Development of Car Coating Materials over the Past Decade for Paint Protection Applications—An Overview on the Different Types of Paint Protections
Abstract
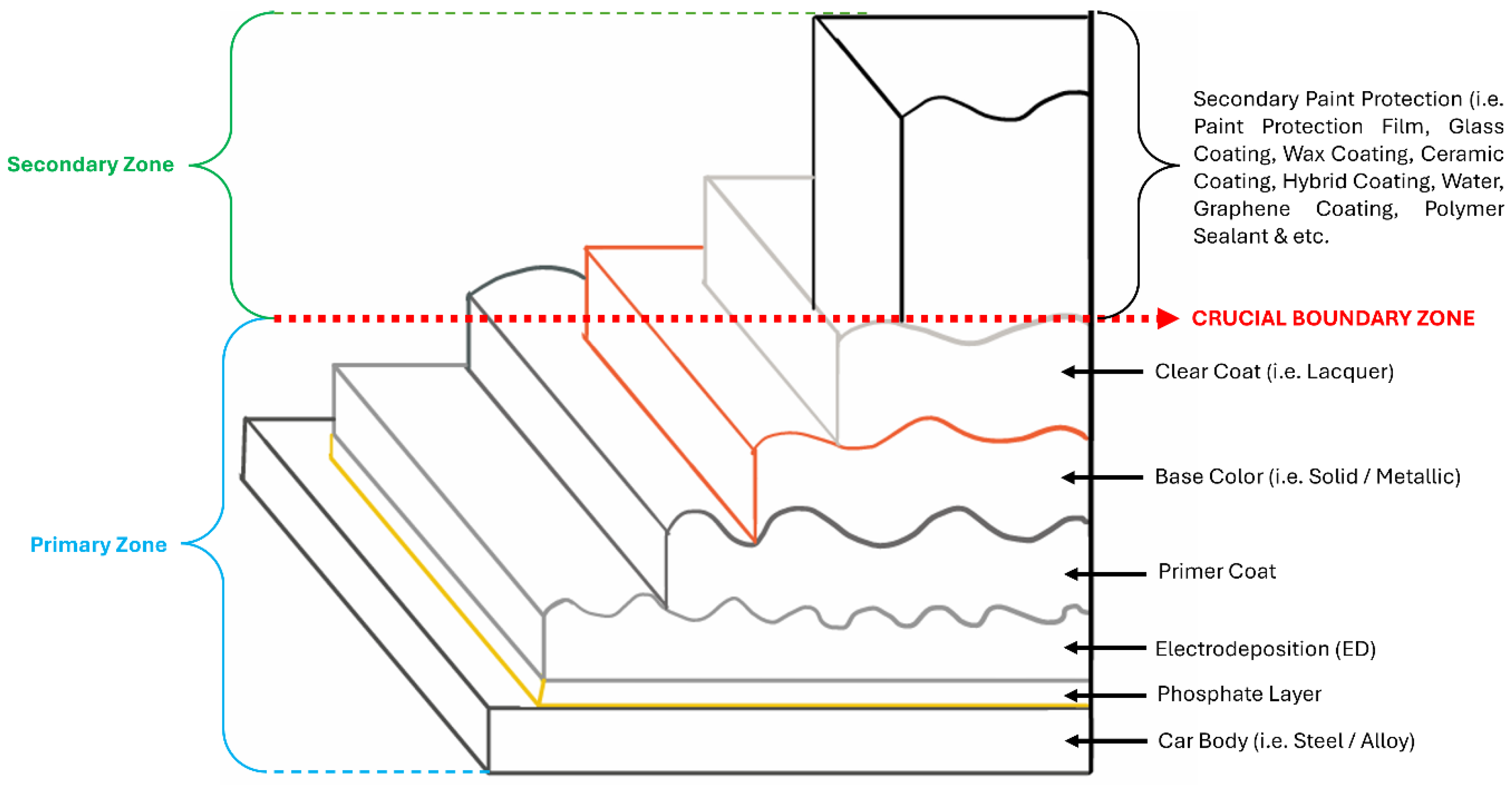
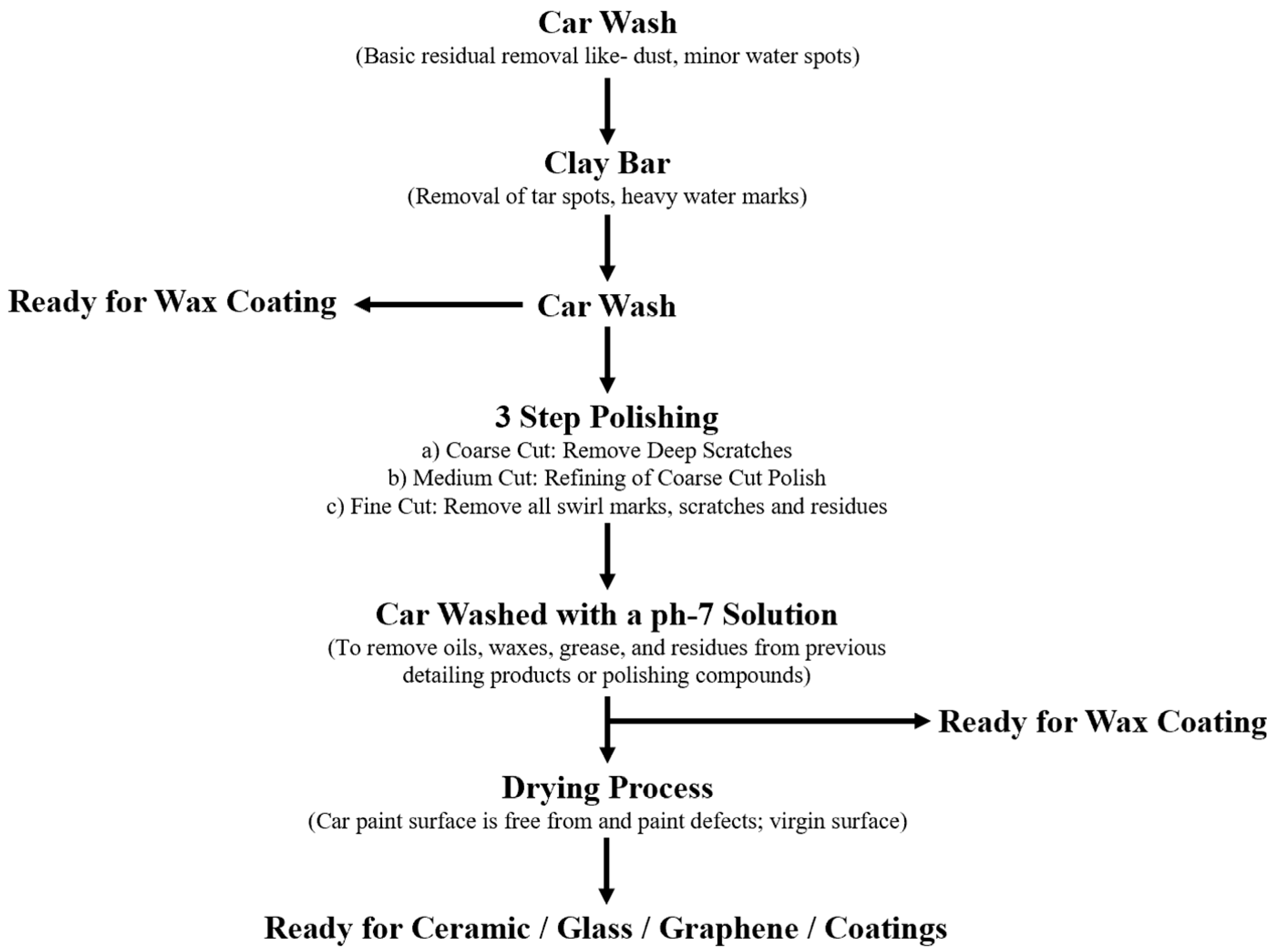
1. Introduction
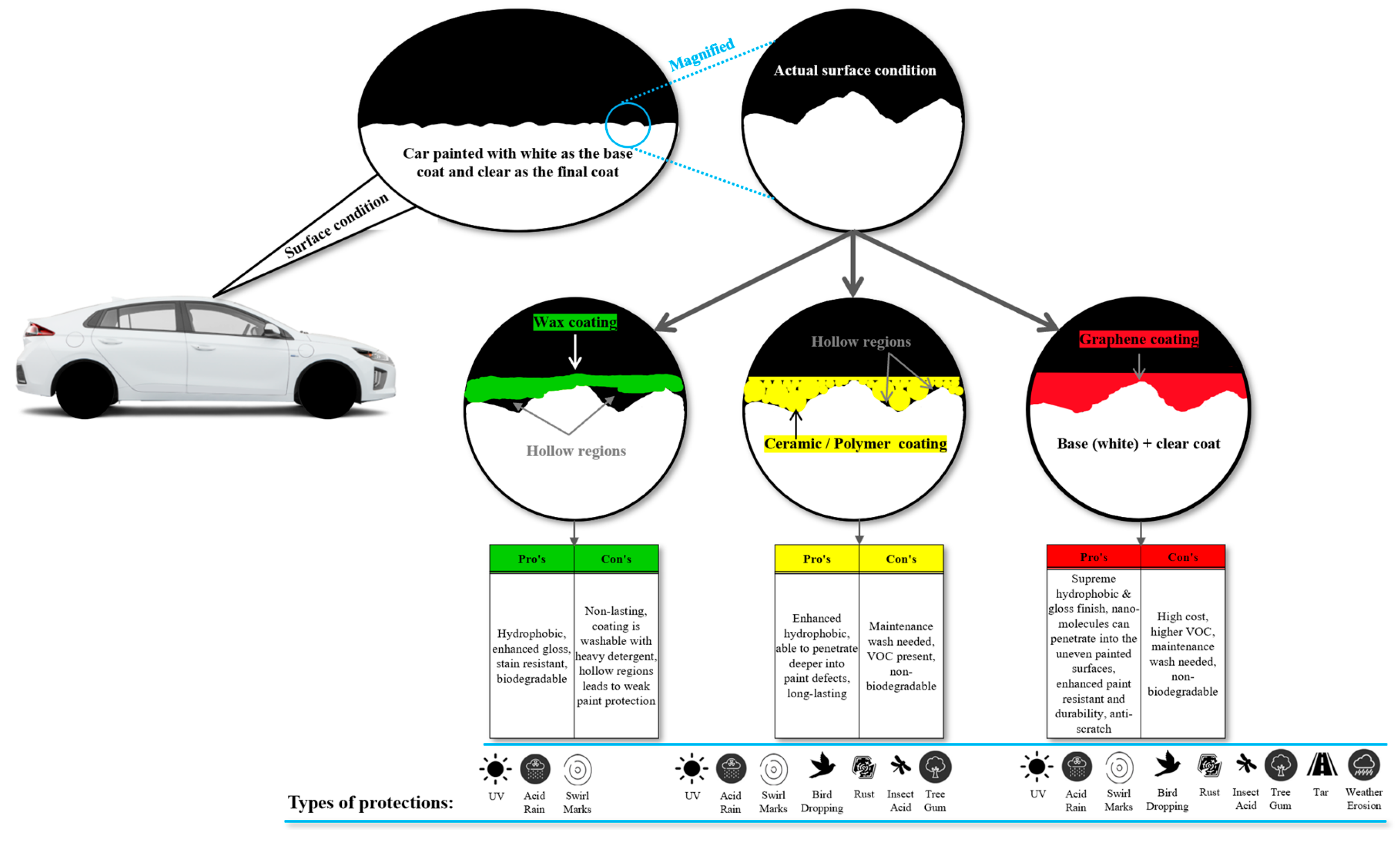
2. Introduction to ‘Wax’ Coating
Review on the Different Types of Wax Coatings by Brands
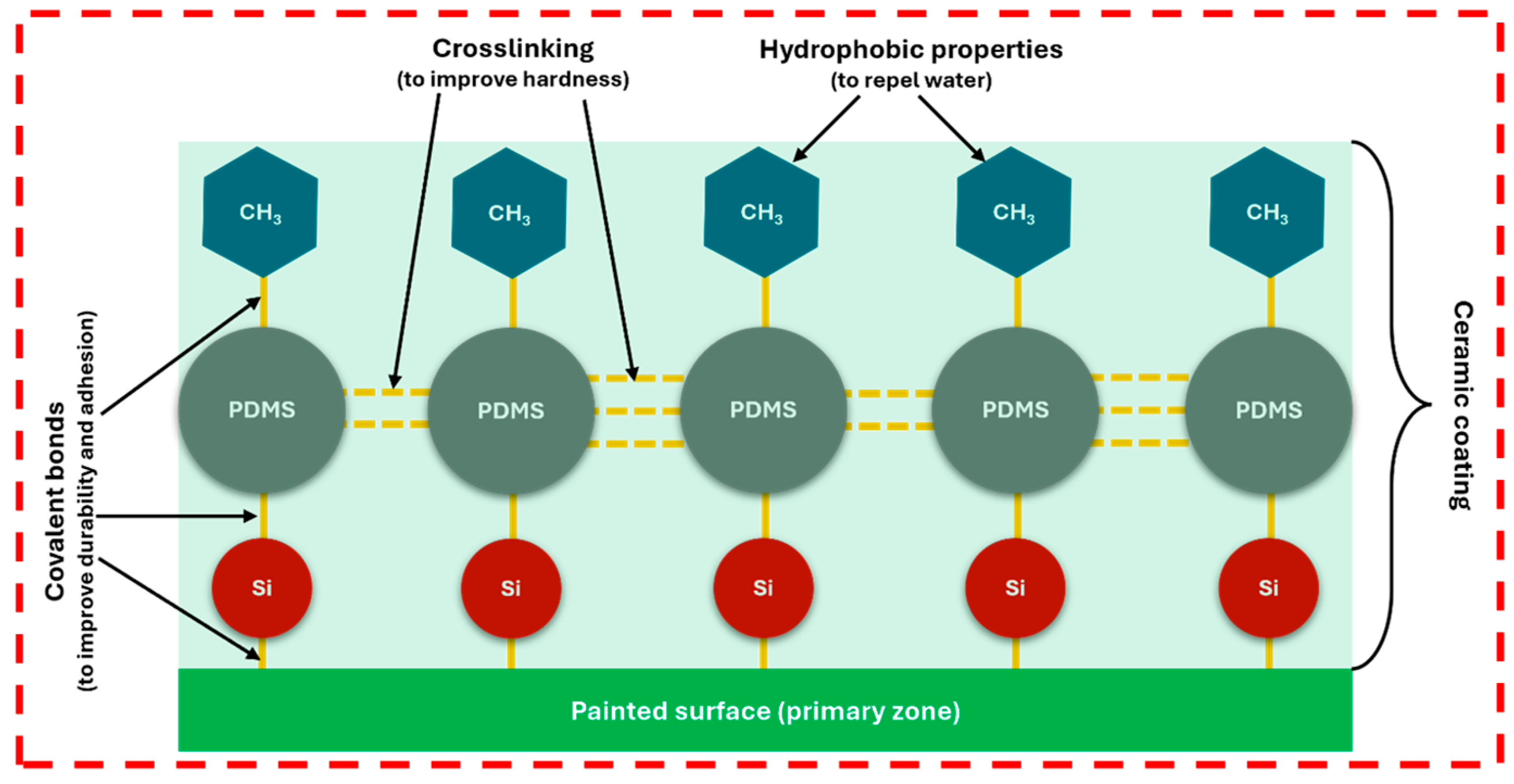
3. Introduction to ‘Ceramic’ Coating
Review on the Different Types of Ceramic Coatings by Brands
4. Other Types of Coating
- (i)
- Polymer coatings/Synthetic sealants
- (ii)
- Graphene coatings
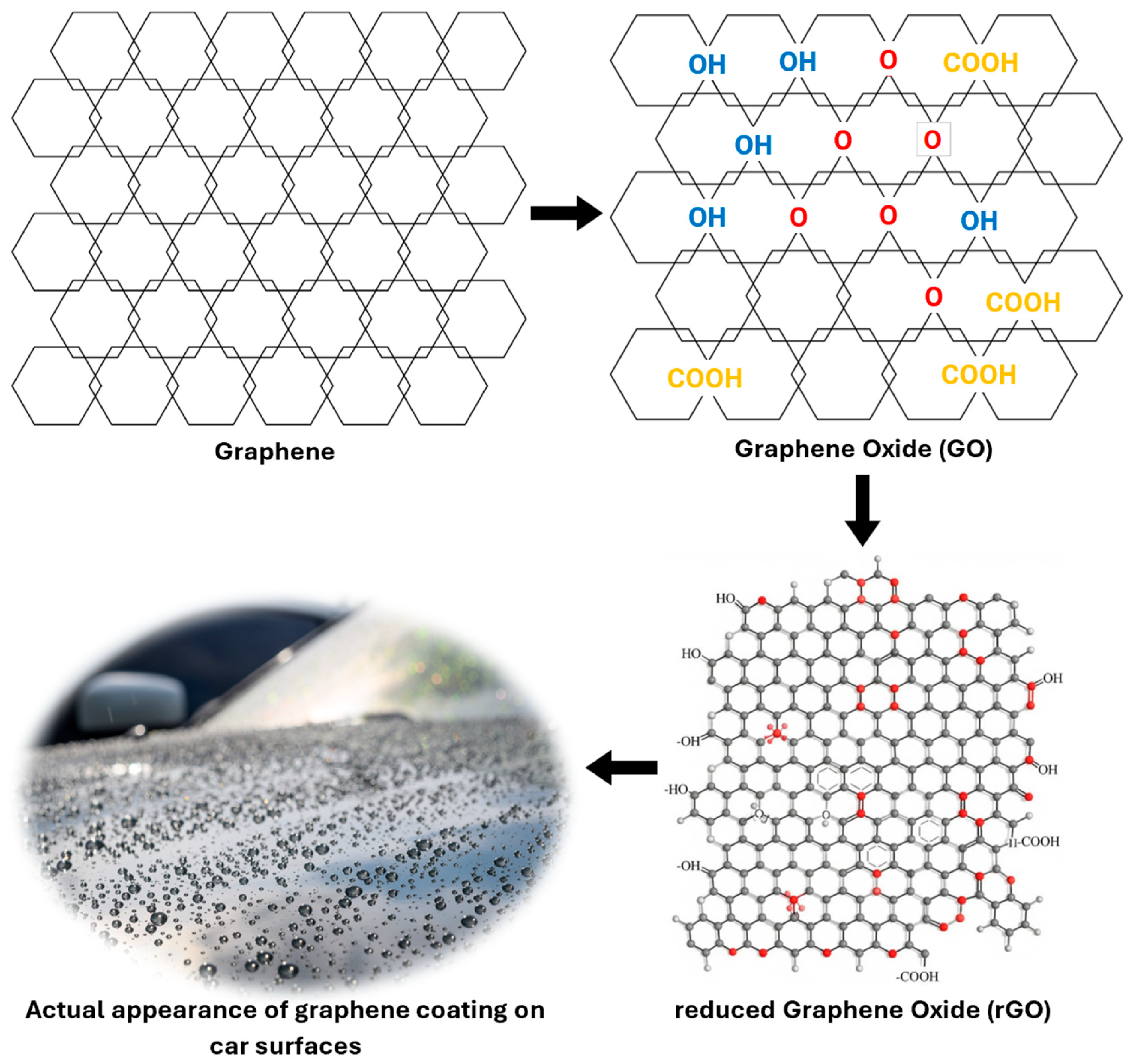
Review on the Different Types of Graphene Coatings by Brands
5. Summary of Reviewed Works
- The cost of paint protection coatings generally follows the order: wax < ceramic << graphene, with wax being the most affordable option and graphene the most expensive.
- Spray-type coatings are generally preferred by car owners due to their ease of use and suitability for DIY application after routine washing. In contrast, liquid-type coatings are typically applied by professional detailers, offering significantly greater durability but at a higher cost. The elevated expense reflects the labour-intensive surface correction required prior to application, a process that may take one to three days depending on weather conditions and curing time.
- Many car owners are aware of the different types of paint protection available. Literature suggests that new car owners, in particular, are more willing to invest in durable and long-lasting coatings, as their vehicles typically exhibit minimal or no surface defects compared to used cars without prior paint protection. Consequently, the overall cost of application is relatively lower, since only limited paint correction work is required by professional detailers.
- The pursuit of an “optimum” coating—one that offers maximum resistance to environmental and mechanical defects—has fuelled intense competition among manufacturers. However, a persistent challenge lies in formulating coatings that are both high-performing and environmentally sustainable. When reducing VOC emissions remains critical: while low-VOC formulations are safer for users and less harmful to the environment, they often compromise coating quality and durability. Consequently, manufacturers must balance performance with regulatory compliance, as coatings are subject to strict legislative standards before being released to the market.
