Structural, Sorption, and Regeneration Properties of Poly(methacrylic acid): Poly(4-vinylpyridine) Interpolymer Systems for the Recovery of Rhenium and Molybdenum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Materials and Preparation
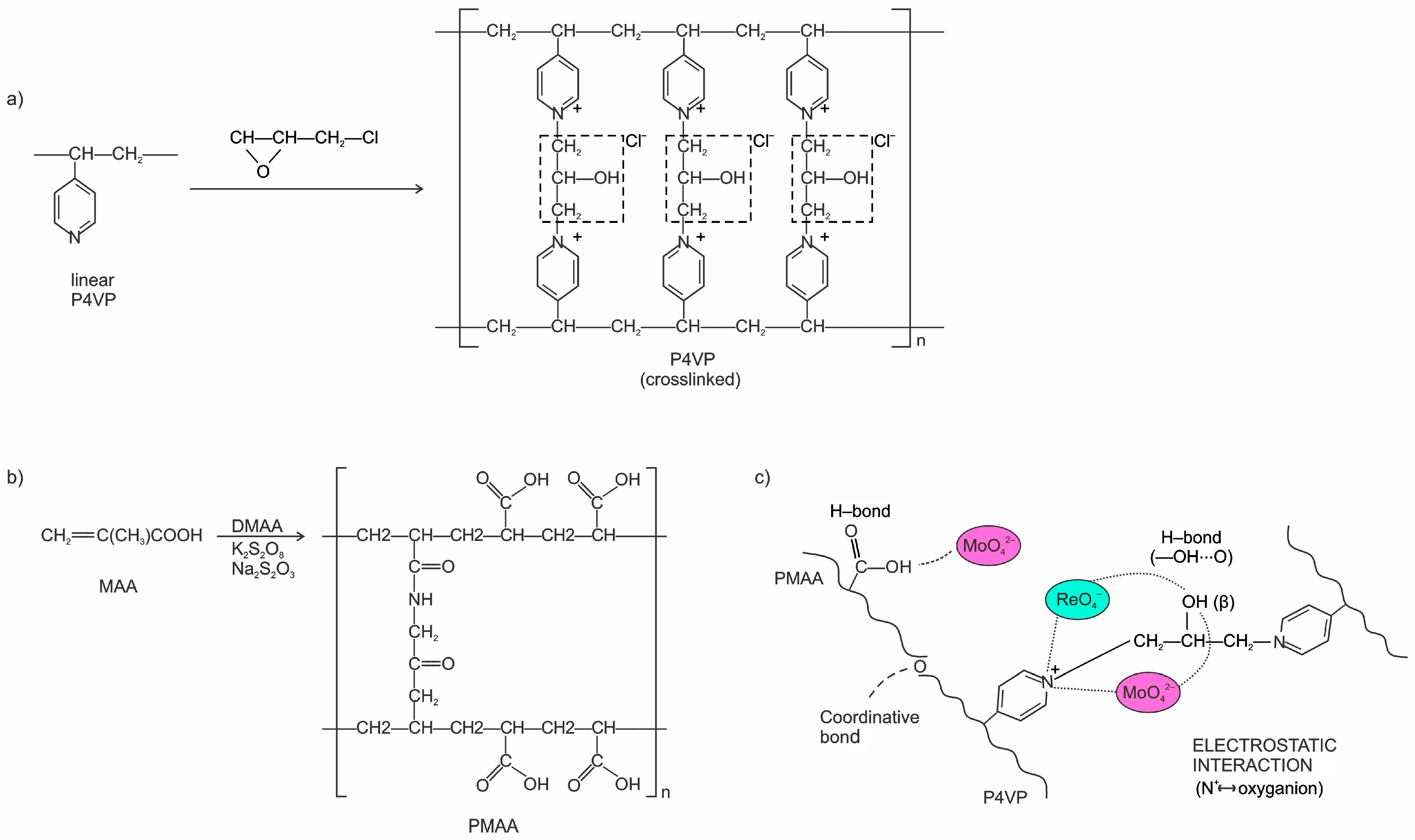
2.1.2. Synthesis of Polymers and Preparation of Interpolymer Systems
2.2. Methods and Equipment
2.2.1. Equipment and Analytical Methods
ICP-AES Analysis
FTIR Spectroscopy
Thermal Analysis (TGA/DSC)
pH Control
2.2.2. Sorption Experiments
2.2.3. Methodology of Desorption Experiments
2.2.4. Binding Degree and Exchange Capacity
2.2.5. Swelling Properties
2.2.6. Influence of pH on the Sorption Efficiency
2.2.7. Kinetics and Isotherm Modeling
Pseudo-First-Order (PFO) Model
Pseudo-Second-Order (PSO) Model
Intraparticle Diffusion (Weber–Morris) Model
Model Selection and Interpretation
Kinetic and Diffusion Modeling with Python and Google Colab
Sorption Isotherms
2.2.8. Mathematical Modeling and Validation of Adsorption Isotherms
Nonlinear Regression and Model Selection
