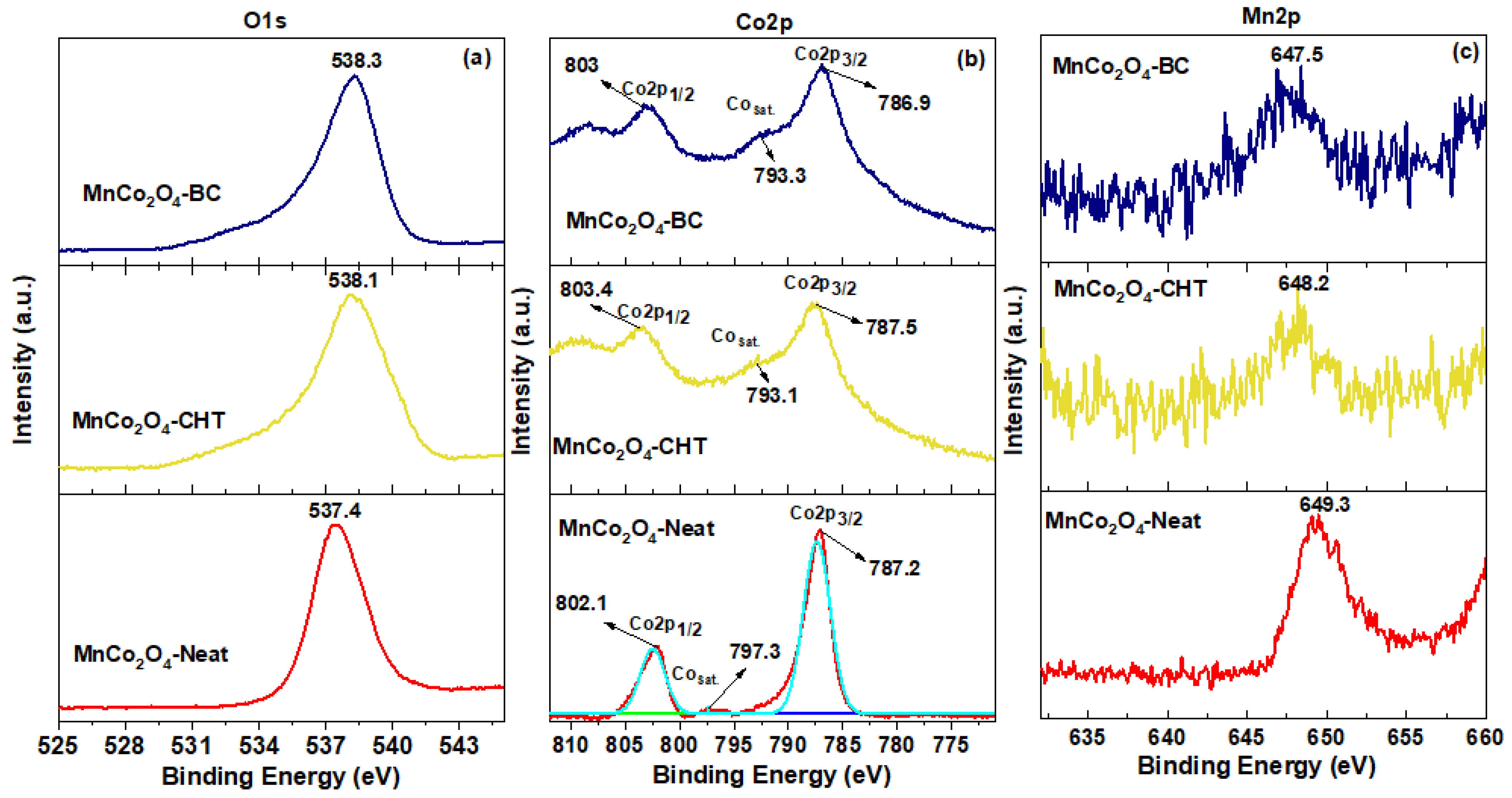
The X-ray photoelectron spectroscopy (XPS) results provide a comprehensive understanding of the surface chemistry and electronic structure of MnCo
2O
4-Neat, MnCo
2O
4-BC, and MnCo
2O
4-CHT, shedding light on the factors driving their photocatalytic performance (
Figure 5). The analysis of the O1s, Co2p, and Mn2p spectra highlights the critical role of biodegradable stabilizers in modifying the surface properties of MnCo
2O
4 and correlates directly with their observed photocatalytic efficiency. The O1s spectra reveal the presence of surface-adsorbed oxygen species, such as hydroxyl groups and chemisorbed water, which are crucial for generating reactive oxygen species (ROS) during photocatalysis. The higher binding energy observed for MnCo
2O
4-CHT and MnCo
2O
4-BC suggests an enhanced abundance of these active oxygen species, likely due to the interaction between the stabilizers and the MnCo
2O
4 framework. This increased reactivity facilitates the adsorption and activation of methylene blue (MB) molecules, contributing to the superior degradation performance observed for MnCo
2O
4-CHT (96% MB degradation) and MnCo
2O
4-BC (65%), compared to MnCo
2O
4-Neat (45%). The Co2p spectra further emphasize the impact of stabilizers on the electronic structure. The increased Co
3+/Co
2+ ratio in MnCo
2O
4-CHT reflects enhanced redox activity, which is critical for efficient charge transfer and reduced electron-hole recombination. Chitosan’s chelating effect likely stabilizes Co
3+ species, while the templating role of cellulose introduces structural modifications that similarly improve the Co
3+ contribution in MnCo
2O
4-BC. These redox-active sites promote photocatalytic reactions by facilitating the transfer of electrons to oxygen molecules, generating ROS to degrade MB. Similarly, the Mn2p spectra highlight the stabilization of Mn
3+ and Mn
4+ states, particularly in MnCo
2O
4-CHT and MnCo
2O
4-BC. The highest binding energy shift in MnCo
2O
4-CHT reflects the presence of a higher proportion of Mn
4+ species, which are critical for enhancing charge carrier dynamics and oxidative degradation. The presence of Mn
4+ improves electron-hole separation, reducing recombination rates and further contributing to the high catalytic efficiency of MnCo
2O
4-CHT. In contrast, MnCo
2O
4-Neat shows lower Mn
4+ contributions, consistent with its lower photocatalytic performance. These XPS findings align closely with the photocatalytic activity of the samples. The superior performance of MnCo
2O
4-CHT is attributed to its optimized surface chemistry, with an abundance of reactive oxygen species, enhanced Co
3+/Co
2+ ratios, and stabilized Mn
4+ states. MnCo
2O
4-BC also exhibits improved activity due to the templating role of cellulose, which enhances surface reactivity. In contrast, the lower activity of MnCo
2O
4-Neat reflects its less favorable surface and electronic properties. In conclusion, the XPS analysis establishes a strong correlation between the surface chemistry of MnCo
2O
4 nanospinels and their photocatalytic efficiency. The use of chitosan and cellulose as stabilizers significantly enhances the catalytic activity by improving the availability of active oxygen species, tuning redox states, and optimizing charge transfer. These findings underline the critical role of biodegradable stabilizers in designing efficient and sustainable photocatalysts for environmental remediation.
