Agar/Carboxymethyl Cellulose Blended Films with Green-Synthesised Silver Nanoparticles as a Sustainable Alternative for Food Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Casting of the Film-Forming Solutions
2.3. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
2.4. Morphological Analysis—Atomic Force Microscopy
2.5. Optical Properties
2.6. Mechanical Properties
2.7. Water Sensitivity
2.8. Thermal Analysis
2.9. Antimicrobial Activity
2.10. Bio-Disintegration
2.11. Statistical Analysis
3. Results and Discussion
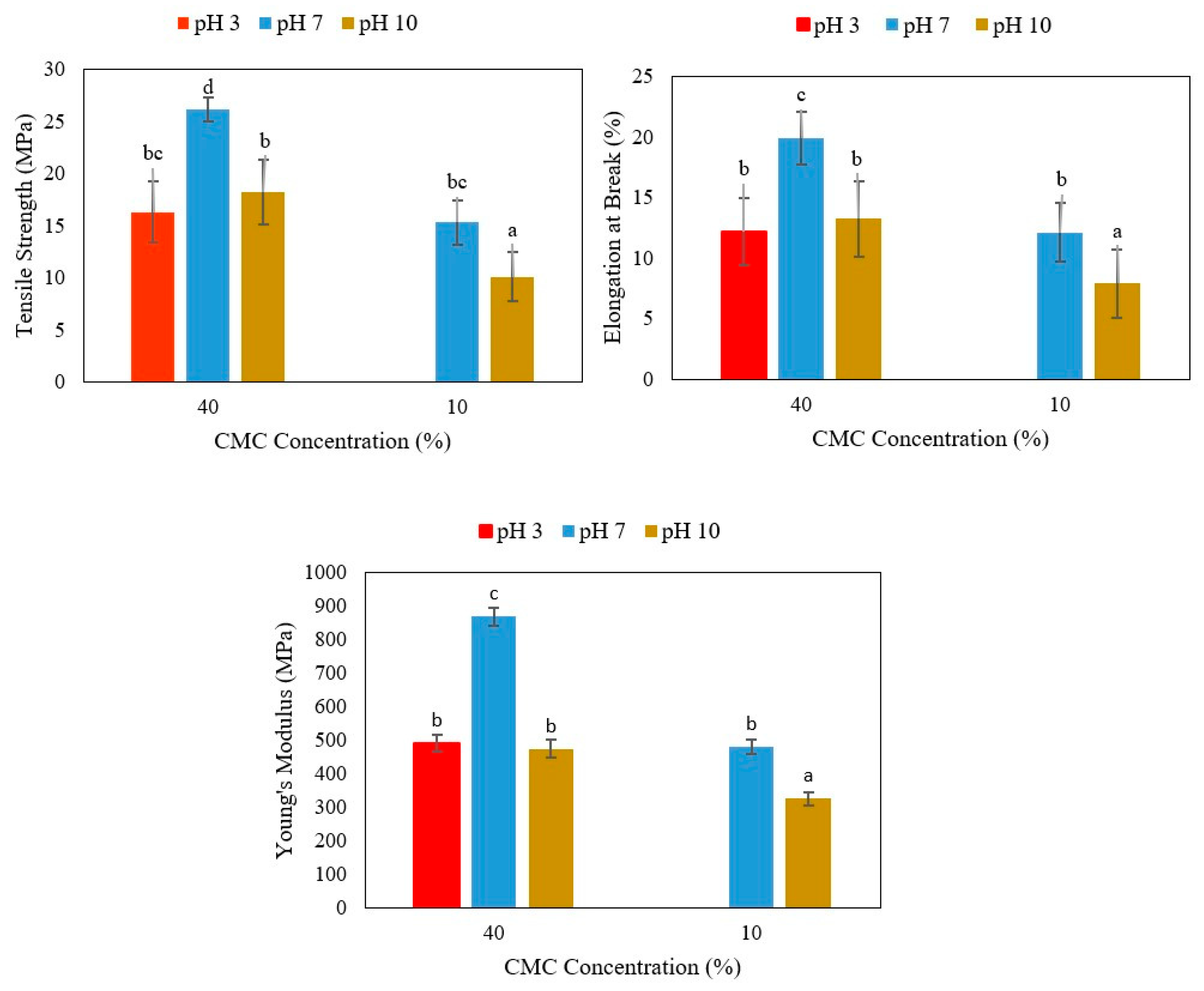
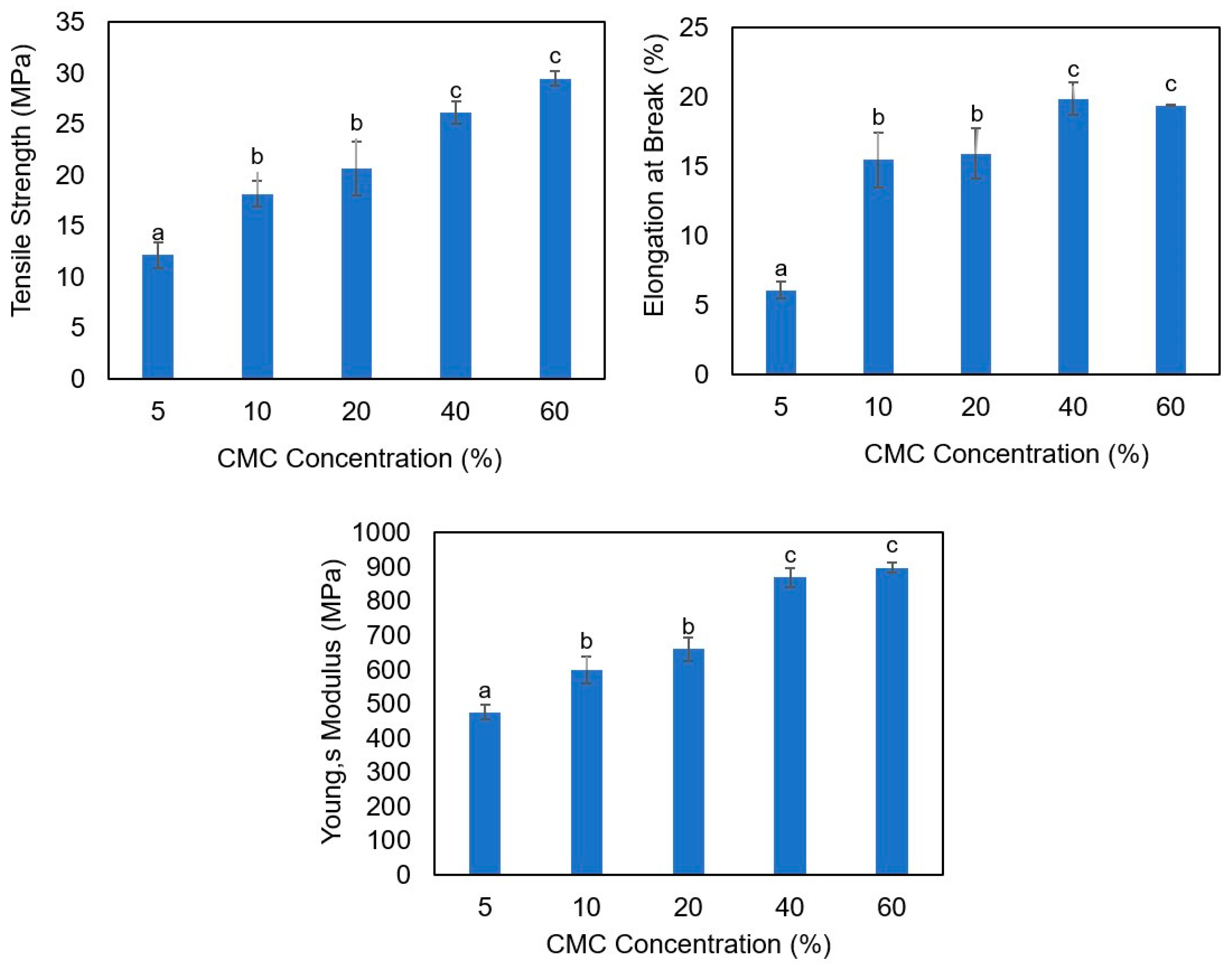
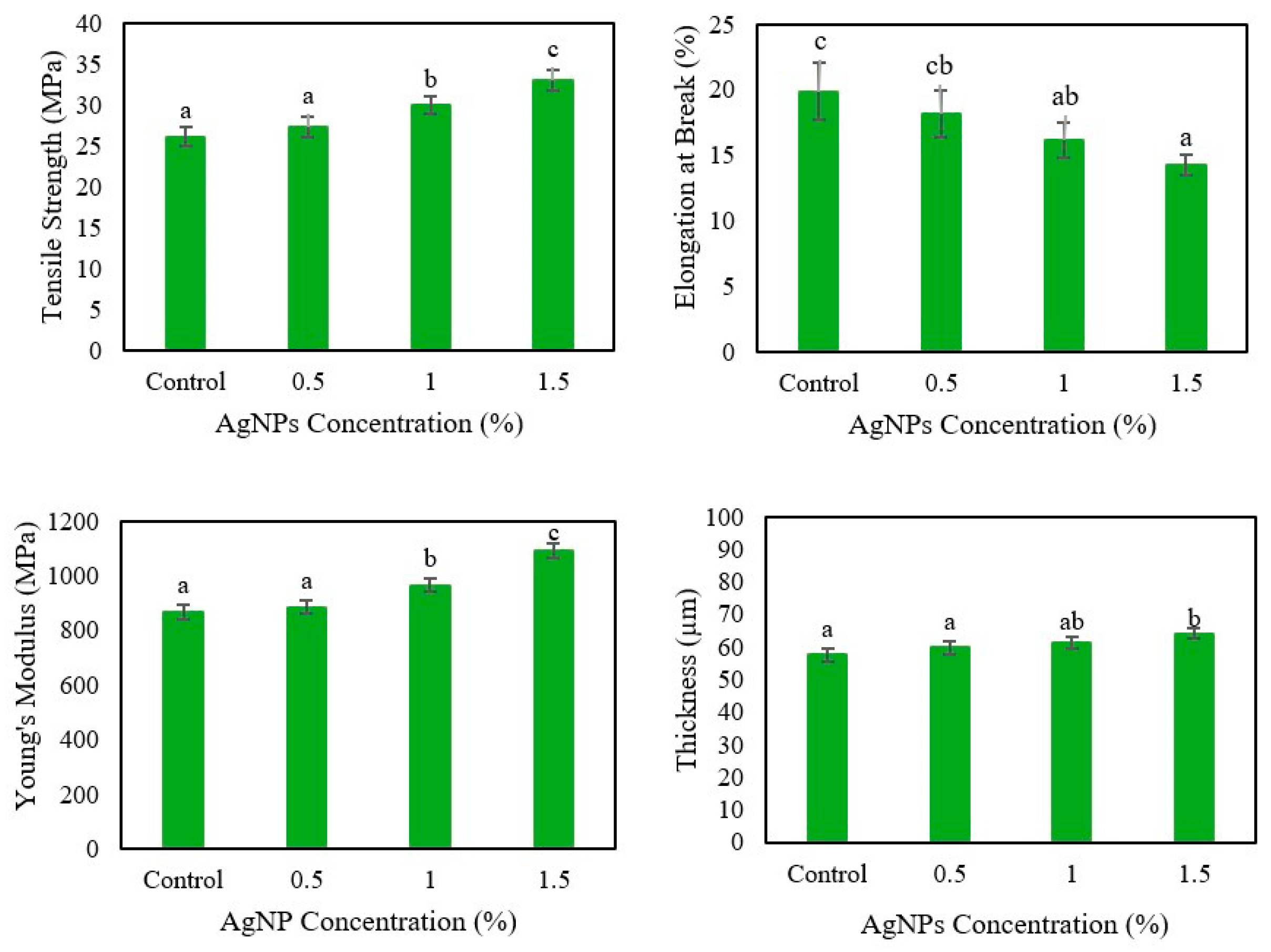
3.1. Mechanical Properties of Agar/Cellulose Films
3.1.1. Effect of Different pH and Cellulose Concentrations
3.1.2. Effect of Different Concentrations of AgNPs
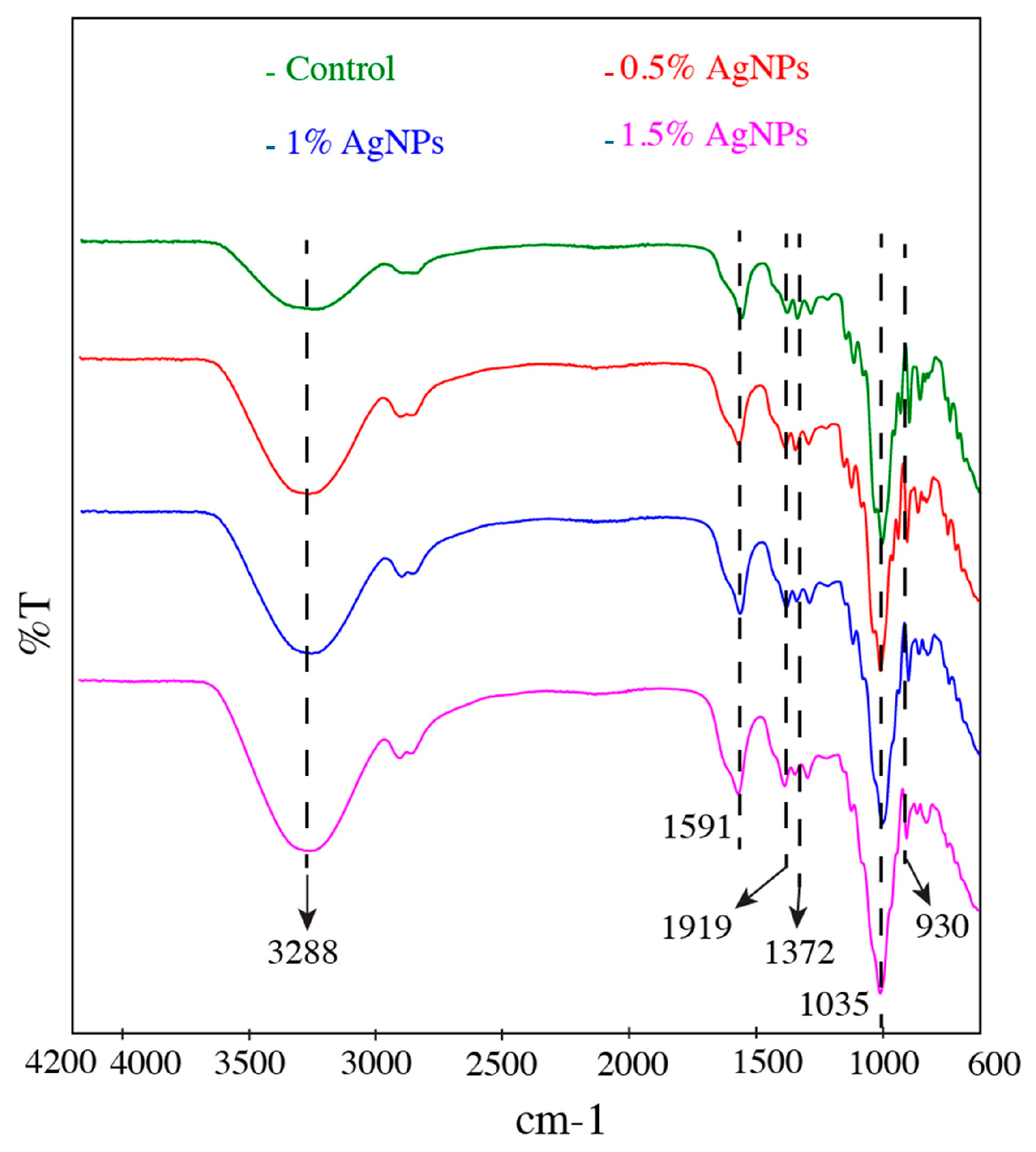
3.2. Film FTIR Analysis
3.3. Morphological Properties of Agar/CMC Films
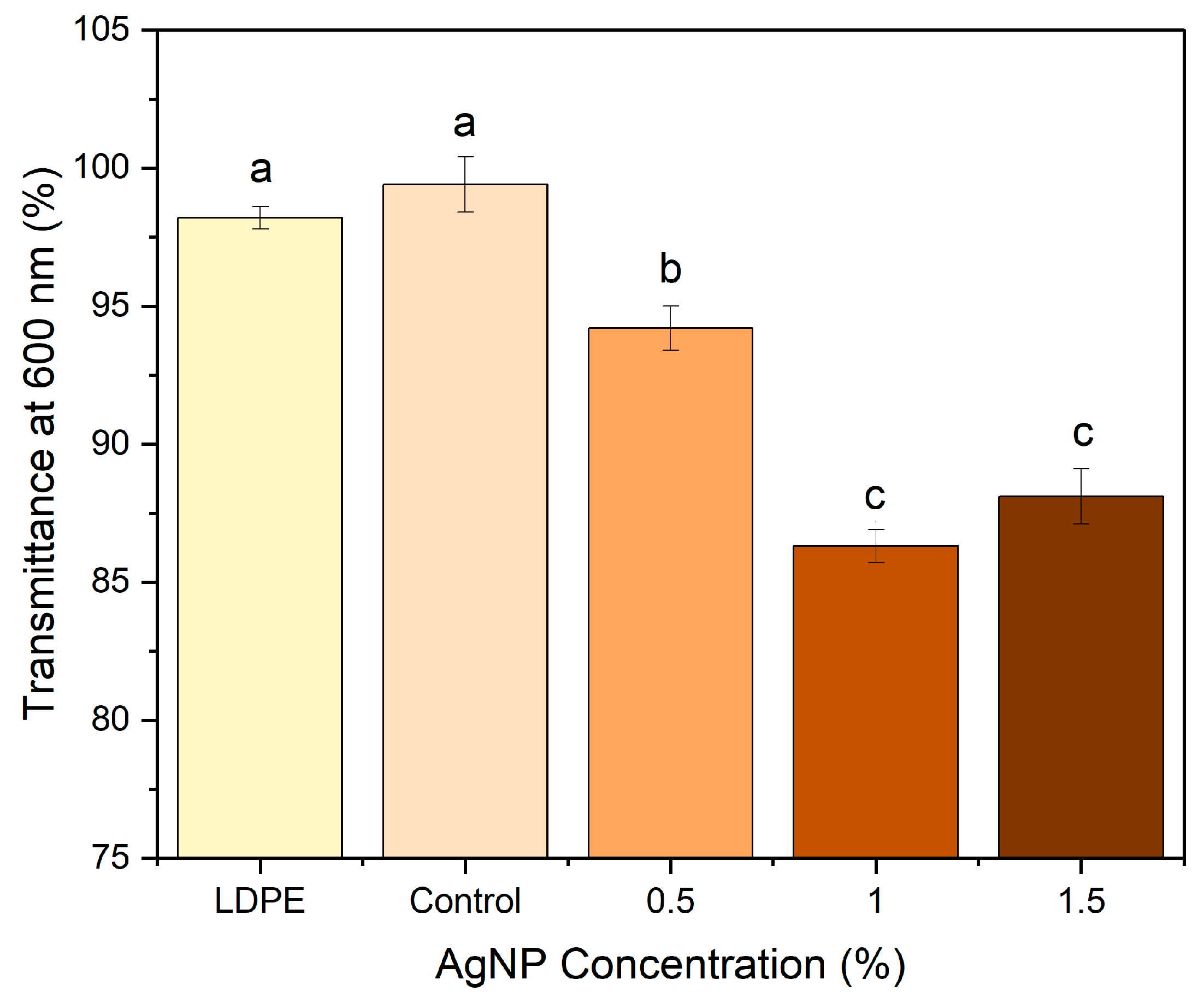
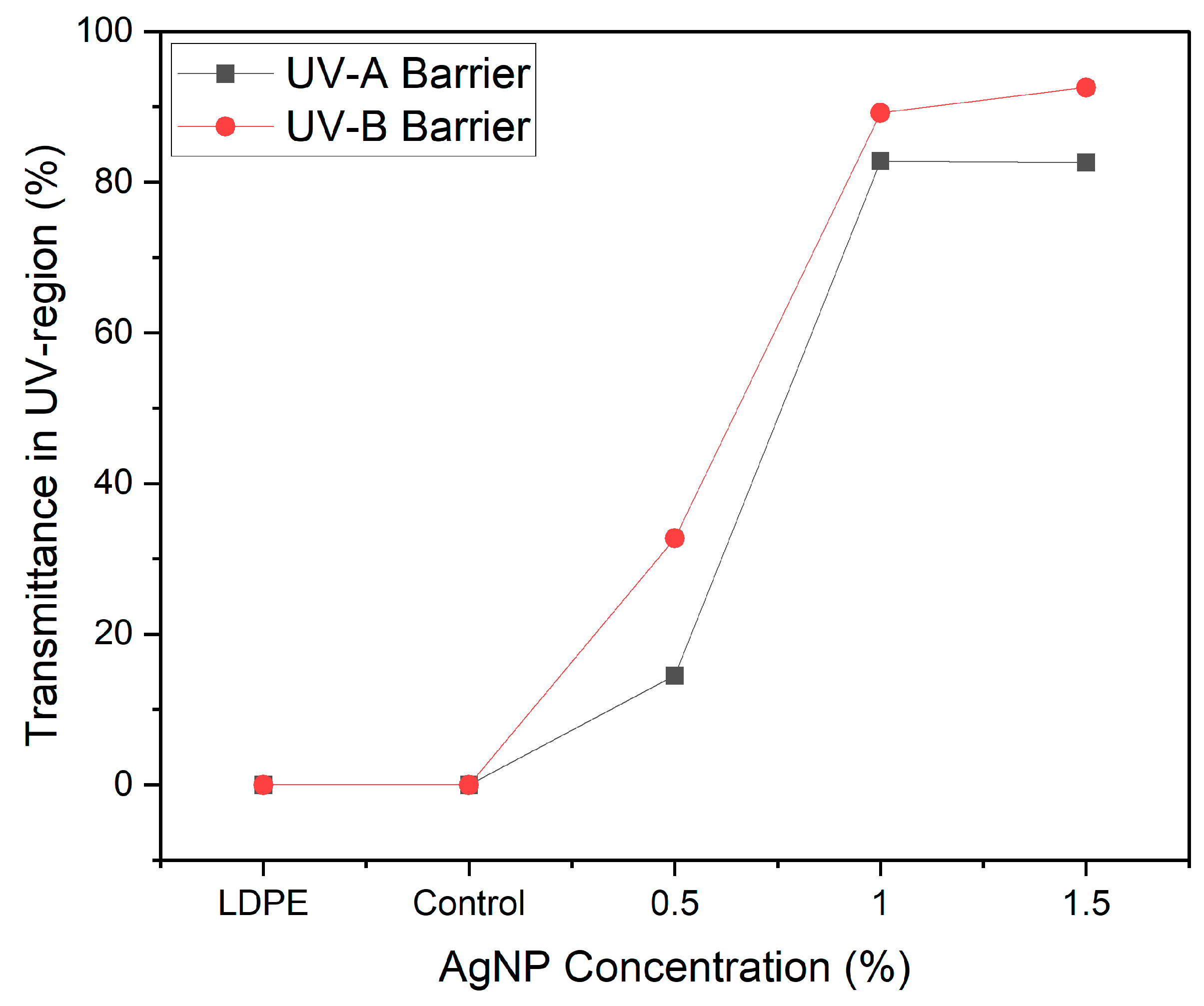
3.4. Film Optical Properties
3.5. Water Sensitivity
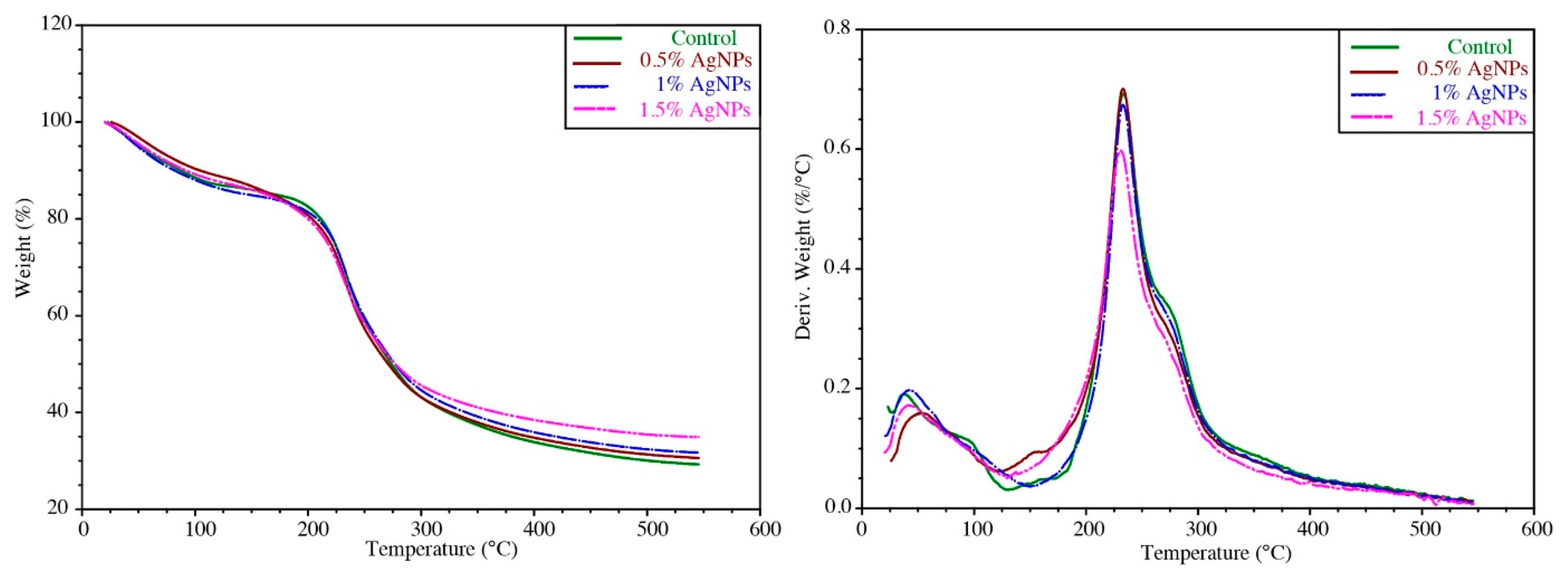
3.6. Thermogravimetric Analysis
3.7. Antimicrobial Activity
3.8. Films Bio-Disintegration
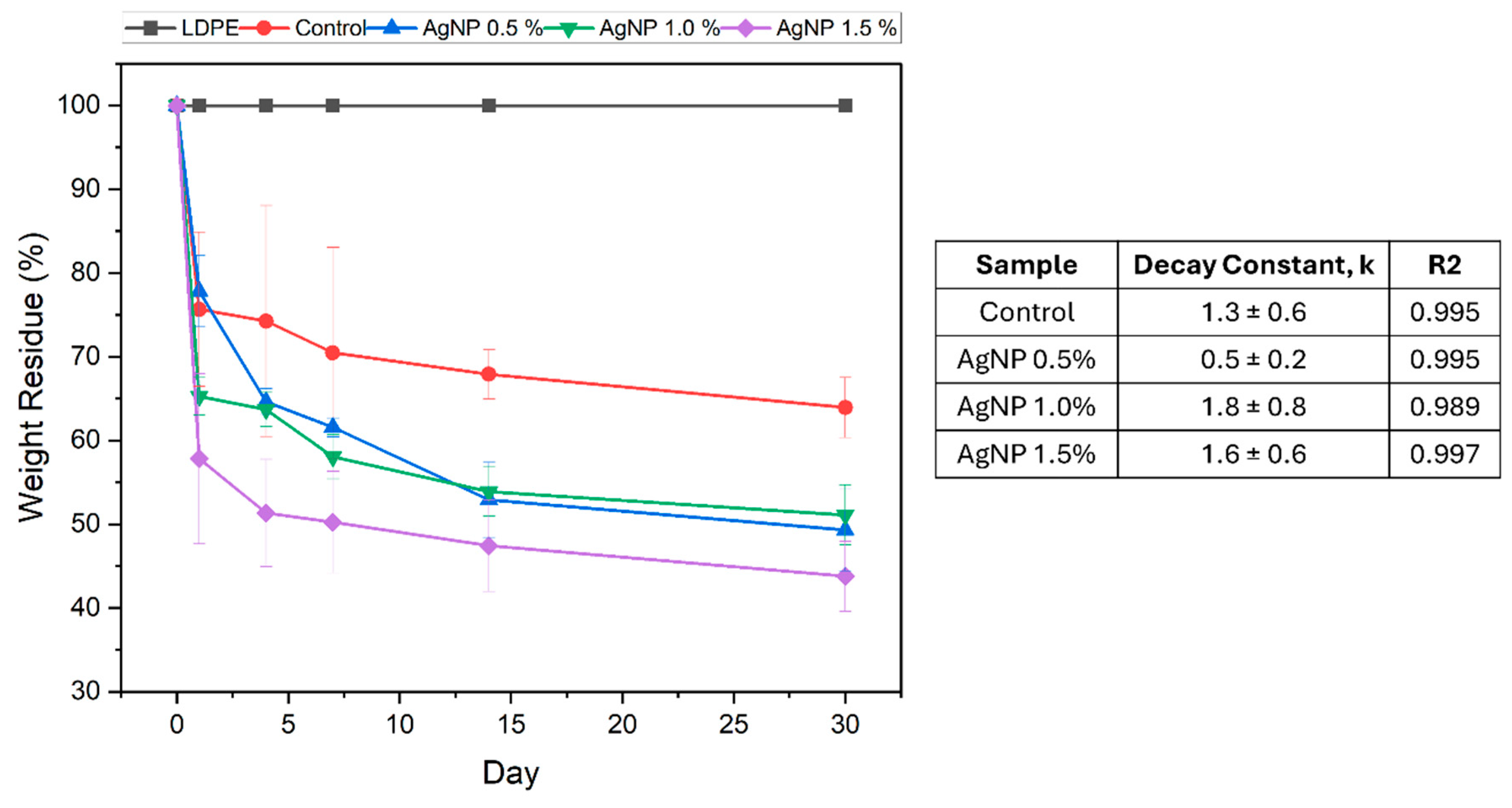
