Recent Advances on Chitosan-Based Nanoparticles for Brain Drug Delivery
Abstract
1. Introduction
2. Mechanisms of Brain Delivery with CNPs
3. Chitosan-Based Nanoparticles: Physical Properties, Preparation Methods and Factors Governing Interpolyelectrolyte Complexes for Brain Applications
3.1. Physicochemical Factors Governing Chitosan-Based Interpolyelectrolyte Complexes (Stoichiometry and PH)
3.2. Key Methods for CNP Synthesis
3.2.1. Ionic Gelation
3.2.2. Complex Coacervation
3.2.3. Polymer–Drug Self-Assembly
3.3. CNPs Shape, Packing Density of Polymer Chains, and Elastic Modulus
4. Therapeutic Applications of CNPs
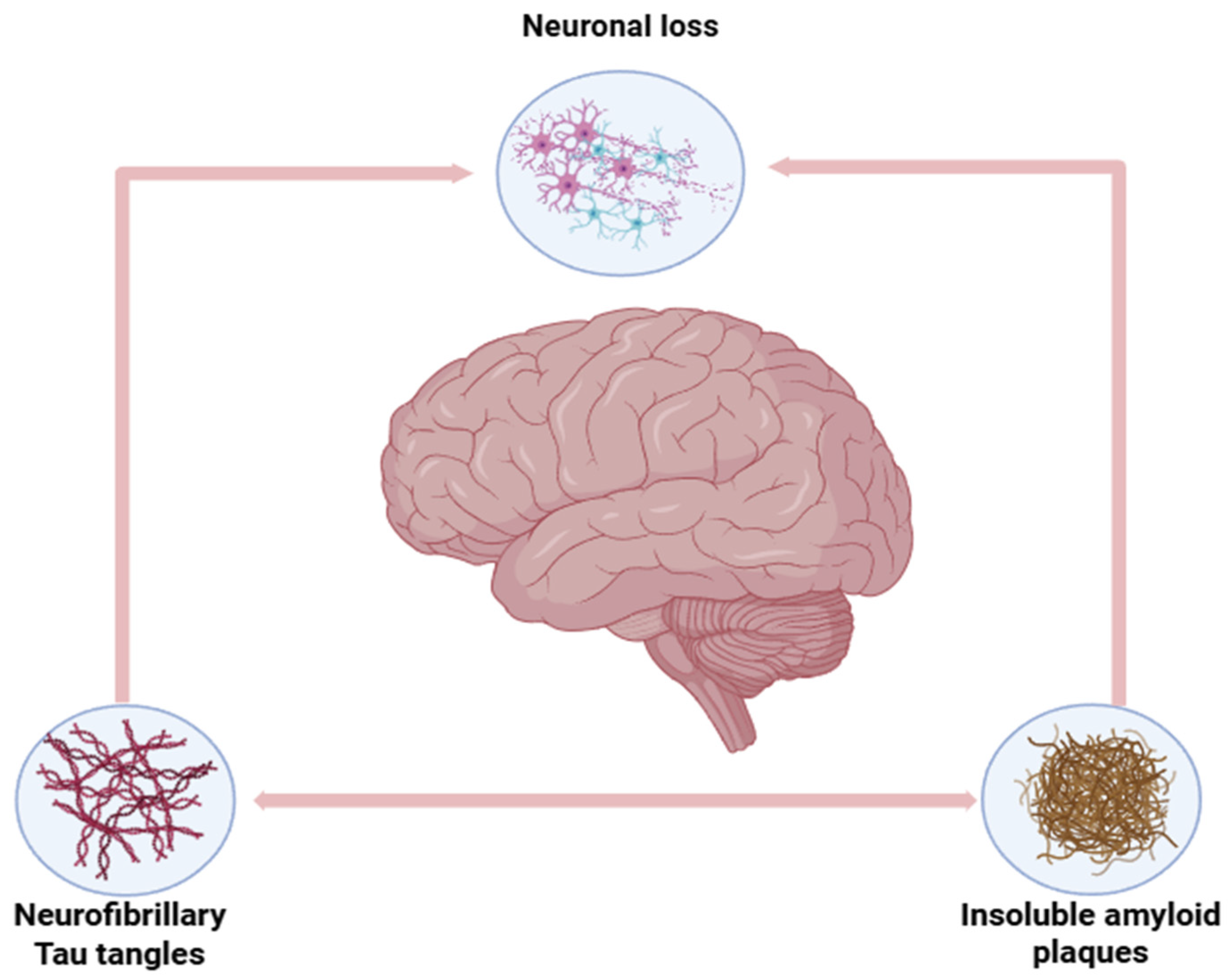
4.1. Treatment of Neuro-Degenerative Diseases: Alzheimer’s, Parkinson’s, and Huntington’s Disease
4.2. Treatment of Brain Cancer
4.3. Treatment of Stroke
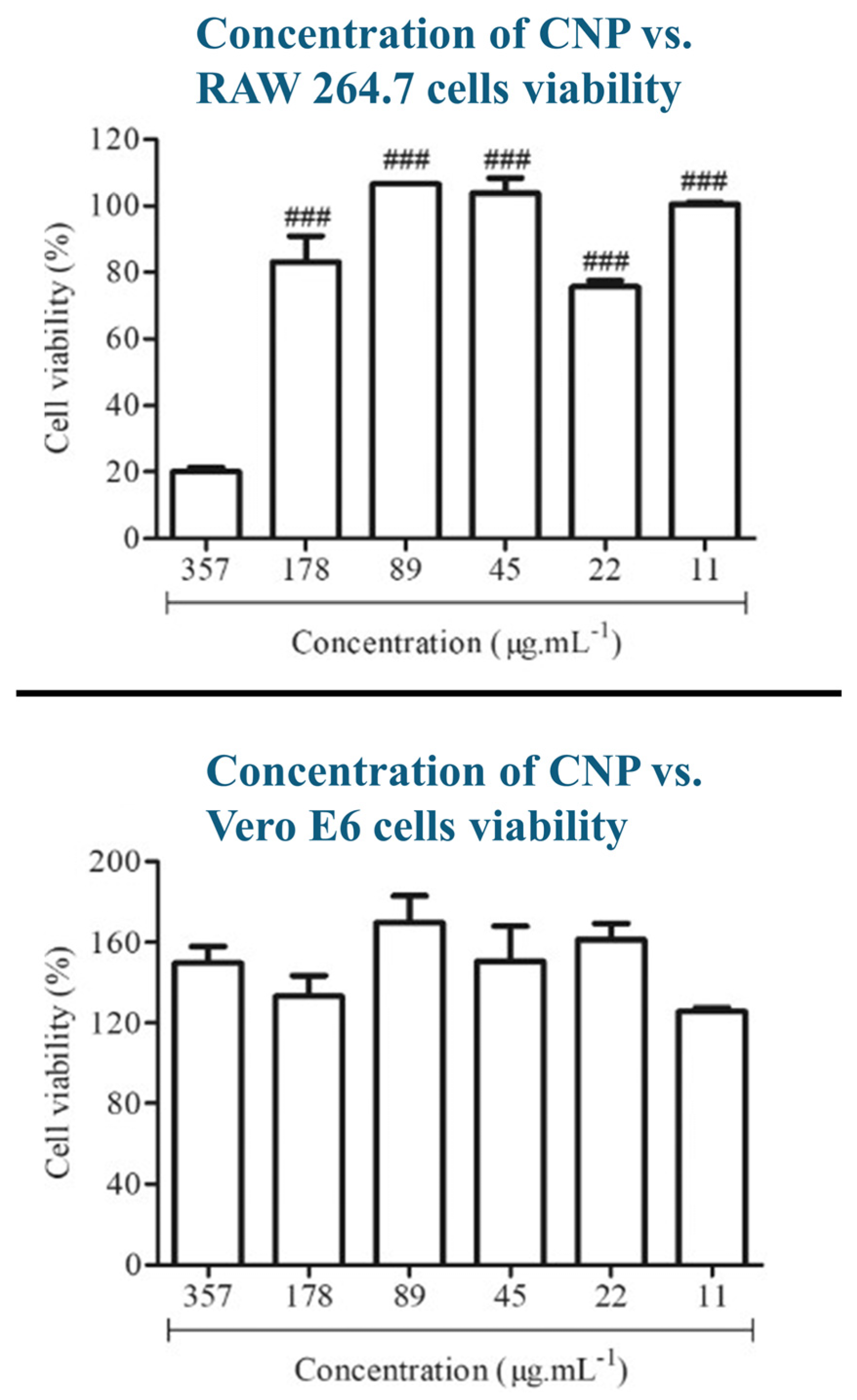
5. In Vitro/in Vivo Studies for Biocompatibility and Cytotoxicity Characterization of CNPs
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | Chitosan |
| CNPs | Chitosan-Based Nanoparticles |
| BBB | Blood–Brain Barrier |
| AD | Alzheimer’s Disease |
| Aβ | Amyloid-beta |
| CIRI | Cerebral Ischemia–Reperfusion Injury |
| CNS | Central Nervous System |
| DD | Degree of Deacetylation |
| DNA | Deoxyribonucleic Acid |
| EGFR | Epidermal Growth Factor Receptor |
| 5-FU | 5-Fluorouracil |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| miRNA | Micro Ribonucleic Acid |
| MMP | Matrix Metalloproteinase |
| P-gp | P-glycoprotein |
| PD | Parkinson’s Disease |
| PLA | Polylactic Acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| ROS | Reactive Oxygen Species |
| siRNA | Small Interfering Ribonucleic Acid |
| sTPP | Sodium Tripolyphosphate |
| TfR | Transferrin Receptor |
| TPP | Tripolyphosphate |
References
- Barnabas, W. Drug targeting strategies into the brain for treating neurological diseases. J. Neurosci. Methods 2019, 311, 133–146. [Google Scholar] [CrossRef]
- Rai, G.; Gauba, P.; Dang, S. Recent advances in nanotechnology for Intra-nasal drug delivery and clinical applications. J. Drug Deliv. Sci. Technol. 2023, 86, 104726. [Google Scholar] [CrossRef]
- Wang, J.; Jia, R.; Wan, W.; Han, H.; Wang, G.; Li, Z.; Li, J. Drug Delivery Targeting Neuroinflammation to Treat Brain Diseases. Bioconjug. Chem. 2024, 35, 1687–1698. [Google Scholar] [CrossRef]
- Li, L.; He, R.; Yan, H.; Leng, Z.; Zhu, S.; Gu, Z. Nanotechnology for the diagnosis and treatment of Alzheimer’s disease: A bibliometric analysis. Nano Today 2022, 47, 101654. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, S.; Jabin, S.; Jadoun, S. Chitosan nanocomposite for tissue engineering and regenerative medicine: A review. Int. J. Biol. Macromol. 2024, 254, 127660. [Google Scholar] [CrossRef] [PubMed]
- Khezri, F.A.N.Z.; Lakshmi, C.S.R.; Bukka, R.; Nidhi, M.; Nargund, S.L. Pharmacokinetic study and brain tissue analysis of Zolmitriptan loaded chitosan nanoparticles in rats by LC-MS method. Int. J. Biol. Macromol. 2020, 142, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Vahab, S.A.; Anjali, K.I.; Sabitha, M.; Kumar, V.S. Exploring chitosan nanoparticles for enhanced therapy in neurological disorders: A comprehensive review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 2151–2167. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Iyer, M.; Elangovan, A.; Sennimalai, R.; Babu, H.W.S.; Thiruvenkataswamy, S.; Krishnan, J.; Yadav, M.K.; Gopalakrishnan, A.V.; Narayanasamy, A.; Vellingiri, B. Chitosan—An alternative drug delivery approach for neurodegenerative diseases. Carbohydr. Polym. Technol. Appl. 2024, 7, 100460. [Google Scholar] [CrossRef]
- Ayub, A.; Wettig, S. An overview of nanotechnologies for drug delivery to the brain. Pharmaceutics 2022, 14, 224. [Google Scholar] [CrossRef]
- Garg, Y.; Kumar, M.; Sharma, G.; Katare, O.P.; Chopra, S.; Bhatia, A. Systematic Designing and Optimization of Polymeric Nanoparticles Using Central Composite Design: A Novel Approach for Nose-to-Brain Delivery of Donepezil Hydrochloride. J. Clust. Sci. 2024, 35, 1007–1019. [Google Scholar] [CrossRef]
- Omidian, H.; Gill, E.J.; Chowdhury, S.D.; Cubeddu, L.X. Chitosan Nanoparticles for Intranasal Drug Delivery. Pharmaceutics 2024, 16, 746. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.; Geetha, K.M.; Wilson, B. Potential applications of nanomedicine for treating Parkinson’s disease. J. Drug Deliv. Sci. Technol. 2021, 66, 102793. [Google Scholar] [CrossRef]
- Nerli, G.; Robla, S.; Bartalesi, M.; Luceri, C.; D’AMbrosio, M.; Csaba, N.; Maestrelli, F. Chitosan coated niosomes for nose-to-brain delivery of clonazepam: Formulation, stability and permeability studies. Carbohydr. Polym. Technol. Appl. 2023, 6, 100332. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Pivoriu, A. Astroglia support, regulate and reinforce brain barriers. Neurobiol. Dis. 2023, 179, 106054. [Google Scholar] [CrossRef]
- Khodaverdi, K.; Bakhshi, A.; Mozafari, M.R.; Naghib, S.M. A review of chitosan-based nanocarriers as drug delivery systems for brain diseases: Critical challenges, outlooks and promises. Int. J. Biol. Macromol. 2024, 278, 134962. [Google Scholar] [CrossRef]