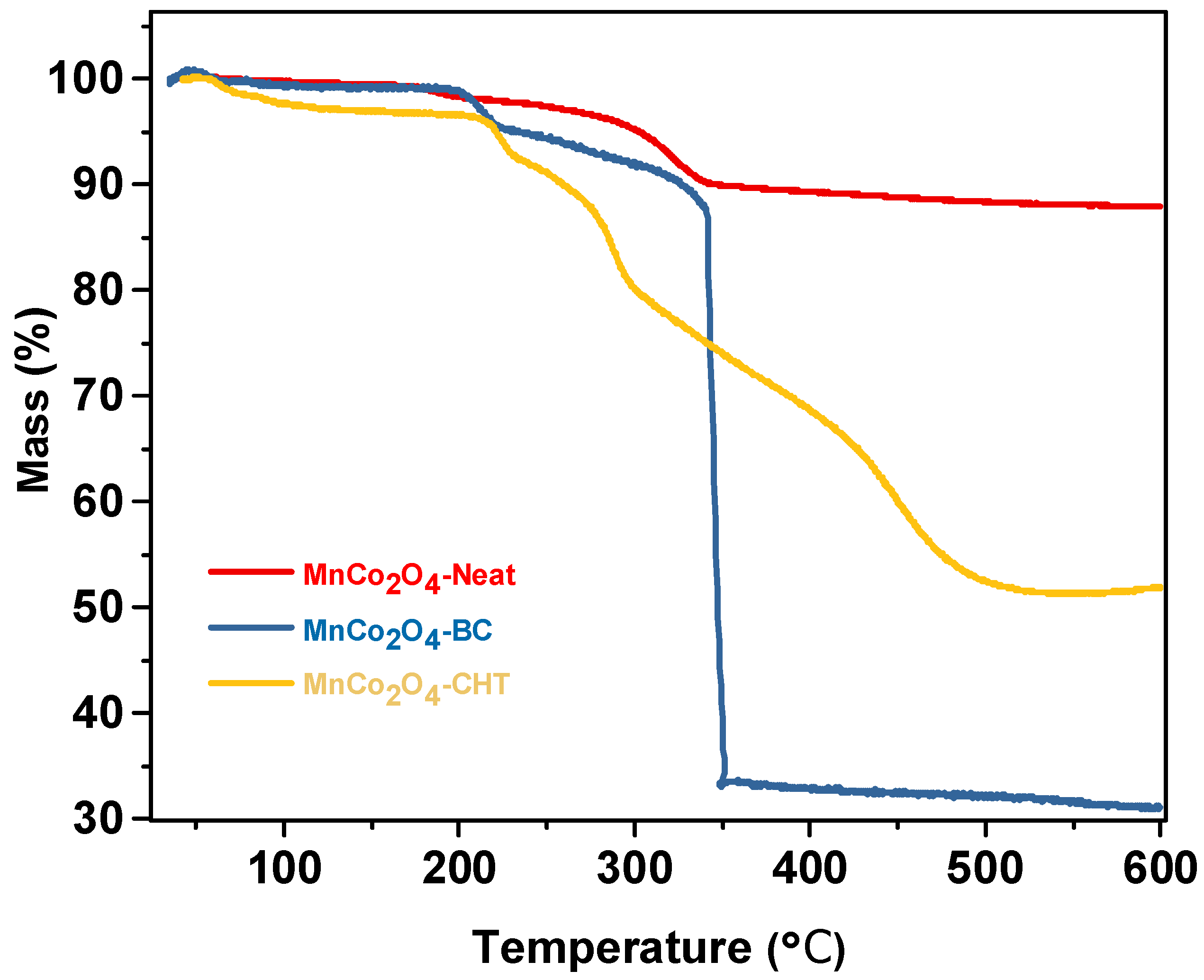
Figure 6 presents the thermogravimetric analysis (TGA) profiles of MnCo
2O
4-Neat, MnCo
2O
4-CHT, and MnCo
2O
4-BC, illustrating their thermal stability and the effect of biodegradable stabilizers on the decomposition behavior of the synthesized materials. The profiles highlight the weight loss percentages as a function of temperature, indicating distinct thermal degradation steps for each sample. The TGA curve for MnCo
2O
4-Neat shows minimal weight loss up to ~200 °C, which can be attributed to the removal of physically adsorbed water and surface-bound moisture. The absence of significant weight loss in this range reflects the lack of organic additives or stabilizers in this sample. Beyond 200 °C, the material exhibits only slight decomposition, indicating good thermal stability, with no major thermal events up to 600 °C. This behavior demonstrates that MnCo
2O
4-Neat retains its structural integrity well at high temperatures. The MnCo
2O
4-BC sample shows a two-step weight loss process. The initial weight loss below 200 °C corresponds to the evaporation of physically adsorbed water and the decomposition of small organic residues introduced by biomass-cellulose. The second and more significant weight loss occurs between 200 °C and 400 °C, which is attributed to the thermal decomposition of cellulose and its derivatives. This substantial weight loss reflects the presence of organic components from the biomass-cellulose stabilizer. Above 400 °C, the decomposition stabilizes, indicating the thermal resilience of the inorganic MnCo
2O
4 framework after the organic components are removed [
41]. The TGA curve for MnCo
2O
4-CHT exhibits a similar two-step degradation profile. The initial weight loss below 200 °C is attributed to the removal of adsorbed water and volatile components. The more pronounced weight loss between 200 °C and 400 °C corresponds to the decomposition of chitosan, including its amine (-NH
2) and hydroxyl (-OH) functional groups. The slower degradation above 400 °C compared to MnCo
2O
4-BC indicates that the chitosan stabilizer contributes to improved thermal stability of the material [
42]. The presence of biodegradable stabilizers (biomass-cellulose and chitosan) significantly affects the thermal degradation profile of MnCo
2O
4. Both MnCo
2O
4-BC and MnCo
2O
4-CHT exhibit higher overall weight loss compared to MnCo
2O
4-Neat due to the decomposition of organic components in the stabilizers. Chitosan, however, appears to enhance thermal stability beyond 400 °C compared to cellulose, potentially due to its stronger interaction with the MnCo
2O
4 framework through chelation and surface modifications. In conclusion, chitosan provides superior stabilization compared to biomass-cellulose, resulting in higher thermal resilience. These findings underline the importance of stabilizers in tailoring the thermal and functional properties of MnCo
2O
4 for advanced catalytic applications.