3.8.1. Soil Bio-Disintegration
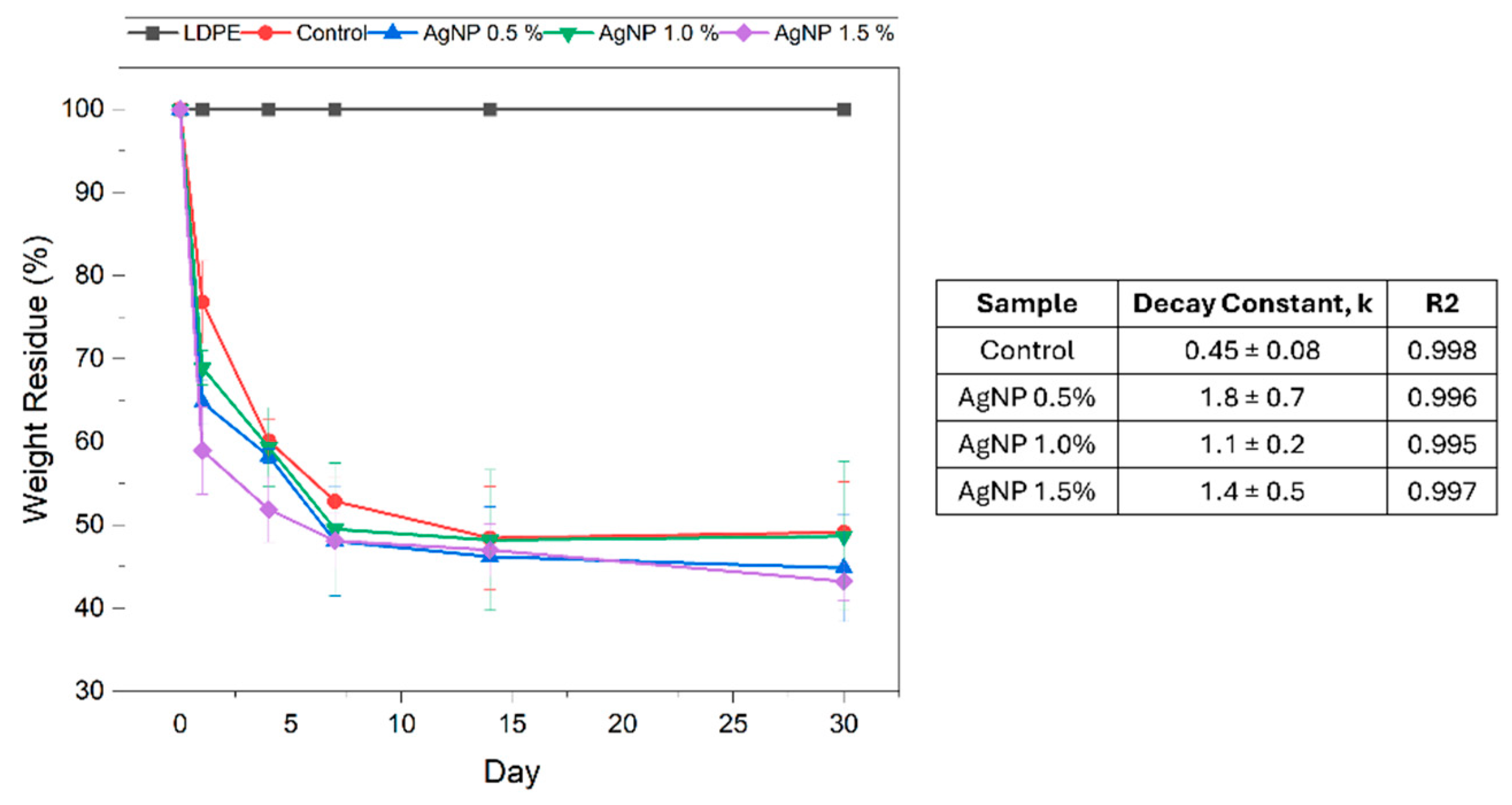
3.8.2. Seawater Bio-Disintegration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tammina, S.K.; Rhim, J.W. Carboxymethylcellulose/agar-based functional film incorporated with nitrogen-doped polyethylene glycol-derived carbon dots for active packaging applications. Chemosphere 2022, 313, 137627. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Cui, Y.; Barnes, M.; Satam, C.; Zhang, S.; Chowdhury, R.A.; Adumbumkulath, A.; Sahin, O.; Miller, C.; Sajadi, S.M.; et al. Multifunctional bio-nanocomposite coatings for perishable fruits. Adv. Mater. 2020, 32, e1908291. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food Wastage Footprint Full-Cost Accounting; FAO: Rome, Italy, 2014. [Google Scholar]
- Du, H.; Sun, X.; Chong, X.; Yang, M.; Zhu, Z.; Wen, Y. A review on smart active packaging systems for food preservation: Applications and future trends. Trends Food Sci. Technol. 2023, 141, 104200. [Google Scholar] [CrossRef]
- Peng, B.; Qin, J.; Li, Y.; Wu, K.; Kuang, Y.; Jiang, F. Recent Advances in Nanomaterials-Enabled Active Food Packaging: Nanomaterials Synthesis, Applications and Future Prospects. Food Control 2024, 163, 110542. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O.; Ashaolu, T.J. Applications of nano-materials in food packaging: A review. J. Food Process. Eng. 2021, 44, e13708. [Google Scholar] [CrossRef]