- Khaledian, S.; Dayani, M.; Fatahian, A.; Fatahian, R.; Martinez, F. Efficiency of lipid-based nano drug delivery systems in crossing the blood–brain barrier: A review. J. Mol. Liq. 2022, 346, 118278. [Google Scholar] [CrossRef]
- Singh, N.; Vishwas, S.; Kaur, A.; Kaur, H.; Kakoty, V.; Khursheed, R.; Chaitanya, M.; Babu, M.R.; Awasthi, A.; Corrie, L.; et al. Harnessing role of sesamol and its nanoformulations against neurodegenerative diseases. Biomed. Pharmacother. 2023, 167, 115512. [Google Scholar] [CrossRef]
- Qiao, R.; Fu, C.; Forgham, H.; Javed, I.; Huang, X.; Zhu, J.; Whittaker, A.K.; Davis, T.P. Magnetic iron oxide nanoparticles for brain imaging and drug delivery. Adv. Drug Deliv. Rev. 2023, 197, 114822. [Google Scholar] [CrossRef]
- Wiranowska, M. Advances in the use of chitosan and chlorotoxin-functionalized chitosan polymers in drug delivery and detection of glioma—A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100427. [Google Scholar] [CrossRef]
- Sari, E.; Anshori, U. Biocoagulant of blood based on chitosan nanoparticle from crustacea. J. Phys. Conf. Ser. 2019, 1246, 012055. [Google Scholar] [CrossRef]
- Yang, H.C.; Hon, M.H. The effect of the degree of deacetylation of chitosan nanoparticles and its characterization and encapsulation efficiency on drug delivery. Polym.-Plast. Technol. Eng. 2010, 49, 1292–1296. [Google Scholar] [CrossRef]
- Liu, C.; Tan, Y.; Liu, C.; Chen, X.; Yu, L. Preparations, characterizations and applications of chitosan-based nanoparticles. J. Ocean Univ. China 2007, 6, 237–243. [Google Scholar] [CrossRef]
- Li, Y.P.; Xu, Y.; Cheng, X.N.; Wu, S.; Liu, H. Preparation of chitosan nanoparticles with fissuaring structure by inverse miniemulsion cross linking method. J. Jiangsu Univ. Nat. Sci. Ed. 2014, 35, 120–124. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, Y.; Yang, L.; Huang, X.; Bao, J. Bile acid conjugated chitosan nanoparticles promote the proliferation and epithelial-mesenchymal transition of hepatocellular carcinoma by regulating the PI3K/Akt/mTOR pathway. Carbohydr. Res. 2024, 545, 109296. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, V.H.B.; Lewandowski, A.; Durai, R.; Gonciarz, W.; Wawrzyniak, P.; Brzezinski, M. Spray-dried tenofovir alafenamide-chitosan nanoparticles loaded oleogels as a long-acting injectable depot system of anti-HIV drug. Int. J. Biol. Macromol. 2022, 222, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Geeva, S.; Narayan, S. Lithium entrapped chitosan nanoparticles to reduce toxicity and increase cellular uptake of lithium. Environ. Toxicol. Pharmacol. 2018, 61, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.N.; Navaid, S.; Waqar, W.; Hussein, D.; Ullah, N.; Khan, M.U.A.; Hussain, Z.; Javed, A. Chitosan-Based Polymeric Nanoparticles as an Efficient Gene Delivery System to Cross Blood Brain Barrier: In Vitro and In Vivo Evaluations. Pharmaceuticals 2024, 17, 169. [Google Scholar] [CrossRef]
- Sahin, A.; Yoyen-Ermis, D.; Caban-Toktas, S.; Horzum, U.; Aktas, Y.; Couvreur, P.; Capan, Y. Evaluation of brain-targeted chitosan nanoparticles through blood–brain barrier cerebral microvessel endothelial cells. J. Microencapsul. 2017, 34, 659–666. [Google Scholar] [CrossRef]
- Pathak, R.; Bhatt, S.; Punetha, V.D.; Punetha, M. Chitosan nanoparticles and based composites as a biocompatible vehicle for drug delivery: A review. Int. J. Biol. Macromol. 2023, 253, 127369. [Google Scholar] [CrossRef]
- Khan, S.; Madni, A.; Shah, H.; Jan, N.; Shafiq, A.; Basit, A.; Rai, N.; Ali, A.; Khan, M.M. Folate decorated lipid chitosan hybrid nanoparticles of 5-fluorouracil for enhanced anticancer efficacy against colon cancer. Int. J. Biol. Macromol. 2022, 222 Pt A, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Chen, Y.; Gu, G.; Miao, Q.; Tan, W.; Li, Q.; Guo, Z. New synthetic adriamycin-incorporated chitosan nanoparticles with enhanced antioxidant, antitumor activities and pH-sensitive drug release. Carbohydr. Polym. 2021, 273, 118623. [Google Scholar] [CrossRef]
- Aziz, S.N.; Badawy, A.A.; Nessem, D.I.; El Malak, N.S.A. Promising nanoparticulate system for topical delivery of diphenhydramine hydrochloride: In-vitro and in-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 55, 101454. [Google Scholar] [CrossRef]
- Salama, A.H.; Salama, A.A.A.; Elhabak, M. Single step nanospray drying preparation technique of gabapentin-loaded nanoparticles-mediated brain delivery for effective treatment of PTZ-induced seizures. Int. J. Pharm. 2021, 602, 120604. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Wang, M.-H. Cerium oxide decorated 5-fluorouracil loaded chitosan nanoparticles for treatment of hepatocellular carcinoma. Int. J. Biol. Macromol. 2022, 216, 52–64. [Google Scholar] [CrossRef]
- Herdiana, Y.; Febrina, E.; Nurhasanah, S.; Gozali, D.; Elamin, K.M.; Wathoni, N. Drug Loading in Chitosan-Based Nanoparticles. Pharmaceutics 2024, 16, 1043. [Google Scholar] [CrossRef]
- Silant’ev, V.E.; Belousov, A.S.; Trukhin, F.O.; Struppul, N.E.; Shmelev, M.E.; Patlay, A.A.; Shatilov, R.A.; Kumeiko, V.V. Rational design of pectin–chitosan polyelectrolyte nanoparticles for enhanced temozolomide delivery in brain tumor therapy. Biomedicines 2024, 12, 1393. [Google Scholar] [CrossRef]