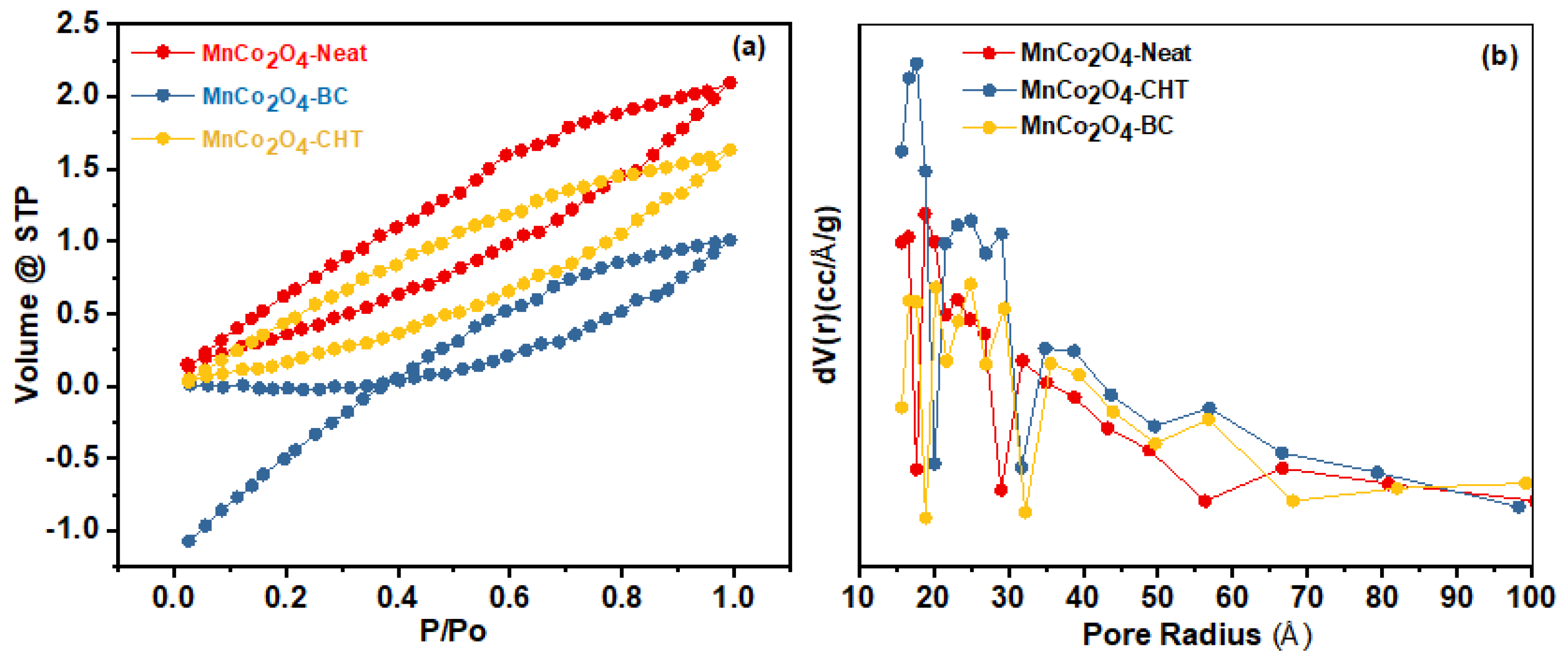
The nitrogen adsorption–desorption isotherms and pore size distribution of MnCo
2O
4-Neat, MnCo
2O
4-CHT, and MnCo
2O
4-BC (
Figure 7) reveal significant differences in surface area and pore characteristics, as summarized in
Table 1. These differences are attributed to the influence of biodegradable stabilizers—chitosan (CHT) and biomass-cellulose (BC)—used during the synthesis process. The analysis provides critical insights into the role of stabilizers in tailoring the textural properties of MnCo
2O
4 nanospinels for optimized catalytic performance. The isotherms in
Figure 7a exhibit a type IV profile with an H3 hysteresis loop for all samples, indicative of mesoporous materials. The MnCo
2O
4-Neat sample, synthesized without any stabilizer, demonstrates the greatest BET surface area (33.44 m
2/g) and a smaller pore size (26.2 nm) compared to the stabilized samples. This result suggests that the absence of stabilizers enables higher surface exposure during synthesis, resulting in smaller pores and a higher surface area. However, the limited structural control may lead to non-uniformity and less organized porosity, as observed in the broader pore distribution in
Figure 7b. For MnCo
2O
4-CHT, the surface area (27.42 m
2/g) is reduced compared to MnCo
2O
4-Neat, but the average pore diameter increases to 29.1 nm. The role of chitosan as a stabilizer is evident in the more uniform pore size distribution and increased pore volume (0.0019 cm
3/g). The chelating ability of chitosan likely facilitates the formation of larger, more organized pores by controlling the aggregation of particles during synthesis. This enhanced structural organization may improve accessibility for catalytic processes, as demonstrated in other studies where chitosan templates increased the porosity and uniformity of nanostructured materials [
43]. Similarly, MnCo
2O
4-BC, synthesized with biomass-cellulose as a stabilizer, exhibits a slightly lower surface area (29.32 m
2/g) than MnCo
2O
4-Neat but shows an increase in pore diameter (27.3 nm) and reduced pore volume (0.0013 cm
3/g). Biomass-cellulose acts as a template to control the particle aggregation and promotes the formation of larger, more accessible pores. The stabilizer’s role in modifying pore size is supported by similar findings in cellulose-stabilized metal oxides, where enhanced porosity was attributed to the templating action of cellulose fibers during the sol-gel process [
41]. The pore size distributions in
Figure 7b further confirm that both chitosan and cellulose stabilize the MnCo
2O
4 nanostructures by preventing excessive pore collapse and aggregation, resulting in larger and more uniform pores compared to MnCo
2O
4-Neat. This structural modification aligns with research on the role of stabilizers in tailoring mesoporous materials for improved adsorption and catalytic properties [
44]. In summary, the use of chitosan and biomass-cellulose as stabilizers introduces significant structural differences in the MnCo
2O
4 samples. While MnCo
2O
4-Neat exhibits the highest surface area, the stabilized samples show more uniform pore structures and larger pore sizes, which may enhance their catalytic efficiency. These findings emphasize the importance of biodegradable stabilizers in optimizing the textural properties of MnCo
2O
4 for applications such as photocatalytic degradation and environmental remediation.