- Dey, A.; Pandey, G.; Rawtani, D. Functionalized nanomaterials driven antimicrobial food packaging: A technological advancement in food science. Food Control 2022, 131, 108469. [Google Scholar] [CrossRef]
- Simbine, E.O.; Rodrigues, L.D.C.; Lapa-Guimarães, J.; Kamimura, E.S.; Corassin, C.H.; de Oliveira, C.A.F. Application of silver nanoparticles in food packages: A review. Food Sci. Technol. 2019, 39, 793–802. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Starch/agar-based functional films integrated with enoki mushroom-mediated silver nanoparticles for active packaging applications. Food Biosci. 2022, 49, 101867. [Google Scholar] [CrossRef]
- Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Silver bionanocomposites as active food packaging: Recent advances & future trends tackling the food waste crisis. Polymers 2023, 15, 4243. [Google Scholar] [CrossRef]
- Marin, S.; Vlasceanu, G.M.; Tiplea, R.E.; Bucur, I.R.; Lemnaru, M.; Marin, M.M.; Grumezescu, A.M. Applications and toxicity of silver nanoparticles: A recent review. Curr. Top. Med. Chem. 2015, 15, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.A.J.; Souza, L.R.R.; Franchi, L.P. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol. Environ. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Porta, R. The plastics sunset and the bio-plastics sunrise. Coatings 2019, 9, 526. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Alrumman, S.A.; Alamri, S.A.; Otaif, K.A.; Mostafa, M.S.; Alfaify, A.M. Bioplastic (poly-3-hydroxybutyrate) production by the marine bacterium Pseudodonghicola xiamenensis through date syrup valorization and structural assessment of the biopolymer. Sci. Rep. 2020, 10, 8815. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, X.; Guan, S.; Dou, Y.; Gao, X. Preparation of lignin containing cellulose nanofibers and its application in PVA nanocomposite films. Int. J. Biol. Macromol. 2020, 158, 1259–1267. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Martínez-Abad, A.; López-Rubio, A. Cost-efficient bio-based food packaging films from unpurified agar-based extracts. Food Packag. Shelf Life 2019, 21, 100367. [Google Scholar] [CrossRef]
