Advances in 3D Bioprinting and Microfluidics for Organ-on-a-Chip Platforms
Abstract
1. Introduction
2. Technological Innovations in 3D Bioprinting
2.1. Evolution of Bioprinting Techniques
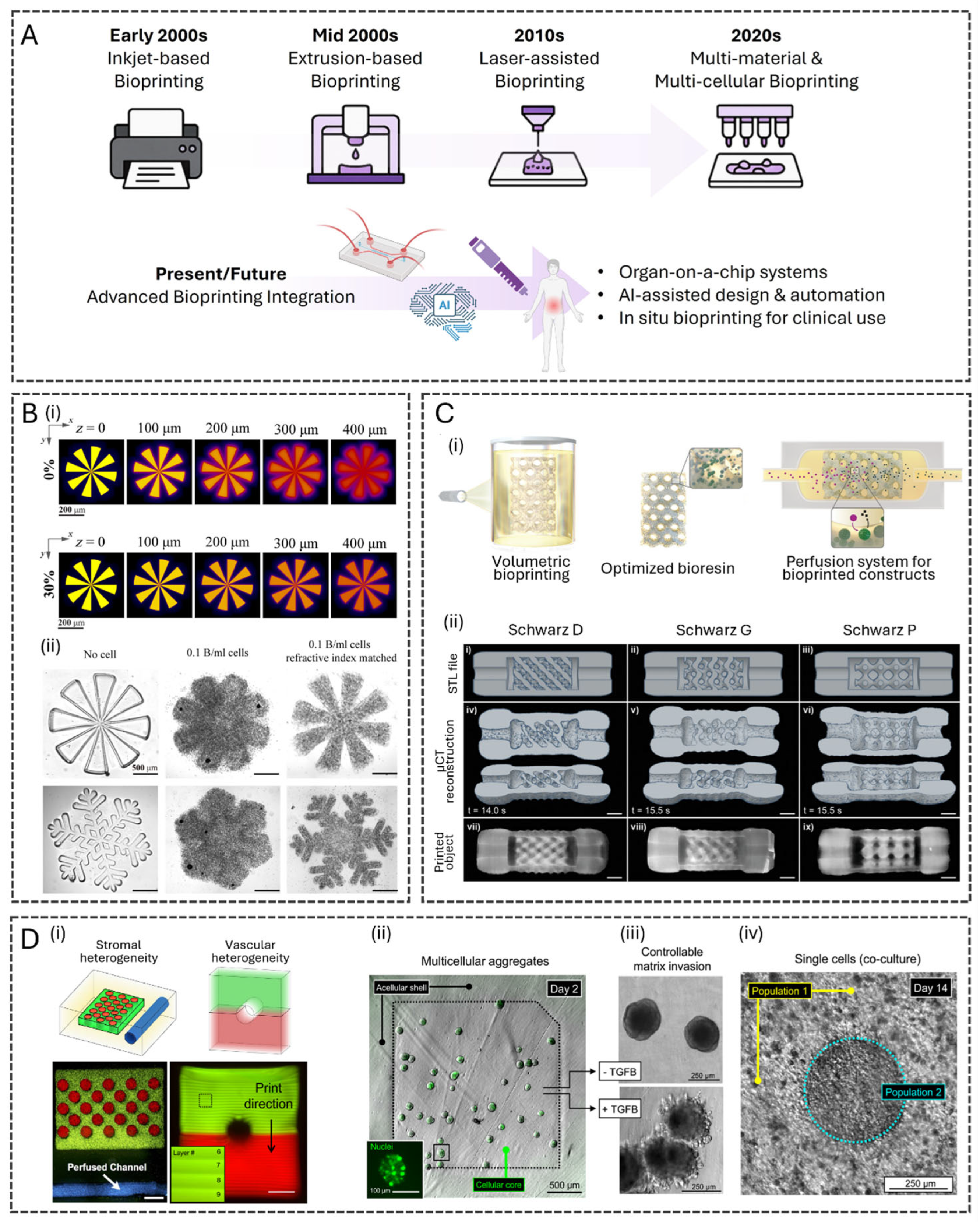
2.2. Advanced Bioinks for 3D Bioprinting
2.3. Bioprinting for Complex Tissue Structures

3. Microfluidic Innovations for Organ-on-a-Chip
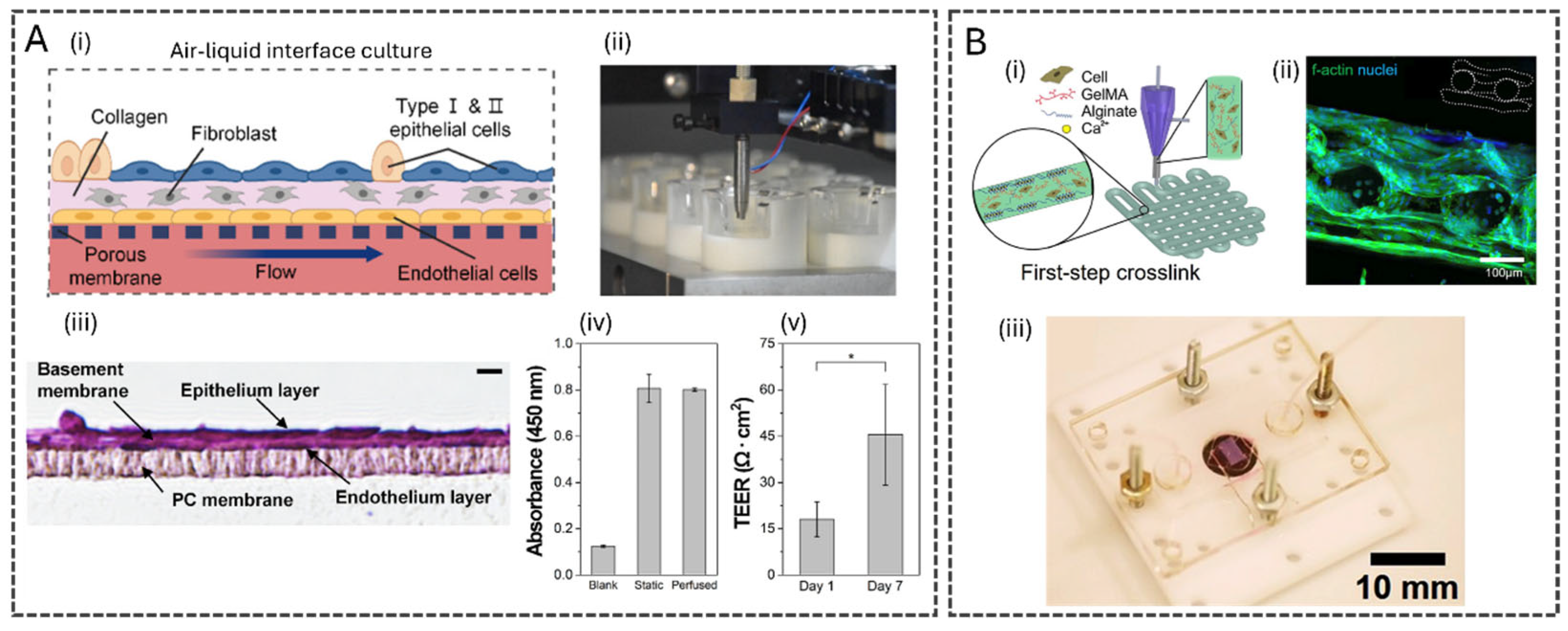
3.1. Advances in Microfluidic Design, Fabrication, and Tissue Integration
3.2. Microfluidics for Multi-Organ Systems
3.3. Quantitative Performance of 3D Bioprinting and Microfluidic Organ-on-a-Chip Systems
3.4. Technological Synergies: Bioprinting Meets Microfluidics
3.5. Co-Culture Systems and Dynamic Tissue Interactions
3.6. Fluidically Enabled Tissue Maturation and Differentiation
4. Applications in Biomedical Research and Therapeutics
4.1. New Approach Methodologies (NAMs) and Organ-on-a-Chip for Ethical and Effective Drug Discovery
4.2. Disease Modeling and Precision Medicine
4.3. Regenerative Medicine and Tissue Engineering
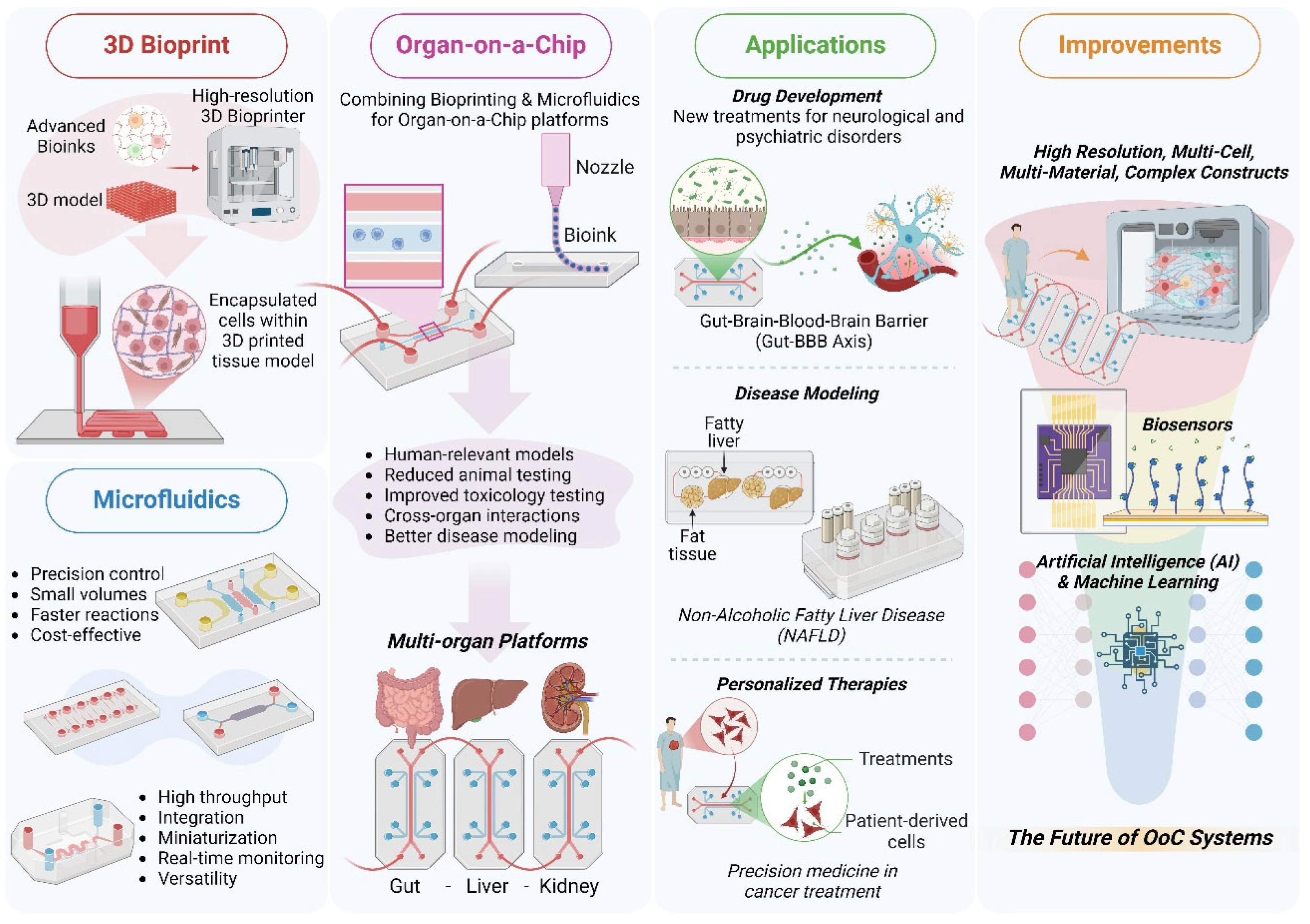
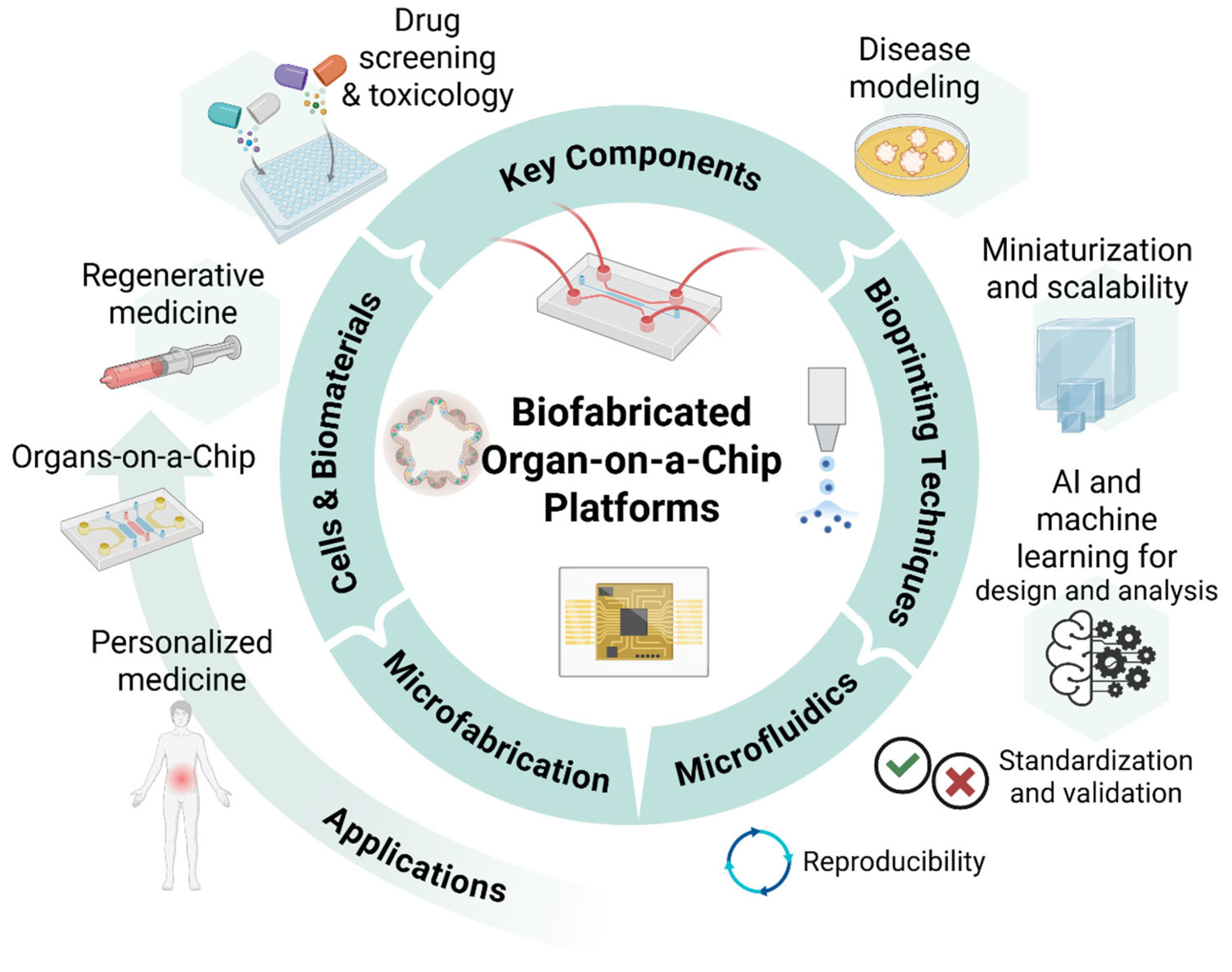
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Shi, W.; Liu, X.; Gu, Z. Recent Advances in 3D-Printing-Based Organ-on-a-Chip. EngMedicine 2024, 1, 100003. [Google Scholar] [CrossRef]
- Rahmani Dabbagh, S.; Rezapour Sarabi, M.; Birtek, M.T.; Mustafaoglu, N.; Zhang, Y.S.; Tasoglu, S. 3D Bioprinted Organ-on-Chips. Aggregate 2023, 4, e197. [Google Scholar] [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Fetah, K.; Tebon, P.; Goudie, M.J.; Eichenbaum, J.; Ren, L.; Barros, N.; Nasiri, R.; Ahadian, S.; Ashammakhi, N.; Dokmeci, M.R.; et al. The Emergence of 3D Bioprinting in Organ-on-Chip Systems. Prog. Biomed. Eng. 2019, 1, 012001. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and Bioprinting Technologies to Make Heterogeneous and Biomimetic Tissue Constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef]
- Nadine, S.; Chung, A.; Diltemiz, S.E.; Yasuda, B.; Lee, C.; Hosseini, V.; Karamikamkar, S.; de Barros, N.R.; Mandal, K.; Advani, S.; et al. Advances in Microfabrication Technologies in Tissue Engineering and Regenerative Medicine. Artif. Organs 2022, 46, E211–E243. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Choudhury, D. Microfluidic Bioprinting for Organ-on-a-Chip Models. Drug Discov. Today 2019, 24, 1248–1257. [Google Scholar] [CrossRef]
- Rothbauer, M.; Eilenberger, C.; Spitz, S.; Bachmann, B.E.M.; Kratz, S.R.A.; Reihs, E.I.; Windhager, R.; Toegel, S.; Ertl, P. Recent Advances in Additive Manufacturing and 3D Bioprinting for Organs-On-A-Chip and Microphysiological Systems. Front. Bioeng. Biotechnol. 2022, 10, 837087. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jang, J.; Kang, H.-W. 3D Bioprinting and Its Application to Organ-on-a-Chip. Microelectron. Eng. 2018, 200, 1–11. [Google Scholar] [CrossRef]
- Amirifar, L.; Besanjideh, M.; Nasiri, R.; Shamloo, A.; Nasrollahi, F.; de Barros, N.R.; Davoodi, E.; Erdem, A.; Mahmoodi, M.; Hosseini, V.; et al. Droplet-Based Microfluidics in Biomedical Applications. Biofabrication 2022, 14, 022001. [Google Scholar] [CrossRef]
- Chen, L.; Yang, C.; Xiao, Y.; Yan, X.; Hu, L.; Eggersdorfer, M.; Chen, D.; Weitz, D.A.; Ye, F. Millifluidics, Microfluidics, and Nanofluidics: Manipulating Fluids at Varying Length Scales. Mater. Today Nano 2021, 16, 100136. [Google Scholar] [CrossRef]
- Amirifar, L.; Shamloo, A.; Nasiri, R.; de Barros, N.R.; Wang, Z.Z.; Unluturk, B.D.; Libanori, A.; Ievglevskyi, O.; Diltemiz, S.E.; Sances, S.; et al. Brain-on-a-Chip: Recent Advances in Design and Techniques for Microfluidic Models of the Brain in Health and Disease. Biomaterials 2022, 285, 121531. [Google Scholar] [CrossRef] [PubMed]
- Virumbrales-Muñoz, M.; Ayuso, J.M. From Microfluidics to Microphysiological Systems: Past, Present, and Future. Organs Chip 2022, 4, 100015. [Google Scholar] [CrossRef]
- Zhao, Y.; Landau, S.; Okhovatian, S.; Liu, C.; Lu, R.X.Z.; Lai, B.F.L.; Wu, Q.; Kieda, J.; Cheung, K.; Rajasekar, S.; et al. Integrating Organoids and Organ-on-a-Chip Devices. Nat. Rev. Bioeng. 2024, 2, 588–608. [Google Scholar] [CrossRef]
- Ermis, M.; Falcone, N.; Roberto de Barros, N.; Mecwan, M.; Haghniaz, R.; Choroomi, A.; Monirizad, M.; Lee, Y.; Song, J.; Cho, H.-J.; et al. Tunable Hybrid Hydrogels with Multicellular Spheroids for Modeling Desmoplastic Pancreatic Cancer. Bioact. Mater. 2023, 25, 360–373. [Google Scholar] [CrossRef]
- Aazmi, A.; Zhang, D.; Mazzaglia, C.; Yu, M.; Wang, Z.; Yang, H.; Huang, Y.Y.S.; Ma, L. Biofabrication Methods for Reconstructing Extracellular Matrix Mimetics. Bioact. Mater. 2024, 31, 475–496. [Google Scholar] [CrossRef] [PubMed]