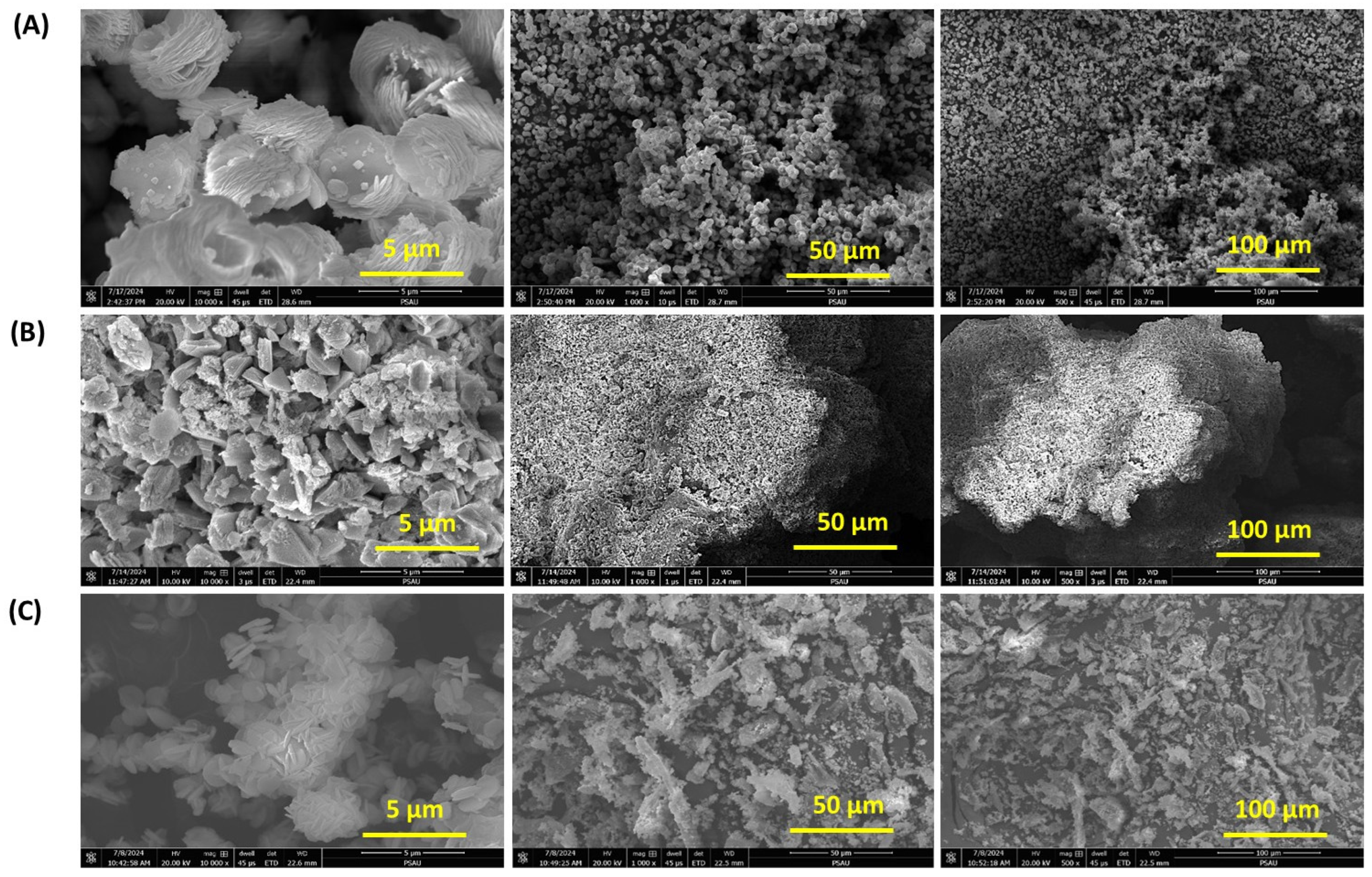
The field emission scanning electron microscopy (FESEM) images in
Figure 8 provide detailed insights into the surface morphology and microstructure of MnCo
2O
4 synthesized with and without biodegradable stabilizers: MnCo
2O
4-Neat, MnCo
2O
4-CHT, and MnCo
2O
4-BC. The variations in morphology among the samples highlight the significant impact of stabilizers—chitosan (CHT) and biomass-cellulose (BC)—on the nucleation and growth of MnCo
2O
4 nanostructures. The FESEM images of MnCo
2O
4-Neat (
Figure 8A) reveal a relatively disordered and aggregated structure with non-uniform particle shapes and sizes. This irregular morphology can be attributed to the absence of stabilizers during synthesis, leading to uncontrolled nucleation and growth. The aggregated particles lack well-defined boundaries, which may negatively impact the surface area and catalytic activity. This observation aligns with previous studies showing that stabilizer-free synthesis often results in non-homogeneous particle distribution due to the lack of templating or chelating agents. In contrast, MnCo
2O
4-CHT (
Figure 8B) demonstrates a more organized structure with smaller, uniformly distributed particles. The chitosan stabilizer facilitates better control over particle size and distribution due to its chelating properties and ability to interact with metal ions during synthesis. The observed morphology suggests that chitosan acts as a template, preventing excessive particle aggregation and promoting the formation of nanostructures with enhanced surface area and porosity. These findings are consistent with previous reports where chitosan was shown to improve the morphology and dispersion of metal oxide nanoparticles for catalytic applications [
43]. The morphology of MnCo
2O
4-BC (
Figure 8C) reveals a fibrous and interconnected structure, characteristic of the templating effect of biomass-cellulose. The cellulose fibers provide a framework for the nucleation and growth of MnCo
2O
4, resulting in a more porous structure with larger surface areas. The observed morphology is consistent with the role of cellulose as a structural template, as reported in similar studies where cellulose-derived frameworks enhanced the structural integrity and porosity of metal oxides [
41]. The fibrous morphology may facilitate better mass transfer and adsorption of target molecules, which is critical for photocatalytic applications. Comparing the three samples, it is evident that the use of biodegradable stabilizers significantly influences the morphology of MnCo
2O
4. The more defined and porous structures observed in MnCo
2O
4-CHT and MnCo
2O
4-BC are likely to enhance their catalytic performance by increasing the available surface area and improving the accessibility of active sites. The stabilizer-induced morphological control highlights the importance of employing templating agents to tailor the structural properties of nanospinels for specific applications. In conclusion, the FESEM analysis demonstrates that the absence of stabilizers in MnCo
2O
4-Neat leads to disordered aggregation, whereas the use of chitosan and cellulose results in more uniform and organized structures. Chitosan yields smaller, well-dispersed nanoparticles, while cellulose produces a fibrous, interconnected network. These findings underscore the role of stabilizers in enhancing the structural and functional properties of MnCo
2O
4, making them more suitable for catalytic and environmental applications.