- Abdollahi, M.; Damirchi, S.; Shafafi, M.; Rezaei, M.; Ariaii, P. Carboxymethyl cellulose-agar biocomposite film activated with summer savory essential oil as an antimicrobial agent. Int. J. Biol. Macromol. 2019, 126, 561–568. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of carboxymethyl cellulose/agar-based functional films hybridized with alizarin and grapefruit seed extract. ACS Appl. Bio Mater. 2021, 4, 4470–4478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, T.; Xia, K.; Liu, X.; Zhang, X. Preparation and application of edible agar-based composite films modified by cellulose nanocrystals. Food Packag. Shelf Life 2022, 34, 100936. [Google Scholar] [CrossRef]
- Macieja, S.; Środa, B.; Zielińska, B.; Roy, S.; Bartkowiak, A.; Łopusiewicz, Ł. Bioactive carboxymethyl cellulose (CMC)-based films modified with melanin and silver nanoparticles (AgNPs)—The effect of the degree of CMC substitution on the in situ synthesis of AgNPs and films’ functional properties. Int. J. Mol. Sci. 2022, 23, 15560. [Google Scholar] [CrossRef]
- ASTM. Standard test method for tensile properties of thin plastic sheeting, D882-02. In Annual Book of ASTM Standard; ASTM: West Conshohocken, PA, USA, 2010. [Google Scholar] [CrossRef]
- ASTM D412; Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension. ASTM International: West Conshohocken, PA, USA, 2021.