- López, E.R.; López, C.S.; Spagnuolo, C.C.; Berardino, B.G.; Alaimo, A.; Pérez, O.E. Chitosan-tricarbocyanine-based nanogels were able to cross the blood–brain barrier showing its potential as a targeted site delivery agent. Pharmaceutics 2024, 16, 964. [Google Scholar] [CrossRef]
- Junior, A.M.M.; Lima, A.M.F.; Martins, G.O.; Tiera, V.A.D.O.; Benderdour, M.; Fernandes, J.C.; Tiera, M.J. Impact of degree of ionization and PEGylation on the stability of nanoparticles of chitosan derivatives at physiological conditions. Mar. Drugs 2022, 20, 476. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Khalil, N. Synthesis and characterization of 1-phenylisatin incorporated chitosan/PEG nanoparticles: Interaction with bovine serum albumin and application in drug delivery. J. Drug Deliv. Sci. Technol. 2023, 86, 104738. [Google Scholar] [CrossRef]
- Herna, H.; Corte, H.; Romero-Montero, A.; Borbolla-Jime, F.V.; Magan, J.J.; Del Prado-Audelo, M.L.; Flora, B.; Go, G.L. Polymeric nanoparticles decorated with fragmented chitosan as modulation systems for neuronal drug uptake. Carbohydr. Polym. 2024, 336, 122121. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Granja, S.; Boni, F.I.; Prezotti, F.G.; Ferreira, L.M.B.; Cury, B.S.F.; Reis, R.M.; Baltazar, F.; Gremião, M.P.D. Modulating chitosan-PLGA nanoparticle properties to design a co-delivery platform for glioblastoma therapy intended for nose-to-brain route. Drug Deliv. Transl. Res. 2020, 10, 1729–1747. [Google Scholar] [CrossRef] [PubMed]
- Hard, S.A.A.A.H.; Shivakumar, H.N.; Redhwan, M.A.M. Development and optimization of in-situ gel containing chitosan nanoparticles for possible nose-to-brain delivery of vinpocetine. Int. J. Biol. Macromol. 2023, 253 Pt 6, 127217. [Google Scholar] [CrossRef]
- Anand, A.; Iyer, B.R.; Ponnusamy, C.; Pandiyan, R.; Sugumaran, A. Design and development of lomustine loaded chitosan nanoparticles for efficient brain targeting. Cardiovasc. Hematol. Agents Med. Chem. 2020, 18, 45–54. [Google Scholar] [CrossRef]
- Singh, S.K.; Hidau, M.K.; Gautam, S.; Gupta, K.; Singh, K.P.; Singh, S.K.; Singh, S. Glycol chitosan functionalized asenapine nanostructured lipid carriers for targeted brain delivery: Pharmacokinetic and teratogenic assessment. Int. J. Biol. Macromol. 2018, 108, 1092–1100. [Google Scholar] [CrossRef]
- Agrawal, P.; Sonali; Singh, R.P.; Sharma, G.; Mehata, A.K.; Singh, S.; Rajesh, C.V.; Pandey, B.L.; Koch, B.; Muthu, M.S. Bioadhesive micelles of d-α-tocopherol polyethylene glycol succinate 1000: Synergism of chitosan and transferrin in targeted drug delivery. Colloids Surf. B Biointerfaces 2017, 152, 277–288. [Google Scholar] [CrossRef]
- Van Woensel, M.; Wauthoz, N.; Rosie, R.; Mathieu, V.; Kiss, R.; Lefranc, F.; Steelant, B.; Dilissen, E.; Van Gool, S.W.; Mathivet, T.; et al. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release 2016, 227, 71–81. [Google Scholar] [CrossRef]
- Ahmad, N.; Al-Ghamdi, M.J.A.; Alnajjad, H.S.M.; Al Omar, B.B.A.; Khan, M.F.; Almalki, Z.S.; Albassam, A.A.; Ullah, Z.; Khalid, M.S.; Ashraf, K. A comparative brain Toxico-Pharmacokinetics study of a developed tannic acid nanoparticles in the treatment of epilepsy. J. Drug Deliv. Sci. Technol. 2022, 76, 103772. [Google Scholar] [CrossRef]
- Khodadadi, M.; Jahromi, G.P.; Meftahi, G.H.; Khodadadi, H.; Hadipour, M.; Ezami, M. Crocin nano-chitosan-coated compound mitigates hippocampal blood-brain barrier disruption, anxiety, and cognitive deficits in chronic immobilization stress-induced rats. Heliyon 2024, 10, e39203. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, T.; Ma, M.; Hu, Y.; Zhang, J. Preparation and evaluation of anti-neuroexcitation peptide (ANEP) loaded N-trimethyl chitosan chloride nanoparticles for brain-targeting. Int. J. Pharm. 2010, 386, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Al-Bayati, K.; Ho, E.A. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv. Transl. Res. 2017, 7, 497–506. [Google Scholar] [CrossRef]
- Kievit, F.M.; Wang, K.; Ozawa, T.; Tarudji, A.W.; Silber, J.R.; Holland, E.C.; Ellenbogen, R.G.; Zhang, M. Nanoparticle-mediated knockdown of DNA repair sensitizes cells to radiotherapy and extends survival in a genetic mouse model of glioblastoma. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Aktas, Y.; Yemisci, M.; Andrieux, K.; Gu, R.N.; Alonso, M.J.; Fernandez-Megia, E.; Carballal, R.N.; Quin, E.; Riguera, R.; Sargon, M.F.; et al. Development and brain delivery of chitosan PEG nanoparticles functionalized with the monoclonal antibody OX26. Bioconjugate Chem. 2005, 16, 1503–1511. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Ashafaq, M.; Samim, M.; Iqbal, Z.; Ahmad, F.J. Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of Cerebral Ischemia. Int. J. Biol. Macromol. 2016, 91, 640–655. [Google Scholar] [CrossRef]
- Thakur, A.; Taranjit, T. Preparation of Chitosan Nanoparticles: A Study of Influencing Factors. AIP Conf. Proc. 2011, 1393, 299–300. [Google Scholar] [CrossRef]