3.2. Photocatalytic Performance of the Prepared Nanospinel MnCo2O4
Spinel oxides, with the general formula AB
2O
4, are characterized by their cubic close-packed oxygen lattice and mixed-metal cation distribution between tetrahedral (A-site) and octahedral (B-site) sites. This unique structure facilitates efficient charge transport and separation, which are critical for enhancing photocatalytic activity. Furthermore, spinel oxides exhibit tunable band gaps, high chemical stability, and strong light absorption, making them particularly effective under visible and UV irradiation. These properties can be further enhanced through doping, structural engineering, and surface modifications. This study focuses on the photocatalytic degradation of MB using spinel-structured MnCo
2O
4 synthesized with and without biodegradable stabilizers. By examining the effects of structural modifications and stabilizer-assisted synthesis on photocatalytic performance, this work aims to provide insights into the design of advanced spinel-based photocatalysts for wastewater treatment applications.
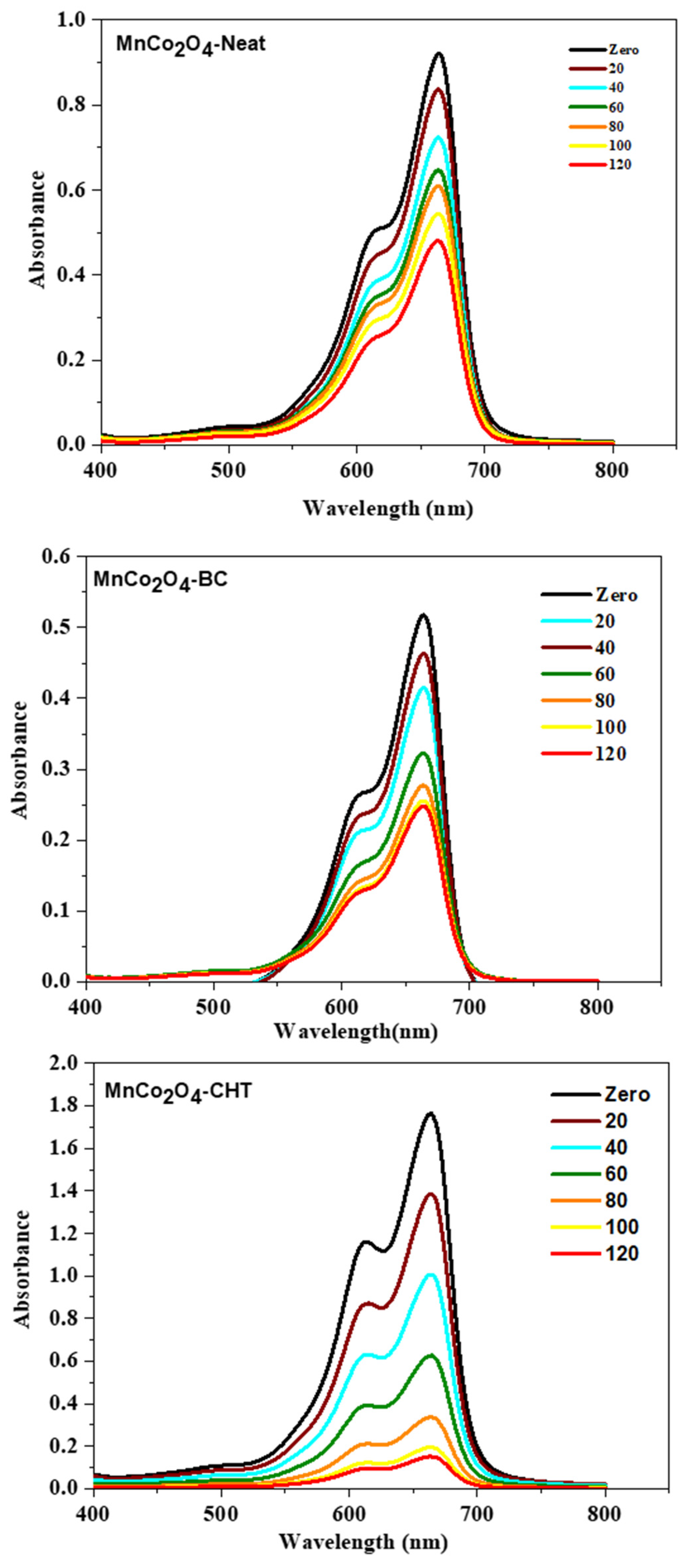
Figure 9 illustrates the time-dependent variations in the absorption spectrum of methylene blue (MB) during photocatalytic degradation under UV-rich solar irradiation, using MnCo
2O
4-Neat, MnCo
2O
4-BC, and MnCo
2O
4-CHT nanospinels at neutral pH (7). The progressive reduction in the intensity of the characteristic MB absorption peak at ~660 nm over 120 min confirms the photocatalysts’ degradation of MB. The comparative performance of the three catalysts highlights the significant influence of stabilizers on the photocatalytic efficiency. For MnCo
2O
4-Neat, the absorption spectra exhibit a gradual decrease in MB intensity, achieving 45% degradation at the end of 120 min. The relatively low photocatalytic efficiency can be attributed to the absence of stabilizers, which limits structural control and the availability of active sites on the catalyst surface. This result aligns with earlier findings where unmodified spinel oxides demonstrated moderate photocatalytic activity due to higher recombination rates of photogenerated charge carriers [
37]. MnCo
2O
4-BC, synthesized with biomass-cellulose as a stabilizer, shows a marked improvement in MB degradation, achieving 65% degradation by the end of 120 min. The enhanced performance can be attributed to the templating effect of cellulose, which improves particle dispersion and increases porosity. These structural improvements facilitate greater light absorption and enhanced interaction with MB molecules, thereby improving photocatalytic activity. Previous studies have demonstrated that cellulose-based stabilizers significantly enhance the structural properties of spinel oxides, enabling higher catalytic performance [
41]. The MnCo
2O
4–CHT catalyst exhibited the highest degradation efficiency, achieving 96% methylene blue (MB) removal within 120 min. This enhanced performance can be attributed to the synergistic interaction between chitosan and the MnCo
2O
4 spinel matrix, which significantly improves both the structural and electronic properties of the catalyst. Chitosan contains abundant amino (–NH
2) and hydroxyl (–OH) functional groups, which serve multiple roles during synthesis and catalysis. These groups coordinate strongly with Mn
2+ and Co
2+ ions, acting as chelating sites that promote uniform nucleation and prevent particle agglomeration, resulting in smaller, well-dispersed nanoparticles with higher active surface areas. Furthermore, the –NH
2 and –OH groups provide electron-donating capabilities that facilitate charge transfer between the chitosan matrix and the MnCo
