Analysis of the Effect of Fabrication Parameters on the Properties of Biopolymer Coatings Deposited on Ti13Zr13Nb Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Material Preparation
2.2. Electrochemical Oxidation of Ti13Zr13Nb Alloy
2.3. Electrophoretic Deposition of Coatings
2.4. Characterization of Microstructure
2.5. Corrosion Studies
2.6. Contact Angle Studies
2.7. Evaluation of the Biological Properties of the Obtained Coatings
2.8. Statistic
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, S.; Tiyyagura, H.R.; Pottathara, Y.B.; Sadasivuni, K.K.; Ponnamma, D.; Douglas, T.E.L.; Skirtach, A.G.; Mohan, M.K. Surface Functionalization of Chitosan as a Coating Material for Orthopaedic Applications: A Comprehensive Review. Carbohydr. Polym. 2021, 255, 117487. [Google Scholar] [CrossRef]
- Tardelli, J.D.C.; Schiavon, M.A.; dos Reis, A.C. Chitosan Coatings on Titanium-Based Implants—From Development to Characterization and Behavior: A Systematic Review. Carbohydr. Polym. 2024, 344, 122496. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Xu, R.; Wang, W.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Electrochemically Deposited Chitosan/Ag Complex Coatings on Biomedical NiTi Alloy for Antibacterial Application. Surf. Coat. Technol. 2013, 232, 370–375. [Google Scholar] [CrossRef]
- Say, Y.; Aksakal, B.; Sinirlioglu, Z.A. Silver/Selenium/Chitosan Co-Substituted Bioceramic Coatings of Ni–Ti Alloy: Antibacterial Efficiency and Cell Viability. Int. J. Appl. Ceram. Technol. 2022, 19, 2701–2712. [Google Scholar] [CrossRef]
- Santosa, S.J.; Hadi, M.; Hatmanto, A.D.; Darmanastri, S.M.; Kusrini, E.; Nugrahaningtyas, K.D.; Usman, A. A Novel Eco-Friendly Method for Synthesizing Silver Nanoparticles (AgNPs)-Decorated Chitosan Film Having High Antibacterial Efficacy. JCIS Open 2025, 20, 100155. [Google Scholar] [CrossRef]