Statistical Evaluation of Fit Quality
Residual and Confidence Analysis
Model Comparison and Validation
2.2.9. Determination of Distribution and Selectivity Coefficients
2.2.10. Statistical Analysis
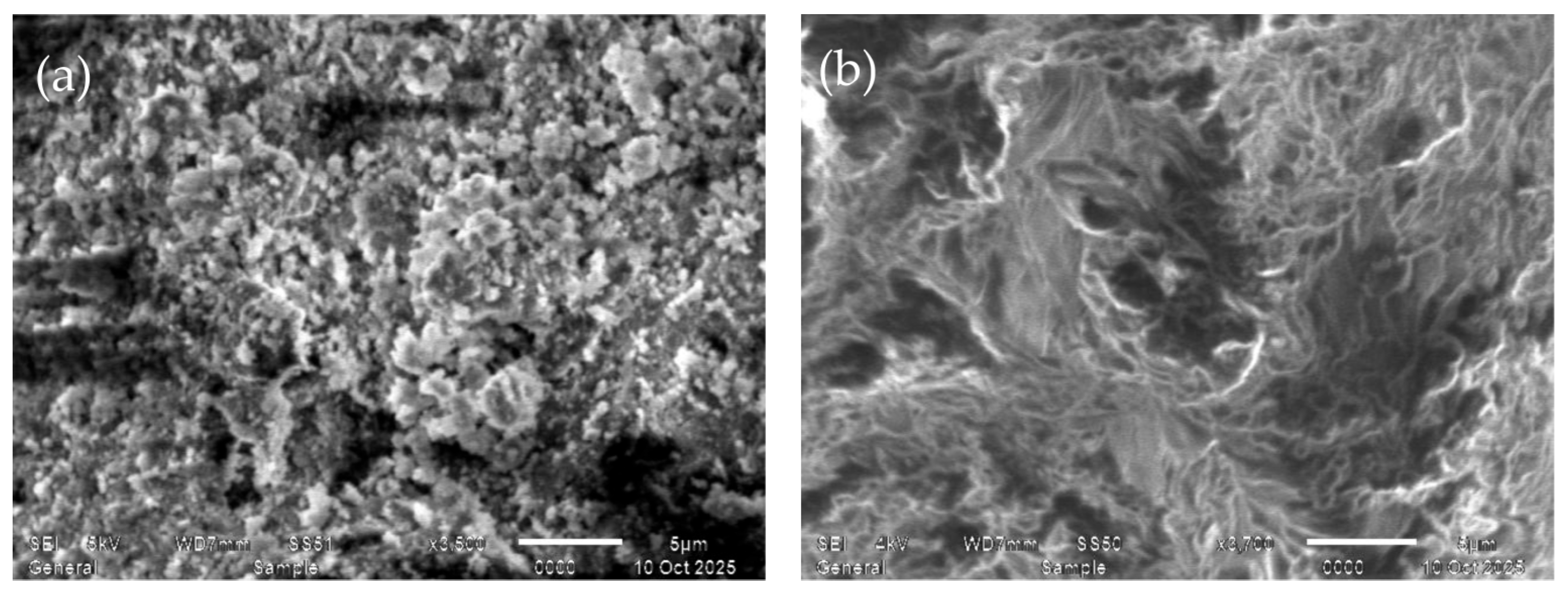
2.2.11. Morphology
3. Results and Discussion
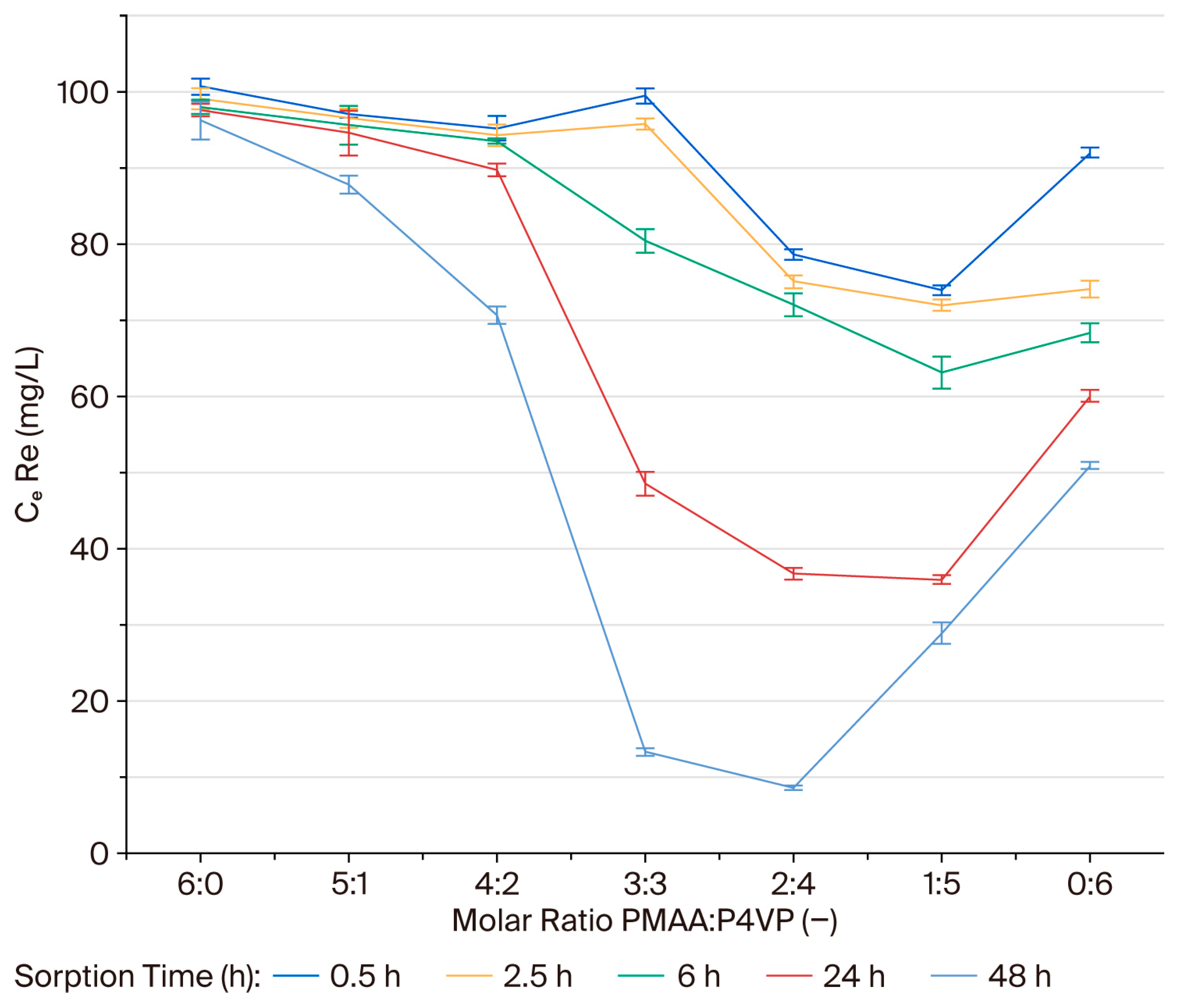
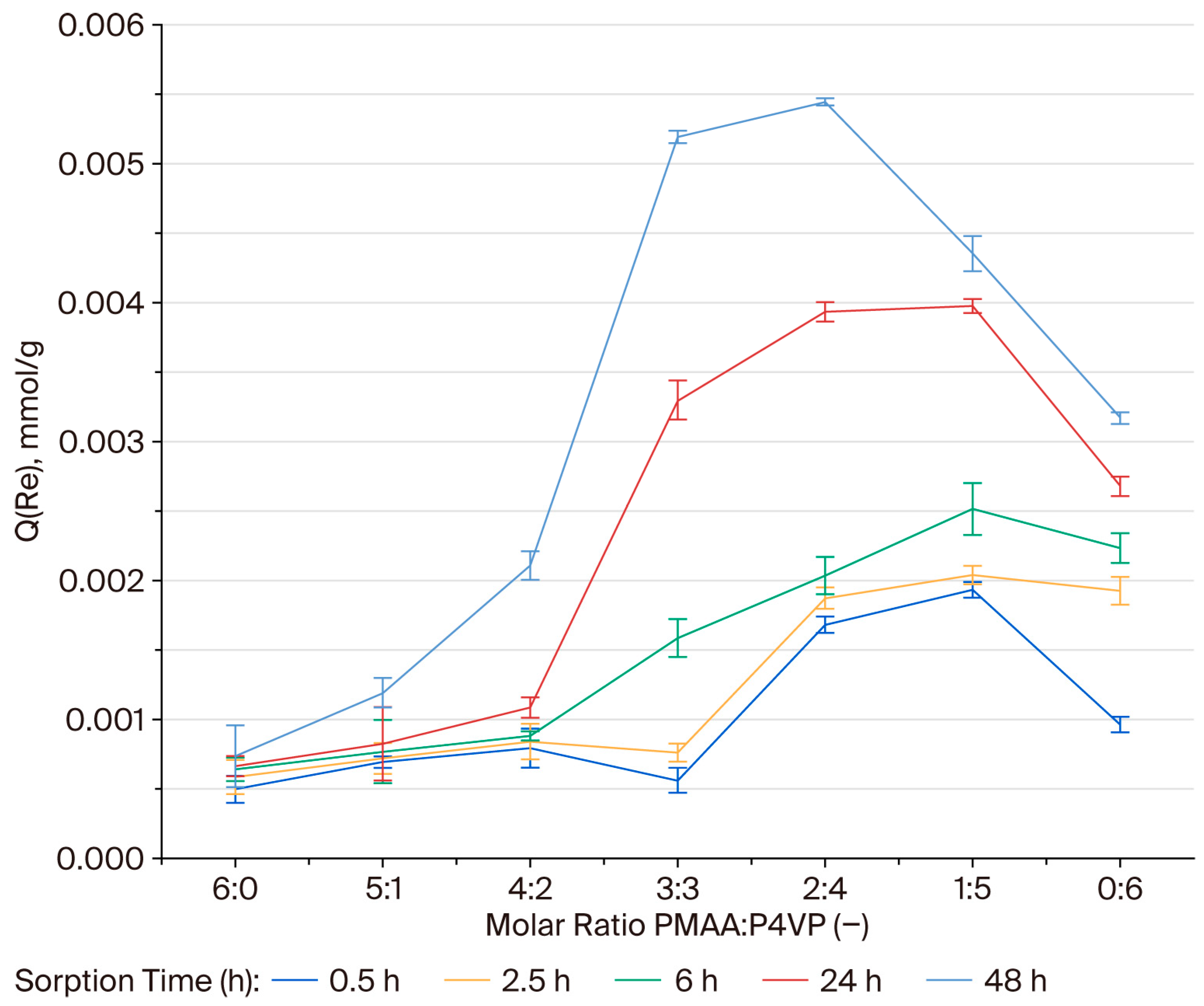
3.1. Effect of Interpolymer Composition on Rhenium Sorption
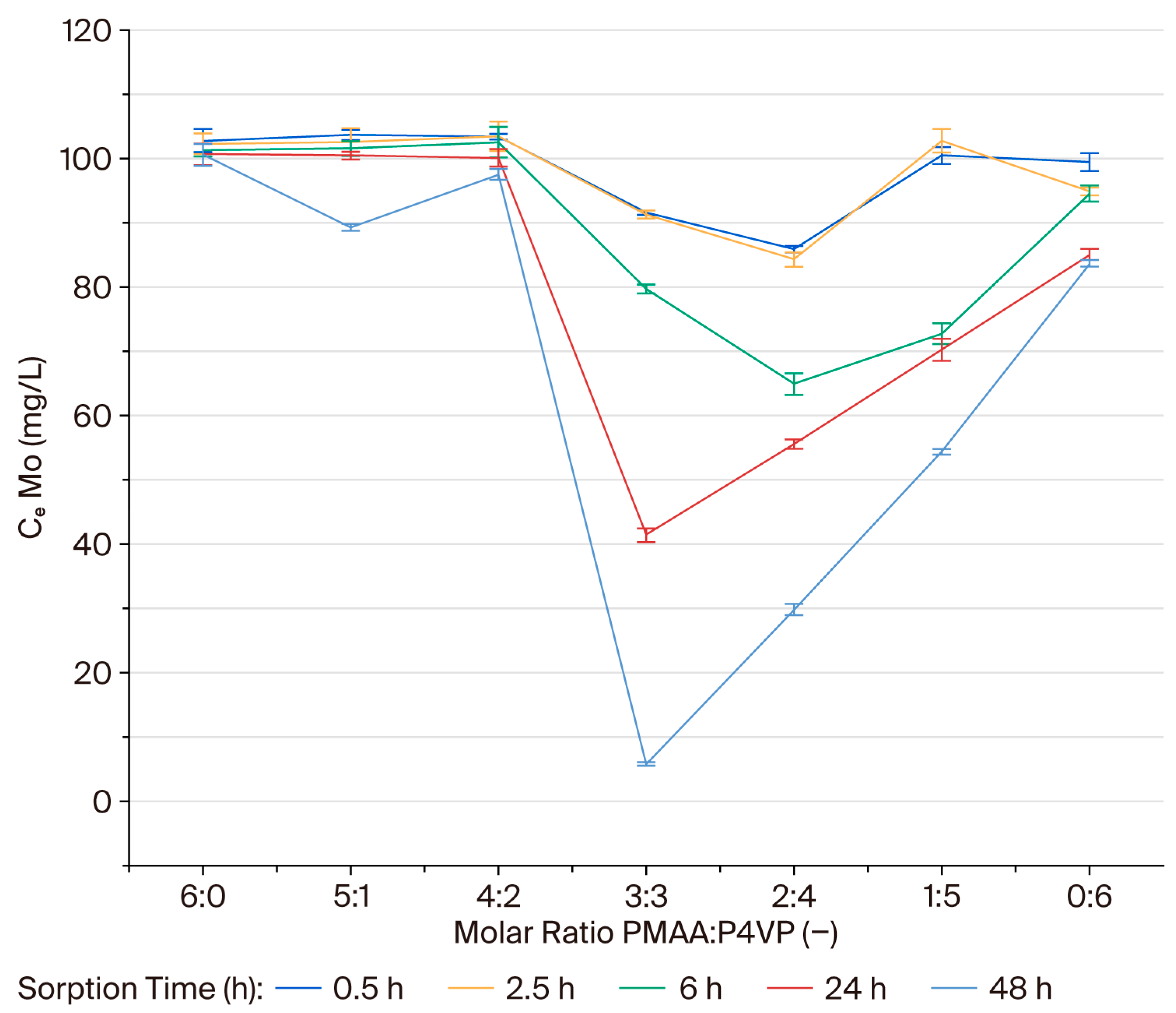
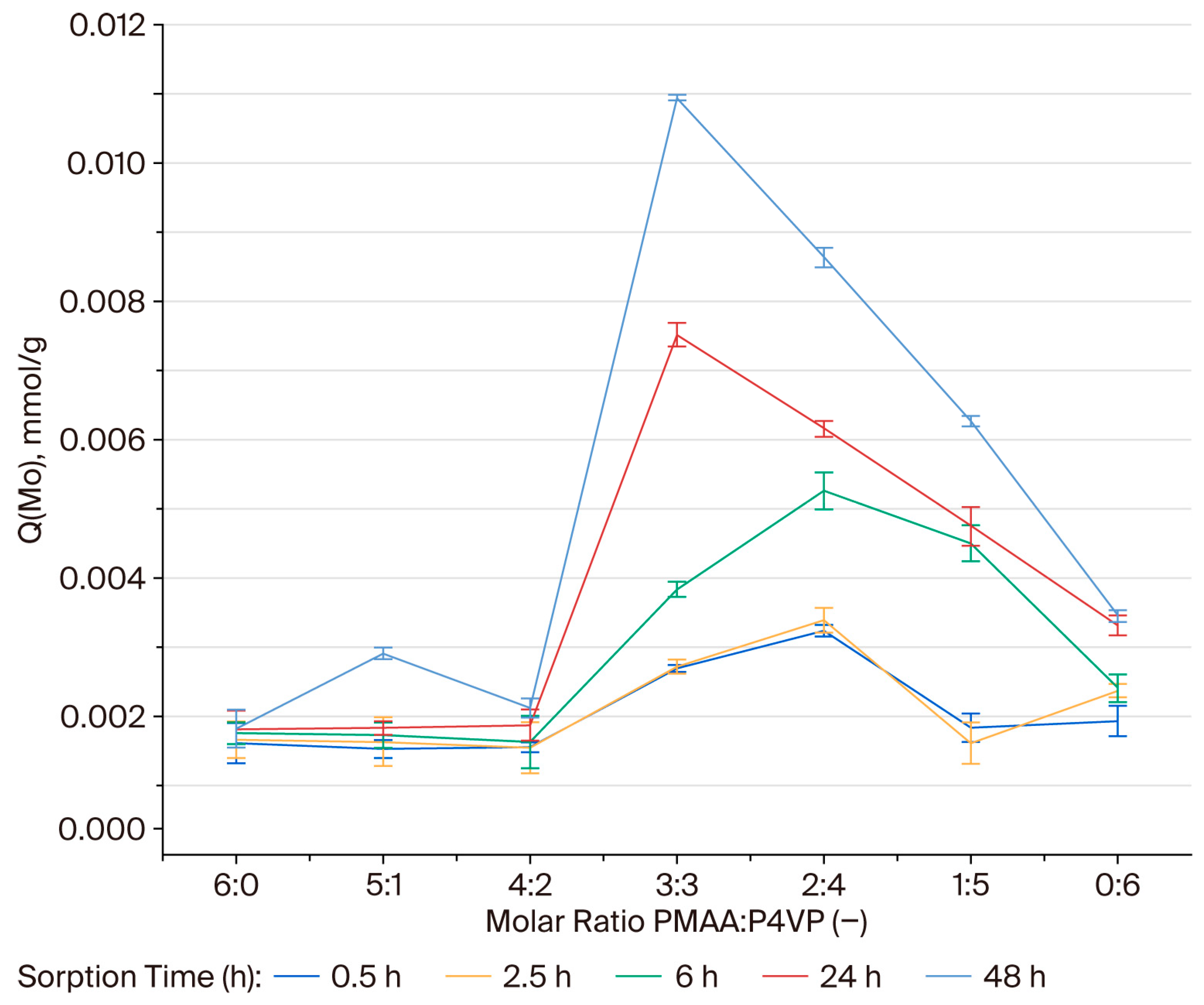
3.2. Effect of the Interpolymer Composition on Molybdenum Sorption
3.3. Mechanistic Interpretation of Sorption Kinetics
3.4. Sorption Efficiency (η) of Rhenium Ions on the PMAA–P4VP Interpolymer System
3.5. Sorption Efficiency of the PMAA–P4VP Interpolymer System for Molybdenum Ions
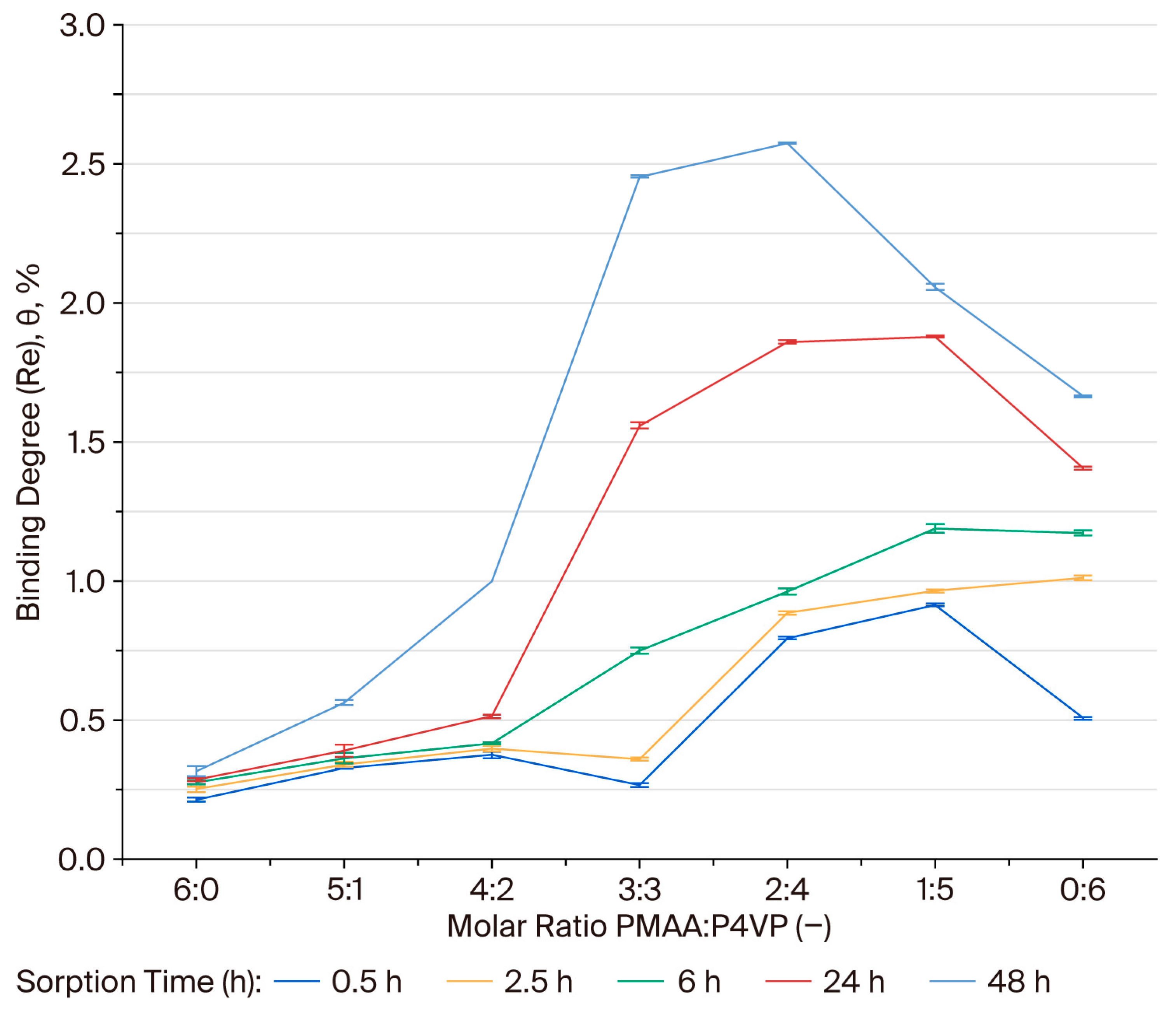
3.6. Binding Degree (θ) of Rhenium Ions by the PMAA–P4VP Interpolymer System
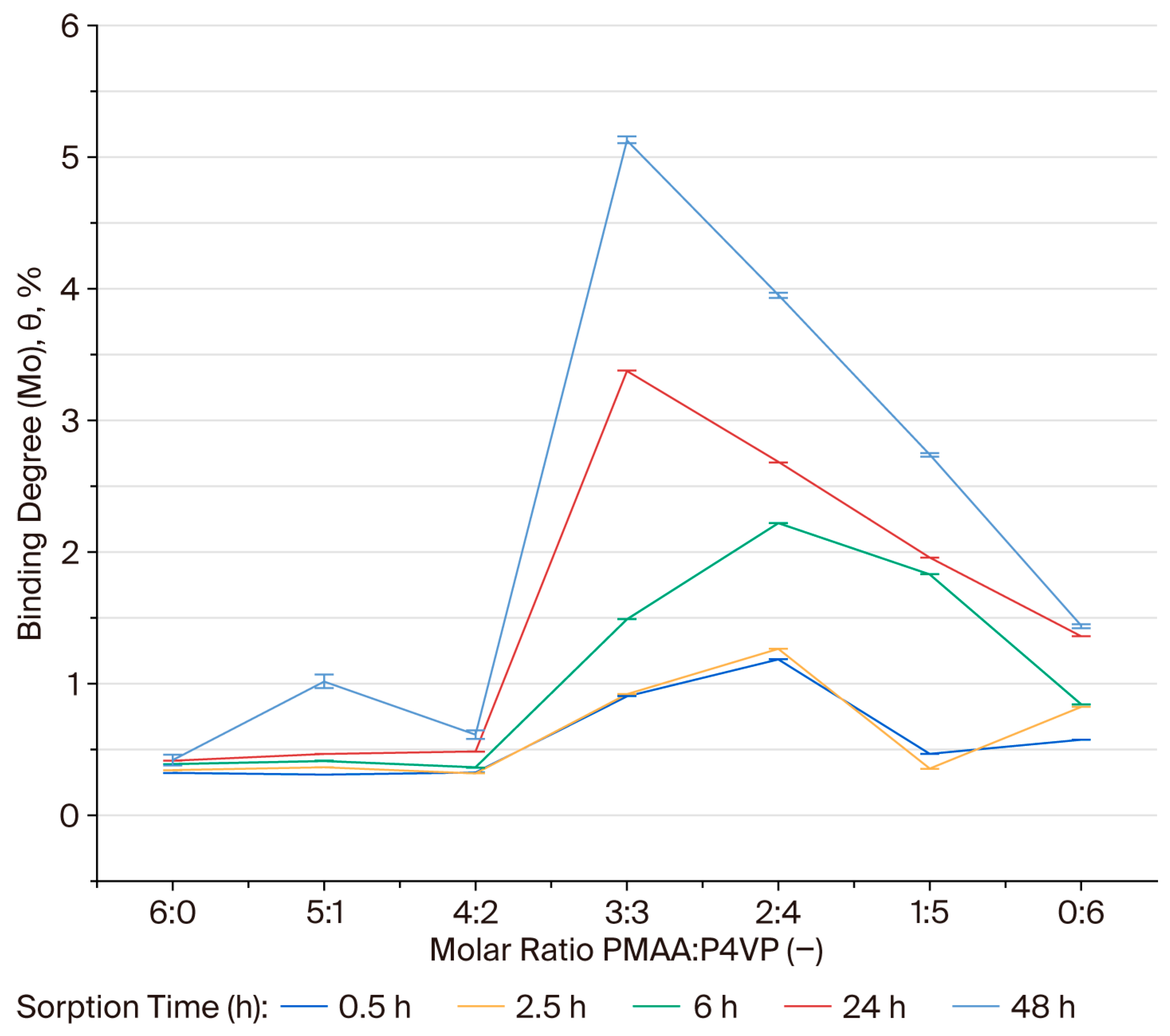
3.7. Binding Degree (θ) of Molybdenum Ions by the PMAA–P4VP Interpolymer System