2O
4 surface, thereby accelerating the separation of photogenerated electron–hole pairs and suppressing recombination losses. This improved charge mobility enhances the generation of reactive oxygen species (ROS), such as •OH and •O
2− radicals, which drive efficient dye degradation. Additionally, the chitosan backbone serves as a templating framework, stabilizing the spinel structure and optimizing the electronic band alignment for efficient light absorption. These synergistic effects collectively explain the superior photocatalytic activity of MnCo
2O
4–CHT, consistent with previous findings that demonstrate the role of chitosan functionalization in improving charge separation and enhancing photocatalytic efficiency in metal oxide nanocomposites [
43,
44]. The comparative results of the three catalysts underscore the critical role of stabilizers in enhancing the photocatalytic properties of MnCo
2O
4 nanospinels. The progressive improvement in performance from MnCo
2O
4-Neat to MnCo
2O
4-BC and MnCo
2O
4-CHT highlights the importance of the structural and electronic modifications imparted by the stabilizers. MnCo
2O
4-CHT, in particular, demonstrates exceptional efficiency due to its enhanced charge carrier dynamics and optimized morphology, making it a promising candidate for practical photocatalytic applications. These findings reinforce the potential of using biodegradable stabilizers to tailor the properties of spinel oxides for environmental remediation.
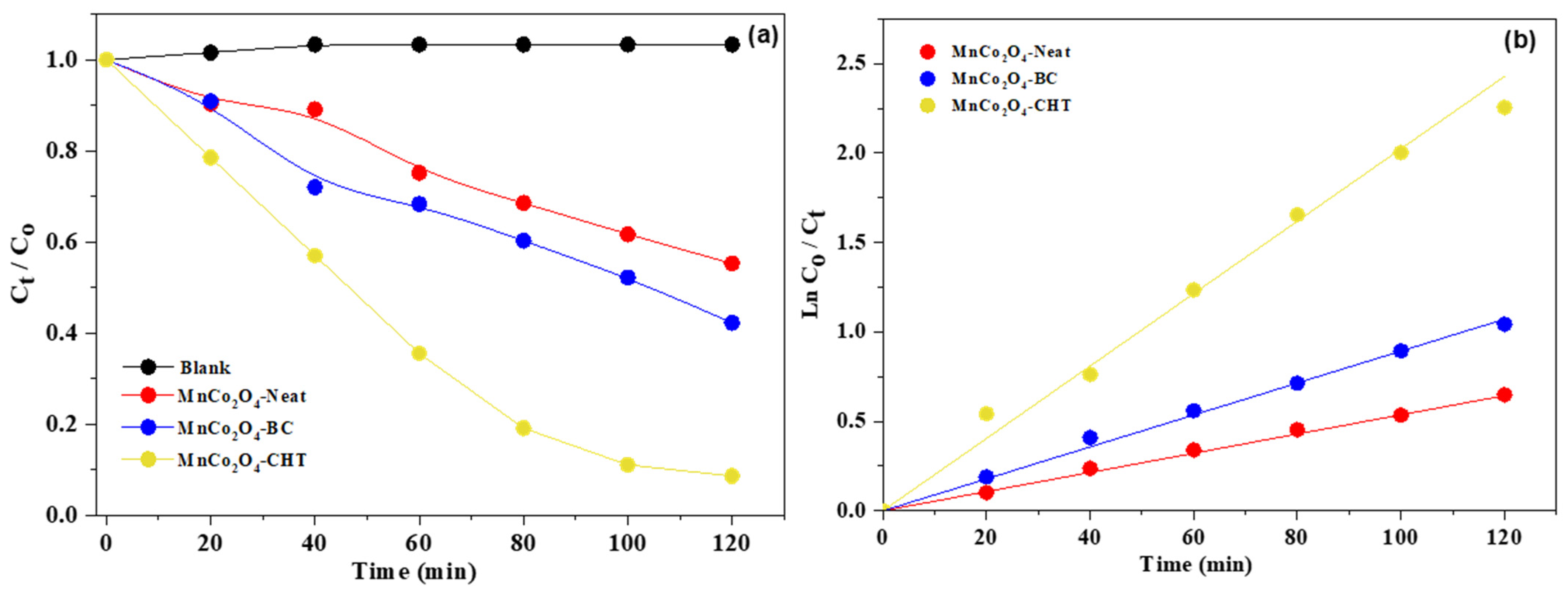
Figure 10 and the accompanying kinetic parameters (
Table 2) provide a comprehensive analysis of the photocatalytic degradation of methylene blue (MB) under UV-rich solar irradiation at pH 7 using MnCo
2O
4-Neat, MnCo
2O
4-BC, and MnCo
2O
4-CHT nanospinels. The data illustrate the degradation efficiency (C
t/C
o vs. time), the kinetic behavior (ln(C
o/C
t) vs. time), and the apparent rate constants (k
app) for the three photocatalysts. These results highlight the influence of biodegradable stabilizers on the catalytic performance of MnCo
2O
4 and the underlying kinetics of MB degradation. The plot of C
t/C
o vs. time (
Figure 10a) demonstrates that MnCo
2O
4-CHT achieves the highest degradation efficiency, removing 96% of MB within 120 min. In comparison, MnCo
2O
4-BC degrades 65% of MB, while MnCo
2O
4-Neat achieves 45% degradation in the same time frame. These trends are further supported by the kinetic study (ln(C
o/C
t) vs. time vs.,
Figure 10b), which shows a linear relationship for all samples, confirming that the degradation process follows pseudo-first-order kinetics. The kinetic parameters in the table reinforce these observations. MnCo
2O
4-CHT exhibits the highest apparent rate constant (k
app = 0.0203 min
−1) and the shortest half-life (t
1/2 = 34.2 min), indicating superior catalytic activity compared to MnCo
2O
4-BC (k
app = 0.0091 min
−1, t
1/2 = 77.9 min) and MnCo
2O
4-Neat (k
app = 0.0054 min
−1, t
1/2 = 128.3 min). These results highlight the role of stabilizers in enhancing photocatalytic performance by improving the material’s structural and electronic properties. The superior performance of MnCo
2O
4-CHT can be attributed to the strong interaction between chitosan and the MnCo
2O
4 framework, which enhances charge separation and reduces electron-hole recombination. Chitosan’s functional groups create additional reactive sites, facilitating efficient interaction with MB molecules. These findings align with previous studies showing that chitosan significantly improves the photocatalytic efficiency of metal oxides by modifying surface properties and reducing recombination rates. MnCo