- In some cases, coatings are misleadingly marketed to maximize profit—for example, products advertised as ceramic coatings may in fact be wax-based. Such misrepresentation often results in buyers paying two or more times the actual value of the product. To avoid these pitfalls, consumers are strongly advised to purchase coatings from reputable brands, certified detailers, or trusted retail outlets.
- Some coatings lack a valid brand or legitimate manufacturer and are often generically labelled, for example, as “glass-ceramic coatings.” When such unverified products are purchased online or off-the-shelf and applied to vehicle surfaces, they may contain harmful chemicals that degrade the clear coat, progressively thinning it with repeated use. This leaves the underlying paint vulnerable to environmental exposure, increasing the risk of cracks, chips, and eventually rust formation.
- In some cases, car detailers prioritize profit over quality, leading to inadequate surface decontamination and preparation prior to coating application. Consequently, defects such as tar spots, scratches, and swirl marks often remain visible beneath the applied layer. While specialized chemicals can be used to strip the coating and correct these flaws, large-scale or repeated use may negatively interact with advanced coatings such as ceramic, glass, or graphene resulting in smearing and an uneven finish that diminishes gloss and visual appeal under light.
- It should be noted that coating longevity is strongly influenced by hardness, which is a key determinant of durability. As hardness increases, the coating becomes increasingly difficult and sometimes nearly impossible to remove, while the use of chemical removers may further compromise surface quality. This underscores the importance of proper surface preparation prior to application. Notably, two detailers using the same brand of coating may deliver very different outcomes depending on the rigor of their preparation process, even if their service costs vary. Ultimately, inadequate surface preparation undermines the protective performance of the coating and diminishes the final aesthetic appearance of the vehicle under visible light.