3.8. Effective Dynamic Exchange Capacity of the PMAA–P4VP Interpolymer System Toward Rhenium Ions
3.9. Effective Dynamic Exchange Capacity of the PMAA–P4VP Interpolymer System Toward Molybdenum Ions
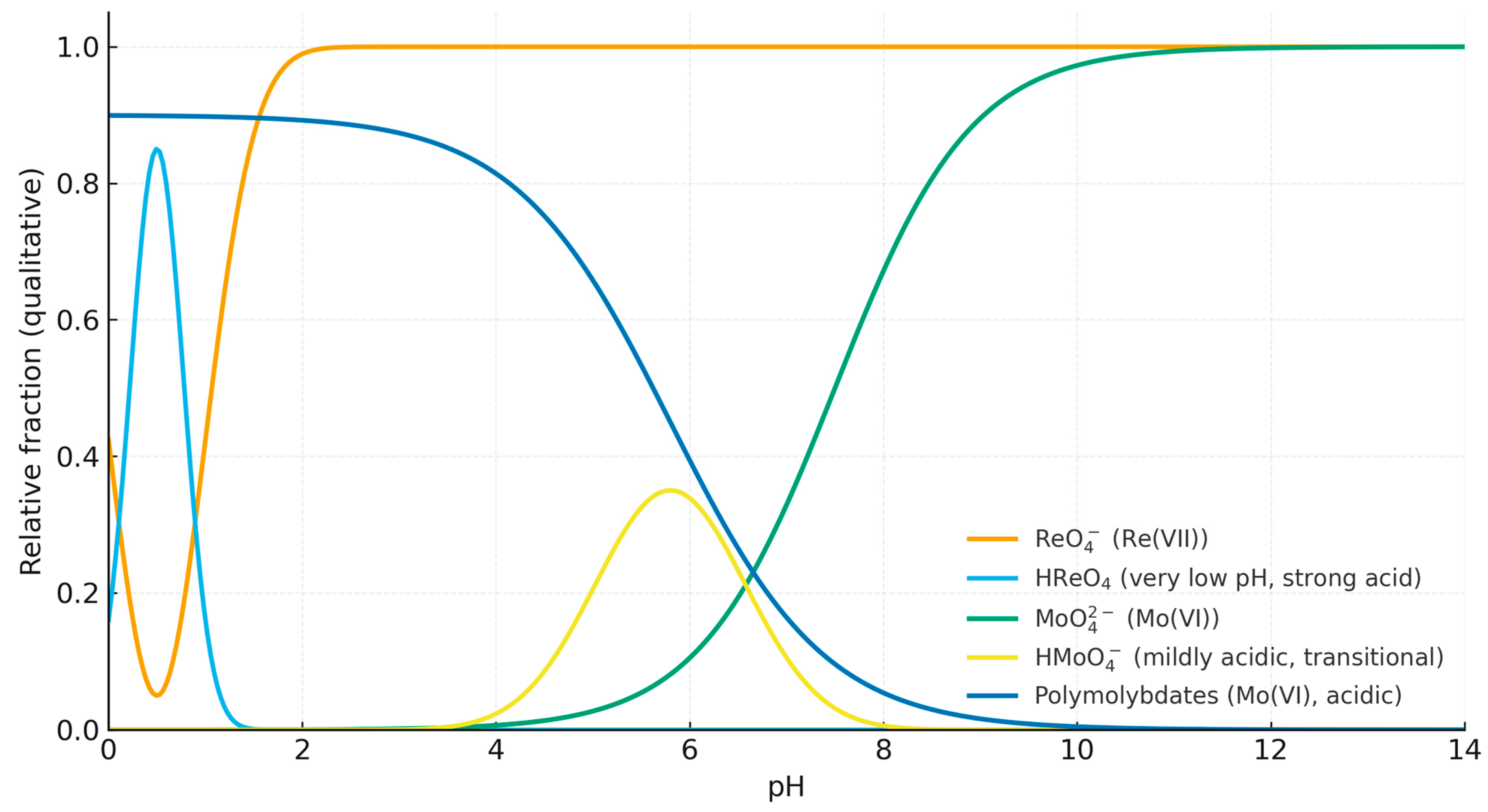
3.10. Effect of pH on Sorption Efficiency of Rhenium and Molybdenum Ions
3.11. Selectivity of Re(VII) and Mo(VI) Sorption
3.12. Desorption of Rhenium and Molybdenum Ions from PMAA–P4VP Interpolymer Systems
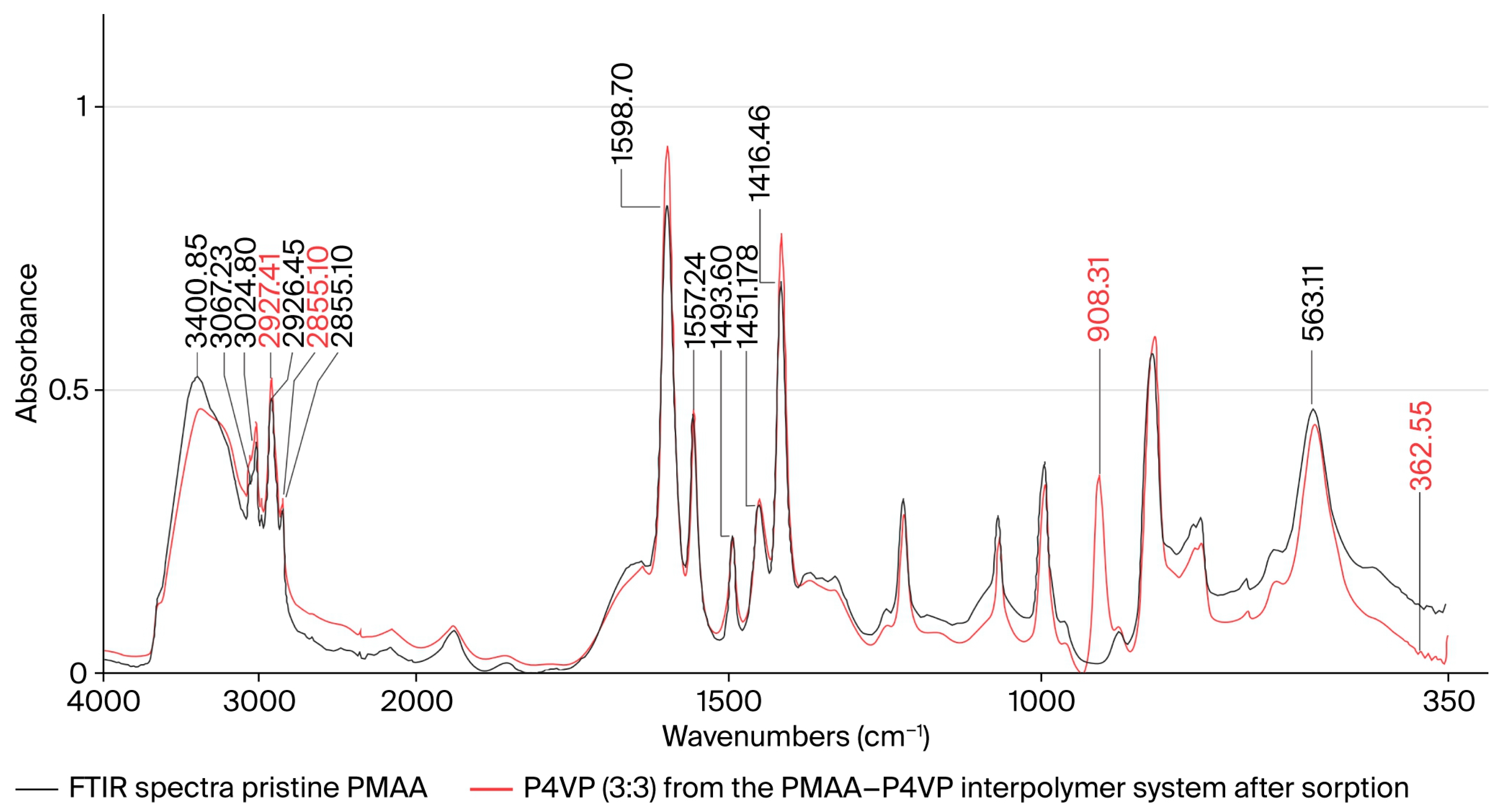
3.13. FTIR Spectroscopic Study of the PMAA–P4VP Interpolymer System
3.14. FTIR Spectroscopic Study of P4VP and PMAA–P4VP System After Metal Sorption
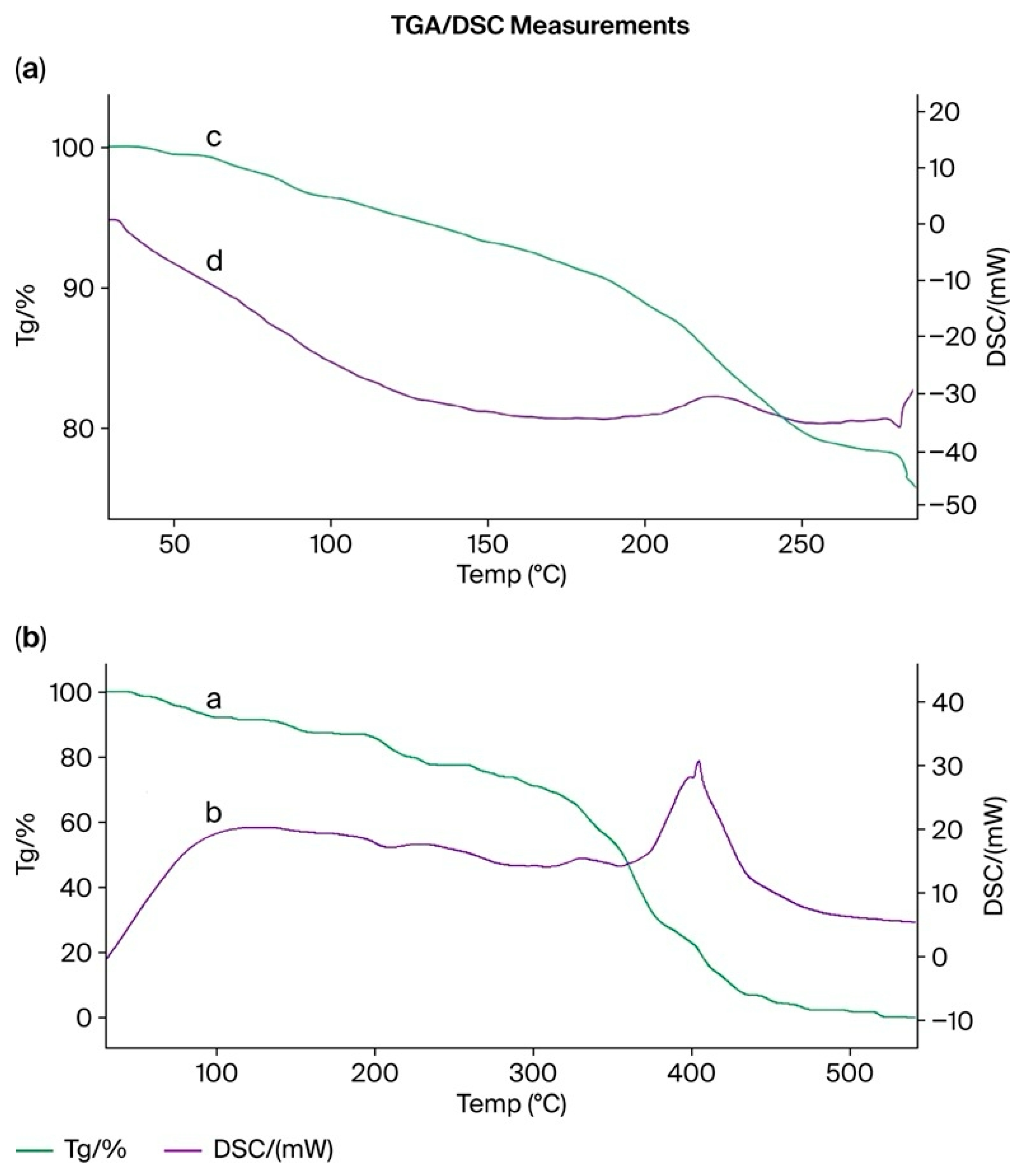
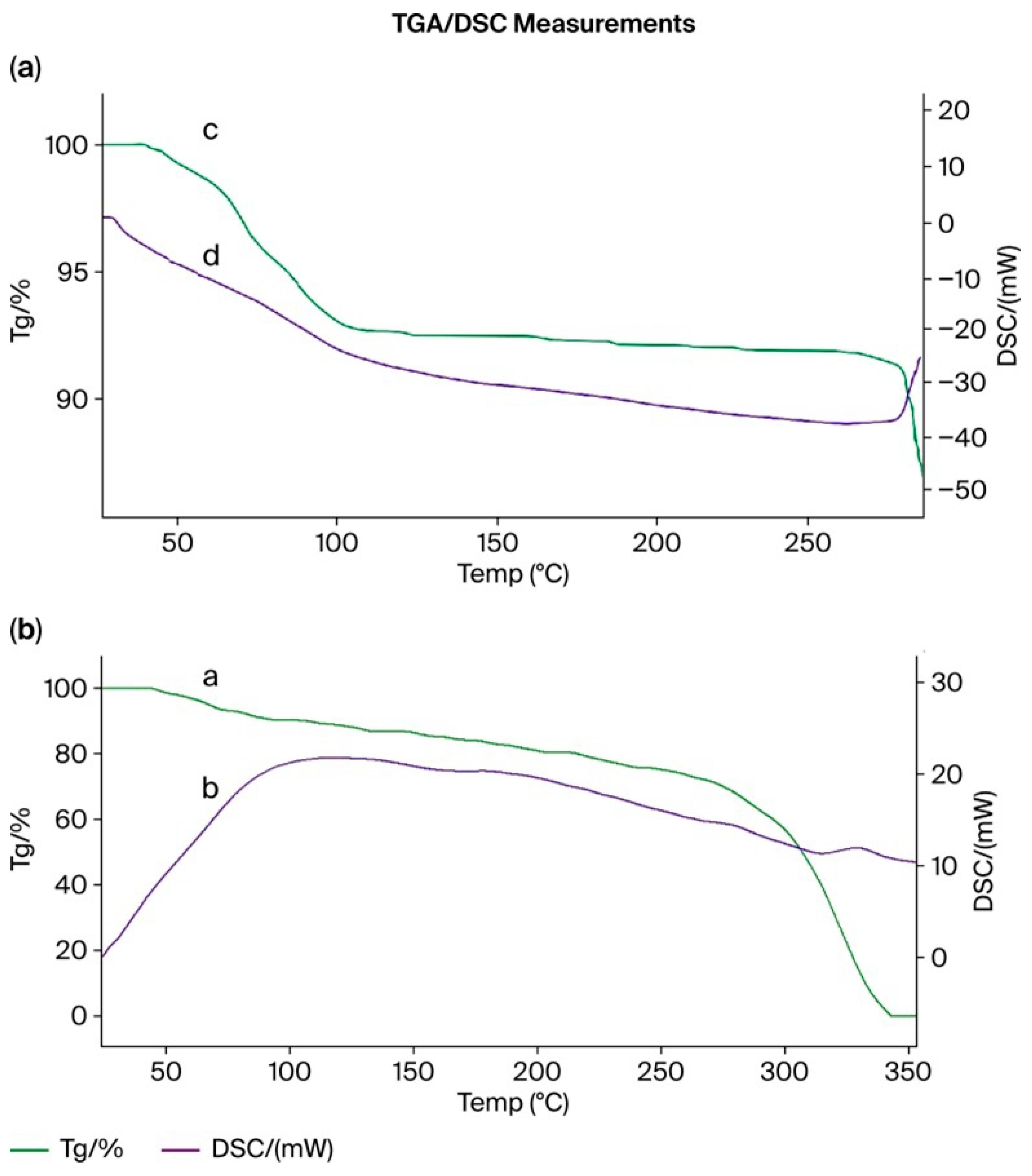
3.15. Thermogravimetric (TGA) and Differential Scanning Calorimetry (DSC) Analysis of the PMAA–P4VP (3:3) Interpolymer System and Its Complexes with Rhenium and Molybdenum
3.16. Modeling of Sorption Isotherms: Linear and Nonlinear Approaches
3.17. Kinetics (Preliminary Analysis)
3.18. Surface Morphology