- Parchen, G.P.; Quaillet, M.; de Freitas, R.A.; Hillaireau, H. Chitosan-based nano-objects for drug delivery: A review of their chemical modifications, supramolecular organization and biological fate. RSC Pharm. 2025, 2, 1292–1322. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef]
- Lin, A.H.; Liu, Y.M.; Ping, Q.N. Free amino groups on the surface of chitosan nanoparticles and its characteristics. Yao Xue Xue Bao (Acta Pharm. Sin.) 2007, 42, 323–328. (In Chinese) [Google Scholar] [PubMed]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, H.; Kjøniksen, A.L.; Hiorth, M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lapitsky, Y. Monovalent salt enhances colloidal stability during the formation of chitosan/tripolyphosphate microgels. Langmuir 2011, 27, 10392–10399. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan-based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Ciro, Y.; Rojas, J.; Alhajj, M.J.; Carabali, G.A.; Salamanca, C.H. Production and Characterization of Chitosan–Polyanion Nanoparticles by Polyelectrolyte Complexation Assisted by High-Intensity Sonication for the Modified Release of Methotrexate. Pharmaceuticals 2020, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Lankalapalli, S.; Kolapalli, V.R.M. Polyelectrolyte Complexes: A Review of Their Applicability in Drug Delivery Technology. Indian J. Pharm. Sci. 2009, 71, 481–487. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Park, K.; Kang, E.; Lee, S.; Nam, H.Y.; Kim, K.; Park, J.H.; Chi, D.Y.; Park, R.W.; et al. Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials 2008, 29, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Han, H.; Jeon, S.; Yoon, H.Y.; Kim, H.; Kwon, I.C.; Kim, K. Deep tumor penetration of doxorubicin-loaded glycol chitosan nanoparticles using high-intensity focused ultrasound. Pharmaceutics 2020, 12, 974. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, Y.; Sun, W.; Cao, J.; Liang, Y.; Han, S.; Wang, X.; Sun, Y. Self-Assembled chitosan/phospholipid nanoparticles: From fundamentals to preparation for advanced drug delivery. Drug Deliv. 2020, 27, 200–215. [Google Scholar] [CrossRef]
- Adwan, S.; Obeidi, T.; Al-Akayleh, F. Chitosan nanoparticles embedded in in situ gel for nasal delivery of imipramine hydrochloride: Short-term stage development and controlled release evaluation. Polymers 2024, 16, 3062. [Google Scholar] [CrossRef]
- Barua, S.; Yoo, J.-W.; Kolhar, P.; Wakankar, A.; Gokarn, Y.R.; Mitragotri, S. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 3270–3275. [Google Scholar] [CrossRef]
- Nowak, M.; Brown, T.D.; Graham, A.; Helgeson, M.E.; Mitragotri, S. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioeng. Transl. Med. 2020, 5, e10153. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, J.; Park, H.; Lee, M. Chitosan-based nanoparticles as a sustained protein release carrier for tissue engineering applications. J. Biomed. Mater. Res. A 2012, 100, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, M.; Gao, W.; Sun, Y.; Dong, X. Coassembled Chitosan–Hyaluronic Acid Nanoparticles as a Theranostic Agent Targeting Alzheimer’s β-Amyloid. ACS Appl. Mater. Interfaces 2021, 13, 55879–55889. [Google Scholar] [CrossRef]
- Noah, N.M.; Ndangili, P.M. Polymeric nanosystems for neurological therapeutics. In Polymeric Nanosystems; Academic Press: Cambridge, MA, USA, 2023; pp. 723–759. [Google Scholar] [CrossRef]
- Al-Sarayra, L.M.S.; Hussein-Al-Ali, S.H.; Haddad, M.K.; Qader, A.A. Gold nanoparticles loaded with chitosan encapsulate donepezil as a novel nanocomposite for Alzheimer’s disease therapy. Mater. Res. 2024, 27, e20230365. [Google Scholar] [CrossRef]
- Fazil, M.; Md, S.; Haque, S.; Kumar, M.; Baboota, S. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef]
- Hanafy, A.; Farid, R.; Helmy, M.; Delivery, S.E.-D. Pharmacological, toxicological and neuronal localization assessment of galan-tamine/chitosan complex nanoparticles in rats: Future potential contribution in Alzheimer’s disease management. Drug Deliv. 2016, 23, 3111–3122. [Google Scholar] [CrossRef]
- Yasir, M.; Zafar, A.; Noorulla, K.; Tura, A.J. Nose to brain delivery of donepezil through surface modified NLCs: Formulation devel-opment, optimization, and brain targeting study. J. Drug Deliv. Sci. Technol. 2022, 75, 103631. [Google Scholar] [CrossRef]
- Shafqat, O.; Rehman, Z.; Shah, M.M.; Ali, S.H.B.; Jabeen, Z.; Rehman, S. Synthesis, structural characterization and in vitro pharmacological properties of betanin-encapsulated chitosan nanoparticles. Chem.-Biol. Interact. 2023, 370, 110291. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Zhang, L.; Hu, C.; Zhou, L.; Cheng, Y.; Liu, Q. Ellagic acid (EA) ameliorates Alzheimer’s disease by reducing A levels, oxidative stress and attenuating inflammation. Eur. J. Pharmacol. 2025, 986, 177099. [Google Scholar] [CrossRef]
- Poumeaud, F.; Mircher, C.; Smith, P.J.; Faye, P.-A.; Sturtz, F.G. Deciphering the links between psychological stress, depression, and neurocognitive decline in patients with Down syndrome. Neurobiol. Stress 2021, 14, 100305. [Google Scholar] [CrossRef]
- Sonne, J.; Reddy, V.; Beato, M.R. Neuroanatomy, substantianigra. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536995/ (accessed on 25 October 2025).