6. Future Research
- Recent studies indicate that combining ceramic coatings with other chemical components to produce hybrid formulations can significantly enhance coating longevity while imparting superhydrophobic properties. Numerous investigations on hybrid coatings have been reported, and continued research is essential to establish an optimal chemical blend that balances performance with user safety and environmental sustainability. In this regard, the development of biodegradable coatings derived from natural plant- and animal-based sources is proposed as a promising direction. However, the challenge remains unaddressed since according to the literature, it is noted that lowering the VOC content of a coating can reduce the longevity of paint protection. Hence, more research is proposed in this area to get a correct formulation of biodegradable hybrid coatings with a justified amount of VOC values for optimum paint protection.
- Most car coatings contain a measurable number of VOCs. Although these coatings generally comply with national regulatory standards, future research should prioritize the development of zero-VOC formulations to minimize their environmental impact and improve user safety.
- Intelligent coatings represent a promising future direction for automotive paint protection. Although this concept remains largely unexplored, the central idea is to enable coatings to self-align at the atomic or molecular level, thereby forming a uniform layer of consistent thickness regardless of the application method. Such a feature would overcome one of the key limitations of current practices, where manual application through rubbing or spreading often results in overlapping layers and non-uniform coating thickness after solidification.
- Spray-type coatings have been widely adopted and have demonstrated excellent water-repellent properties. However, whether applied manually or robotically, the spraying process often produces overlapping layers, leading to uneven coating thickness. This not only compromises coating uniformity but may also add unnecessary weight to the vehicle, with potential implications for fuel efficiency. Consequently, the development of intelligent coatings capable of achieving self-regulated, uniform deposition remains an unresolved research challenge.