- de Barros, N.R.; Gomez, A.; Ermis, M.; Falcone, N.; Haghniaz, R.; Young, P.; Gao, Y.; Aquino, A.-F.; Li, S.; Niu, S.; et al. Gelatin Methacryloyl and Laponite Bioink for 3D Bioprinted Organotypic Tumor Modeling. Biofabrication 2023, 15, 045005. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, T.; Huang, Q.; Wang, Y. From Organ-on-a-Chip to Human-on-a-Chip: A Review of Research Progress and Latest Applications. ACS Sens. 2024, 9, 3466–3488. [Google Scholar] [CrossRef]
- Kim, T.Y.; Choi, J.-W.; Park, K.; Kim, S.; Kim, J.F.; Park, T.-E.; Seo, J. Lubricant-Coated Organ-on-a-Chip for Enhanced Precision in Preclinical Drug Testing. Small 2024, 20, 2402431. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Gu, B.; Yao, C.; Gu, Y.; Xu, W.; Zhang, J.; He, J.; Liu, X.; Li, D. Advancing Intelligent Organ-on-a-Chip Systems with Comprehensive In Situ Bioanalysis. Adv. Mater. 2024, 36, 2305268. [Google Scholar] [CrossRef]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for Organ Regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef]
- Ke, D.; Niu, C.; Yang, X. Evolution of 3D Bioprinting-from the Perspectives of Bioprinting Companies. Bioprinting 2022, 25, e00193. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Haghiashtiani, G.; Hübscher, T.; Kelly, D.J.; Lee, J.M.; Lutolf, M.; McAlpine, M.C.; Yeong, W.Y.; Zenobi-Wong, M.; Malda, J. 3D Extrusion Bioprinting. Nat. Rev. Methods Primers 2021, 1, 75. [Google Scholar] [CrossRef]
- Lam, E.; Yu, F.; Zhu, S.; Wang, Z. 3D Bioprinting for Next-Generation Personalized Medicine. Int. J. Mol. Sci. 2023, 24, 6357. [Google Scholar] [CrossRef]
- Lin, D.; Tang, C.; Wang, J.; Yang, Y.; Yang, H.; Zhou, Y.; Yu, W.; Li, B.; Huang, Q.; Wang, H.; et al. Multiorgan Proteomic Analysis of Infected Animal Models Predict Potential Host Factors for Chikungunya Virus. MedComm 2025, 6, e70013. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In Situ Printing of Mesenchymal Stromal Cells, by Laser-Assisted Bioprinting, for in Vivo Bone Regeneration Applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Mesbah Oskui, S.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective Bioprinting Resolution in Tissue Model Fabrication. Lab Chip 2019, 19, 2019–2037. [Google Scholar] [CrossRef]
- You, S.; Xiang, Y.; Hwang, H.H.; Berry, D.B.; Kiratitanaporn, W.; Guan, J.; Yao, E.; Tang, M.; Zhong, Z.; Ma, X.; et al. High Cell Density and High-Resolution 3D Bioprinting for Fabricating Vascularized Tissues. Sci. Adv. 2025, 9, eade7923. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, 1904209. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Castaño, M.I.; Madsen, A.G.; Madrid-Wolff, J.; Sgarminato, V.; Boniface, A.; Glückstad, J.; Moser, C. Holographic Tomographic Volumetric Additive Manufacturing. Nat. Commun. 2025, 16, 1551. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Lian, L.; Hou, Y.; Li, Z.; Zheng, Z.; Li, G.; Tang, G.; Xie, G.; Xie, M. Advances in Volumetric Bioprinting. Biofabrication 2024, 16, 012004. [Google Scholar] [CrossRef]
- Bernal, P.N.; Bouwmeester, M.; Madrid-Wolff, J.; Falandt, M.; Florczak, S.; Rodriguez, N.G.; Li, Y.; Größbacher, G.; Samsom, R.-A.; van Wolferen, M.; et al. Volumetric Bioprinting of Organoids and Optically Tuned Hydrogels to Build Liver-Like Metabolic Biofactories. Adv. Mater. 2022, 34, 2110054. [Google Scholar] [CrossRef]
- Grigoryan, B.; Sazer, D.W.; Avila, A.; Albritton, J.L.; Padhye, A.; Ta, A.H.; Greenfield, P.T.; Gibbons, D.L.; Miller, J.S. Development, Characterization, and Applications of Multi-Material Stereolithography Bioprinting. Sci. Rep. 2021, 11, 3171. [Google Scholar] [CrossRef]
- Dikyol, C.; Altunbek, M.; Bartolo, P.; Koc, B. Multimaterial Bioprinting Approaches and Their Implementations for Vascular and Vascularized Tissues. Bioprinting 2021, 24, e00159. [Google Scholar] [CrossRef]
- Puistola, P.; Miettinen, S.; Skottman, H.; Mörö, A. Novel Strategy for Multi-Material 3D Bioprinting of Human Stem Cell Based Corneal Stroma with Heterogenous Design. Mater. Today Bio 2024, 24, 100924. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Mille, L.S.; Ching, T.; Luo, Z.; Tang, G.; Garciamendez, C.E.; Lesha, A.; Hashimoto, M.; Zhang, Y.S. Digital Light Processing Based Bioprinting with Composable Gradients. Adv. Mater. 2022, 34, 2107038. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bölükbas, D.A.; Prithiviraj, S.; Da Silva, I.A.N.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-Matrix-Reinforced Bioinks for 3D Bioprinting Human Tissue. Adv. Mater. 2021, 33, 2005476. [Google Scholar] [CrossRef]
- Ruiz-Alonso, S.; Ordoyo-Pascual, J.; Lafuente-Merchan, M.; García-Villén, F.; Sainz-Ramos, M.; Gallego, I.; Del-Burgo, L.S.; Pedraz, J.L. Hydrogel Bioink Formulation for 3D Bioprinting: Sustained Delivery of PDGF-BB and VEGF in Biomimetic Scaffolds for Tendon Partial Rupture Repair. Int. J. Bioprint. 2024, 10, 2632. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Liu, J. Bioprinting of 3D Tissues/Organs Combined with Microfluidics. RSC Adv. 2018, 8, 21712–21727. [Google Scholar] [CrossRef]