- Mishra, S.K.; Ferreira, J.M.F.; Kannan, S. Mechanically Stable Antimicrobial Chitosan-PVA-Silver Nanocomposite Coatings Deposited on Titanium Implants. Carbohydr. Polym. 2015, 121, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, G.; Chinnalagu, D.K.; Chinniah, K.; Mahalingam, S. Biodegradable Multifunctional Fabrication of Silver-Doped Zinc Oxide Nanoparticle on Chitosan/Polyvinyl Alcohol Flexible Film for Effective Antibacterial Potential and Attenuation of Human Liver Carcinoma Cells. Int. J. Biol. Macromol. 2025, 321, 146309. [Google Scholar] [CrossRef]
- Mohamed, A.S.A.; Abdelaziz, A.A.; Abo-Kamar, A.M.; Al-Madboly, L.A.; El-Maradny, Y.A.; El-Fakharany, E.M. Multifunctional Efficacy of the Fabricated Chitosan-Coated Silver Nanoparticles as an Antiviral Agent against SARS-CoV-2: Potent and Safe Mechanistic Insights. Int. J. Biol. Macromol. 2025, 320, 145623. [Google Scholar] [CrossRef]
- Anitha, J.; Muthusankar, A.; Sangeetha, M.; Kishore, V.L.; Sherin, P.J.; Selvakumar, R.; Chandraprakash, K.; Premkumar, T. Chitosan-Coated Silver Nanoparticles Synthesized Using Moringa Oleifera Flower Extract: A Potential Therapeutic Approach against Triple-Negative Breast Cancer. Int. J. Biol. Macromol. 2025, 320, 145995. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, F.; Ali, S.; Pervaiz, A.; Khan, K.; Afsar, T.; Aldisi, D.; Amor, H.; Razak, S. Sericin-Chitosan Conjugated Silver Nanoparticles Protect against 1,2-Dimethylhydrazine-Induced Colon Cancer in Mice by Regulating Metastatic Biomarkers, Prohibiting Dysplasia and Enhancing Antioxidant Potential. Int. J. Biol. Macromol. 2025, 321, 146478. [Google Scholar] [CrossRef]
- Rengasamy, G.; Mahalingam, S. Multifunctional Composite Film of Chitosan/Reduced Graphene Oxide Infused Silver Nanoparticle for Antibacterial and Cytotoxic Effects on HeLa Cells. Int. J. Biol. Macromol. 2025, 321, 146356. [Google Scholar] [CrossRef]
- Karataş, M.; Erzen, B.; Aydoğmuş, E.; Orhan, R. PVA/Chitosan Biofilms Enriched with Biosynthesized Silver Nanoparticles and Tea Tree Oil: Towards Multifunctional and Environmentally Friendly Materials. Int. J. Biol. Macromol. 2025, 312, 144164. [Google Scholar] [CrossRef]