- Zhang, R.; Chen, X.; Cheng, Y.; Chen, Z.; Li, X.; Deng, Y. Recent advances of nanomaterials for intervention in Parkinson’s disease in the context of anti-inflammation. Coord. Chem. Rev. 2024, 502, 215616. [Google Scholar] [CrossRef]
- Martínez, E.O.; Herna, M.E.M.; Castillo-Gonza, J.; Gonza, E.; Mart, M.A.R. Dopamine-loaded chitosan-coated solid lipid nanoparticles as a promise nanocarriers to the CNS. Neuropharmacology 2024, 249, 109871. [Google Scholar] [CrossRef]
- Saha, P.; Singh, P.; Kathuria, H.; Chitkara, D.; Pandey, M.M. Self-assembled lecithin-chitosan nanoparticles improved rotigotine nose-to-brain delivery and brain targeting efficiency. Pharmaceutics 2023, 15, 851. [Google Scholar] [CrossRef]
- Sardoiwala, M.N.; Karmakar, S.; Choudhury, S.R. Chitosan nanocarrier for FTY720 enhanced delivery retards Parkinson’s disease via PP2A-EzH2 signaling in vitro and ex vivo. Carbohydr. Polym. 2021, 254, 117435. [Google Scholar] [CrossRef]
- Fihurka, O.; Sava, V.; Sanchez-Ramos, J. Dual-function hybrid nanoparticles with gene silencing and anti-inflammatory effects. Nanomedicine 2022, 17, 577–590. [Google Scholar] [CrossRef]
- Wahyuningtyas, D.; Chen, W.H.; Huang, C.H.; He, Y.J.; Huang, J.J.T. Biocompatible inhibitor based on chitosan and amphiphilic peptide against mutant Huntingtin toxicity. ChemBioChem 2019, 20, 2133–2140. [Google Scholar] [CrossRef]
- Kesharwani, P.; Halwai, K.; Jha, S.K.; Al Mughram, M.H.; Almujri, S.S.; Almalki, W.H.; Sahebkar, A. Folate-engineered chitosan nanoparticles: Next-generation anticancer nanocarriers. Mol. Cancer 2024, 23, 244. [Google Scholar] [CrossRef]
- Rahman, M.H.; Mondal, M.I.H. Stability, challenges, and prospects of chitosan for the delivery of anticancer drugs and tissue regenerative growth factors. Heliyon 2024, 10, e39879. Available online: https://www.cell.com/heliyon/fulltext/S2405-8440(24)15910-5 (accessed on 25 October 2025). [CrossRef] [PubMed]
- Ruman, U.; Buskaran, K.; Pastorin, G.; Masarudin, M.J.; Fakurazi, S.; Hussein, M.Z. Synthesis and characterization of chitosan-based nanodelivery systems to enhance the anticancer effect of sorafenib drug in hepatocellular carcinoma and colorectal adenocarcinoma cells. Nanomaterials 2021, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Gabold, B.; Adams, F.; Brameyer, S.; Jung, K.; Ried, C.L.; Merdan, T.; Merkel, O.M. Transferrin-modified chitosan nanoparticles for targeted nose-to-brain delivery of proteins. Drug Deliv. Transl. Res. 2023, 13, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Lara-Velazquez, M.; Alkharboosh, R.; Norton, E.S.; Ramirez-Loera, C.; Freeman, W.D.; Guerrero-Cazares, H.; Estrada, R.S. Chitosan-based non-viral gene and drug delivery systems for brain cancer. Front. Neurol. 2020, 11, 740. [Google Scholar] [CrossRef]
- Yousaf, F.; Iqbal, S.; Fatima, N.; Kousar, T.; Rahim, M.S.M. Multi-class disease detection using deep learning and human brain medical imaging. Biomed. Signal Process. Control 2023, 85, 104875. [Google Scholar] [CrossRef]
- Abbasi, H.; Orouskhani, M.; Asgari, S.; Zadeh, S.S. Automatic brain ischemic stroke segmentation with deep learning: A review. Neurosci. Inform. 2023, 3, 100145. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Zhu, Z.; Sun, Y. Improved neuroprotective effects of gallic acid-loaded chitosan nanoparticles against ischemic stroke. Rejuvenation Res. 2019, 2019, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Nagareddy, R.; Kim, J.-H.; Kim, J.-H.; Thomas, R.G.; Choi, K.-H.; Jeong, Y.-Y. Reactive oxygen species-responsive chitosan–bilirubin nanoparticles loaded with statin for treatment of cerebral ischemia. Biomater. Res. 2024, 28, 0097. [Google Scholar] [CrossRef] [PubMed]
- Frigaard, J.; Jensen, J.L.; Galtung, H.K.; Hiorth, M. The potential of chitosan in nanomedicine: An overview of the cytotoxicity of chitosan-based nanoparticles. Front. Pharmacol. 2022, 13, 880377. [Google Scholar] [CrossRef]
- Torres-Re, M.; Gláucia-Silva, F.; Soares, K.S.R.; de Souza, L.B.F.C.; Damasceno, I.Z.; dos Santos-Silva, E.; Lacerda, A.F.; Chaves, G.M.; da Silva-Júnior, A.A.; Fernandes-Pedrosa, M.d.F. Biodegradable cross-linked chitosan nanoparticles improve anti-Candida and anti-biofilm activity of TistH, a peptide identified in the venom gland of the Tityus stigmurus scorpion. Mater. Sci. Eng. C 2019, 103, 109830. [Google Scholar] [CrossRef]
- Zoe, L.H.; David, S.R.; Rajabalaya, R. Chitosan nanoparticle toxicity: A comprehensive literature review of in vivo and in vitro assessments for medical applications. Toxicol. Rep. 2023, 11, 83–106. [Google Scholar] [CrossRef]