- Fritschen, A.; Lindner, N.; Scholpp, S.; Richthof, P.; Dietz, J.; Linke, P.; Guttenberg, Z.; Blaeser, A. High-Scale 3D-Bioprinting Platform for the Automated Production of Vascularized Organs-on-a-Chip. Adv. Healthc. Mater. 2024, 13, 2304028. [Google Scholar] [CrossRef] [PubMed]
- Silvani, G.; Basirun, C.; Wu, H.; Mehner, C.; Poole, K.; Bradbury, P.; Chou, J. A 3D-Bioprinted Vascularized Glioblastoma-on-a-Chip for Studying the Impact of Simulated Microgravity as a Novel Pre-Clinical Approach in Brain Tumor Therapy. Adv. Ther. 2021, 4, 2100106. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Uzel, S.G.M.; Nam, L.L.; Ahrens, J.H.; Truby, R.L.; Damaraju, S.; Lewis, J.A. Biomanufacturing of Organ-Specific Tissues with High Cellular Density and Embedded Vascular Channels. Sci. Adv. 2025, 5, eaaw2459. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
- Gehlen, J.; Qiu, W.; Schädli, G.N.; Müller, R.; Qin, X.-H. Tomographic Volumetric Bioprinting of Heterocellular Bone-like Tissues in Seconds. Acta Biomater. 2023, 156, 49–60. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef]
- Song, H.; Hong, Y.; Lee, H. Rapid Automated Production of Tubular 3D Intestine-on-a-Chip with Diverse Cell Types Using Coaxial Bioprinting. Lab Chip 2025, 25, 90–101. [Google Scholar] [CrossRef]
- Avelino, T.M.; Harb, S.V.; Adamoski, D.; Oliveira, L.C.M.; Horinouchi, C.D.S.; Azevedo, R.J.d.; Azoubel, R.A.; Thomaz, V.K.; Batista, F.A.H.; d’Ávila, M.A.; et al. Unveiling the Impact of Hypodermis on Gene Expression for Advancing Bioprinted Full-Thickness 3D Skin Models. Commun. Biol. 2024, 7, 1437. [Google Scholar] [CrossRef]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.-S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically Patterned Biomimetic Human IPSC-Derived Hepatic Model via Rapid 3D Bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef]
- Tofani, L.B.; Avelino, T.M.; de Azevedo, R.J.; Elias, G.B.; Ganzerla, M.D.; Terra, M.F.; Rodrigues, V.K.T.; Rabelo, R.S.; Harb, S.V.; Figueira, A.C.M. Biofabricated 3D Intestinal Models as an Alternative to Animal-Based Approaches for Drug Toxicity Assays. Tissue Eng. Regen. Med. 2025, 22, 181–194. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Review of Recent Metamaterial Microfluidic Sensors. Sensors 2018, 18, 232. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Miranda, C.C.; Cabral, J.M.S. Modeling the Human Body on Microfluidic Chips. Trends Biotechnol. 2021, 39, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Kam, D.; Rulf, O.; Reisinger, A.; Lieberman, R.; Magdassi, S. 3D Printing by Stereolithography Using Thermal Initiators. Nat. Commun. 2024, 15, 2285. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Q.; Wang, J. A Review of Stereolithography: Processes and Systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Stokes, K.; Clark, K.; Odetade, D.; Hardy, M.; Goldberg Oppenheimer, P. Advances in Lithographic Techniques for Precision Nanostructure Fabrication in Biomedical Applications. Discov. Nano 2023, 18, 153. [Google Scholar] [CrossRef]
- Rein, C.; Toner, M.; Sevenler, D. Rapid Prototyping for High-Pressure Microfluidics. Sci. Rep. 2023, 13, 1232. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.B.; Wu, Q.; Yazbeck, J.; Liu, C.; Okhovatian, S.; Radisic, M. Beyond Polydimethylsiloxane: Alternative Materials for Fabrication of Organ-on-a-Chip Devices and Microphysiological Systems. ACS Biomater. Sci. Eng. 2021, 7, 2880–2899. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, A.; Paolelli, X.; De Vitis, E.; Selicato, N.; Gervaso, F.; Gigli, G.; Moroni, L.; Polini, A. VAT Photopolymerization 3D Printing Optimization of High Aspect Ratio Structures for Additive Manufacturing of Chips towards Biomedical Applications. Addit. Manuf. 2022, 60, 103200. [Google Scholar] [CrossRef]
- Bosmans, C.; Ginés Rodriguez, N.; Karperien, M.; Malda, J.; Moreira Teixeira, L.; Levato, R.; Leijten, J. Towards Single-Cell Bioprinting: Micropatterning Tools for Organ-on-Chip Development. Trends Biotechnol. 2024, 42, 739–759. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Yoon, S.; Kilicarslan You, D.; Jeong, U.; Lee, M.; Kim, E.; Jeon, T.-J.; Kim, S.M. Microfluidics in High-Throughput Drug Screening: Organ-on-a-Chip and C. Elegans-Based Innovations. Biosensors 2024, 14, 55. [Google Scholar] [CrossRef]
- Soragni, C.; Queiroz, K.; Ng, C.P.; Stok, A.; Olivier, T.; Tzagkaraki, D.; Heijmans, J.; Suijker, J.; de Ruiter, S.P.M.; Olczyk, A.; et al. Phenotypic Screening in Organ-on-a-Chip Systems: A 1537 Kinase Inhibitor Library Screen on a 3D Angiogenesis Assay. Angiogenesis 2024, 27, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Azizgolshani, H.; Coppeta, J.R.; Vedula, E.M.; Marr, E.E.; Cain, B.P.; Luu, R.J.; Lech, M.P.; Kann, S.H.; Mulhern, T.J.; Tandon, V.; et al. High-Throughput Organ-on-Chip Platform with Integrated Programmable Fluid Flow and Real-Time Sensing for Complex Tissue Models in Drug Development Workflows. Lab Chip 2021, 21, 1454–1474. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Wang, H.; An, G.; Wu, H.; Huang, W. A High-Throughput, Open-Space and Reusable Microfluidic Chip for Combinational Drug Screening on Tumor Spheroids. Lab Chip 2021, 21, 3924–3932. [Google Scholar] [CrossRef] [PubMed]