- Self-healing coatings have been reported to restore minor damages such as light scratches and swirl marks. However, a major limitation is their dependence on external stimuli—typically heat—to activate the healing process. This requirement restricts their effectiveness in colder regions where sunlight and UV exposure are limited. To address this, the concept of an “e-self-healing” coating has been proposed, wherein a mild electrical current induces molecular realignment, allowing the coating to recover its original properties. This mechanism relies on the ability of conductive components within the coating to reorganize under electrical stimulation. Nevertheless, further research is required, as such coatings are commonly formulated with conductive materials—such as iron (Fe)—which may increase the overall weight of the vehicle.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Sparer, J.; Woskie, S.R.; Cullen, M.R.; Chung, J.S.; Holm, C.T.; Redlich, C.A. Qualitative assessment of isocyanate skin exposure in auto body shops: A pilot study. Am. J. Ind. Med. 2000, 37, 265–274. [Google Scholar] [CrossRef]
- Khanna, A.S. (Ed.) High-Performance-Organic-Coatings; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Standeven, H. The Development of Decorative Gloss Paints in Britain and the United States C. 1910–1960. J. Am. Inst. Conserv. 2006, 45, 51–65. [Google Scholar] [CrossRef]
- Streitberger, H.-J.; Dössel, K.-F. (Eds.) Automotive Paints and Coatings, 2nd ed.; Wiley-VCH: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Tudose, I.V.; Mouratis, K.; Ionescu, O.N.; Romanitan, C.; Pachiu, C.; Popescu, M.; Khomenko, V.; Butenko, O.; Chernysh, O.; Kenanakis, G.; et al. Novel Water-Based Paints for Composite Materials Used in Electromagnetic Shielding Applications. Nanomaterials 2022, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Geffen, C.A.; Rothenberg, S. Suppliers and environmental innovation the automotive paint process. Int. J. Oper. Prod. Manag. 2000, 20, 166–186. [Google Scholar] [CrossRef]
- Kwaambwa, H. A Review of Current and Future Challenges in Paints and Coatings Chemistry. Prog. Multidiscip. Res. J. 2013, 3, 75–101. [Google Scholar]
- Hughes, A.E.; Mol, M.J.; Zheludkevich, M.L.; Buchheit, R.G. (Eds.) Active Protective Coatings New-Generation Coatings for Metals; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Caruso, M.M.; Delafuente, D.A.; Ho, V.; Sottos, N.R.; Moore, J.S.; White, S.R. Solvent-promoted self-healing epoxy materials. Macromolecules 2007, 40, 8830–8832. [Google Scholar] [CrossRef]
- Siyang, W.; Marek, W.U. Self-healing polymers. Nat. Rev. Mater. 2020, 2, 562–583. [Google Scholar] [CrossRef]
- Baghdachi, J.; Hernandez, H.R.P.; Templeman, C.G. Self-Stratifying Automotive Topcoat Compositions and Processes. U.S. Patent Number 7,863,375, 8 April 2011. [Google Scholar]
- Ohno, M. Painting of plastic automotive parts and panels. Int. J. Mater. Prod. Technol. 1988, 3, 285–306. [Google Scholar]
- Gómez, O.; Perales, E.; Chorro, E.; Burgos, F.J.; Viqueira, V.; Vilaseca, M.; Martínez-Verdú, F.M.; Pujol, J. Visual and instrumental assessments of color differences in automotive coatings. Color Res. Appl. 2016, 41, 384–391. [Google Scholar] [CrossRef]
- Iost, A.; Bigot, R. Hardness of coatings. Surf. Coat. Technol. 1996, 80, 117–120. [Google Scholar] [CrossRef]
- Pashova, S. Application of Plant Waxes in Edible Coatings. Coatings 2023, 13, 911. [Google Scholar] [CrossRef]
- Junio, E.J.M.R.; Stephen, J.R.V.; Muthuvel, M.; Roy, A.; Rodrigue, P.d.A.; Mendonça Filho, M.J.A.d.; Araújo Teixeira, R.; Barbosa, A.d.P.; Benjamin, S.R. Chemistry, Biological Activities, and Uses of Carnauba Wax. In Gums, Resins and Latexes of Plant Origin; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–23. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Sotoudeh, F.; Mousavi, S.M.; Karimi, N.; Lee, B.J.; Abolfazli-Esfahani, J.; Manshadi, M.K.D. Natural and synthetic superhydrophobic surfaces: A review of the fundamentals, structures, and applications. Alex. Eng. J. 2023, 68, 587–609. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Neinhuis, C. Superhydrophobic hierarchically structured surfaces in biology: Evolution, structural principles and biomimetic applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20160191. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Z.; Liu, W. Biomimetic Superhydrophobic Materials Construct from Binary Structure: A Review on Design, Properties, and Applications. Adv. Mater. Interfaces 2023, 10, 2201847. [Google Scholar] [CrossRef]
- Avossa, J.; Bifulco, A.; Amendola, E.; Gesuele, F.; Oscurato, S.L.; Gizaw, Y.; Mensitieri, G.; Branda, F. Forming nanostructured surfaces through Janus colloidal silica particles with nanowrinkles: A new strategy to superhydrophobicity. Appl. Surf. Sci. 2019, 465, 73–81. [Google Scholar] [CrossRef]
- Placido, F.; Birney, R.; Kavanagh, J. Investigation of automotive detailing products by ellipsometry and contact angle analysis. Acta Phys. Pol. A 2009, 116, 712–714. [Google Scholar] [CrossRef]
- Sweeney, D. Available online: https://www.torquedetail.com/blogs/auto-detailing/how-long-does-wax-last-on-a-car (accessed on 11 October 2025).
- Poozesh, S.; Akafuah, N.; Saito, K. Effects of automotive paint spray technology on the paint transfer efficiency—A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2018, 232, 282–301. [Google Scholar] [CrossRef]
- Anthony, C. Turtle Wax Review: Foam Wash, Seal N Shine, Scratch Remover, Detail Wipes & Odor-X (2025), Automo Blog (2024). Available online: https://www.automoblog.com/turtle-wax-review/ (accessed on 8 January 2025).
- Wax & Dry Spray Car Wax by Turtle Wax. Available online: https://www.turtlewax.com/products/wax-dry-spray-wax-26-fl?_pos=10&_sid=73c04eb0d&_ss=r (accessed on 15 January 2025).
- Super Hard Shell Liquid Car Wax by Turtle Wax. Available online: https://www.turtlewax.com/products/super-hard-shell-liquid-car-wax (accessed on 15 January 2025).
- Glossome Wax by Phoenix E.O.D. Available online: https://phoenixeod.com/products/glossome-wax?srsltid=AfmBOop35RrE8D1PJMsMDGM-JZiqZKEFvO7Gfa4QcgrEdYi2sHa52zxU (accessed on 15 January 2025).
- Pinnacle Crystal Mist Detail Spray by Pinnaclewax. Available online: https://pinnaclewax.com/pincrysmis.html?srsltid=AfmBOooPTw_iBpadQjhF2dRUclSpfqf5Lw4grdXeXiVHZ814NdS4CJcr (accessed on 15 January 2025).
- Meguiar’s Deep Crystal Carnauba Wax by Meguiar’s. Available online: https://www.meguiars.com/automotive/products/meguiarsr-deep-crystaltm-carnauba-wax-a2216-16-oz-liquid (accessed on 16 January 2025).
- Spray Wax Quick Coat by Shine Armour. Available online: https://www.shinearmor.com/products/best-car-wax (accessed on 16 January 2025).
- Butter Wet Wax by Chemical Guys. Available online: https://www.chemicalguys.com/products/butter-wet-wax-warm-deep-carnauba-shine?srsltid=AfmBOopwXp7gpx4vYt4qbn7fjtueYFUo_kJu62e7CF6k-HDK2xmRA9Qw (accessed on 16 January 2025).
- Ominent Sdn Bhd. Company No.: (1127091-U), The Science of Ceramic Coatings, IGL Coatings. 2025. Available online: https://iglcoatings.com/ (accessed on 14 August 2025).