- Tayel, A.A.; Ebaid, A.M.; Otian, A.M.; Mahrous, H.; El Rabey, H.A.; Salem, M.F. Application of Edible Nanocomposites from Chitosan/Fenugreek Seed Mucilage/Selenium Nanoparticles for Protecting Lemon from Green Mold. Int. J. Biol. Macromol. 2024, 273, 133109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, B.; Zeng, X.; Zhang, X.; Ren, B.; Yang, X. Bentonite and Gallotannin-Mediated Silver Nanoparticle-Modified Chitosan Biofilms for Food Preservation. Int. J. Biol. Macromol. 2025, 320, 146100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Jin, W.; Xu, Y.; Mei, F.; Cheng, D.; Cai, G.; Wang, X. Biomass-Based Food Packaging Film of Chitosan/Flavonoids/Silver Nanoparticle for Shelf Life Extension of Food. Colloids Surf. A Physicochem. Eng. Asp. 2025, 727, 138154. [Google Scholar] [CrossRef]
- Zhuo, M.; Liu, C.; Wang, Q.; Wang, Z.; Wang, Y.; Yu, F.; Zhang, Y. Catharanthus Roseus Extract-Assisted Silver Nanoparticles Chitosan Films with High Antioxidant and Antimicrobial Properties for Fresh Food Preservation. Int. J. Biol. Macromol. 2025, 309, 142771. [Google Scholar] [CrossRef]
- Lieu, M.D.; Nguyen, H.H.; Huynh, N.A.T.; Tuyen Ha, P.K.; Dang, T.K.T.; Nguyen, T.H. Green Synthesized Silver Nanoparticles by Avocado Leaves Incorporating Chitosan Edible Coating for Avocado Preservation under Ambient Conditions. Biocatal. Agric. Biotechnol. 2025, 67, 103650. [Google Scholar] [CrossRef]
- Silva, A.O.; Cunha, R.S.; Hotza, D.; Machado, R.A.F. Chitosan as a Matrix of Nanocomposites: A Review on Nanostructures, Processes, Properties, and Applications. Carbohydr. Polym. 2021, 272, 118472. [Google Scholar] [CrossRef]
- Lin, M.H.; Wang, Y.H.; Kuo, C.H.; Ou, S.F.; Huang, P.Z.; Song, T.Y.; Chen, Y.C.; Chen, S.T.; Wu, C.H.; Hsueh, Y.H.; et al. Hybrid ZnO/Chitosan Antimicrobial Coatings with Enhanced Mechanical and Bioactive Properties for Titanium Implants. Carbohydr. Polym. 2021, 257, 117639. [Google Scholar] [CrossRef]