- Cliffe, F.E.; Madden, C.; Costello, P.; Devitt, S.; Mukkunda, S.R.; Keshava, B.B.; Fearnhead, H.O.; Vitkauskaite, A.; Dehkordi, M.H.; Chingwaru, W.; et al. Mera: A Scalable High Throughput Automated Micro-Physiological System. SLAS Technol. 2023, 28, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nishikawa, M.; Kutsuzawa, N.; Tokito, F.; Kobayashi, T.; Kurniawan, D.A.; Shioda, H.; Cao, W.; Shinha, K.; Nakamura, H.; et al. Advancements in Microphysiological Systems: Exploring Organoids and Organ-on-a-Chip Technologies in Drug Development -Focus on Pharmacokinetics Related Organs-. Drug Metab. Pharmacokinet. 2025, 60, 101046. [Google Scholar] [CrossRef]
- Özkayar, G.; Lötters, J.C.; Tichem, M.; Ghatkesar, M.K. Toward a Modular, Integrated, Miniaturized, and Portable Microfluidic Flow Control Architecture for Organs-on-Chips Applications. Biomicrofluidics 2022, 16, 021302. [Google Scholar] [CrossRef]
- Aazmi, A.; Zhou, H.; Li, Y.; Yu, M.; Xu, X.; Wu, Y.; Ma, L.; Zhang, B.; Yang, H. Engineered Vasculature for Organ-on-a-Chip Systems. Engineering 2022, 9, 131–147. [Google Scholar] [CrossRef]
- de Haan, P.; Mulder, J.-P.S.H.; Lötters, J.C.; Verpoorte, E. A Highly Stable, Pressure-Driven, Flow Control System Based on Coriolis Mass Flow Sensors for Organs-on-Chips. Flow Meas. Instrum. 2024, 97, 102576. [Google Scholar] [CrossRef]
- Carvalho, V.; Gonçalves, I.M.; Rodrigues, N.; Sousa, P.; Pinto, V.; Minas, G.; Kaji, H.; Shin, S.R.; Rodrigues, R.O.; Teixeira, S.F.C.F.; et al. Numerical Evaluation and Experimental Validation of Fluid Flow Behavior within an Organ-on-a-Chip Model. Comput. Methods Programs Biomed. 2024, 243, 107883. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Pan, Y.; Zhou, D.; Liu, Y.; Yin, Y.; Yang, J.; Wang, Y.; Song, Y. Emerging Trends in Organ-on-a-Chip Systems for Drug Screening. Acta Pharm. Sin. B 2023, 13, 2483–2509. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Karnam, G.; Madgula, V. Liver-on-Chips for Drug Discovery and Development. Mater. Today Bio 2024, 27, 101143. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Bañobre-López, M.; Minas, G.; Teixeira, S.F.C.F.; Lima, R.; Rodrigues, R.O. The Integration of Spheroids and Organoids into Organ-on-a-Chip Platforms for Tumour Research: A Review. Bioprinting 2022, 27, e00224. [Google Scholar] [CrossRef]
- Shik Mun, K.; Arora, K.; Huang, Y.; Yang, F.; Yarlagadda, S.; Ramananda, Y.; Abu-El-Haija, M.; Palermo, J.J.; Appakalai, B.N.; Nathan, J.D.; et al. Patient-Derived Pancreas-on-a-Chip to Model Cystic Fibrosis-Related Disorders. Nat. Commun. 2019, 10, 3124. [Google Scholar] [CrossRef] [PubMed]
- Aceves, J.O.; Heja, S.; Kobayashi, K.; Robinson, S.S.; Miyoshi, T.; Matsumoto, T.; Schäffers, O.J.M.; Morizane, R.; Lewis, J.A. 3D Proximal Tubule-on-Chip Model Derived from Kidney Organoids with Improved Drug Uptake. Sci. Rep. 2022, 12, 14997. [Google Scholar] [CrossRef]
- Yang, J.; Hirai, Y.; Iida, K.; Ito, S.; Trumm, M.; Terada, S.; Sakai, R.; Tsuchiya, T.; Tabata, O.; Kamei, K. Integrated-Gut-Liver-on-a-Chip Platform as an in Vitro Human Model of Non-Alcoholic Fatty Liver Disease. Commun. Biol. 2023, 6, 310. [Google Scholar] [CrossRef]
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered Human Blood–Brain Barrier Platform for Understanding Nanoparticle Transport Mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef]
- Liu, Y.; Kamran, R.; Han, X.; Wang, M.; Li, Q.; Lai, D.; Naruse, K.; Takahashi, K. Human Heart-on-a-Chip Microphysiological System Comprising Endothelial Cells, Fibroblasts, and IPSC-Derived Cardiomyocytes. Sci. Rep. 2024, 14, 18063. [Google Scholar] [CrossRef]
- Kumar, D.; Nadda, R.; Repaka, R. Advances and Challenges in Organ-on-Chip Technology: Toward Mimicking Human Physiology and Disease in Vitro. Med. Biol. Eng. Comput. 2024, 62, 1925–1957. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, S.H.; Choi, N.; Sung, J.H. Construction of Pancreas–Muscle–Liver Microphysiological System (MPS) for Reproducing Glucose Metabolism. Biotechnol. Bioeng. 2019, 116, 3433–3445. [Google Scholar] [CrossRef]
- Ortiz-Collazos, S.; Sousa-Batista, A.J.; Balbino, T.A. Engineering Microfluidic Devices to Mimic Signaling Cascades in Continuous-Flow Cell Culture as Multiorgan Microphysiological Systems. Biochem. Eng. J. 2024, 211, 109475. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef]
- Kida, R.; Rajendran, A.; Tsugane, M.; Duclos-Vallée, J.-C.; Mahe, M.M.; Bensalem, S.; Suzuki, H.; Pioufle, B. Le Gut-Liver Interaction Study on an All-Polydimethylsiloxane Microfluidic Device Integrating Intestinal Paracellular Permeability Assay. Talanta Open 2024, 9, 100289. [Google Scholar] [CrossRef]