3.19. Comparison with Literature Data
3.20. Limitations and Future Work
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Analysis and Comparison of Water Purification Strategies
| Method | Principle of Action | Advantages | Limitations | References |
|---|---|---|---|---|
| Membrane technologies (MF, UF, NF, RO) | Physical separation based on pore size and pressure | High efficiency, modularity | Membrane fouling, high cost | [19,20] |
| Chemical cleaning of membranes | Dissolution of contaminants using acids, alkalis, and oxidants | Rapid restoration of performance | Possible membrane damage, chemical waste | [19] |
| Photocatalytic treatment | Generation of reactive radicals (OH) under light | Effective against organic pollutants | Requires light and a photocatalyst, a slow process | [15] |
| Electrochemical treatment | Electric field destruction of pollutants or stimulation of reactions | No reagents, targeted effect | High energy consumption, complex equipment | [21] |
| Biological cleaning | Use of enzymes or bacteria to degrade contaminants | Environmentally friendly, mild conditions | Long reaction time, sensitivity to environmental conditions | [21] |
| Sorption (adsorption/absorption) | Capture of pollutants on the surface or within the sorbent structure | Simplicity, availability, and high selectivity | Sorbent saturation, need for regeneration | [22] |
References
- Shen, L.; Tesfaye, F.; Li, X.; Lindberg, D.; Taskinen, P. Review of rhenium extraction and recycling technologies from primary and secondary resources. Miner. Eng. 2021, 161, 106719. [Google Scholar] [CrossRef]
- Werner, T.T.; Mudd, G.M.; Jowitt, S.M.; Huston, D. Rhenium mineral resources: A global assessment. Resour. Policy 2023, 82, 103441. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Rhenium. In Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024; pp. 134–136. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-rhenium.pdf (accessed on 30 July 2025).