2O
4-BC also shows improved catalytic performance compared to MnCo
2O
4-Neat, although its efficiency is lower than that of MnCo
2O
4-CHT. The biomass-cellulose stabilizer improves particle dispersion and introduces porosity, increasing the availability of active sites for the photocatalytic reaction. The templating effect of cellulose has been shown to enhance the structural integrity and porosity of spinel oxides, contributing to better adsorption and catalytic activity. In contrast, MnCo
2O
4-Neat exhibits the lowest catalytic efficiency due to the absence of stabilizers, resulting in less controlled morphology, lower surface area, and higher charge recombination rates. Previous reports have highlighted the limitations of unstabilized spinel oxides in achieving high catalytic performance, primarily due to structural irregularities and recombination of photogenerated carriers. To ensure that the observed methylene blue (MB) degradation resulted solely from photocatalytic activity rather than adsorption or photolysis, appropriate control experiments were performed under identical conditions. In the adsorption control test, the catalyst suspensions were stirred in the dark for 30 min to establish adsorption–desorption equilibrium prior to illumination. Only a negligible decrease (<5%) in MB concentration was detected, indicating minimal dye adsorption on the catalyst surface. In the photolysis control test, an MB solution without a catalyst was exposed to UV-rich solar light for 120 min, showing an insignificant change (<3%) in absorbance, confirming that direct photolysis of MB under UV-rich solar light was negligible. These results demonstrate that the observed dye degradation in the presence of MnCo
2O
4-based catalysts originates predominantly from photocatalytic reactions, validating the real contribution of the MnCo
2O
4 nanospinels and their biopolymer-stabilized composites to the degradation process.
In conclusion, the photocatalytic performance and kinetic analysis clearly demonstrate the significant impact of biodegradable stabilizers on MB degradation. MnCo2O4-CHT shows the most promising results due to its enhanced electronic and structural properties, followed by MnCo2O4-BC and MnCo2O4-Neat. These findings highlight the potential of stabilizer-assisted synthesis to optimize spinel oxides for efficient environmental remediation applications.
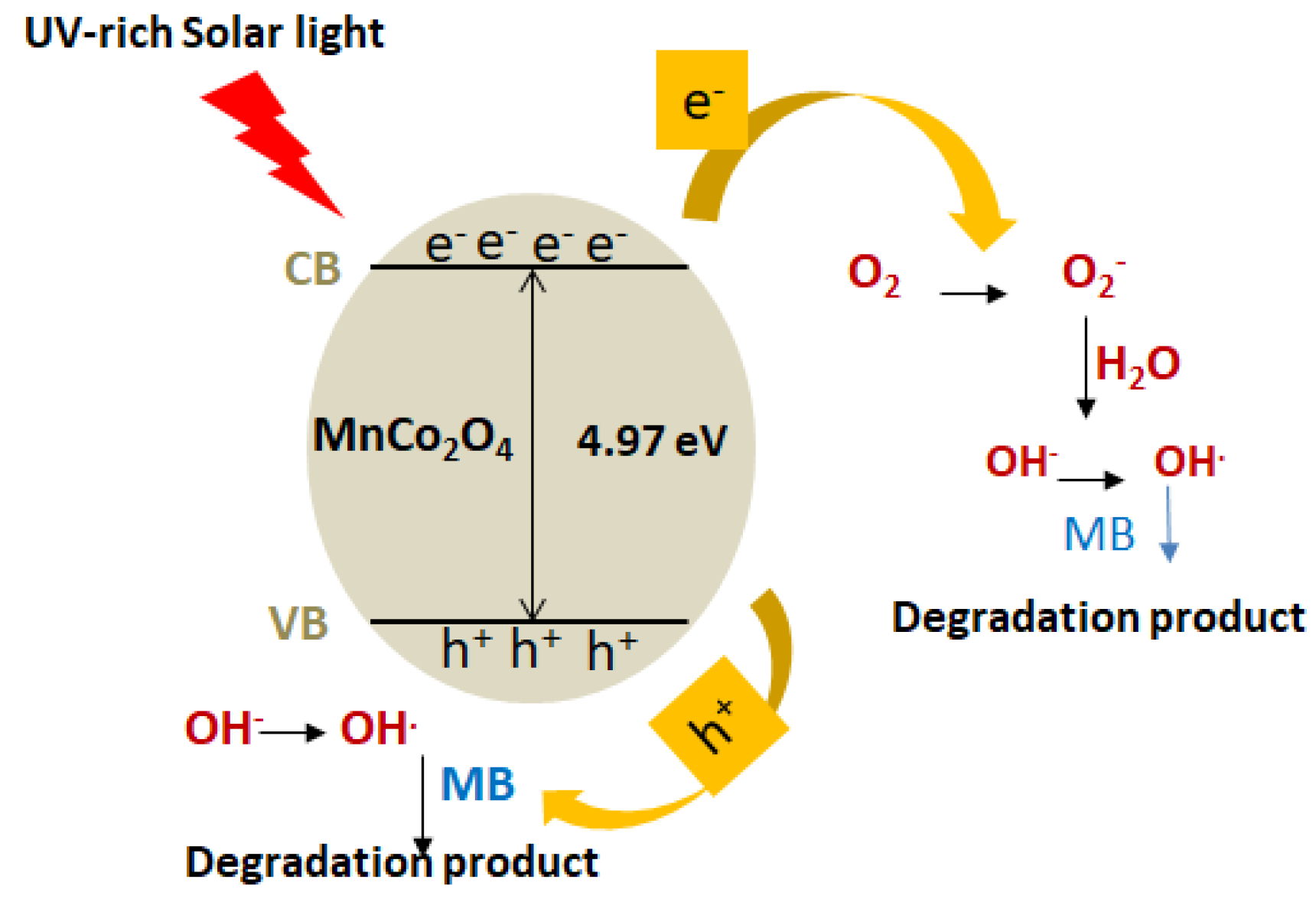
3.3. Photochemical Mechanism of MB Degradation
The photocatalytic degradation of methylene blue (MB) using the MnCo
2O
4–CHT nanocomposite proceeds through a light-induced redox mechanism involving photoexcitation, charge separation, and the generation of reactive oxygen species (ROS). Upon irradiation with UV-rich solar light, photons with energy equal to or greater than the band gap energy (E
g = 4.97 eV) excite electrons (e
−) from the valence band (VB) to the conduction band (CB) of MnCo
2O
4, leaving behind holes (h
+) in the VB. The chitosan matrix, rich in amino (–NH
2) and hydroxyl (
–OH) functional groups, facilitates efficient charge transfer between the Mn and Co sites and suppresses electron–hole recombination through coordination and electron-donating interactions. The photogenerated electrons in the CB migrate to the catalyst surface, where they react with adsorbed O
2 molecules to form superoxide radicals (
•O
2−). These radicals can undergo further protonation and transformation to yield hydroxyl radicals (
•OH), powerful oxidizing species that decompose MB molecules into smaller, non-toxic intermediates and, ultimately, into CO
2 and H
2O. Simultaneously, the VB holes (h