- Kozelskaya, A.I.; Früh, A.; Rutkowski, S.; Goreninskii, S.I.; Verzunova, K.N.; Soldatova, E.A.; Dorozhko, E.V.; Frueh, J.; Bakina, O.V.; Buldakov, M.A.; et al. Antibacterial Double-Layer Calcium Phosphate/Chitosan Composite Coating on Metal Implants for Tissue Engineering. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135652. [Google Scholar] [CrossRef]
- Hidayat, M.I.; Hardiansyah, A.; Khoiriah, K.; Yulianti, E.; Wardhani, R.A.K.; Fahrialdi, F.; Yusuf, M.R.I. Composite Films Based on Chitosan Incorporating Molybdenum Disulfide Nanosheets and Zinc Oxide Nanoparticles with Potential Antibacterial Application. Food Chem. 2025, 477, 143480. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Thomas, B.; Sudheep, N.M.; Muhammed Shamil, K.V.; Anjana, R.; Krishna, A.; Thomas, J.B.; Krishnankutty, R.E. Development of Garlic Extract-Loaded Zinc Oxide Nanoparticle/Chitosan/Polyvinyl Alcohol Nanocomposite-Based Active Eco-Friendly Packaging Films for Extending Shelf-Life of Fish Fillets. Int. J. Biol. Macromol. 2025, 327, 147273. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, P.; Chetia, J.; Sharma, M.; Badwaik, L.S. Shelf Life Extension of Grapes through Chitosan Coating Reinforced Zinc Oxide Nanoparticles Containing Phytocompounds from Lemon Pomace. Sci. Hortic. 2025, 342, 114018. [Google Scholar] [CrossRef]
- Wei, X.; Li, Q.; Wu, C.; Sun, T.; Li, X. Preparation, Characterization and Antibacterial Mechanism of the Chitosan Coatings Modified by Ag/ZnO Microspheres. J. Sci. Food Agric. 2020, 100, 5527–5538. [Google Scholar] [CrossRef]
- Wei, X.; Li, Q.; Hao, H.; Yang, H.; Li, Y.; Sun, T.; Li, X. Preparation, Physicochemical and Preservation Properties of Ti/ZnO/in Situ SiOx Chitosan Composite Coatings. J. Sci. Food Agric. 2020, 100, 570–577. [Google Scholar] [CrossRef]
- bin Anwar Fadzil, A.F.; Pramanik, A.; Basak, A.K.; Prakash, C.; Shankar, S. Role of Surface Quality on Biocompatibility of Implants—A Review. Ann. 3D Print. Med. 2022, 8, 100082. [Google Scholar] [CrossRef]