- Borawski, A.; Tarasiuk, W. Comparative analysis of protective coatings of car paints. IOP Conf. Ser. Mater. Sci. Eng. 2018, 421, 032004. [Google Scholar] [CrossRef]
- Tyassmadi, A.T.; Iqbal, M. The effect of type of nano ceramic coating brand on thickness, sticking power, and corrosion rate on the vehicle body. Seybold Rep. J. 2022, 17, 879–890. [Google Scholar] [CrossRef]
- Huang, W.F.; Xiao, Y.L.; Huang, Z.J.; Tsui, G.C.P.; Yeung, K.W.; Tang, C.Y.; Liu, Q. Super-hydrophobic polyaniline-TiO2 hierarchical nanocomposite as anticorrosion coating. Mater. Lett. 2020, 258, 126822. [Google Scholar] [CrossRef]
- Khuje, S.; Sun, J.; Yang, C.; Wang, Z.; Zhu, L.; Li, T.; Valentino, G.; Ku, N.; Bujanda, A.; Yu, J.; et al. Pyrolyzed preceramic precursors to compositionally complex ceramics. Matter 2025, 8, 102285. [Google Scholar] [CrossRef]
- Hanagadakar, M.S.; Kulkarni, R.M. Environmental impacts and benefits of ceramic coatings. In Advanced Ceramic Coatings: Fundamentals, Manufacturing, and Classification; Elsevier: Amsterdam, The Netherlands, 2023; pp. 461–487. [Google Scholar] [CrossRef]
- Chate, G.R.; Kulkarni, R.M.; Nikhil, N.R.; Chate, V.R.; Patel, G.M.; Sollapur, S.; Shettar, M. Ceramic material coatings: Emerging future applications. In Advanced Ceramic Coatings for Emerging Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–17. [Google Scholar] [CrossRef]
- Dong, Z.; Zhu, H.; Hang, Y.; Liu, G.; Jin, W. Polydimethylsiloxane (PDMS) Composite Membrane Fabricated on the Inner Surface of a Ceramic Hollow Fiber: From Single-Channel to Multi-Channel. Engineering 2020, 6, 89–99. [Google Scholar] [CrossRef]
- Salazar-Hernández, C.; Salazar-Hernández, M.; Mendoza-Miranda, J.M.; Elorza-Rodríguez, E. Anticorrosive SiO2-PDMS ceramic coating: Effect of viscosity and functional group on the siloxane chain. J. Exp. Syst. 2024, 30, e41130107. [Google Scholar] [CrossRef]
- Zhu, Q. Ceramic Films and Coatings: Properties and Applications. Coatings 2024, 14, 483. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, L.; Liang, X. Properties of Ceramic Coating on Heating Surface of Waste Incineration Boiler Prepared by Slurry Method. Materials 2022, 15, 4574. [Google Scholar] [CrossRef]
- Wraps, A.S. Ceramic Coating Curing Time: What You Need to Know for Optimal Results. 2025. Available online: https://www.automotivespecialtywraps.com/ceramic-coating-curing-time-what-you-need-to-know-for-optimal-results? (accessed on 15 November 2025).
- Detailing, P.A. The Impact of Weather Conditions on Ceramic Coating: Effects on Durability and Protection. 2025. Available online: https://www.presidentialdetailing.net/the-impact-of-weather-conditions-on-ceramic-coating (accessed on 15 November 2025).
- Solutions, D. Ceramic Coating Cure Time: Secrets to a Showroom-Ready Shine. 2025. Available online: https://www.baltimoredetail.com/ceramic-coating-cure-time-secrets-to-a-showroom-ready-shine? (accessed on 15 November 2025).
- Jiménez-López, A.M.; Hincapié-Llanos, G.A. Identification of factors affecting the reduction of VOC emissions in the paint industry: Systematic literature review-SLR. Prog. Org. Coat. 2022, 170, 106945. [Google Scholar] [CrossRef]
- Köse, R.; Urtekin, L.; Ceylan, A.; Salman, S.; Findik, F. Three types of ceramic coating applicability in automotive industry for wear resistance purpose. Ind. Lubr. Tribol. 2005, 57, 140–144. [Google Scholar] [CrossRef]
- Aimovi-Pavlovi, Z.; Prsti, A.; Andri, L.; Miloevi, V.; Milievi, S. Ceramic Coating for Cast House Application. In Ceramic Coatings—Applications in Engineering; InTech: Rang-du-Fliers, France, 2012. [Google Scholar] [CrossRef]
- Xuebai, L.; Fengwei, Z.; Lu, L.; Xuefeng, S.; Fangyuan, Z.; Xiaoqing, C.; Xuewu, L. Robust bioinspired ceramic-based superhydrophobic Al2O3-STA@WPU composite coating via air spraying: A strategy in enhancing mechanical stability and corrosion resistance for steel 45. J. Mater. Res. Technol. 2025, 37, 3089–3104. [Google Scholar] [CrossRef]
- Barthwal, S.; Uniyal, S.; Barthwal, S. Nature-Inspired Superhydrophobic Coating Materials: Drawing Inspiration from Nature for Enhanced Functionality. Micromachines 2024, 15, 391. [Google Scholar] [CrossRef]
- Carbon Force Ceramic Protective Paint Coating System. Available online: https://www.chemicalguys.com.sg/products/carbon-force-ceramic-protective-paint-coating-system (accessed on 24 January 2025).
- EZPZ—Ceramic Coating For Cars. Available online: https://ethoscarcare.com/products/ceramic-coating (accessed on 28 January 2025).
- CQUARTZ CQ UK 3.0 | CARPRO. Available online: https://carpro.global/product/cq_uk/ (accessed on 24 January 2025).
- AH-CRCPAINT Rapid Ceramic Paint Sealant | Cerakote Ceramic Coatings. Available online: https://www.cerakote.com/shop/cerakote-coating/AH-CRCPAINT/rapid-ceramic-paint-sealant (accessed on 24 January 2025).
- CMX® Ceramic Spray Coating | Mothers® Polish. Available online: https://mothers.com/products/cmx-ceramic-spray-coating-01024?srsltid=AfmBOoovbApC3qz4WZl1Fh4kF_774XpSEz5dLgpXfXn9T3t-i_0C3hrv (accessed on 24 January 2025).
- Meguiar’s Ultimate Ceramic Coating—Ultra-Durable Cutting-Edge Ceramic Protection with Excellent Water Beading While Also Increasing Gloss, Slickness, and Concealing Minor Paint Defects—8oz Spray | Meguiar’s. Available online: https://www.meguiars.com/automotive/products/meguiars-ultimate-ceramic-coating-ultra-durable-cutting-edge-ceramic-protection (accessed on 28 January 2025).
- Nexgen Ceramic Spray Coating | Rated 5 Stars | Nexgen. Available online: https://getnexgen.com/products/nexgen-ceramic-spray?srsltid=AfmBOoq94KJ022Kul4BzMDekBbvbKDWqRgv2908puaabuDSbB8KU8ULI (accessed on 28 January 2025).
- Tian, Y.; Huang, H.; Wang, W.; Ma, Y.; He, X.; Zhang, L.; Sheng, X.; Zhang, X. Corrosion resistant nanoscale polymer-based coatings. In Polymer-Based Nanoscale Materials for Surface Coatings; Elsevier: Amsterdam, The Netherlands, 2023; pp. 547–584. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Surface Preparation of Thermoplastics, Thermosets, and Elastomers. In Handbook of Adhesives and Surface Preparation: Technology, Applications and Manufacturing; William Andrew: Norwich, NY, USA, 2011; pp. 107–134. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, J.; Zhang, J.; Huang, Z.; Qi, D. Review of research on thermoplastic self-healing polyurethanes. React. Funct. Polym. 2024, 199, 105886. [Google Scholar] [CrossRef]
- 3M, Paint Protection Film SGH6, 84830, 8 mil, Transparent. Available online: https://www.3m.com/3M/en_US/p/d/v000097086/ (accessed on 28 September 2025).
- Cai, G.; Wang, H.; Jiang, D.; Dong, Z. Degradation of fluorinated polyurethane coating under UVA and salt spray. Part I: Corrosion resistance and morphology. Prog. Org. Coat. 2018, 123, 337–349. [Google Scholar] [CrossRef]
- Mirror Glaze Synthetic Sealant. 2025. pp. 3–12. Available online: https://www.meguiars.lv/public/LIETOSANAS_INSTRUKCIJA/M21_Professional_Synthetic_Sealant_2.0_.pdf (accessed on 14 August 2025).
- Nirmal, U.; Hashim, J.; Ahmad, M.M.H.M. A Review on Tribological Performance of Natural Fibre Polymeric Composites. Tribol. Int. 2014, 83, 77–104. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ming, H.P. An Introduction to Graphene and the 2010 Nobel Physics Prize. 2011. Available online: http://nobelprize.org/nobel_prizes/physics/laureates/2010/press.html (accessed on 15 October 2025).
- Mathkar, A.; Tozier, D.; Cox, P.; Ong, P.; Galande, C.; Balakrishnan, K.; Reddy, A.L.M.; Ajayan, P.M. Controlled, stepwise reduction and band gap manipulation of graphene oxide. J. Phys. Chem. Lett. 2012, 3, 986–991. [Google Scholar] [CrossRef]
- Bui, H.T.T.; Hoang, A.N.; Le, C.M. Study on the Effect of Graphene on Characteristics of Inorganic Polymer Paint. ACS Omega 2025, 10, 5503–5516. [Google Scholar] [CrossRef]
- Fallahazad, P. Rational and key strategies toward enhancing the performance of graphene/silicon solar cells. Mater. Adv. 2023, 4, 1876–1899. [Google Scholar] [CrossRef]
- Topbestpicks, The Top 5 Best Graphene Coating in 2025, YouTube. 2025. Available online: https://www.youtube.com/watch?v=GlvTt0Y_Ayo (accessed on 20 August 2025).