- Ding, P.; Liu, H.; Zhu, X.; Chen, Y.; Zhou, J.; Chai, S.; Wang, A.; Zhang, G. Thiolated chitosan encapsulation constituted mucoadhesive nanovaccine confers broad protection against divergent influenza A viruses. Carbohydr. Polym. 2024, 328, 121689. [Google Scholar] [CrossRef]
- Wani, T.U.; Pandith, A.H.; Sheikh, F.A. Polyelectrolytic nature of chitosan: Influence on physicochemical properties and synthesis of nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 65, 102730. [Google Scholar] [CrossRef]
- Moradikhah, F.; Doosti-Telgerd, M.; Shabani, I.; Soheili, S.; Dolatyar, B.; Seyedjafari, E. Microfluidic fabrication of alendronate-loaded chitosan nanoparticles for enhanced osteogenic differentiation of stem cells. Life Sci. 2020, 254, 117768. [Google Scholar] [CrossRef]
- Çakır-Koç, R.; Çalık, H.; Mutlu, B.; Ay, H.F.; Karavelioğlu, Z.; Aslan-Polat, B.; Pençeci, B. Nanotoxicity in neural regenerative medicine. In Neural Regenerative Nanomedicine; Academic Press: Cambridge, MA, USA, 2020; pp. 259–283. [Google Scholar] [CrossRef]
- Khan, Z.; Maqsood, Q.; Baradoke, A.; Ferreira, L.F.R.; Franco, M.; Schmidt, J.E.; Hussain, N. Environmental and toxicological implications of chitosan nanostructures. In Advances in Chemical Pollution, Environmental Management and Protection; Elsevier: Amsterdam, The Netherlands, 2024; Volume 10, pp. 137–172. [Google Scholar] [CrossRef]
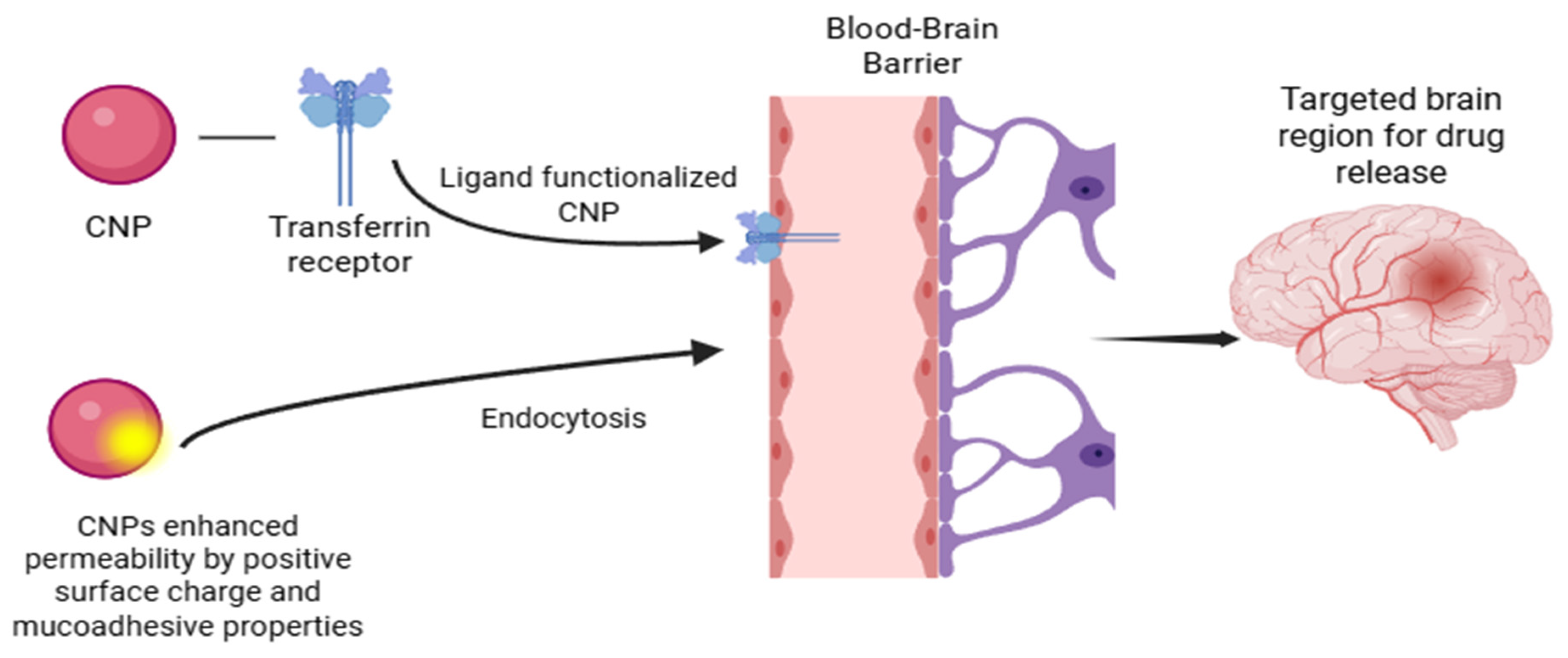
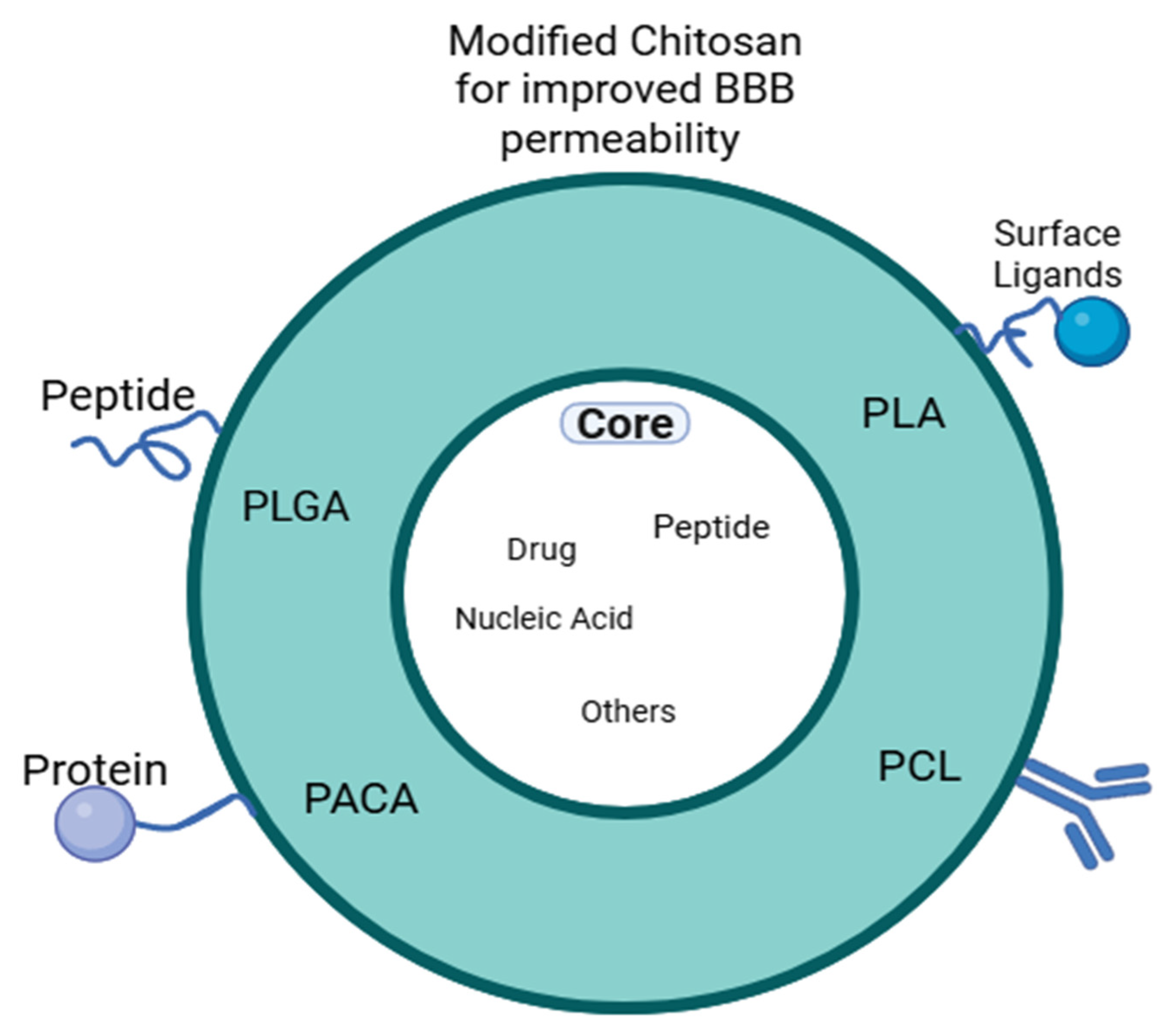

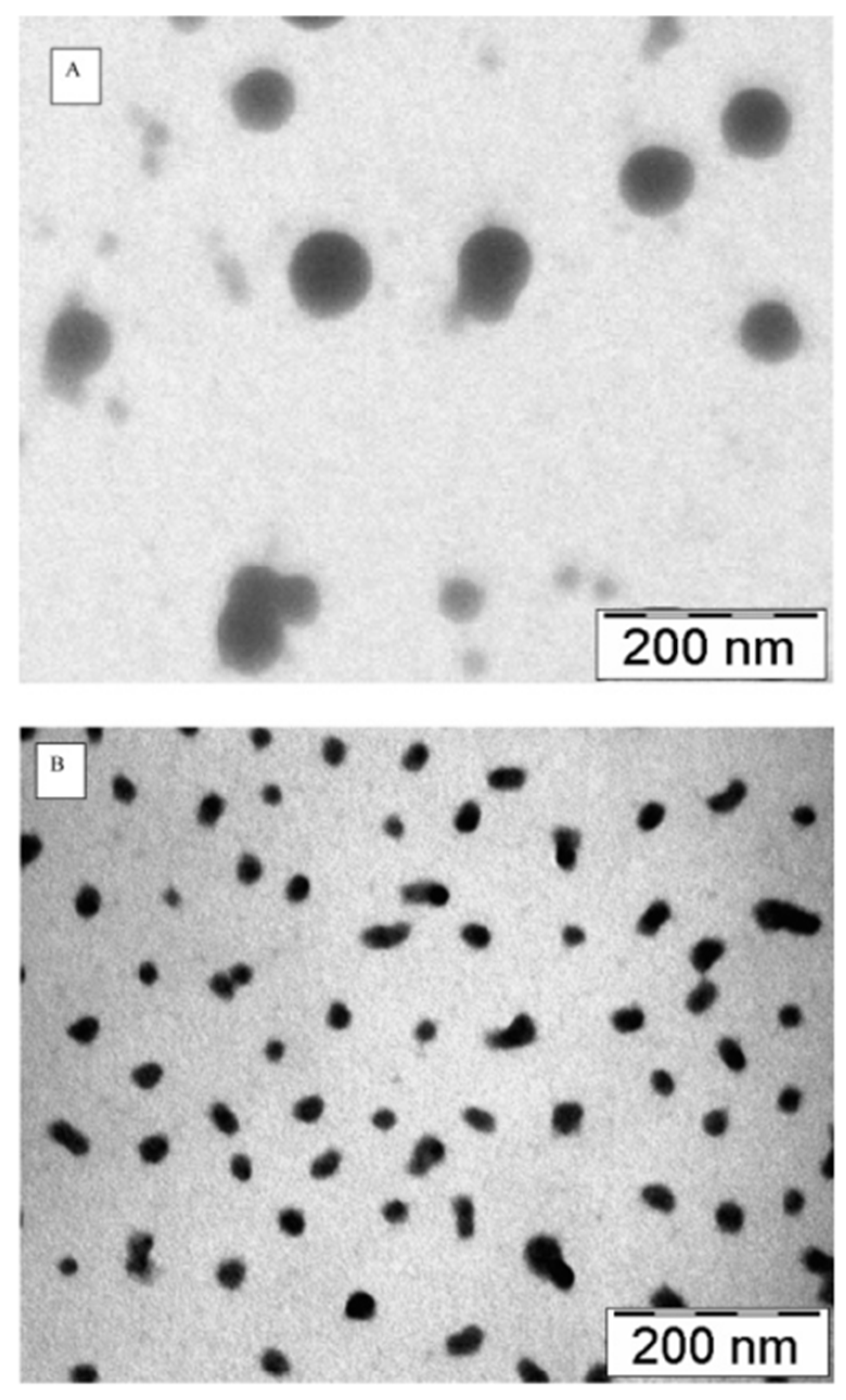
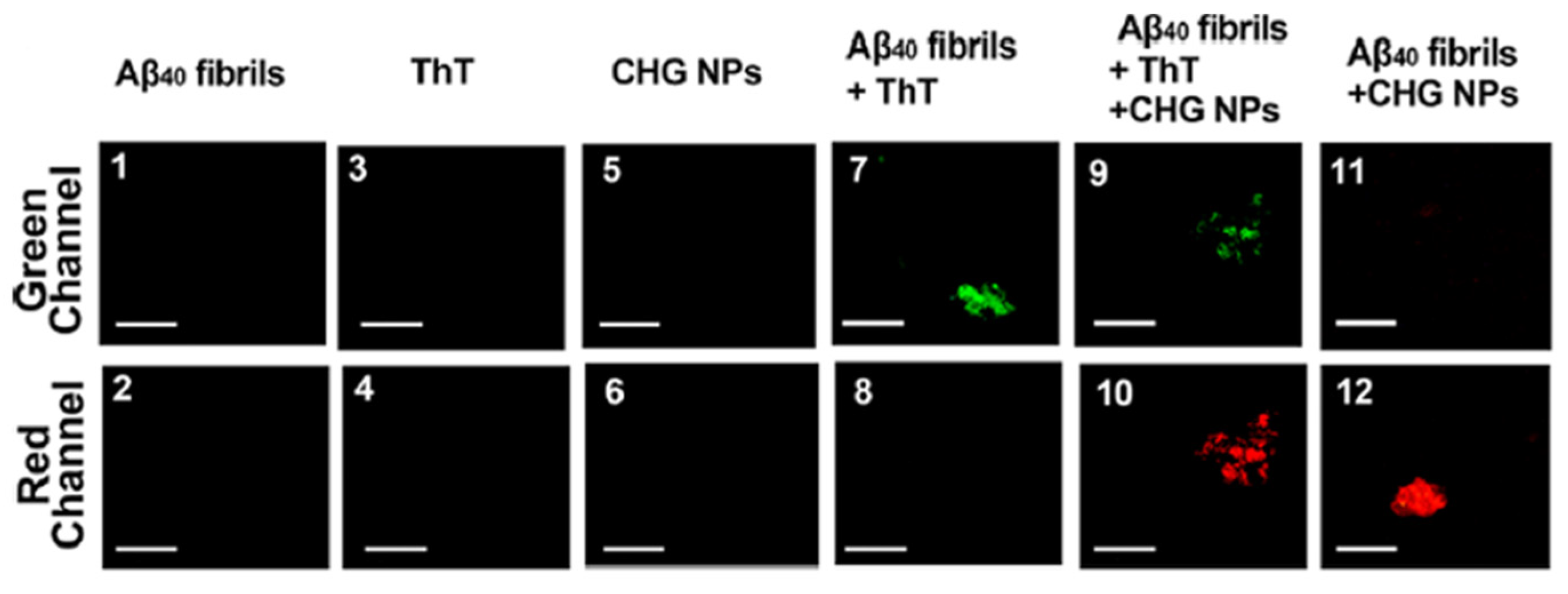
| Route | Description/Mechanism | Advantages | Limitations | Ref |
|---|---|---|---|---|
| Oral | The drug is administered through the gastrointestinal tract and absorbed into the systemic circulation before reaching the brain via the bloodstream. | Convenient and non-invasive for patients. Suitable for chronic administration. | Undergoes first-pass metabolism, which reduces the amount of drug reaching the brain. Low brain targeting efficiency | [11] |
| Intranasal | The drug is administered through the nasal cavity and absorbed into the brain through the olfactory pathway. | Avoids first-pass metabolism. Rapid drug onset and direct brain targeting. Chitosan provides mucoadhesion, prolonging. residence time and improving absorption. | Limited drug volume per dose. Mucociliary clearance can lower the drug retention. Larger particle sizes (>300 nm) can hinder mucosal transport and uptake. | [13,14] |
| Intravenous | Direct injection of the drug into the systemic circulation for rapid distribution to body tissues. | Rapid systemic distribution provides controlled dosing. | Possible systemic side effects. Requires clinical administration. | [12] |
| Nanoparticle Composition/Encapsulated Drug | Size (nm) | Zeta Potential (mV) | Entrapment Efficiency (%) | Drug Delivery Route | Key Findings | Ref. |
|---|---|---|---|---|---|---|
| PLGA with fragmented chitosan (CS) coating | 211.9 ± 14.04 | +7.1 ± 2.3 38 | 34.37 | - | Enhanced drug delivery across the BBB, 17.18% drug loading; coated PLGA NPs for neuronal cells | [41] |
| PLGA and oligomeric chitosan (OCS) with CTX conjugation | 213–875 (Optimal 258) | +37 (optimal) | 75.69 to 93.23 (Optimal 88%) | Nasal | Nasal co-delivery of CHC and CTX to the brain; high positive charge and stability optimized by emulsification | [42] |
| Chitosan nanoparticles for VIN delivery | 130.6 ± 8.38 | +40.81 ± 0.11 | - | Intranasal | High brain delivery efficacy with intranasal administration; enhanced stability due to high zeta potential | [43] |
| Chitosan nanoparticles for lomustine | 190 to 255 | - | 77.12 to 88.74 | - | Diffusion-controlled release over 8 h; optimized by Box- Behnken design | [44] |
| Glycol chitosan-coated lipid carrier (GC-ANLC) | 184.2 ± 5.59 | +18.83 ± 1.18 | 83.52 ± 2.59 | Intranasal | 2.3- to 4-fold higher brain bioavailability in rats; high biocompatibility with nasal epithelial cells | [45] |
| TPGS-conjugated chitosan (TPGS-CS) micelles, TfR-targeted, loaded with docetaxel (DTX) | 16.12 ± 2.2 | 1.11 ± 0.57 | 98.9 | Intravenous (iv) | 2.9- to 4.1-fold higher bioavailability in vivo; 97- to 248-fold increase in vitro cytotoxicity against glioma cells; effective targeting of TfR—overexpressed glioma | [46] |
| Chitosan nanoparticles encapsulating anti-Gal-1 siRNA | 141 ± 5 | +32 | 81 ± 3 | Intranasal | Protected siRNA from degradation; enhanced nasal retention and CNS penetration; downregulated Gal-1 expression; inhibited GBM tumor progression | [47] |