- Cappellini, C.; Malandruccolo, A.; Kiem, S.; Abeni, A. Analysis of Ti-6Al-4V Micro-Milling Resulting Surface Roughness for Osteointegration Enhancement. Mater. Res. Proc. 2023, 28, 1255–1264. [Google Scholar]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The Influence of Surface Texture and Wettability on Initial Bacterial Adhesion on Titanium and Zirconium Oxide Dental Implants. Int. J. Implant. Dent. 2017, 3, 32. [Google Scholar] [CrossRef]
- Pan, M.; Weng, Z.; Liu, J. Effect of Positive Bias on Properties of Chitosan Coating Prepared on Micro-Arc Oxidation Surface of Ti–6Al–4V Alloy by Electrophoretic Deposition. Mater. Chem. Phys. 2022, 275, 125257. [Google Scholar] [CrossRef]
- Hiromoto, S. 4—Corrosion of Metallic Biomaterials. In Metals for Biomedical Devices, 2nd ed.; Niinomi, M., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2019; pp. 131–152. ISBN 978-0-08-102666-3. [Google Scholar]
- Chandrasekaran, A.; Dakshanamoorthy, A. Synthesis and Characterization of Nano-Hydroxyapatite (n-HAP) Using the Wet Chemical Technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Kesmez, Ö. Preparation of Anti-Bacterial Biocomposite Nanofibers Fabricated by Electrospinning Method. J. Turk. Chem. Soc. Sect. A Chem. 2020, 7, 125–142. [Google Scholar] [CrossRef]
- Anjaneyulu, U.; Swaroop, V.K.; Vijayalakshmi, U. Preparation and Characterization of Novel Ag Doped Hydroxyapatite-Fe3O4-Chitosan Hybrid Composites and in Vitro Biological Evaluations for Orthopaedic Applications. RSC Adv. 2016, 6, 10997–11007. [Google Scholar] [CrossRef]
- Yang, S.; Shao, D.; Wang, X.; Hou, G.; Nagatsu, M.; Tan, X.; Ren, X.; Yu, J. Design of Chitosan-Grafted Carbon Nanotubes: Evaluation of How the -OH Functional Group Affects Cs+ Adsorption. Mar. Drugs 2015, 13, 3116–3131. [Google Scholar] [CrossRef]
- Qasim, I.; Mumtaz, M.; Nadeem, K.; Abbas, S.Q. Zinc Nanoparticles at Intercrystallite Sites of (Cu0.5Tl0.5)Ba2Ca3Cu4O12-δ Superconductor. J. Nanomater. 2016, 2016, 9781790. [Google Scholar] [CrossRef]
- Pawłowski, Ł.; Rościszewska, M.; Majkowska-Marzec, B.; Jażdżewska, M.; Bartmański, M.; Zieliński, A.; Tybuszewska, N.; Samsel, P. Influence of Surface Modification of Titanium and Its Alloys for Medical Implants on Their Corrosion Behavior. Materials 2022, 15, 7556. [Google Scholar] [CrossRef]