- Yang, J.; Imamura, S.; Hirai, Y.; Tsuchiya, T.; Tabata, O.; Kamei, K. Gut-Liver-Axis Microphysiological System for Studying Cellular Fluidic Shear Stress and Inter-Tissue Interaction. Biomicrofluidics 2022, 16, 044113. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Kim, D.; Sung, J.H. A Gut-Brain Axis-on-a-Chip for Studying Transport across Epithelial and Endothelial Barriers. J. Ind. Eng. Chem. 2021, 101, 126–134. [Google Scholar] [CrossRef]
- Ferrari, E.; Visone, R.; Monti, E.; Torretta, E.; Moretti, M.; Occhetta, P.; Rasponi, M. LivHeart: A Multi Organ-on-Chip Platform to Study Off-Target Cardiotoxicity of Drugs Upon Liver Metabolism. Adv. Mater. Technol. 2023, 8, 2201435. [Google Scholar] [CrossRef]
- Fedi, A.; Vitale, C.; Fato, M.; Scaglione, S. A Human Ovarian Tumor & Liver Organ-on-Chip for Simultaneous and More Predictive Toxo-Efficacy Assays. Bioengineering 2023, 10, 270. [Google Scholar] [CrossRef]
- Indolfo, N.D.C.; Ganzerla, M.D.; Doratioto, T.R.; Avelino, T.M.; Tofani, L.B.; Peroni, L.A.; Rabelo, R.S.; Arroteia, K.F.; Figueira, A.C.M. Combining a Microphysiological System of Three Organ Equivalents and Transcriptomics to Assess Toxicological Endpoints for Cosmetic Ingredients. Lab Chip 2023, 23, 5092–5106. [Google Scholar] [CrossRef]
- Ganzerla, M.D.; Indolfo, N.D.C.; Oliveira, L.C.M.; Doratioto, T.R.; Avelino, T.M.; de Azevedo, R.J.; Tofani, L.B.; Terra, M.F.; Elias, G.B.; de Sousa, I.L.; et al. Unveiling the Intricacies of BPA and BPS: Comprehensive Insights into Its Toxic Effects Using a Cutting-Edge Microphysiological System. Toxicol. Vitr. 2024, 98, 105849. [Google Scholar] [CrossRef] [PubMed]
- Shinha, K.; Nihei, W.; Ono, T.; Nakazato, R.; Kimura, H. A Pharmacokinetic–Pharmacodynamic Model Based on Multi-Organ-on-a-Chip for Drug–Drug Interaction Studies. Biomicrofluidics 2020, 14, 044108. [Google Scholar] [CrossRef]
- Slaughter, V.L.; Rumsey, J.W.; Boone, R.; Malik, D.; Cai, Y.; Sriram, N.N.; Long, C.J.; McAleer, C.W.; Lambert, S.; Shuler, M.L.; et al. Validation of an Adipose-Liver Human-on-a-Chip Model of NAFLD for Preclinical Therapeutic Efficacy Evaluation. Sci. Rep. 2021, 11, 13159. [Google Scholar] [CrossRef] [PubMed]
- Fois, C.A.M.; Schindeler, A.; Valtchev, P.; Dehghani, F. Dynamic Flow and Shear Stress as Key Parameters for Intestinal Cells Morphology and Polarization in an Organ-on-a-Chip Model. Biomed. Microdevices 2021, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, Y.; Kang, D.; Kwak, T.; Lee, H.-R.; Jung, S. 3D Inkjet-Bioprinted Lung-on-a-Chip. ACS Biomater. Sci. Eng. 2023, 9, 2806–2815. [Google Scholar] [CrossRef]
- Lee, H.; Cho, D.-W. One-Step Fabrication of an Organ-on-a-Chip with Spatial Heterogeneity Using a 3D Bioprinting Technology. Lab Chip 2016, 16, 2618–2625. [Google Scholar] [CrossRef]
- Bhusal, A.; Dogan, E.; Nguyen, H.-A.; Labutina, O.; Nieto, D.; Khademhosseini, A.; Miri, A.K. Multi-Material Digital Light Processing Bioprinting of Hydrogel-Based Microfluidic Chips. Biofabrication 2022, 14, 014103. [Google Scholar] [CrossRef] [PubMed]
- Mountcastle, S.E.; Cox, S.C.; Sammons, R.L.; Jabbari, S.; Shelton, R.M.; Kuehne, S.A. A Review of Co-Culture Models to Study the Oral Microenvironment and Disease. J. Oral Microbiol. 2020, 12, 1773122. [Google Scholar] [CrossRef]
- Miki, Y.; Ono, K.; Hata, S.; Suzuki, T.; Kumamoto, H.; Sasano, H. The Advantages of Co-Culture over Mono Cell Culture in Simulating in Vivo Environment. J. Steroid Biochem. Mol. Biol. 2012, 131, 68–75. [Google Scholar] [CrossRef]
- Chen, P.; Rodda, A.E.; Parkington, H.C.; Forsythe, J.S. 13—Electrospun Scaffolds for Neural Tissue Engineering. In Electrospun Materials for Tissue Engineering and Biomedical Applications; Uyar, T., Kny, E., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 299–320. ISBN 978-0-08-101022-8. [Google Scholar]
- Loukelis, K.; Koutsomarkos, N.; Mikos, A.G.; Chatzinikolaidou, M. Advances in 3D Bioprinting for Regenerative Medicine Applications. Regen. Biomater. 2024, 11, rbae033. [Google Scholar] [CrossRef]
- Moghimi, N.; Hosseini, S.A.; Dalan, A.B.; Mohammadrezaei, D.; Goldman, A.; Kohandel, M. Controlled Tumor Heterogeneity in a Co-Culture System by 3D Bio-Printed Tumor-on-Chip Model. Sci. Rep. 2023, 13, 13648. [Google Scholar] [CrossRef]
- Vis, M.A.M.; Ito, K.; Hofmann, S. Impact of Culture Medium on Cellular Interactions in in Vitro Co-Culture Systems. Front. Bioeng. Biotechnol. 2020, 8, 911. [Google Scholar] [CrossRef]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-Culture Systems and Technologies: Taking Synthetic Biology to the next Level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef] [PubMed]
- Juste-Lanas, Y.; Hervas-Raluy, S.; García-Aznar, J.M.; González-Loyola, A. Fluid Flow to Mimic Organ Function in 3D in Vitro Models. APL Bioeng. 2023, 7, 031501. [Google Scholar] [CrossRef]