- Leszczyńska-Sejda, K.; Palmowski, A.; Ochmański, M.; Benke, G.; Grzybek, A.; Orda, S.; Goc, K.; Malarz, J.; Kopyto, D. Recycling of Rhenium from Superalloys and Manganese from Spent Batteries to Produce Manganese(II) Perrhenate Dihydrate. Recycling 2024, 9, 36. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Molybdenum. In Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024; pp. 108–110. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-molybdenum.pdf (accessed on 30 July 2025).
- Kenzhaliyev, B.K. Innovative technologies providing enhancement of nonferrous, precious, rare and rare earth metals extraction. Kompleks. Ispolzovanie Miner. Syr’a 2019, 3, 64–75. [Google Scholar] [CrossRef]
- Berdikulova, F.A.; Ikhlasova, A.T. Study of the process of rhenium extraction from lead slime. Kompleks. Ispolzovanie Miner. Syr’a 2020, 3, 22–27. [Google Scholar] [CrossRef]
- Shodiev, A.N.; Khamidov, S.B.; Turobov, S.N. Investigation of sorption technology for extracting molybdenum and rhenium from waste. Univers. Tech. Sci. Electron. Sci. J. 2020, 11. Available online: https://7universum.com/ru/tech/archive/item/10938 (accessed on 7 August 2025).