- Bartmański, M.; Wekwejt, M.; Urbanowicz, K.; Mielewczyk-Gryń, A.; Gajowiec, G.; Pałubicka, A.; Michno, A.; Serafin, P.K.; Koszałka, P. Chitosan-Nanogold and Chitosan-Nanozinc Electrodeposited Coatings for Biomedical Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2025, 113, e35571. [Google Scholar] [CrossRef]
- Bartmański, M.; Pawłowski, Ł.; Knabe, A.; Mania, S.; Banach-Kopeć, A.; Sakowicz-Burkiewicz, M.; Ronowska, A. The Effect of Marginal Zn2+ Excess Released from Titanium Coating on Differentiation of Human Osteoblastic Cells. ACS Appl. Mater. Interfaces 2024, 16, 48412–48427. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, P.; Wu, J. Effect of Exposure of Osteoblast-like Cells to Low-Dose Silver Nanoparticles: Uptake, Retention and Osteogenic Activity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.E.; Hofstetter, W.; Siebenrock, K.A.; Landmann, R.; Klenke, F.M. In Vitro Cytotoxicity of Silver Nanoparticles on Osteoblasts and Osteoclasts at Antibacterial Concentrations. Nanotoxicology 2013, 7, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Ding, P.; Li, G.; Lu, E.; Zhao, Z. Hydroxyapatite Nanoparticles Facilitate Osteoblast Differentiation and Bone Formation within Sagittal Suture during Expansion in Rats. Drug Des. Devel Ther. 2021, 15, 905–917. [Google Scholar] [CrossRef]
- Torres, F.C.L.; De Sousa, E.M.B.; Cipreste, M.F. A Brief Review on Hydroxyapatite Nanoparticles Interactions with Biological Constituents. J. Biomater. Nanobiotechnol 2022, 13, 24–44. [Google Scholar] [CrossRef]
- Klinge, L.; Kluy, L.; Spiegel, C.; Siemers, C.; Groche, P.; Coraça-Huber, D. Nanostructured Ti-13Nb-13Zr Alloy for Implant Application—Material Scientific, Technological, and Biological Aspects. Front. Bioeng. Biotechnol. 2023, 11, 1255947. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, V.; Szymczyk-Ziółkowska, P.; Rusińska, M.; Poradowski, D.; Janeczek, M.; Ziółkowski, G.; Dybała, B. Study of Cytotoxic Activity of Ti–13Nb–13Zr Medical Alloy with Different Surface Finishing Techniques. J. Mater. Sci. 2021, 56, 17747–17767. [Google Scholar] [CrossRef]