- Thompson, C.L.; Fu, S.; Heywood, H.K.; Knight, M.M.; Thorpe, S.D. Mechanical Stimulation: A Crucial Element of Organ-on-Chip Models. Front. Bioeng. Biotechnol. 2020, 8, 602646. [Google Scholar] [CrossRef]
- Yahyazadeh Shourabi, A.; Kashaninejad, N.; Saidi, M.S. An Integrated Microfluidic Concentration Gradient Generator for Mechanical Stimulation and Drug Delivery. J. Sci. Adv. Mater. Devices 2021, 6, 280–290. [Google Scholar] [CrossRef]
- Ingber, D.E. Human Organs-on-Chips for Disease Modelling, Drug Development and Personalized Medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Bakuova, N.; Toktarkan, S.; Dyussembinov, D.; Azhibek, D.; Rakhymzhanov, A.; Kostas, K.V.; Kulsharova, G. Design, Simulation, and Evaluation of Polymer-Based Microfluidic Devices via Computational Fluid Dynamics and Cell Culture “On-Chip”. Biosensors 2023, 13, 754. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Seo, J.-H.; Garud, K.S.; Park, S.W.; Lee, M.-Y. Numerical Approach-Based Simulation to Predict Cerebrovascular Shear Stress in a Blood-Brain Barrier Organ-on-a-Chip. Biosens. Bioelectron. 2021, 183, 113197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Marucci, L.; Homer, M.E. In Silico Modelling of Organ-on-a-Chip Devices: An Overview. Front. Bioeng. Biotechnol. 2025, 12, 1520795. [Google Scholar] [CrossRef] [PubMed]
- Dornhof, J.; Kieninger, J.; Muralidharan, H.; Maurer, J.; Urban, G.A.; Weltin, A. Microfluidic Organ-on-Chip System for Multi-Analyte Monitoring of Metabolites in 3D Cell Cultures. Lab Chip 2022, 22, 225–239. [Google Scholar] [CrossRef]
- van der Zalm, A.J.; Barroso, J.; Browne, P.; Casey, W.; Gordon, J.; Henry, T.R.; Kleinstreuer, N.C.; Lowit, A.B.; Perron, M.; Clippinger, A.J. A Framework for Establishing Scientific Confidence in New Approach Methodologies. Arch. Toxicol. 2022, 96, 2865–2879. [Google Scholar] [CrossRef] [PubMed]
- Schmeisser, S.; Miccoli, A.; von Bergen, M.; Berggren, E.; Braeuning, A.; Busch, W.; Desaintes, C.; Gourmelon, A.; Grafström, R.; Harrill, J.; et al. New Approach Methodologies in Human Regulatory Toxicology—Not If, but How and When! Env. Int. 2023, 178, 108082. [Google Scholar] [CrossRef]
- Thakare, K.; Jerpseth, L.; Pei, Z.; Elwany, A.; Quek, F.; Qin, H. Bioprinting of Organ-on-Chip Systems: A Literature Review from a Manufacturing Perspective. J. Manuf. Mater. Process. 2021, 5, 91. [Google Scholar] [CrossRef]
- Sunildutt, N.; Parihar, P.; Chethikkattuveli Salih, A.R.; Lee, S.H.; Choi, K.H. Revolutionizing Drug Development: Harnessing the Potential of Organ-on-Chip Technology for Disease Modeling and Drug Discovery. Front. Pharmacol. 2023, 14, 1139229. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, L.; Ramsden, D.; Leite, S.B.; Beken, S.; Bonzo, J.A.; Brown, P.; Candarlioglu, P.L.; Chan, T.S.; Chen, E.; Choi, C.K.; et al. Considerations from an International Regulatory and Pharmaceutical Industry (IQ MPS Affiliate) Workshop on the Standardization of Complex In Vitro Models in Drug Development. Adv. Biol. 2024, 8, 2300131. [Google Scholar] [CrossRef] [PubMed]
- Vulto, P.; Joore, J. Adoption of Organ-on-Chip Platforms by the Pharmaceutical Industry. Nat. Rev. Drug Discov. 2021, 20, 961–962. [Google Scholar] [CrossRef]
- Rumsey, J.W.; Lorance, C.; Jackson, M.; Sasserath, T.; McAleer, C.W.; Long, C.J.; Goswami, A.; Russo, M.A.; Raja, S.M.; Gable, K.L.; et al. Classical Complement Pathway Inhibition in a “Human-On-A-Chip” Model of Autoimmune Demyelinating Neuropathies. Adv. Ther. 2022, 5, 2200030. [Google Scholar] [CrossRef]
- Milani, N.; Parrott, N.; Ortiz Franyuti, D.; Godoy, P.; Galetin, A.; Gertz, M.; Fowler, S. Application of a Gut–Liver-on-a-Chip Device and Mechanistic Modelling to the Quantitative in Vitro Pharmacokinetic Study of Mycophenolate Mofetil. Lab Chip 2022, 22, 2853–2868. [Google Scholar] [CrossRef]
- Rigal, S.; Casas, B.; Kanebratt, K.P.; Wennberg Huldt, C.; Magnusson, L.U.; Müllers, E.; Karlsson, F.; Clausen, M.; Hansson, S.F.; Leonard, L.; et al. Normoglycemia and Physiological Cortisone Level Maintain Glucose Homeostasis in a Pancreas-Liver Microphysiological System. Commun. Biol. 2024, 7, 877. [Google Scholar] [CrossRef]
- Cairns, J.; Leonard, E.; Khan, K.; Parks, C.; Maglennon, G.; Zhang, B.; Lazic, S.E.; Ewart, L.; David, R. Optimal Experimental Design for Efficient Toxicity Testing in Microphysiological Systems: A Bone Marrow Application. Front. Pharmacol. 2023, 14, 1142581. [Google Scholar] [CrossRef]
- Gracia, B.; Michelle, M.-A. The Future of Drug Development: Leveraging EMA Support for Use of Alternative Nonclinical Approaches. Paraxel. 2024. Available online: https://www.parexel.com/insights/blog/the-future-of-drug-development-leveraging-ema-support-for-use-of-alternative-nonclinical-approaches (accessed on 24 October 2025).
- NIH. NIH to Prioritize Human-Based Research Technologies; NIH: Bethesda, MD, USA, 2025.
- FDA. News FDA Announces Plan to Phase Out Animal Testing Requirement for Monoclonal Antibodies and Other Drugs; FDA: Silver Spring, MD, USA, 2025.