- Zhan-fang, C.; Hong, Z.; Zhao-hui, Q. Solvent extraction of rhenium from molybdenum in alkaline solution. Hydrometallurgy 2009, 97, 153–157. [Google Scholar] [CrossRef]
- Khoshnevisan, A.; Yoozbashizadeh, H.; Mohammadi, M.; Abazarpoor, A.; Mohammadi, M.; Maarefvand, M. Separation of rhenium and molybdenum from molybdenite leach liquor by the solvent extraction method. Miner. Met. Process 2013, 30, 53–58. [Google Scholar] [CrossRef]
- Abdollahi, H.; Noaparast, M.; Shafaei, S.Z.; Manafi, Z.; Erust, C.; Akcil, A. Acidic Leaching with Chlorate as Oxidizing Agent to Extract Mo and Re from Molybdenite Flotation Concentrate in a Copper Plant. Sep. Sci. Technol. 2015, 50, 2396–2404. [Google Scholar] [CrossRef]
- Khabiyev, A.T.; Baigenzhenov, O.S.; Akbarov, M.S.; Sydykanov, M.M. Study of the possibility of molybdenum recovery from sulfate solutions on the anionite Lewatit MP62W5. Kompleks. Ispolzovanie Miner. Syr’a 2020, 2, 46–51. [Google Scholar] [CrossRef]
- Lindstrom, R.E.; Scheiner, B.J. Extraction of Molybdenum and Rhenium from Concentrates by Electrooxidation; Report of Investigations No. 7802; U.S. Department of the Interior, Bureau of Mines: Washington, DC, USA, 1973.
- Liu, B.; Zhang, B.; Han, G.; Wang, M.; Huang, Y.; Su, S.; Xue, Y.; Wang, Y.D. Clean separation and purification for strategic metals of molybdenum and rhenium from minerals and waste alloy scraps—A review. Resour. Conserv. Recycl. 2022, 181, 106232. [Google Scholar] [CrossRef]
- Rajendran, D.S.; Devi, E.G.; Subikshaa, V.S.; Sethi, P.; Patil, A.; Chakraborty, A.; Venkataraman, S.; Kumar, V.V. Recent advances in various cleaning strategies to control membrane fouling: A comprehensive review. Clean Technol. Environ. Policy 2025, 27, 649–664. [Google Scholar] [CrossRef]
- Truong, H.T.; Nguyen, T.H.; Lee, M.S. Separation of molybdenum(VI), rhenium(VII), tungsten(VI), and vanadium(V) by solvent extraction. Hydrometallurgy 2017, 171, 298–305. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mahanty, S.; Das, P.; Chaudhuri, P.; Das, S. Batch adsorption and process optimization for sequestration of Cr(VI) from aqueous solution using biofilm forming filamentous fungus Aspergillus niger BSC-1. J. Water Process Eng. 2022, 50, 103325. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Grozdov, D.; Vergel, K.; Nekhoroshkov, P.; Rodlovskaya, E. Treatment of Rhenium-Containing Effluents Using Environmentally Friendly Sorbent, Saccharomyces cerevisiae Biomass. Materials 2021, 14, 4763. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, M.A.; Tieama, H.A. A Review of Membranes Classifications, Configurations, Surface Modifications, Characteristics and Its Applications in Water Purification. Chem. Biomol. Eng. 2017, 2, 57–82. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kumar, A.; Indhur, R.; Sheik, A.G.; Krishna, S.B.N.; Kumari, S.; Bux, F. A review on conventional and novel adsorbents to boost the sorption capacity of heavy metals: Current status, challenges and future outlook. Environ. Technol. Rev. 2024, 13, 521–543. [Google Scholar] [CrossRef]
- Diniz, V.; Volesky, B. Desorption of lanthanum, europium and ytterbium from Sargassum. Purif. Technol. 2006, 50, 71–76. [Google Scholar] [CrossRef]
- Gaete, J.; Molina, L.; Alfaro, I.; Yañez, J.; Valenzuela, F.; Basualto, C. Recovery and separation of rhenium and molybdenum from aqueous solutions that simulate mine waters using magnetite nanoparticles functionalized with amine-derivative groups. Miner. Eng. 2019, 136, 66–76. [Google Scholar] [CrossRef]
- Batueva, T.; Scherban, M.; Kondrashova, N.; Chekanova, L.G. Sorption of rhenium (VII) and molybdenum (VI) by modified mesoporous silicas. Sci. Technol. 2021, 57, 532–541. [Google Scholar] [CrossRef]
- Fathi, M.; Mahmoudian, M.; Alorro, R.D.; Chegini, M. Crosslinked Polydiallyldimethylammonium Chloride Adsorbent for the Selective Separation of Rhenium Ions from Pregnant Leach Solutions. Materials 2024, 17, 2737. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tran, V.A.K.; Tran, L.B.; Phan, P.T.; Nguyen, M.T.; Bach, L.G.; Padungthon, S.; Ta, C.K.; Nguyen, N.H. Synthesis of cation exchange resin-supported iron and magnesium oxides/hydroxides composite for nitrate removal in water. Chin. J. Chem. Eng. 2021, 32, 378–384. [Google Scholar] [CrossRef]
- Jumadilov, T.K.; Baishibekov, A.M.; Fischer, D.E. Investigation of the efficiency of rhenium ion sorption using ion-exchange resins polyacrylic acid:poly-4-vinylpyridine in various ratios. Chem. J. Kazakhstan 2024, 3, 36–44. [Google Scholar] [CrossRef]
- Jumadilov, T.; Utesheva, A.; Grazulevicius, J.; Imangazy, A. Selective Sorption of Cerium Ions from Uranium-Containing Solutions by Remotely Activated Ion Exchangers. Polymers 2023, 15, 816. [Google Scholar] [CrossRef]
- Dyussembayeva, G.; Jumadilov, T.; Mukataeva, Z.; Suleimenova, M.; Grazulevicius, J. Characteristics of Mutual Activation of an Intergel System Based on Hydrogel Polymethacrylic Acid and Poly-4-Vinyl Pyridine. Chem. J. Kazakhstan 2024, 2, 94–104. [Google Scholar] [CrossRef]
- N’diaye, A.D.; Kankou, M.S. Modeling of adsorption isotherms of pharmaceutical products onto various adsorbents: A Short Review. J. Mater. Environ. Sci. 2020, 11, 1264–1276. [Google Scholar]
- Jumadilov, T.; Totkhuskyzy, B.; Imangazy, A.; Gražulevičius, J. Anomalous Sorption of Yttrium Ions by the Mutual Activated Hydrogels in the Interpolymer System of Poly(Methacrylic Acid) and Poly(4-Vinylpyridine). Chem. Chem. Technol. 2023, 17, 52–59. [Google Scholar] [CrossRef]
- Junkers, L.S.; Skjærvø, S.L.; Aalling-Frederiksen, O.; Hansen, C.T.; Juelsholt, M.; Lefeld, N.; Pittkowski, R.K.; Magnard, N.P.L.; Kirsch, A.; Jensen, K.M. Linking Transition Metal Molybdate Formation to Polyoxometalate and Chloride Complex Speciation: An In Situ X-Ray Total Scattering Study. Chem. Eur. 2025, 3, e202500062. [Google Scholar] [CrossRef]
- Popov, V.; Menushenkov, A.; Yastrebtsev, A.; Molokova, A.; Pisarev, A.; Khramov, E.; Zubavichus, Y.; Shchetinin, I.; Ponkratov, K.; Tsarenko, N.; et al. The synthesis and studies of crystal/local structures and morphology of hydrated molybdenum oxides. J. Solid State Chem. 2021, 301, 122356. [Google Scholar] [CrossRef]
- Zou, Y.; Yan, L.; Maillet, B.; Sidi-Boulenouar, R.; Brochard, L.; Coussot, P. Critical Role of Boundary Conditions in Sorption Kinetics Measurements. Langmuir 2023, 39, 18866–18879. [Google Scholar] [CrossRef] [PubMed]
- Barbero, G.; Scarfone, A.M.; Evangelista, L.R. The Kinetics of Sorption–Desorption Phenomena: Local and Non-Local Kinetic Equations. Molecules 2022, 27, 7601. [Google Scholar] [CrossRef] [PubMed]
- Chorover, J.; Brusseau, M. Kinetics of Sorption—Desorption. In Kinetics of Water-Rock Interaction; Brantley, S., Kubicki, J., White, A., Eds.; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Plazinski, W.; Dziuba, J.; Rudzinski, W. Modeling of sorption kinetics: The pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption 2013, 19, 1055–1064. [Google Scholar] [CrossRef]