- Peng, Y.; Song, C.; Yang, C.; Guo, Q.; Yao, M. Low Molecular Weight Chitosan-Coated Silver Nanoparticles Are Effective for the Treatment of MRSA-Infected Wounds. Int. J. Nanomed. 2017, 12, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Putthanuparp, T.; Rirattanapong, P.; Ruangsawasdi, N. The Cytotoxicity of Silver Nanoparticles on Human Gingival Fibroblast Cells: An in Vitro Study. Mahidol Dent. J. 2023, 43, 107–114. [Google Scholar]
- Cierech, M.; Wojnarowicz, J.; Kolenda, A.; Krawczyk-Balska, A.; Prochwicz, E.; Woźniak, B.; Łojkowski, W.; Mierzwińska-Nastalska, E. Zinc Oxide Nanoparticles Cytotoxicity and Release from Newly Formed PMMA–ZNO Nanocomposites Designed for Denture Bases. Nanomaterials 2019, 9, 1318. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Sanders, J.G.F.; Andrée, L.; van Oirschot, B.A.J.A.; Plachokova, A.S.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; Yang, F. Development of Zinc-Containing Chitosan/Gelatin Coatings with Immunomodulatory Effect for Soft Tissue Sealing around Dental Implants. Tissue Eng. Regen. Med. 2025, 22, 57–75. [Google Scholar] [CrossRef]
- Maheo, A.R.; Vithiya B, S.M.; Arul Prasad T, A.; Mangesh, V.L.; Perumal, T.; Al-Qahtani, W.H.; Govindasamy, M. Cytotoxic, Antidiabetic, and Antioxidant Study of Biogenically Improvised Elsholtzia Blanda and Chitosan-Assisted Zinc Oxide Nanoparticles. ACS Omega 2023, 8, 10954–10967. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Golus, J.; Kolmas, J.; Wojcik, M.; Kolodynska, D.; Przekora, A. Noncytotoxic Zinc-Doped Nanohydroxyapatite-Based Bone Scaffolds with Strong Bactericidal, Bacteriostatic, and Antibiofilm Activity. Biomater. Adv. 2022, 139, 213011. [Google Scholar] [CrossRef]
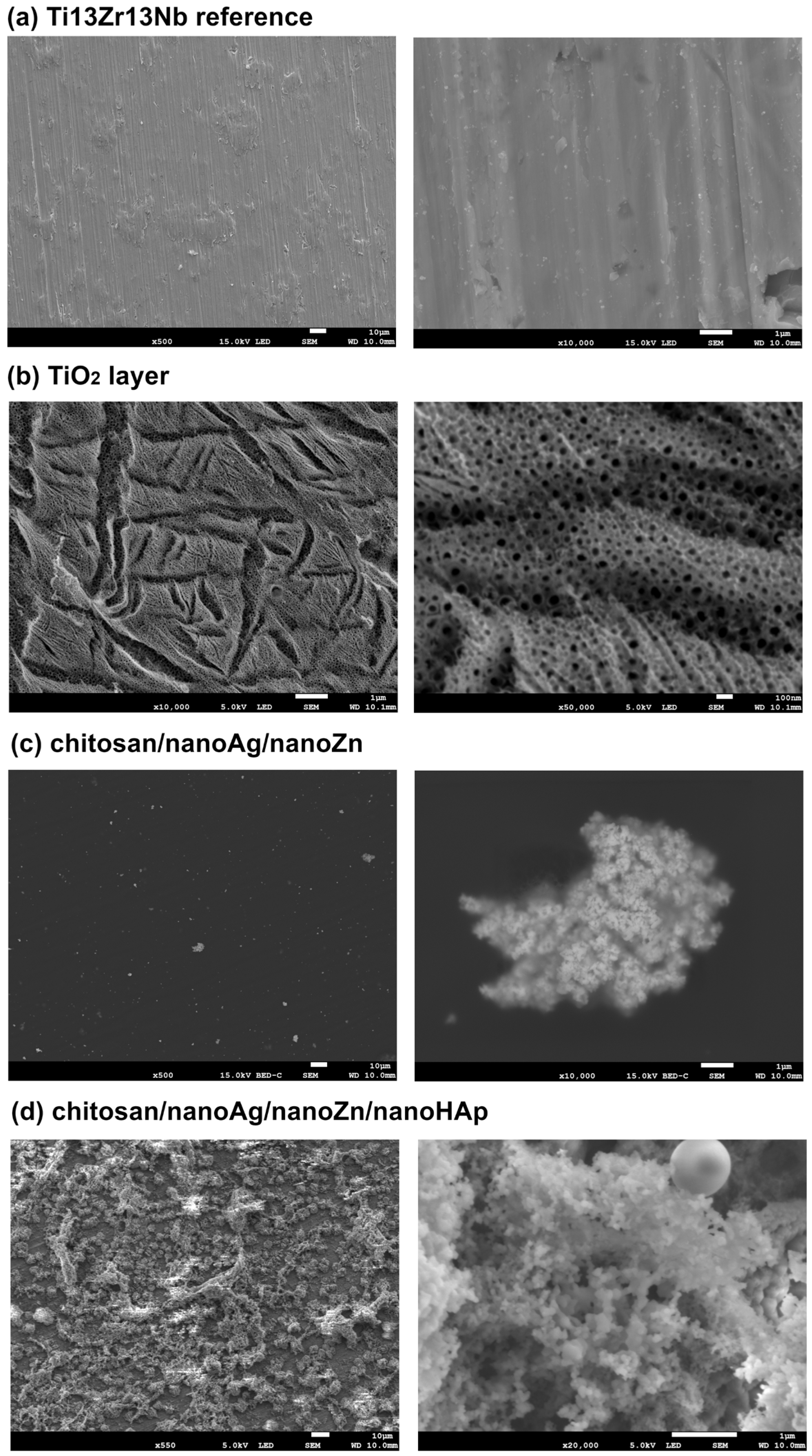

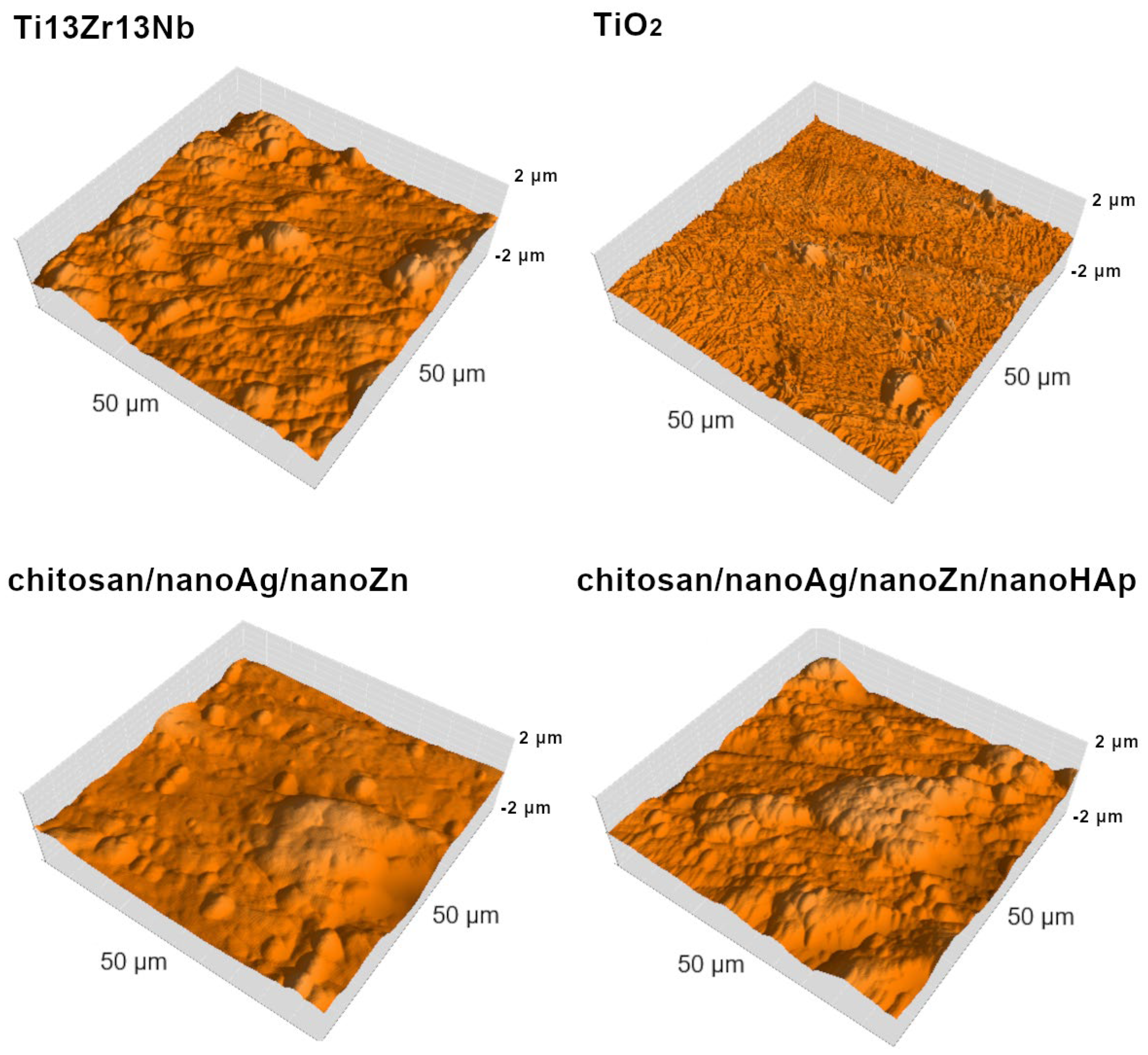




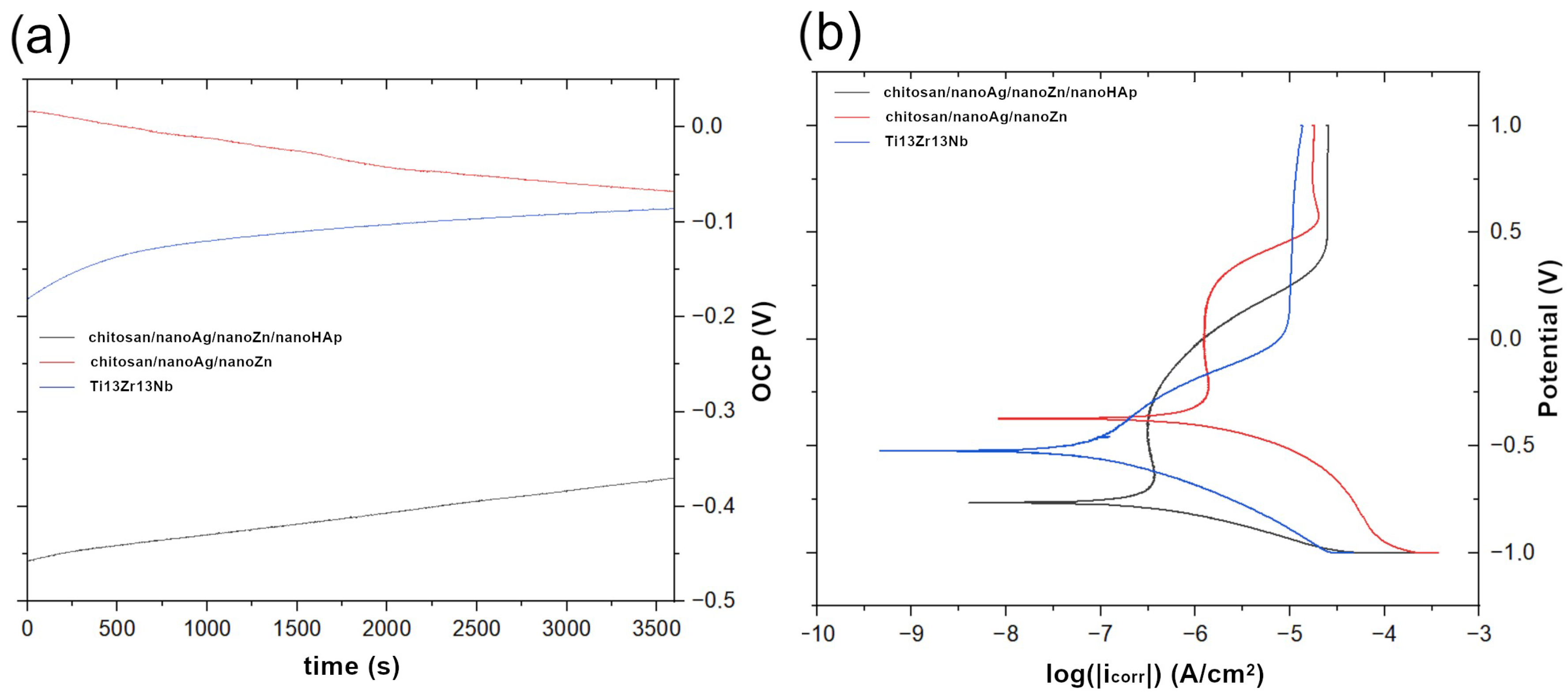


| Element | Nb | Zr | Fe | C | N | O | Ti |