- Hutson, M.S.; Alexander, P.G.; Allwardt, V.; Aronoff, D.M.; Bruner-Tran, K.L.; Cliffel, D.E.; Davidson, J.M.; Gough, A.; Markov, D.A.; McCawley, L.J.; et al. Organs-on-Chips as Bridges for Predictive Toxicology. Appl. Vitr. Toxicol. 2016, 2, 97–102. [Google Scholar] [CrossRef]
- Marx, U.; Akabane, T.; Andersson, T.B.; Baker, E.; Beilmann, M.; Beken, S.; Brendler-Schwaab, S.; Cirit, M.; David, R.; Dehne, E.-M.; et al. Biology-Inspired Microphysiological Systems to Advance Patient Benefit and Animal Welfare in Drug Development. ALTEX Altern. Anim. Exp. 2020, 37, 365–394. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, R.; Wang, Y.; Deng, P.; Song, T.; Zhang, M.; Wang, P.; Zhang, X.; Cui, K.; Tao, T.; et al. SARS-CoV-2 Induced Intestinal Responses with a Biomimetic Human Gut-on-Chip. Sci. Bull. 2021, 66, 783–793. [Google Scholar] [CrossRef]
- Freag, M.S.; Namgung, B.; Reyna Fernandez, M.E.; Gherardi, E.; Sengupta, S.; Jang, H.L. Human Nonalcoholic Steatohepatitis on a Chip. Hepatol. Commun. 2021, 5, 217–233. [Google Scholar] [CrossRef]
- Bengoa-Vergniory, N.; Faggiani, E.; Ramos-Gonzalez, P.; Kirkiz, E.; Connor-Robson, N.; Brown, L.V.; Siddique, I.; Li, Z.; Vingill, S.; Cioroch, M.; et al. CLR01 Protects Dopaminergic Neurons in Vitro and in Mouse Models of Parkinson’s Disease. Nat. Commun. 2020, 11, 4885. [Google Scholar] [CrossRef]
- Maulana, T.I.; Teufel, C.; Cipriano, M.; Roosz, J.; Lazarevski, L.; van den Hil, F.E.; Scheller, L.; Orlova, V.; Koch, A.; Hudecek, M.; et al. Breast Cancer-on-Chip for Patient-Specific Efficacy and Safety Testing of CAR-T Cells. Cell Stem Cell 2024, 31, 989–1002.e9. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-G.; Jeong, Y.H.; Kim, Y.; Choi, Y.-J.; Moon, H.E.; Park, S.H.; Kang, K.S.; Bae, M.; Jang, J.; Youn, H.; et al. A Bioprinted Human-Glioblastoma-on-a-Chip for the Identification of Patient-Specific Responses to Chemoradiotherapy. Nat. Biomed. Eng. 2019, 3, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Beaurivage, C.; Kanapeckaite, A.; Loomans, C.; Erdmann, K.S.; Stallen, J.; Janssen, R.A.J. Development of a Human Primary Gut-on-a-Chip to Model Inflammatory Processes. Sci. Rep. 2020, 10, 21475. [Google Scholar] [CrossRef]
- Liu, J.; Lu, R.; Zheng, X.; Hou, W.; Wu, X.; Zhao, H.; Wang, G.; Tian, T. Establishment of a Gut-on-a-Chip Device with Controllable Oxygen Gradients to Study the Contribution of Bifidobacterium Bifidum to Inflammatory Bowel Disease. Biomater. Sci. 2023, 11, 2504–2517. [Google Scholar] [CrossRef]
- Özkan, A.; Merry, G.E.; Chou, D.B.; Posey, R.R.; Stejskalova, A.; Sperry, M.; Horvath, V.; Ferri, L.E.; Carlotti, E.; McDonald, C.; et al. Human Organ Chips Reveal New Inflammatory Bowel Disease Drivers. medRxiv 2025. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, D.; Zeng, J.; Fu, Q.; Chen, Z.; Sun, X.; Yang, Y.; Li, S.; Chen, M. Application of 3D-Printed Tissue-Engineered Skin Substitute Using Innovative Biomaterial Loaded with Human Adipose-Derived Stem Cells in Wound Healing. Int. J. Bioprint. 2023, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Gao, M.; Xing, J.; Wang, C.; Zhao, P.; Zhang, H.; Qu, J. Experimental Study on Repair of Cartilage Defects in the Rabbits with GelMA-MSCs Scaffold Prepared by Three-Dimensional Bioprinting. Int. J. Bioprint. 2023, 9, 662. [Google Scholar] [CrossRef]
- Zeng, J.; Jia, L.; Wang, D.; Chen, Z.; Liu, W.; Yang, Q.; Liu, X.; Jiang, H. Bacterial Nanocellulose-Reinforced Gelatin Methacryloyl Hydrogel Enhances Biomechanical Property and Glycosaminoglycan Content of 3D-Bioprinted Cartilage. Int. J. Bioprint. 2022, 9, 631. [Google Scholar] [CrossRef]
- Song, S.; Liu, X.; Huang, J.; Zhang, Z. Neural Stem Cell-Laden 3D Bioprinting of Polyphenol-Doped Electroconductive Hydrogel Scaffolds for Enhanced Neuronal Differentiation. Biomater. Adv. 2022, 133, 112639. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Shrike Zhang, Y.; Shin, S.R.; Calzone, G.; et al. A Liver-on-a-Chip Platform with Bioprinted Hepatic Spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D Microfibrous Scaffolds for Engineering Endothelialized Myocardium and Heart-on-a-Chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef]
- Pontiggia, L.; Van Hengel, I.A.J.; Klar, A.; Rütsche, D.; Nanni, M.; Scheidegger, A.; Figi, S.; Reichmann, E.; Moehrlen, U.; Biedermann, T. Bioprinting and Plastic Compression of Large Pigmented and Vascularized Human Dermo-Epidermal Skin Substitutes by Means of a New Robotic Platform. J. Tissue Eng. 2022, 13, 20417314221088510. [Google Scholar] [CrossRef]
- Huan, Y.; Zhou, D.; Wu, X.; He, X.; Chen, H.; Li, S.; Jia, B.; Dou, Y.; Fei, X.; Wu, S.; et al. 3D Bioprinted Autologous Bone Particle Scaffolds for Cranioplasty Promote Bone Regeneration with Both Implanted and Native BMSCs. Biofabrication 2023, 15, 025016. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, K.; Man, W.; Zheng, J.; Cao, Z.; Yang, C.-Y.; Kim, K.; Yang, S.; Hou, Z.; Wang, G.; et al. 3D Bio-Printed Living Nerve-like Fibers Refine the Ecological Niche for Long-Distance Spinal Cord Injury Regeneration. Bioact. Mater. 2023, 25, 160–175. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Zhu, J.; Liu, F.; Xue, Y.; Huang, Y.; Zhu, B.; Wu, D.; Pan, H.; Gong, T.; et al. Hyaluronic Acid Methacrylate/Pancreatic Extracellular Matrix as a Potential 3D Printing Bioink for Constructing Islet Organoids. Acta Biomater. 2023, 165, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xue, R.; Zhao, Y.; Ramsay, K.; Wang, E.Y.; Savoji, H.; Veres, T.; Cartmell, S.H.; Radisic, M. Automated Fabrication of a Scalable Heart-on-a-Chip Device by 3D Printing of Thermoplastic Elastomer Nanocomposite and Hot Embossing. Bioact. Mater. 2024, 33, 46–60. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Shakur, R.; Horne, J.P.; Dickinson, S.C.; Armstrong, C.T.; Lau, K.; Kadiwala, J.; Lowe, R.; Seddon, A.; Mann, S.; et al. Artificial Membrane-Binding Proteins Stimulate Oxygenation of Stem Cells during Engineering of Large Cartilage Tissue. Nat. Commun. 2015, 6, 7405. [Google Scholar] [CrossRef]
- Farris, A.L.; Lambrechts, D.; Zhou, Y.; Zhang, N.Y.; Sarkar, N.; Moorer, M.C.; Rindone, A.N.; Nyberg, E.L.; Perdomo-Pantoja, A.; Burris, S.J.; et al. 3D-Printed Oxygen-Releasing Scaffolds Improve Bone Regeneration in Mice. Biomaterials 2022, 280, 121318. [Google Scholar] [CrossRef]
- Pamies, D.; Ekert, J.; Zurich, M.-G.; Frey, O.; Werner, S.; Piergiovanni, M.; Freedman, B.S.; Keong Teo, A.K.; Erfurth, H.; Reyes, D.R.; et al. Recommendations on Fit-for-Purpose Criteria to Establish Quality Management for Microphysiological Systems and for Monitoring Their Reproducibility. Stem Cell Rep. 2024, 19, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Nakipoglu, M.; Hartung, T. Lessons and Insights from the First Microphysiological World Summit. J. Craniofacial Surg. Open 2023, 1, e0011. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Cui, H.; Chen, H.; Wang, Y.; Du, X. Programmed Shape-Morphing Scaffolds Enabling Facile 3D Endothelialization. Adv. Funct. Mater. 2018, 28, 1801027. [Google Scholar] [CrossRef]
- Ni, C.; Chen, D.; Yin, Y.; Wen, X.; Chen, X.; Yang, C.; Chen, G.; Sun, Z.; Wen, J.; Jiao, Y.; et al. Shape Memory Polymer with Programmable Recovery Onset. Nature 2023, 622, 748–753. [Google Scholar] [CrossRef]
- Yue, L.; Sun, X.; Yu, L.; Li, M.; Montgomery, S.M.; Song, Y.; Nomura, T.; Tanaka, M.; Qi, H.J. Cold-Programmed Shape-Morphing Structures Based on Grayscale Digital Light Processing 4D Printing. Nat. Commun. 2023, 14, 5519. [Google Scholar] [CrossRef]
- Lai, J.; Liu, Y.; Lu, G.; Yung, P.; Wang, X.; Tuan, R.S.; Li, Z.A. 4D Bioprinting of Programmed Dynamic Tissues. Bioact. Mater. 2024, 37, 348–377. [Google Scholar] [CrossRef] [PubMed]