+) directly oxidize MB molecules or react with surface-adsorbed H
2O or OH
− ions to generate additional
•OH radicals. The combined oxidative attack by
•O
2− and
•OH species, supported by the high surface area and charge-separation efficiency imparted by chitosan, results in rapid degradation of MB within 120 min. The following steps can represent the overall photocatalytic process (
Figure 11):
- 1.
Photon absorption and excitation:
MnCo2O4 + hν → e−(CB) + h+(VB)
- 2.
Charge transfer and stabilization by chitosan:
Chitosan (–NH2/–OH) → acts as an electron donor/acceptor, suppressing e−–h+ recombination.
- 3.
ROS generation:
e− + O2 → •O2−
•O2− + H+ → HO2• → H2O2 → •OH
h+ + H2O/OH− → •OH
- 4.
Pollutant degradation:
•OH/•O2− + MB → Intermediates → CO2 + H2O
The chitosan’s functional groups enhance the catalyst’s surface charge distribution and electron transport, making MnCo2O4–CHT more efficient than unstabilized MnCo2O4. This synergistic effect between the spinel oxide and the biopolymer matrix is responsible for the 96% degradation efficiency observed.
Table 3 compares the photocatalytic performance of the MnCo
2O
4 nanospinels synthesized in this study with previously reported cobalt-based spinel photocatalysts used for methylene blue (MB) degradation [
45,
46,
47,
48,
49,
50,
51]. The data highlight the significant enhancement achieved by incorporating biodegradable stabilizers—particularly chitosan (CHT), into the MnCo
2O
4 system. The MnCo
2O
4–CHT catalyst developed in this work achieved a 96% degradation efficiency within 120 min, with an apparent rate constant (k
app = 0.0203 min
−1) and a band gap of 4.97 eV under UV-rich solar irradiation. This performance is comparable to or surpasses several previously reported cobalt spinel photocatalysts, including CoFe
2O
4 (99% in 60 min, 2.2–2.3 eV) [
46], and Co
xNi
1−xFe
2O
4 (92% in 100 min, 2.2–2.32 eV) [
48]. Although these materials operate under visible light due to their narrower band gaps, the MnCo
2O
4–CHT catalyst demonstrates exceptional efficiency even under UV-rich conditions without requiring dopants or co-catalysts. The superior performance of MnCo
2O
4–CHT can be attributed to the synergistic effect of chitosan, whose amino (–NH
2) and hydroxyl (–OH) functional groups enhance metal-ion coordination, charge separation, and ROS generation, thereby enabling more effective photocatalytic reactions. In comparison, MnCo
2O
4–BC achieved 65% degradation (k
app = 0.0091 min
−1), confirming the beneficial but less pronounced effect of cellulose as a stabilizer. The unmodified MnCo
2O
4–Neat exhibited only 45% degradation, highlighting the critical role of stabilizers in tailoring the surface and electronic properties to improve activity. While some reported materials such as MnCo
2O
4.
5 NPs [
45] achieve complete degradation using chemical oxidants like peroxymonosulfate (PMS), the MnCo
2O
4–CHT catalyst attains comparable performance without external oxidants, demonstrating a greener and more sustainable photocatalytic route. Moreover, despite its relatively larger band gap, MnCo
2O
4–CHT maintains high activity due to enhanced surface reactivity and efficient electron–hole separation facilitated by chitosan stabilization. Overall, the comparison confirms that stabilizer-assisted MnCo
2O
4 nanospinels, particularly those synthesized with chitosan, offer a promising alternative to conventional doped or composite cobalt spinels. Their high degradation rate, excellent stability, and environmentally friendly synthesis approach make them viable candidates for practical wastewater treatment and large-scale photocatalytic applications.