|---|---|---|---|---|---|---|---|
| wt.% | 13.0 | 13.0 | 0.05 | 0.04 | 0.019 | 0.11 | remainder |
| Specimens Description | Electrochemical Oxidation | Coating Deposition Parameters |
|---|---|---|
| Ti13Zr13Nb | - | - |
| TiO2 | H3PO4 + HF; 20 V; 20 min | - |
| chitosan/nanoAg/nanoZn | 1 g/L chitosan + 0.05 g/L AgNPs + 0.05 g/L ZnNPs; 20 V; 1 min | |
| chitosan/nanoAg/nanoZn/nanoHAp | 1 g/L chitosan + 0.05 g/L AgNPs + 0.05 g/L ZnNPs + 2.5 g/L HApNPs; 20 V; 1 min |
| Chitosan/nanoHAp/nanoAg/nanoZn | Chitosan/nanoAg/nanoZn | ||
|---|---|---|---|
| Wavenumber (cm−1) | Bond/Functional Group | Wavenumber (cm−1) | Bond/Functional Group |
| 3638 | –O–H | 3363 | –O–H/N–H |
| 3560–3000 | –O–H/N–H | 3285 | –O–H/N–H |
| 2930 | C–H | 2920 | C–H |
| 1605 | C=O | 2875 | C–H |
| 1536 | N–H | 1645 | C=O |
| 1448 | CO32− | 1567 | N-H |
| 1413 | CH2/CH3 | 1423 | CH2/CH3 |
| 1153 | C–O–C | 1377 | CH2/CH3 |
| 1085 | PO43− | 1320 | C–N |
| 1064 | C–O | 1259 | –O–H |
| 1030 | C–O | 1153 | C–O–C |
| 950 | P–O | 1066 | C–O |
| 870 | CO32− | 1028 | C–O |
| - | - | 896 | C-H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartmański, M.; Sionek, K. Analysis of the Effect of Fabrication Parameters on the Properties of Biopolymer Coatings Deposited on Ti13Zr13Nb Alloy. Polymers 2025, 17, 3136. https://doi.org/10.3390/polym17233136
Bartmański M, Sionek K. Analysis of the Effect of Fabrication Parameters on the Properties of Biopolymer Coatings Deposited on Ti13Zr13Nb Alloy. Polymers. 2025; 17(23):3136. https://doi.org/10.3390/polym17233136
Chicago/Turabian StyleBartmański, Michał, and Kamila Sionek. 2025. "Analysis of the Effect of Fabrication Parameters on the Properties of Biopolymer Coatings Deposited on Ti13Zr13Nb Alloy" Polymers 17, no. 23: 3136. https://doi.org/10.3390/polym17233136
APA StyleBartmański, M., & Sionek, K. (2025). Analysis of the Effect of Fabrication Parameters on the Properties of Biopolymer Coatings Deposited on Ti13Zr13Nb Alloy. Polymers, 17(23), 3136. https://doi.org/10.3390/polym17233136