- ShineArmor, Graphene Ceramic Coating by SHINE ARMOR-5th Place, Amazon. Available online: https://www.amazon.com/dp/B08YS5T1T1?tag=vis-new-08-20 (accessed on 20 August 2025).
- Adam’sPolishes, Advanced Graphene Ceramic Spray Coating by Adam’s Polishes-3rd Place, Amazon. Available online: https://www.amazon.com/dp/B09KTK4KB4?tag=vis-new-08-20&th=1 (accessed on 20 August 2025).
- Products, Graphene Nano Spray Coating by 303 Products-2nd Place, Amazon. Available online: https://www.amazon.com/dp/B08K3N5QP5?tag=vis-new-08-20&th=1 (accessed on 20 August 2025).
- TorqueDetail, Graphene Burst Coat—Graphene Ceramic Coating Spray by Torque Detail, Amazon. Available online: https://www.amazon.com/Graphene-Burst-Coat-Protection-Application/dp/B09R8ZLPT4?&linkCode=sl1&tag=productreview065-20&linkId=5384f493e647ddee18ec9641184b52e4&language=en_US&ref_=as_li_ss_tl (accessed on 20 August 2025).
- Adam’sPolishes, Advanced Graphene Ceramic Coating—10H Graphene Coating by Adam’s Polishes, Amazon. Available online: https://www.amazon.com/Adams-Advanced-Graphene-Ceramic-Coating/dp/B099KN8Z22?th=1&linkCode=sl1&tag=productreview065-20&linkId=da29af0d65a0a5d6bc610d6dc6bc5ede&language=en_US&ref_=as_li_ss_tl (accessed on 20 August 2025).
- Weiruixin, Advanced Graphene Coating 12H by Weiruixin, Amazon. Available online: https://www.amazon.com/Ceramic-Coating-Hydrophobicty-Protection-Warranty/dp/B088H2X1B3/ref=sims_dp_d_dex_popular_subs_t3_v6_d_sccl_2_9/142-8495159-7598116?pd_rd_w=50FyL&content-id=amzn1.sym.e94802a9-3b18-4cbd-b410-204abb9c6aed&pf_rd_p=e94802a9-3b18-4cbd-b410-204abb9c6aed&pf_rd_r=2CGHC3WA0YY91CBJ1PCF&pd_rd_wg=ixhp0&pd_rd_r=bec3d507-e003-4b76-a183-84025386a12e&pd_rd_i=B088H2X1B3&psc=1 (accessed on 20 August 2025).
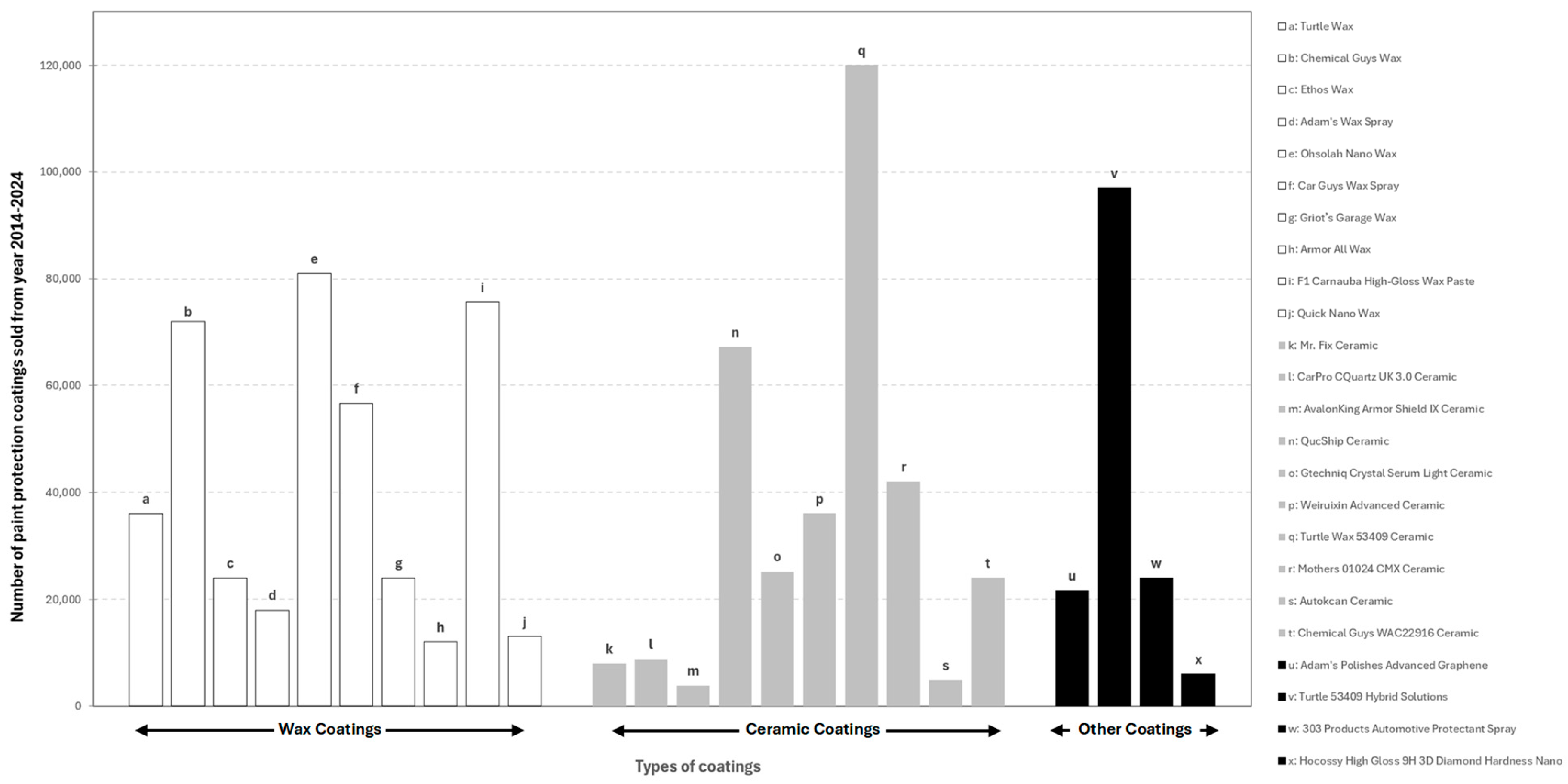
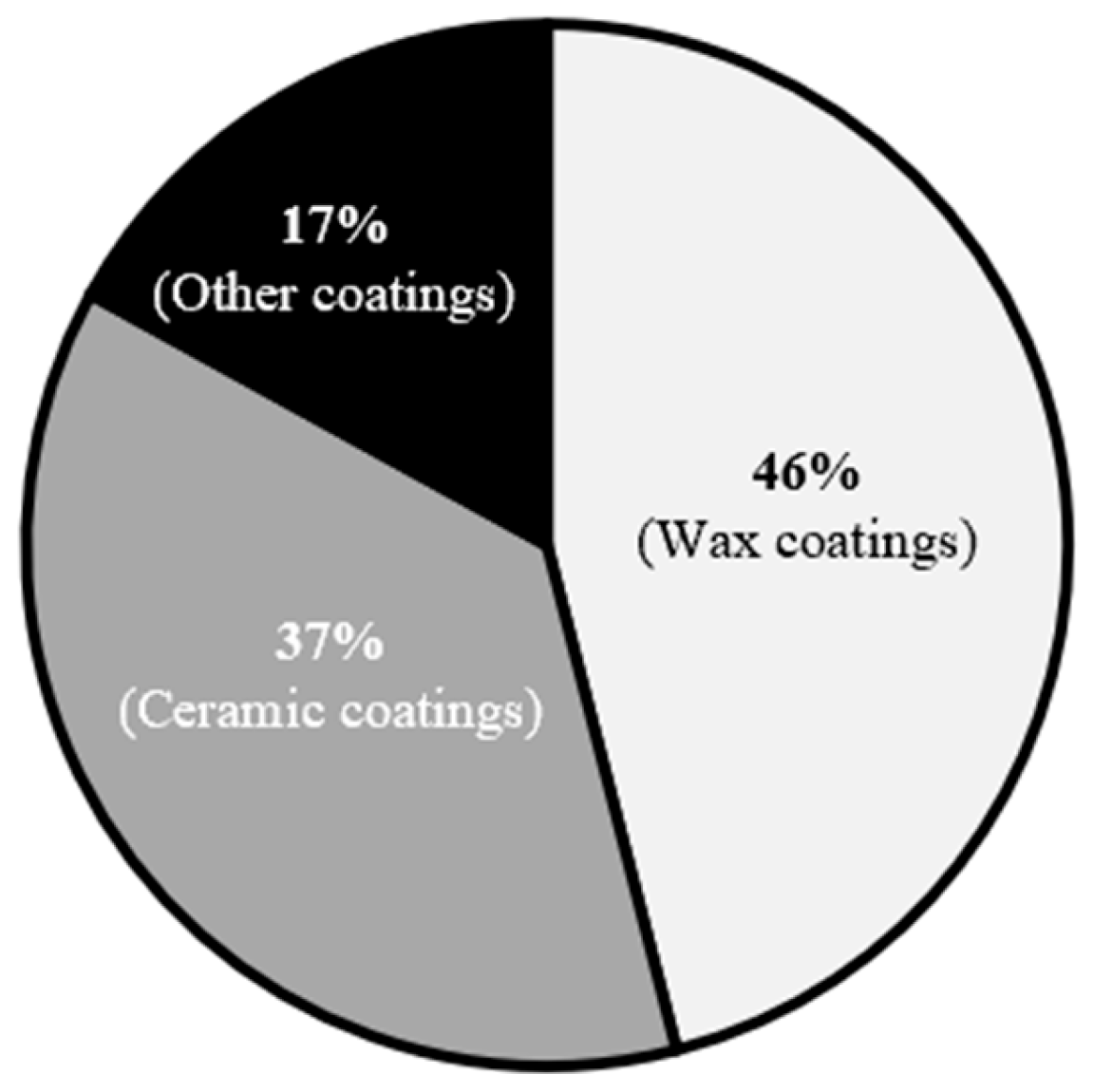
| Product | Product Characteristics | Manufacturer | Average Paint Protection Time |
|---|---|---|---|
 Wax & Dry Spray Car Wax | Two in one, wash and wax spray type. Advanced drying agents repel water from wet surfaces to cut back on drying time. Minute Wax Shine technology adds a layer of instant shine and protection. Product contains Acetaldehyde and Ethylene Glycol which can cause cancer and birth defects [26]. | Tuttle Wax | 2–4 weeks |
 Super Hard Shell Liquid Car Wax | Shake before use. Comes in liquid form and provides protection from light scratches and swirls. Safe on most paint surfaces. Application by microfiber cloth via apply and buff. Buffing is done after 1 min of waiting time after coating. Contains Acrylamide which can cause cancer and birth defects [27]. | Tuttle Wax | 48 weeks |
 Glossome Wax | Shake before use and surface preparation should be free from any defects. Super-long-lasting synthetic sealer. Long lasting hydrophobic deep gloss. Mixture of Teflon, resins, polymers, and synthetic and organic waxes. Provides hyper-water beading and protection. Application is by applying, wait 5 min before buffing. Protects the color and the brightness of the vehicle’s paint with UV protection. Product may cause eye irritation, respiratory irritation and gastrointestinal irritation if wrongly used [28]. | Phoenix E.O.D. where E.O.D stands for ‘Evolve or Die’ | 36 weeks |
 Pinnacle Crystal Mist Detail Spray | Formulated with restructured gloss-enhancing crystalline polymers, nourishing oils, and real Brazilian carnauba wax. Provide instant burst of shine and slickness. Crystal Mist lubricates the paint surface to remove light contamination such as this, without scratching or marring the finish. Paint will appear clean, vibrant and brilliantly glossy. Meets all Volatile Organic Compound (VOC) standards. Best used on black and red paints [29]. | Pinnaclewax | 1–2 weeks |
 Meguiar’s Deep Crystal Carnauba Wax | High quality carnauba wax for long-lasting protection to clear coat and single stage paint. Creates brilliant look shine and locks it in with an extremely durable wax barrier. Offers protection against effects by the sunlight. Recommended product after 3 stages polishing namely to seal in rich gloss with brilliance and create a protective coating. Application is by applying, wait 2–3 min before buffing [30]. | Meguiar’s | 4 weeks |
 Spray Wax Quick Coat | Application is by spray on and wipe off. Contains Brazilian Carnauba for deep gloss finish look. Leaves no streaks and no residue. Protection against UV Rays and environmental contaminants. Safe for all car surfaces. Regular use is recommended for continuous paint protection [31]. | Shine Armor | 6 weeks |