| Tannic acid-loaded PLGA nanoparticles coated with chitosan (2% and 4%) | Uncoated: 105.7 ± 11.02; Coated: 117.2 ± 3.09 | Uncoated: −22.3 ± 2.3; Coated: +21.6 ± 1.09 | Uncoated: 69.31 ± 5.89; Coated: 73.94 ± 4.28 up to 74.64 ± 4.91 | Intranasal | Higher brain bioavailability and therapeutic efficacy in epilepsy models; enhanced mucoadhesion and brain targeting; safe based on toxicological evaluation | [48] |
| Crocin nano-chitosan-coated compound (CNCC) | 175 ± 5 | - | 85 | - | Improved memory, learning, and anxiety indicators; upregulated NMDA receptor subunits and BBB tight junction proteins; more effective than intact crocin or chitosan | [49] |
| N-trimethyl chitosan chloride (TMC) nanoparticles loaded with anti-neuroexcitation peptide (ANEP) | 255 | +32 | 80.63 | Intravenous | Enhanced brain distribution by absorption-mediated transcytosis; effectively delivered ANEP to the brain | [50] |
| Dual antibody-modified chitosan nanoparticles (anti-Tf and anti-B2) loaded with siRNA | 235.7 ± 10.2 | +22.88 ± 1.78 | 61.9 | - | Enhanced cellular uptake and gene silencing efficiency in astrocytes; significantly improved knockdown of HIV replication compared to non-modified and single-antibody-modified nanoparticles | [51] |
| Chitosan-PEG-PEI copolymer nanoparticles functionalized with chlorotoxin and loaded with anti-Ape1 siRNA | 48.5 ± 4.0 | +13 ± 3.4 | - | Intravenously through the tail vein in the mouse model | Reduced Ape1 expression and increased GBM radiosensitivity; 40% Ape1 activity reduction in tumor tissue; doubled survival extension in GBM mouse models when combined with radiotherapy | [52] |
| Chitosan nanospheres conjugated with PEG and anti-caspase peptide Z-DEVD- FMK, modified with OX26 monoclonal antibody using SA-biotin technique | 149.73 ± 1.85 | +16.06 ± 3.43 | 31.13 ± 1.61 | Intravenous | Localized in brain tissue and outside intravascular compartment, successfully delivered Z- DEVD- FMK to brain tissue as confirmed by electron microscopy | [53] |
| Rutin-encapsulated-chitosan nanoparticles (RUT-CS-NPs) prepared by ionic gelation | 92.28 ± 2.96 | +31.04 ± 1.91 | 84.98 ± 4.18 | Intranasal | For particle size less than 100 nm, enhanced nasal permeability (>80% in 24 h), 3-fold higher brain uptake, and increased bioavailability compared to free rutin; reduced cerebral infarction volume in cerebral ischemia models | [54] |
| Method | Mechanism | Size/Zeta | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| Ionic Gelation | Mix Chitosan with multivalent anion (e.g., TPP), forming ionic crosslinks. | Size: 40–300 nm (e.g., 40 nm to 250 nm). Zeta: 20 to 50 mV typically (highly positive). | Very simple and mild (aqueous, no covalent crosslinkers); good encapsulation efficiency for small/ionic drugs; easily scaled. | Salt-sensitive (may swell/dissociate in physiological buffer); less stable if diluted; hard to load very large or hydrophobic drugs; sometimes broad size distribution. | [12,56,57,58,59,60,61] |
| Complex Coacervation (Polyelectrolyte Complex) | Mix CS (poly-cation) with an anionic polymer (e.g., alginate, HA, DNA) to form a polyion complex. | Size: 100–300 nm (e.g., 260 nm reported). Zeta: low positive or near neutral; can be adjusted by charge ratio. | No chemical crosslinkers; can encapsulate charged biomolecules (DNA, peptides) under mild conditions; uses biocompatible polyanions. | Highly sensitive to charge ratio—1:1 mixing can give neutral aggregates; pH/ionic strength must be controlled; larger particles; limited loading for neutral/hydrophobic drugs. | [28,62,63,64] |
| Self-Assembly (Polymer–Drug) | Use amphiphilic CS derivatives or polymer–drug conjugates that spontaneously assemble (e.g., hydrophobic grafts such as cholanic acid). | Size: 150–300 nm (e.g., 230 nm or 284 nm). Zeta: high positive (e.g., +30 to +50 mV) due to CS shell. | Encapsulates hydrophobic drugs well (high loading efficiency); no extraneous crosslinker needed; mild processing. | Requires polymer modification (synthesis time); assembly is sensitive to the degree of substitution; controlling size/composition can be tricky. | [65,66,67] |
| Alzheimer’s Disease | ||||
|---|---|---|---|---|
| CNP Composition | Drug | Aim/Target | Findings | Ref. |
| Chitosan-coated PLGA nanoparticles conjugated with a novel anti-Amyloid antibody | Anti-amyloid-beta antibody | Amyloid-beta protein | Enhanced uptake at the BBB and better targeting of the Amyloid Beta proteins in vitro | [73] |
| Chitosan nanoparticles cross-linked with glutaraldehyde | Hyaluronic acid | Amyloid-beta (Aβ) protein | Was able to detect and inhibit amyloid-beta fibrillization in vitro and in vivo | [72] |