- Yarali, E.; Mirzaali, M.J.; Ghalayaniesfahani, A.; Accardo, A.; Diaz-Payno, P.J.; Zadpoor, A.A. 4D Printing for Biomedical Applications. Adv. Mater. 2024, 36, 2402301. [Google Scholar] [CrossRef] [PubMed]
- Shaw, I.; Ali, Y.S.; Nie, C.; Zhang, K.; Chen, C.; Xiao, Y. Integrating Artificial Intelligence and Microfluidics Technology for Psoriasis Therapy: A Comprehensive Review for Research and Clinical Applications. Adv. Intell. Syst. 2024, 7, 2400558. [Google Scholar] [CrossRef]
- Galan, E.A.; Zhao, H.; Wang, X.; Dai, Q.; Huck, W.T.S.; Ma, S. Intelligent Microfluidics: The Convergence of Machine Learning and Microfluidics in Materials Science and Biomedicine. Matter 2020, 3, 1893–1922. [Google Scholar] [CrossRef]
- Mencattini, A.; Rizzuto, V.; Antonelli, G.; Di Giuseppe, D.; D’Orazio, M.; Filippi, J.; Comes, M.C.; Casti, P.; Vives Corrons, J.L.; Garcia-Bravo, M.; et al. Machine Learning Microfluidic Based Platform: Integration of Lab-on-Chip Devices and Data Analysis Algorithms for Red Blood Cell Plasticity Evaluation in Pyruvate Kinase Disease Monitoring. Sens. Actuators A Phys. 2023, 351, 114187. [Google Scholar] [CrossRef]
- Jeznach, O.; Tabakoglu, S.; Zaszczyńska, A.; Sajkiewicz, P. Review on Machine Learning Application in Tissue Engineering: What Has Been Done so Far? Application Areas, Challenges, and Perspectives. J. Mater. Sci. 2024, 59, 21222–21250. [Google Scholar] [CrossRef]
- Eş, I.; Ece, E.; Hacıosmanoğlu, N.; Güngen, M.A.; Inci, F. Chapter 5—Artificial Intelligence–Assisted Organ-on-Chip Systems. In Smart Organ-on-Chip Devices; Balbino, T.A., Bartolo, P., Charelli, L., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 61–69. ISBN 978-0-443-13403-6. [Google Scholar]
- Obeid, P.J.; Yammine, P.; El-Nakat, H.; Kassab, R.; Tannous, T.; Nasr, Z.; Maarawi, T.; Dahdah, N.; El Safadi, A.; Mansour, A.; et al. Organ-On-A-Chip Devices: Technology Progress and Challenges. ChemBioChem 2024, 25, e202400580. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Bai, H.; Wang, H.; Hao, S.; Ding, Y.; Peng, B.; Zhang, J.; Li, L.; Huang, W. An Overview of Organs-on-Chips Based on Deep Learning. Research 2025, 2022, 9869518. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Mekkattu, M.; Katzschmann, R.K. Sustainable Biofabrication: From Bioprinting to AI-Driven Predictive Methods. Trends Biotechnol. 2025, 43, 290–303. [Google Scholar] [CrossRef]
- Movčana, V.; Strods, A.; Narbute, K.; Rūmnieks, F.; Rimša, R.; Mozoļevskis, G.; Ivanovs, M.; Kadiķis, R.; Zviedris, K.G.; Leja, L.; et al. Organ-On-A-Chip (OOC) Image Dataset for Machine Learning and Tissue Model Evaluation. Data 2024, 9, 28. [Google Scholar] [CrossRef]
- Li, J.; Liang, W.; Chen, Z.; Li, X.; Gu, P.; Liu, A.; Chen, P.; Li, Q.; Mei, X.; Yang, J.; et al. OOCDB: A Comprehensive, Systematic, and Real-Time Organs-on-a-Chip Database. Genom. Proteom. Bioinform. 2023, 21, 243–258. [Google Scholar] [CrossRef]
- De Spirito, M.; Palmieri, V.; Perini, G.; Papi, M. Bridging the Gap: Integrating 3D Bioprinting and Microfluidics for Advanced Multi-Organ Models in Biomedical Research. Bioengineering 2024, 11, 664. [Google Scholar] [CrossRef]
- Knowlton, S.; Yenilmez, B.; Tasoglu, S. Towards Single-Step Biofabrication of Organs on a Chip via 3D Printing. Trends Biotechnol. 2016, 34, 685–688. [Google Scholar] [CrossRef]
- Cognetti, J.S.; Moen, M.T.; Brewer, M.G.; Bryan, M.R.; Tice, J.D.; McGrath, J.L.; Miller, B.L. A Photonic Biosensor-Integrated Tissue Chip Platform for Real-Time Sensing of Lung Epithelial Inflammatory Markers. Lab Chip 2023, 23, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Aleman, J.; Kilic, T.; Mille, L.S.; Shin, S.R.; Zhang, Y.S. Microfluidic Integration of Regeneratable Electrochemical Affinity-Based Biosensors for Continual Monitoring of Organ-on-a-Chip Devices. Nat. Protoc. 2021, 16, 2564–2593. [Google Scholar] [CrossRef] [PubMed]
- Aydogmus, H.; Hu, M.; Ivancevic, L.; Frimat, J.-P.; van den Maagdenberg, A.M.J.M.; Sarro, P.M.; Mastrangeli, M. An Organ-on-Chip Device with Integrated Charge Sensors and Recording Microelectrodes. Sci. Rep. 2023, 13, 8062. [Google Scholar] [CrossRef]
- Palasantzas, V.E.J.M.; Tamargo-Rubio, I.; Le, K.; Slager, J.; Wijmenga, C.; Jonkers, I.H.; Kumar, V.; Fu, J.; Withoff, S. IPSC-Derived Organ-on-a-Chip Models for Personalized Human Genetics and Pharmacogenomics Studies. Trends Genet. 2023, 39, 268–284. [Google Scholar] [CrossRef]
- Gautier Contributing Editor, L.J. CRISPR in 3D: Innovations in Disease Modelling and Personalized Medicine. Biotechniques 2024, 76, 473–478. [Google Scholar] [CrossRef]
- Bas-Cristóbal Menéndez, A.; Du, Z.; van den Bosch, T.P.P.; Othman, A.; Gaio, N.; Silvestri, C.; Quirós, W.; Lin, H.; Korevaar, S.; Merino, A.; et al. Creating a Kidney Organoid-Vasculature Interaction Model Using a Novel Organ-on-Chip System. Sci. Rep. 2022, 12, 20699. [Google Scholar] [CrossRef]
- Khorsandi, D.; Yang, J.-W.; Foster, S.; Khosravi, S.; Hosseinzadeh Kouchehbaghi, N.; Zarei, F.; Lee, Y.B.; Runa, F.; Gangrade, A.; Voskanian, L.; et al. Patient-Derived Organoids as Therapy Screening Platforms in Cancer Patients. Adv. Healthc. Mater. 2024, 13, 2302331. [Google Scholar] [CrossRef]
- Ding, S.; Hsu, C.; Wang, Z.; Natesh, N.R.; Millen, R.; Negrete, M.; Giroux, N.; Rivera, G.O.; Dohlman, A.; Bose, S.; et al. Patient-Derived Micro-Organospheres Enable Clinical Precision Oncology. Cell Stem Cell 2022, 29, 905–917.e6. [Google Scholar] [CrossRef]

| Platform Type | Technique/Model | Key Quantitative Parameters | Reference |
|---|---|---|---|
| 3D bioprinting | Volumetric (VBP) hepatic organoids | Resolution ≈ 50 µm; viability > 90%; albumin synthesis ↑ 2.3× vs. 2D | [32] |
| 3D bioprinting | Extrusion-based cardiac patch | Perfusable channels 250 µm Ø; synchronous contraction > 90% cells | [44] |
| 3D bioprinting | Intestine-on-a-chip | Flow 15 nL min−1; MUC-2 expression ↑ 3× vs. static; villi ≈ 300 µm | [47] |
| Microfluidic OoC | BBB-on-a-chip | Flow 16 µL min−1; shear ≈ 4 dyn cm−2; TEER ≈ 250 Ω·cm2 | [78] |
| Microfluidic OoC | Gut–liver chip (NAFLD model) | Flow 15 nL min−1; lipid accumulation ↑ after 7 days; albumin ↑ 1.5× | [77] |
| Multi-OoC | Liver–heart system | Albumin ≈ 15 µg mL−1 (day 7); spontaneous beating > 70 bpm | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Barros, N.R.; Harb, S.V.; da Silva Horinouchi, C.D.; Tofani, L.B.; dos Santos, D.M.; Elias, G.B.; Velho, J.C.M.; de Aguiar, A.C.; Sant’Ana, M.; Figueira, A.C.M. Advances in 3D Bioprinting and Microfluidics for Organ-on-a-Chip Platforms. Polymers 2025, 17, 3078. https://doi.org/10.3390/polym17223078
de Barros NR, Harb SV, da Silva Horinouchi CD, Tofani LB, dos Santos DM, Elias GB, Velho JCM, de Aguiar AC, Sant’Ana M, Figueira ACM. Advances in 3D Bioprinting and Microfluidics for Organ-on-a-Chip Platforms. Polymers. 2025; 17(22):3078. https://doi.org/10.3390/polym17223078
Chicago/Turabian Stylede Barros, Natan Roberto, Samarah Vargas Harb, Cintia Delai da Silva Horinouchi, Larissa Bueno Tofani, Daniela Mayra dos Santos, Giovanna Blazutti Elias, Julia Carnelos Machado Velho, Ana Carolina de Aguiar, Monielle Sant’Ana, and Ana Carolina Migliorini Figueira. 2025. "Advances in 3D Bioprinting and Microfluidics for Organ-on-a-Chip Platforms" Polymers 17, no. 22: 3078. https://doi.org/10.3390/polym17223078
APA Stylede Barros, N. R., Harb, S. V., da Silva Horinouchi, C. D., Tofani, L. B., dos Santos, D. M., Elias, G. B., Velho, J. C. M., de Aguiar, A. C., Sant’Ana, M., & Figueira, A. C. M. (2025). Advances in 3D Bioprinting and Microfluidics for Organ-on-a-Chip Platforms. Polymers, 17(22), 3078. https://doi.org/10.3390/polym17223078