 Butter Wet Wax | Formulated from a blend of natural Carnauba, polymers and resins. Can be used on top of ceramic coating for an extra layer of shine and protection. Not recommended for matte finishes. Resistance against UVA and UVB rays. Application method is by wipe on-wipe off method. Can be applied on wet or dry surface and under direct sunlight [32]. | Chemical Guys | 3–4 weeks |
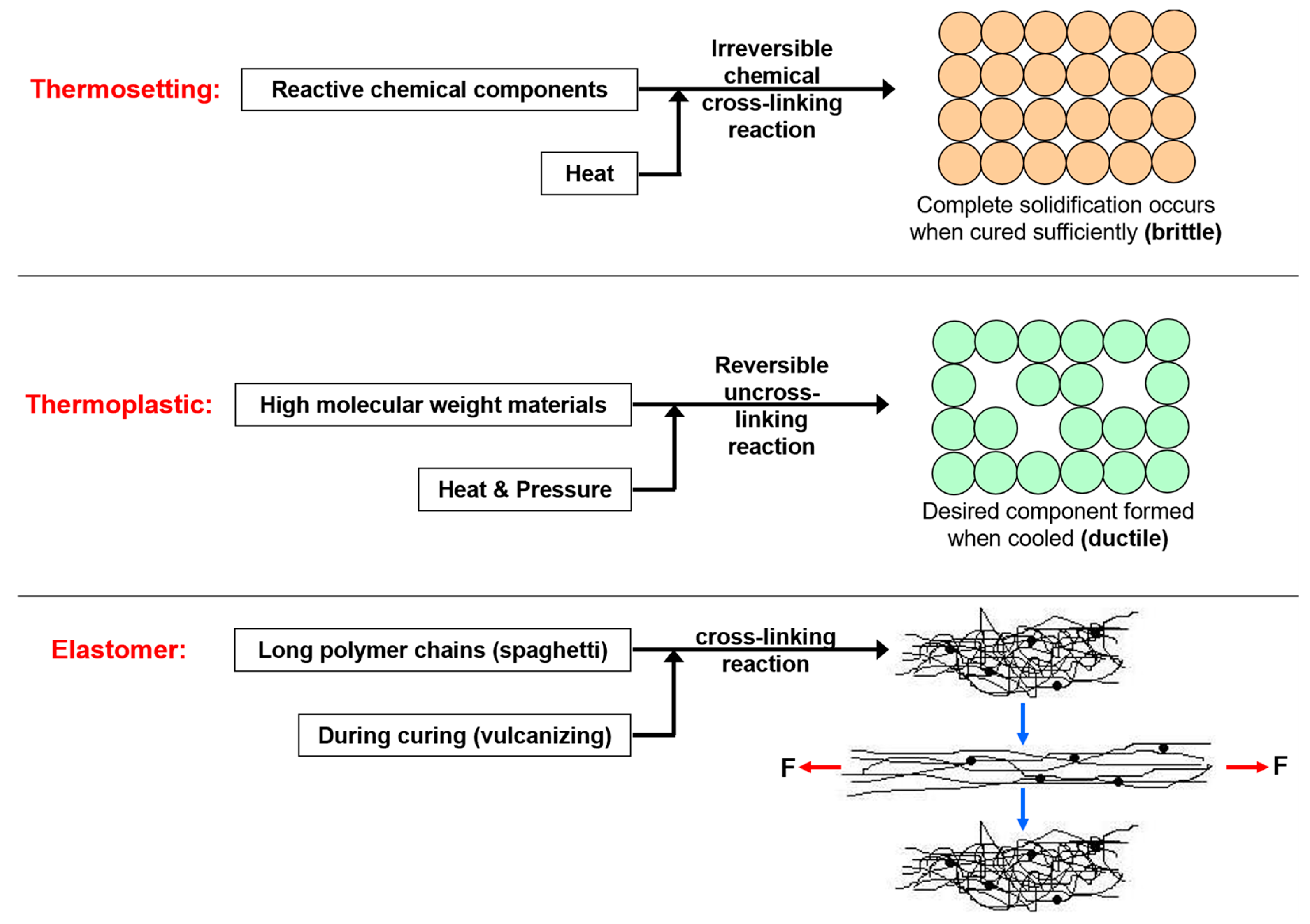
| Schematic View | Surface Treatment | Contact Angle to Surface | Characteristics | Range of Hydrophilicity | Range of Hydrophobicity |
|---|---|---|---|---|---|
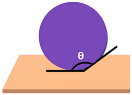 | Surface treated with high end ceramic coating (Ex: PDMS–Si–CH3) | 150o < θ < 180o | Superhydrophobic (i.e., highly water repellent, form full water droplets that beads from the surface) |  |  |
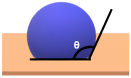 | Surface treated with normal ceramic coating (Ex: Si-O-Si) | 90o < θ < 150o | Hydrophobic (i.e., average water repellent, form ¾ water droplets that beads from the surface, leaving minimum water marks on the surface) | ||
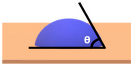 | Surface has no any sort of coating | 10o < θ < 90o | Hydrophilic (i.e., average water attraction, little or no beading from the surface leaving water marks) | ||
 | Surface has no any sort of coating | 0o < θ < 10o | Superhydrophilic (i.e., highly attracted to water, 100% in contact with surface and does not bead) | ||
Note:  —Blue colour represents water; —Blue colour represents water;  —Beige colour represents painted surface with a given condition; treated/untreated —Beige colour represents painted surface with a given condition; treated/untreated | |||||
| Product | Product Characteristics | Manufacturer | Average Paint Protection Time |
|---|---|---|---|
 Carbon Force Ceramic Coating | Offers DIY detailers professional-grade protection with its liquid formula and easy application. Using ceramic nanotechnology, it creates a durable, bonded layer that shields against environmental contaminants, UV rays, and wear and tear for up to 5 years. The coating seals imperfections, creating a smooth, glossy finish that repels water and contaminants, minimizing maintenance. With added anti-scratch technology, Carbon Force enhances shine and provides long-lasting protection, making it a compelling choice for car enthusiasts [52]. | Chemical Guy | 4–5 years |
 EZPZ Ceramic Coating | EZPZ Ceramic Polymer Coating offers a DIY-friendly approach to ceramic car coatings. Its Polymersilazane formula provides up to 3 years of hydrophobic protection, repelling water and contaminants for a cleaner, glossier finish. The flexible co-polymers allow for easier application and a longer work time, even in challenging conditions. Designed as a topcoat, EZPZ is compatible with other coatings and promises professional results without the hassle [53]. | Ethos | 2–3 years |
 CQUK Ceramic Quartz Coating | With broad pH tolerance (3–14), and wide application temperature range (3–40°C/40F–100F) ensure robust protection against various environmental factors and ease of use. The formula’s high silica-quartz content creates a hard, protective layer, enhancing gloss and offering resistance to chemicals, salt, and UV damage. The simple wipe-on/wipe-off application further adds to its user-friendliness [54]. | CARPRO™ | 1–2 years |
 Rapid Ceramic Paint Sealant | Promises a stunning, easy-to-maintain finish, boasting exceptional gloss and shine, extreme hydrophobicity repelling water and dirt, and unsurpassed slickness. The coating simplifies washing and drying, while its application process is quick and easy, involving spraying and wiping. Coating’s durability, lasting for months due to its true inorganic ceramic technology [55]. | CERAKOTE® | 1–2 years |
 CMX Ceramic Spray Coating | Its simple spray-and-wipe application, suitable for both wet and dry surfaces, promises a showroom shine in minutes. The coating bonds to the paint, creating a protective layer that enhances shine and repels contaminants. While optimal results are achieved on clean, defect-free surfaces, the ease of application and potential for multiple coats make it an appealing option for car owners seeking convenient and effective ceramic protection [56]. | Mothers® Polish | 1–2 years |