| Chitosan nanoparticles coated with gold | Donepezil | To inhibit acetylcholinesterase to slow AD progression | Shown potential for AD treatment as it showed a desired controlled release of the drug | [74] |
| chitosan nanoparticles (CS- RHT NPs) | Rivastigmine | To improve bioavailability and brain uptake of rivastigmine for Alzheimer’s disease treatment by intranasal delivery. | High encapsulation efficiency (85.3%) and sustained release over 24 h; Improved nasal mucosa permeability and brain targeting efficiency (355%); Direct nose-to-brain transport (71.8%) with enhanced brain deposition. | [75] |
| Galantamine hydrobromide—chitosan complex nanoparticles (CX-NP2) | Galantamine hydrobromide (GH) | To investigate if GH/chitosan complexation improves therapeutic potential for Alzheimer’s disease (AD) without altering pharmacological or toxicological profiles. | CX-NP2 significantly decreased brain acetylcholinesterase (AChE) protein level and activity compared to oral and nasal GH solutions; No toxicity or histopathological abnormalities were observed; Nanoparticles localized intracellularly within brain neurons, confirming their potential for intranasal AD management. | [76] |
| Donepezil (DPZ)-loaded nanostructured lipid carriers (NLCs) coated with chitosan (CH) | Donepezil (DPZ) | To enhance brain delivery of donepezil through the intranasal route using CH-coated NLCs. | Optimized formulation had 192.5 nm particle size, 89.85% entrapment efficiency, and 0.298 PDI; Bioavailability was 2.02-fold higher intranasally and 2.41-fold higher than intravenous delivery; showed 321.21% drug targeting efficiency and 74.55% nose-to-brain transport. | [77] |
| Parkinson’s Disease | ||||
| CNP composition | Drug | Aim/Target | Findings | Ref. |
| Chitosan nanoparticles | FTY720 (PP2A activator) | Phosphorylated-alpha-synuclein (pSer129) | Reduced levels of pSer129 alpha-synuclein, indicating neuroprotection against Parkinson’s Disease | [85] |
| Chitosan-coated solid lipid | Dopamine | To mitigate motor symptoms of Parkinson’s Disease | Enhancing dopamine bioavailability in the brain and reduced motor symptoms | [83] |
| Lecithin-chitosan nanoparticle | Dopamine-agonist rotigotine | To treat PD and restless leg syndrome | It demonstrated improved brain drug delivery (through the nasal route) and targeting efficiency | [84] |
| Huntington’s Disease | ||||
| CNP composition | Drug | Aim/Target | Findings | Ref. |
| Chitosan/amphiphilic peptides complex | Amphiphilic peptides | Mutant huntingtin protein (mHTT) | Nanocomposite was able to penetrate the cells, inhibit mHTT aggregation, and reduce their toxicity | [87] |
| Hybrid-chitosan-based nanocarriers | Small interfering RNA (siRNA) | To reduce the mHTT levels and inflammation in the stem cells of the mouse | It showed an effective reduction in mHTT and inflammation | [86] |
| Brain Tumor | ||||
| CNP composition | Drug | Aim/Target | Findings | Ref. |
| Folate-coated chitosan nanoparticles | Sorafenib | Human hepatocellular carcinoma and colorectal adenocarcinoma cells | Enhanced drug delivery to cancer cells, improving targeting efficiency against liver and colorectal cancers. | [90] |
| Transferrin-coated chitosan nanoparticles | Protein (Not specified) | Human glioblastoma cells in vitro | Enhanced targeting of cancer cells and increased cellular uptake | [91] |
| Ischemic Stroke | ||||
| Drug | Aim/Target | Findings | Ref. | |
| Bilirubin-coated chitosan nanoparticles | Atorvastatin | Ischemic stroke regions (anti-inflammatory and antioxidant targeting) | Reduced pro-inflammatory cytokines (TNF-α, IL-1β) and increased antioxidant enzyme activity, lowering oxidative stress | [96] |
| O-carboxymethyl-coated chitosan nanoparticles | Gallic acid | Ischemic regions | Significantly reduce the levels of pro-inflammatory cytokines and enhanced activity of antioxidant enzymes | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezzaki, C.; Chaari, A.; Al-Othman, A. Recent Advances on Chitosan-Based Nanoparticles for Brain Drug Delivery. Polymers 2025, 17, 3055. https://doi.org/10.3390/polym17223055
Ezzaki C, Chaari A, Al-Othman A. Recent Advances on Chitosan-Based Nanoparticles for Brain Drug Delivery. Polymers. 2025; 17(22):3055. https://doi.org/10.3390/polym17223055
Chicago/Turabian StyleEzzaki, Chihab, Anas Chaari, and Amani Al-Othman. 2025. "Recent Advances on Chitosan-Based Nanoparticles for Brain Drug Delivery" Polymers 17, no. 22: 3055. https://doi.org/10.3390/polym17223055
APA StyleEzzaki, C., Chaari, A., & Al-Othman, A. (2025). Recent Advances on Chitosan-Based Nanoparticles for Brain Drug Delivery. Polymers, 17(22), 3055. https://doi.org/10.3390/polym17223055