- Dag, D.; Jung, J.; Zhao, Y. Development and characterization of cellulose nanofiber reinforced hydroxypropyl methylcellulose films functionalized with propolis-loaded zein nanoparticles and its application for cheddar cheese storage. Int. J. Biol. Macromol. 2024, 261, 129790. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Zannini, D.; Santagata, G.; Giosafatto, C.V.L. Cardoon seed oil cake proteins as substrate for microbial transglutaminase: Their application as matrix for bio-based packaging to extend the shelf-life of peanuts. Food Hydrocoll. 2023, 147, 109339. [Google Scholar] [CrossRef]
- ASTM. Standard test methods for water vapor transmission of materials, E96-95. In ASTM. Annual Book of ASTM Standards; American Society for Testing and Materials: Philadephia, PA, USA, 1995; pp. 1–12. [Google Scholar]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packag. Shelf Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Klein, S.E.; Alzagameem, A.; Rumpf, J.; Korte, I.; Kreyenschmidt, J.; Schulze, M. Antimicrobial activity of lignin-derived polyurethane coatings prepared from unmodified and demethylated lignins. Coatings 2019, 9, 494. [Google Scholar] [CrossRef]
- Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Advancing food preservation: Sustainable Green-AgNPs bionanocomposites in paper-starch flexible packaging for prolonged shelf life. Polymers 2024, 16, 941. [Google Scholar] [CrossRef]
- Roy, S.; Chawla, R.; Santhosh, R.; Thakur, R.; Sarkar, P.; Zhang, W. Agar-based edible films and food packaging application: A comprehensive review. Trends Food Sci. Technol. 2023, 141, 104198. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Varriale, S.; Porta, R.; Naviglio, D.; Spennato, M.; Gardossi, L.; Giosafatto, C.V.L.; Pezzella, C. A biorefinery approach for the conversion of Cynara cardunculus biomass to active films. Food Hydrocolloids 2022, 122, 107099. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation and characterization of agar/lignin/silver nanoparticles composite films with ultraviolet light barrier and antibacterial properties. Food Hydrocoll. 2017, 71, 76–84. [Google Scholar] [CrossRef]
- Wardana, A.A.; Widyaningsih, T.D. Development of edible films from tapioca starch and agar, enriched with red cabbage (Brassica oleracea) as a sausage deterioration bio-indicator. IOP Conf. Ser. Earth Environ. Sci. 2017, 109, 012031. [Google Scholar] [CrossRef]
- Roy, S.; Shankar, S.; Rhim, J.W. Melanin-mediated synthesis of silver nanoparticle and its use for the preparation of carrageenan-based antibacterial films. Food Hydrocoll. 2019, 88, 237–246. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Rahman, R.A.; Jokar, M.; Darroudi, M. Silver/poly(lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int. J. Nanomed. 2010, 5, 573–579. [Google Scholar] [CrossRef]
- Di Muzio, L.; Cairone, F.; Cesa, S.; Sergi, C.; Tirillò, J.; Angiolella, L.; Giammarino, A.; Giusiano, G.; Petralito, S.; Casadei, M.A.; et al. Gellan gum-based nanocomposites films containing bio reduced silver nanoparticles: Synthesis, characterisation and antifungal activity. Carbohydr. Polym. Technol. Appl. 2024, 7, 100485. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Volery, P.; Besson, R.; Schaffer-Lequart, C. Characterization of commercial carrageenans by Fourier transform infrared spectroscopy using single-reflection attenuated total reflection. J. Agric. Food Chem. 2004, 52, 7457–7463. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Rhim, J.-W. Properties and characterization of agar/CuNP bionanocomposite films prepared with different copper salts and reducing agents. Carbohydr. Polym. 2014, 114, 484–492. [Google Scholar] [CrossRef] [PubMed]
- El Miri, N.; Abdelouahdi, K.; Barakat, A.; Zahouily, M.; Fihri, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films reinforced with cellulose nanocrystals: Rheology of film-forming solutions, transparency, water vapor barrier and tensile properties of films. Carbohydr. Polym. 2015, 129, 156–167. [Google Scholar] [CrossRef]