 Ultimate Ceramic Coating | Meguiar’s Ultimate Ceramic Coating offers DIY-friendly, professional-grade ceramic protection for your car. This easy-to-apply spray coating enhances gloss, slickness, and water beading, while also concealing minor paint imperfections. Safe for various surfaces, including paint, trim, and PPF, it provides durable protection similar to professional ceramic coatings, but with a simplified application process. Proper surface preparation and maintenance are recommended for optimal results [57]. | Meguiar’s | 3–4 years |
 Ceramic Spray Silicon Dioxide | Offers a user-friendly approach to long-lasting car protection. Its graphene-infused formula promises up to a year of protection against UV rays, water spots, and contaminants, while enhancing gloss and depth. The spray-on application simplifies the process, eliminating the need for professional detailing expertise. The coating’s thermal properties allow for application in direct sunlight without streaking or spotting and also help prevent hard water spots. Safe for various surfaces like paint, glass, wheels, and trim [58]. | Nexgen | 1–2 years |
| Product | Product Characteristics | Manufacturer | Average Paint Protection Time |
|---|---|---|---|
 Spray type Graphene Ceramic Coating | Infuses graphene and ceramic to form a powerful coating that enhances vehicles protection, slickness, and durability. Powerful hydrophobic properties and high resistance to harmful UV rays, water, dirt, grimes, dust and debris. Can be used on all exterior vehicle surfaces such as wheel, glass, headlights, plastic, trim & etc. DIY friendly [73]. | Shine Armor | 6–12 months |
 Graphene Ceramic Spray Coating | Infused with UV tracers that activate with UV blue light, coating become more visible in glossy looks under sunlight. Safe to use on painted surfaces, wheels, glass, headlights, plastic trim, bed liners, tonneau covers, canvas tops, floor mats, chrome and unfinished metal. DIY friendly [74]. | Adam’s Polishes | 18–20 months |
 Graphene Nano Spray Coating | High resistance against UV rays, water spotting, fading & cracking. Has enhanced gloss finished properties. Formulated from graphene -oxide nano coating that can be applied on car’s paint, trim, & windows. DIY friendly [75]. | 303 Products | ±12 months |
 Graphene Burst Coat Coating | Repels any weather, chemical or roadside contaminants. Graphene Burst’s proprietary formula blends nano silica dioxide particles providing endless glass shine. Hydrophilic surface so water sheets off instead of beading. Improved resistance against stains & scratches. Has high contact angle and lower sliding angle; i.e., less stains, scratches & containments. DIY friendly [76]. | Torque Detail | ±12 months |
 Advanced Graphene Ceramic Coating | A clear, nanocrystalline coating that protects vehicle from weather, chemicals, and UV rays while rejecting water, dirt and other deposits. 10H hardness and more than 7 years of protection. Has higher scratch and stain resistance features. Can be applied on paint, glass, headlights, chrome, wheels and trims. Used by professional car detailers [77]. | Adam’s Polishes | 84 months |
 Advanced Graphene Coating | Updated version coating hardness increases to 12H so that it lasts longer. Superior resistance from salt fog corrosion, bird’s dropping, UV light, scratches, crushed stones and iron filings. Outstanding water beading that makes water almost jump off the surface. Used by professional car detailers. Used by professional car detailers [78]. | Weiruixin | ±120 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nirmal, U.; Musa, M.A.; Yuhazri, M.Y.; Ahmad, M.M.H.M. Development of Car Coating Materials over the Past Decade for Paint Protection Applications—An Overview on the Different Types of Paint Protections. Polymers 2025, 17, 3114. https://doi.org/10.3390/polym17233114
Nirmal U, Musa MA, Yuhazri MY, Ahmad MMHM. Development of Car Coating Materials over the Past Decade for Paint Protection Applications—An Overview on the Different Types of Paint Protections. Polymers. 2025; 17(23):3114. https://doi.org/10.3390/polym17233114
Chicago/Turabian StyleNirmal, Umar, M. A. Musa, Mohd Yaakob Yuhazri, and M. M. H. Megat Ahmad. 2025. "Development of Car Coating Materials over the Past Decade for Paint Protection Applications—An Overview on the Different Types of Paint Protections" Polymers 17, no. 23: 3114. https://doi.org/10.3390/polym17233114
APA StyleNirmal, U., Musa, M. A., Yuhazri, M. Y., & Ahmad, M. M. H. M. (2025). Development of Car Coating Materials over the Past Decade for Paint Protection Applications—An Overview on the Different Types of Paint Protections. Polymers, 17(23), 3114. https://doi.org/10.3390/polym17233114