- Agarwal, S.; Hoque, M.; Bandara, N.; Pal, K.; Sarkar, P. Synthesis and characterization of tamarind kernel powder-based antimicrobial edible films loaded with geraniol. Food Packag. Shelf Life 2020, 26, 100562. [Google Scholar] [CrossRef]
- Ahmadi, F.; Lackner, M. Green synthesis of silver nanoparticles from Cannabis sativa: Properties, synthesis, mechanistic aspects, and applications. ChemEngineering 2024, 8, 64. [Google Scholar] [CrossRef]
- Song, X.; Wei, X.; Liu, L.; Liu, Y. Gelatin/agar pH-indicator film based on cranberry extract loaded with linalool nanoparticle: Survey on physical, antimicrobial, and antioxidant properties. Int. J. Biol. Macromol. 2024, 268, 131767. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Ibáñez-Ibáñez, P.F.; Giosafatto, C.V.L.; del Castillo-Santaella, T.; Rodríguez-Valverde, M.A.; Maldonado-Valderrama, J. Surface activity of protein extracts from seed oil by-products and wettability of developed bioplastics. Food Hydrocoll. 2023, 145, 109091. [Google Scholar] [CrossRef]
- Dash, K.K.; Kumar, A.; Kumari, S.; Malik, M.A. Silver nanoparticle incorporated flaxseed protein-alginate composite films: Effect on physicochemical, mechanical, and thermal properties. J. Polym. Environ. 2021, 29, 3649–3659. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Rossi-Marquez, G.; Mariniello, L.; Kadivar, M.; Arabestani, A. Microstructure and properties of bitter vetch (Vicia ervilia) protein films reinforced by microbial transglutaminase. Food Hydrocoll. 2015, 50, 102–107. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Tocopherol-mediated synthesis of silver nanoparticles and preparation of antimicrobial PBAT/silver nanoparticles composite films. LWT-Food Sci. Technol. 2016, 72, 149–156. [Google Scholar] [CrossRef]
- Sarıcaoglu, F.T.; Turhan, S. Physicochemical, antioxidant and antimicrobial properties of mechanically deboned chicken meat protein films enriched with various essential oils. Food Packag. Shelf Life 2020, 25, 100527. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Agar-based antioxidant composite films incorporated with melanin nanoparticles. Food Hydrocoll. 2019, 94, 391–398. [Google Scholar] [CrossRef]
- Ahuja, D.; Kumar, L.; Kaushik, A. Thermal stability of starch bionanocomposites films: Exploring the role of esterified cellulose nanofibers isolated from crop residue. Carbohydr. Polym. 2021, 255, 117466. [Google Scholar] [CrossRef] [PubMed]
- Mahuwala, A.A.; Hemant, V.; Meharwade, S.D.; Deb, A.; Chakravorty, A.; Grace, A.N.; Raghavan, V. Synthesis and characterization of starch/agar nanocomposite films for food packaging application. IET Nanobiotechnol. 2020, 14, 809–814. [Google Scholar] [CrossRef]
- Liu, C.; Shen, Y.; Yang, M.; Chi, K.; Guo, N. Hazard of staphylococcal enterotoxins in food and promising strategies for natural products against virulence. J. Agric. Food Chem. 2022, 70, 2450–2465. [Google Scholar] [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; García, M.A.; Arce, V.B. Nanocomposite films with silver nanoparticles synthesized in situ: Effect of corn starch content. Food Hydrocoll. 2019, 97, 105200. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Liu, B.; Dong, Q. One-step analysis for Listeria monocytogenes growth in ready-to-eat braised beef at dynamic and static conditions. J. Food Prot. 2019, 82, 1820–1827. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Castro, L.E.N.; da Rosa, C.G.; da Rosa Almeida, A.; Maciel-Silva, F.W.; Kempe, P.R.G.; de Oliveira, A.L.R.; Forster-Carneiro, T.; Bertoldi, F.C.; Barreto, P.L.M.; et al. Production of nanocomposite films functionalized with silver nanoparticles bioreduced with rosemary (Rosmarinus officinalis L.) essential oil. J. Agric. Food Res. 2023, 11, 100479. [Google Scholar] [CrossRef]
- Bashir, A.; Ali, K.; Bux, K.; Farid, N.; Khaireabadi, M.; Hassan, K.A.; Hussain, A.; Fatima, K.; Mehmood, S.; Haider, S.A.; et al. Molecular characterization, purification, and mode of action of enterocin KAE01 from lactic acid bacteria and its in silico analysis against MDR/ESBL Pseudomonas aeruginosa. Genes 2022, 13, 2333. [Google Scholar] [CrossRef] [PubMed]
- Mirpoor, S.F.; Corrado, I.; Di Girolamo, R.; Dal Poggetto, G.; Panzella, L.; Borselleca, E.; Pezzella, C.; Giosafatto, C.V.L. Manufacture of active multilayer films made of functionalized pectin coated by polyhydroxyalkanoates: A fully renewable approach to active food packaging. Polymer 2023, 281, 126136. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Xie, J.; Xin, L.; Hu, X.; Cheng, W.; Liu, W.; Wang, Z. Technical application of safety and cleaner production technology by underground coal gasification in China. J. Clean. Prod. 2020, 250, 119487. [Google Scholar] [CrossRef]
- Hong, S.-I.; Cho, Y.; Rhim, J.-W. Effect of agar/AgNP composite film packaging on refrigerated beef loin quality. Membranes 2021, 11, 750. [Google Scholar] [CrossRef]
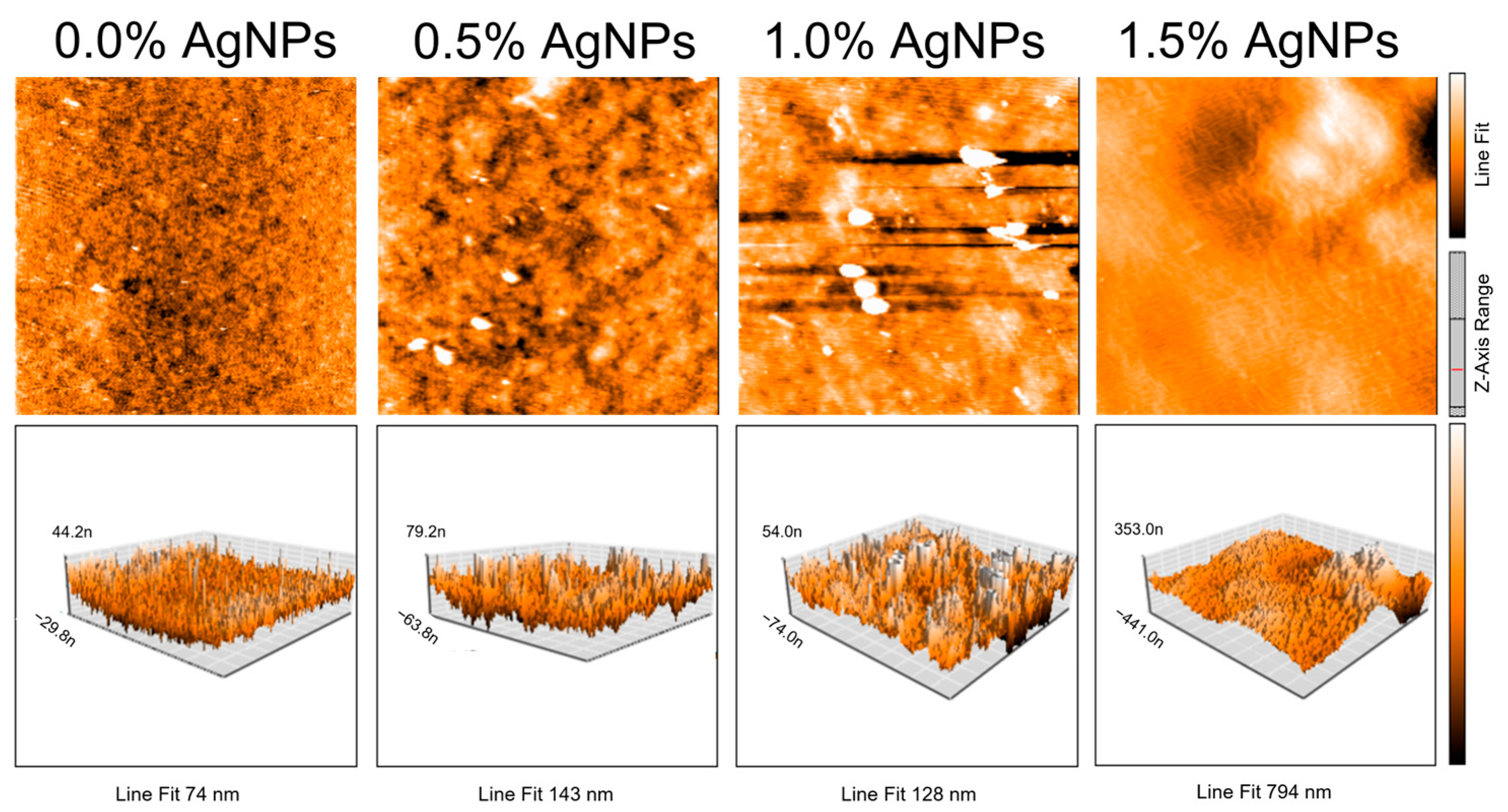
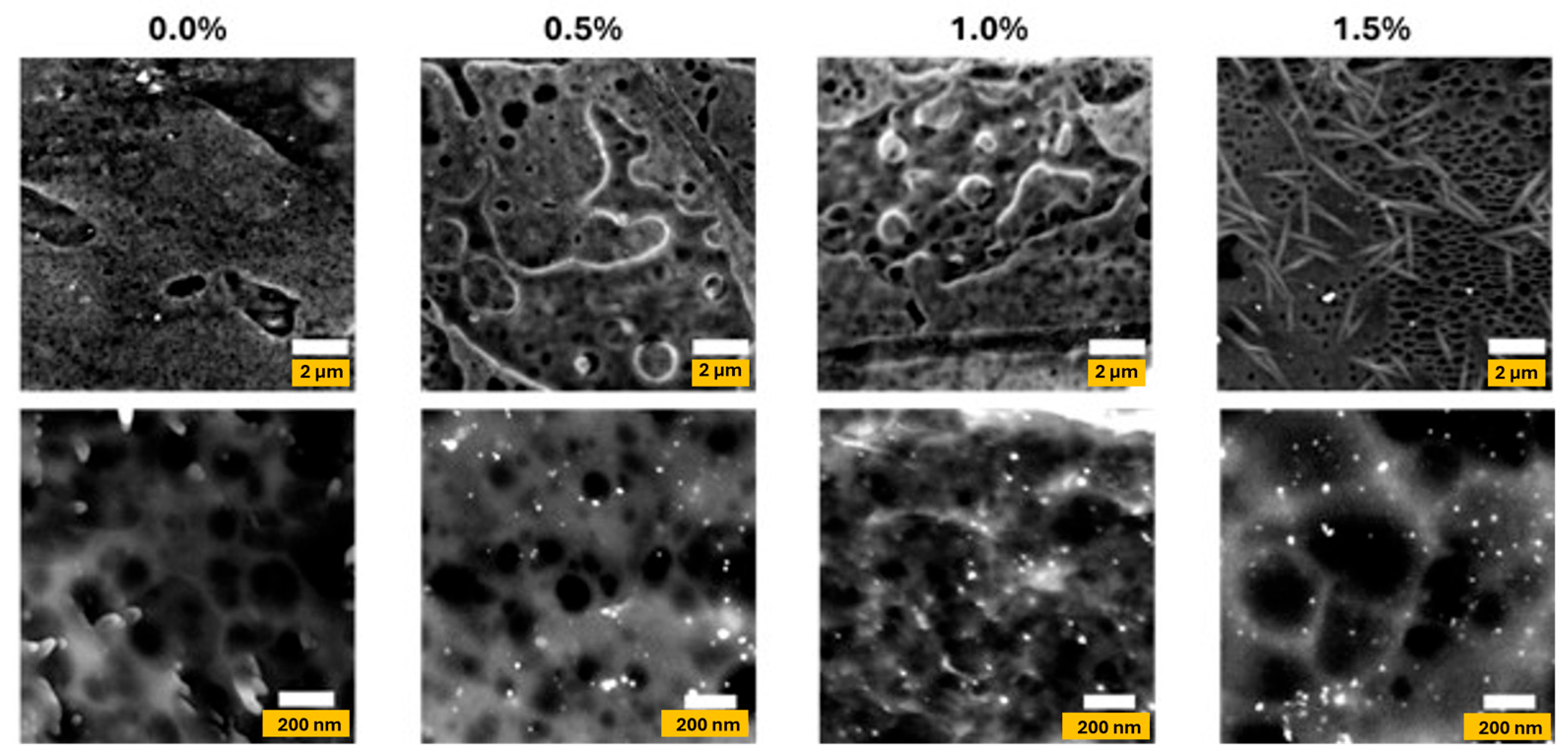

| AgNP Concentration (%) | Roughness Ra (nm) | Roughness Rq (nm) |
|---|---|---|
| 0 | 8.92 ± 1.24 a | 11.65 ± 2.29 e |
| 0.5 | 15.98 ± 2.07 b | 20.16 ± 3.07 f |
| 1.0 | 22.86 ± 3.81 c | 28.37 ± 5.20 g |
| 1.5 | 36.92 ± 4.26 d | 39.99 ± 14.24 g |
| AgNP Concentration (%) | WVP (×10−9 g m/m2 Pa s) | Swelling Ratio (%) | Moisture Content (%) | Solubility (%) |
|---|---|---|---|---|
| Control | 1.24 ± 0.08 c | 1558.4 ± 69.48 c | 17.2 ± 0.81 a | 79.2 ± 3.48 c |
| 0.5 | 1.09 ± 0.05 ab | 1451.63 ± 75.69 bc | 16.74 ± 0.51 a | 75.1 ± 2.43 bc |
| 1.0 | 0.92 ± 0.06 a | 1338.8 ± 76.94 ab | 16.8 ± 0.92 a | 71.24 ± 1.84 ab |
| 1.5 | 0.98 ± 0.04 ab | 1228.9 ± 65.27 a | 17 ± 0.73 a | 67.33 ± 2.41 a |
| AgNPs Concentration (%) | Control | 0.5 | 1.0 | 1.5 |
|---|---|---|---|---|
| Listeria monocytogenes 1043S | No inhibition | 0.097 ± 0.008 | 0.112 ± 0.005 | |
| Staphylococcus aureus NCTC 8532 | No inhibition | 0.120 ± 0.012 | ||
| Escherichia coli ATCC 25922 | No inhibition | 0.136 ± 0.007 | ||
| Pseudomonas aerujinosa NCTC 10322 | No inhibition | 0.105 ± 0.010 | ||
| Sample | Day 1 | Day 4 | Day 14 | Day 30 |
|---|---|---|---|---|
| Control |  |  |  |  |
| 0.5% AgNPs |  |  |  |  |
| 1.0% AgNPs |  |  |  |  |
| 1.5% AgNPs |  |  |  |  |
| Sample | Day 1 | Day 4 | Day 14 | Day 30 |
|---|---|---|---|---|
| Control |  |  |  |  |
| 0.5% AgNPs |  |  |  |  |
| 1.0% AgNPs |  |  |  |  |
| 1.5% AgNPs |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirpoor, S.F.; Massironi, A.; Winning, D.; Lignou, S.; Ghawi, S.K.; Trotta, F.; Charalampopoulos, D. Agar/Carboxymethyl Cellulose Blended Films with Green-Synthesised Silver Nanoparticles as a Sustainable Alternative for Food Packaging Applications. Polymers 2025, 17, 3126. https://doi.org/10.3390/polym17233126
Mirpoor SF, Massironi A, Winning D, Lignou S, Ghawi SK, Trotta F, Charalampopoulos D. Agar/Carboxymethyl Cellulose Blended Films with Green-Synthesised Silver Nanoparticles as a Sustainable Alternative for Food Packaging Applications. Polymers. 2025; 17(23):3126. https://doi.org/10.3390/polym17233126
Chicago/Turabian StyleMirpoor, Seyedeh Fatemeh, Alessio Massironi, Danielle Winning, Stella Lignou, Sameer Khalil Ghawi, Federico Trotta, and Dimitris Charalampopoulos. 2025. "Agar/Carboxymethyl Cellulose Blended Films with Green-Synthesised Silver Nanoparticles as a Sustainable Alternative for Food Packaging Applications" Polymers 17, no. 23: 3126. https://doi.org/10.3390/polym17233126
APA StyleMirpoor, S. F., Massironi, A., Winning, D., Lignou, S., Ghawi, S. K., Trotta, F., & Charalampopoulos, D. (2025). Agar/Carboxymethyl Cellulose Blended Films with Green-Synthesised Silver Nanoparticles as a Sustainable Alternative for Food Packaging Applications. Polymers, 17(23), 3126. https://doi.org/10.3390/polym17233126