- Fang, D.; Zhuang, X.; Huang, L.; Zhang, Q.; Shen, Q.; Jiang, L.; Xu, X.; Ji, F. Developing the new kinetics model based on the adsorption process: From fitting to comparison and prediction. Sci. Total Environ. 2020, 725, 138490. [Google Scholar] [CrossRef]
- Musah, M.; Azeh, Y.; Mathew, J.; Umar, M.; Abdulhamid, Z.; Muhammad, A. Adsorption Kinetics and Isotherm Models: A Review. Caliphate J. Sci. Technol. 2020, 4, 20–26. [Google Scholar] [CrossRef]
- Brandani, S. Effects of nonlinear equilibrium on zero length column experiments. Chem. Eng. Sci. 1998, 53, 2791–2798. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, K. Modeling phase or chemical reaction equilibrium with an equation of state, an unrealistic modeling. J. Mol. Liq. 2024, 403, 124833. [Google Scholar] [CrossRef]
- Rao, S.S. Finite element methods. In Encyclopedia of Vibration; Elsevier: Amsterdam, The Netherlands, 2001; pp. 530–544. [Google Scholar] [CrossRef]
- Sidorov, N.; Sidorov, D.; Li, Y. Nonlinear systems’ equilibrium points: Branching, blow-up and stability. J. Phys. Conf. Ser. 2019, 1268, 012065. [Google Scholar] [CrossRef]
- Khandaker, S.; Willott, J.D.; Webber, G.B.; Wanless, E.J. Adsorption of polyacrylamides on mineral oxides: Effect of solution pH and polymer molecular weight. Miner. Eng. 2024, 206, 108547. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Dziubla, T.D.; Hilt, J.Z. The Impact of Solution Ionic Strength, Hardness, and pH on the Sorption Efficiency of Polychlorinated Biphenyls in Magnetic Nanocomposite Microparticle (MNM) Gels. Gels 2023, 9, 344. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.Q.; Xu, T.C.; Yang, L.J.; Zhang, Y.Y.; Jin, H.J. Sorption Characteristics and Separation of Rhenium Ions from Aqueous Solutions Using Modified Nano-Al2O3. Ind. Eng. Chem. Res. 2012, 51, 5577–5584. [Google Scholar] [CrossRef]
- Fathi, M.B.; Rezai, B.; Alamdari, E.K.; Alorro, R.D. Studying effects of ion exchange resin structure and functional groups on Re(VII) adsorption onto Purolite A170 and Dowex 21K. J. Min. Environ. 2019, 9, 243–254. [Google Scholar] [CrossRef]
- Wu, C.-H.; Lo, S.-L.; Lin, C.-F. Competitive adsorption of molybdate, chromate, sulfate, selenate, and selenite on γ-Al2O3. Colloids Surf. A Physicochem. Eng. Asp. 2000, 166, 251–259. [Google Scholar] [CrossRef]
- Gamal, R.; Rizk, S.E.; El-Hefny, N.E. The adsorptive removal of Mo(VI) from aqueous solution by a synthetic magnetic chromium ferrite nanocomposite using a nonionic surfactant. J. Alloys Comp. 2021, 853, 157039. [Google Scholar] [CrossRef]
- Fallah, N.; Taghizadeh, M.; Hassanpour, S. Selective adsorption of Mo(VI) ions from aqueous solution using a surface-grafted Mo(VI) ion imprinted polymer. Polymer 2018, 144, 80–91. [Google Scholar] [CrossRef]
- Brion-Roby, R.; Gagnon, J.; Nosrati, S.; Deschênes, J.-S.; Chabot, B. Adsorption and desorption of molybdenum(VI) in con-taminated water using a chitosan sorbent. J. Water Proc. Eng. 2018, 23, 13–19. [Google Scholar] [CrossRef]
- Lian, J.; Zhou, F.; Chen, B.; Yang, M.; Wang, S.; Liu, Z.; Niu, S. Enhanced adsorption of molybdenum(VI) onto drinking water treatment residues modified by thermal treatment and acid activation. J. Clean. Product. 2020, 244, 118719. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sangeetha, D. Removal of molybdate from water by adsorption onto ZnCl2 activated coir pith carbon. Biores. Technol. 2006, 97, 1194–1200. [Google Scholar] [CrossRef]
- Silverstein, R.; Bassler, G.; Morrill, T. Spectrometric Identification of Organic Compounds; Translated from English; Mir: Moscow, Russia, 1977; p. 592. [Google Scholar]
- Semushin, A.M.; Yakovlev, V.A.; Ivanova, E.V. Infrared Absorption Spectra of Ion-Exchange Materials: Handbook; Chemistry: Leningrad, Russia, 1980; p. 96. [Google Scholar]
- Laskorin, B.N. (Ed.) Ion-Exchange Materials for Hydrometallurgy, Wastewater Treatment and Water Preparation: Handbook; VNIIKhT: Moscow, Russia, 1983; p. 207. [Google Scholar]
- Bolshakov, G.F.; Glebovskaya, E.A.; Kaplan, Z.G. Infrared Spectra and X-Ray Diffraction Patterns of Heteroorganic Compounds; Chemistry Publishing House, Leningrad Branch: Leningrad, Russia, 1967; p. 224. [Google Scholar]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds; Translated from English; Mir: Moscow, Russia, 1966; p. 412. [Google Scholar]
- Hobbollahi, E.; List, M.; Monkowius, U. Poly(4-vinylpyridine) as ligand for Au(I) and Zn(II) cations: Luminescent metal-containing polymers. Monatsh. Chem. 2019, 150, 877–883. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Malachite Green Adsorption by Rattan Sawdust: Isotherm, Kinetic, and Mechanism Modeling. J. Hazard. Mater. 2008, 159, 574–579. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, Kinetics, Mechanism, and Process Design for the Sorption of Methylene Blue onto Rice Husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Doke, K.M.; Khan, E.M. Equilibrium, Kinetic, and Diffusion Mechanism of Cr(VI) Adsorption onto Activated Carbon Derived from Wood Apple Shell. Arab. J. Chem. 2017, 10, S252–S260. [Google Scholar] [CrossRef]
- An, B. Cu(II) and As(V) Adsorption Kinetic Characteristic of the Multifunctional Amino Groups in Chitosan. Processes 2020, 8, 1194. [Google Scholar] [CrossRef]
- Kalbasi, R.J.; Mosaddegh, N. Characterization and Catalytic Performance of Poly(4-vinylpyridine) Supported on Mesoporous Carbon: Comparison with Poly(4-vinylpyridine) Supported on Mesoporous Silica. J. Porous Mater. 2012, 19, 557–565. [Google Scholar] [CrossRef]
- Kurajica, S.; Minga, I.; Blazic, R.; Muzina, K.; Tominac, P. Adsorption and Degradation Kinetics of Methylene Blue on As-Prepared and Calcined Titanate Nanotubes. Athens J. Sci. 2018, 5, 7–22. [Google Scholar] [CrossRef]
- Dahri, M.K.; Kooh, M.R.R.; Lim, L.B. Application of Casuarina equisetifolia Needle for the Removal of Methylene Blue and Malachite Green Dyes from Aqueous Solution. Alex. Eng. J. 2016, 55, 875–884. [Google Scholar] [CrossRef]
- Campos, N.F.; Barbosa, C.M.B.M.; Rodríguez-Díaz, J.M.; Duarte, M.M.M.B. Removal of Naphthenic Acids Using Activated Charcoal: Kinetic and Equilibrium Studies. J. Chem. Technol. Biotechnol. 2018, 93, 2069–2078. [Google Scholar] [CrossRef]
- Felıx, N.; Ienna, N.; Ikelle, İ.; Anselm, O.; Nwabueze, E.; Emeka, İ.; Clinton, O. Activated Plantain Peel Biochar as Adsorbent for Sorption of Zinc(II) Ions: Equilibrium and Kinetics Studies. J. Turk. Chem. Soc. Sect. A Chem. 2018, 5, 1257–1270. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Kurmysheva, A.Y.; Vedenyapina, M.D.; Kulaishin, S.A.; Podrabinnik, P.; Pinargote, N.W.S.; Smirnov, A.; Metel, A.S.; Bartolomé, J.F.; Grigoriev, S.N. Adsorption Removal of Mo(VI) from an Aqueous Solution by Alumina with the Subsequent Regeneration of the Adsorbent. Int. J. Mol. Sci. 2023, 24, 8700. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, W.; Zheng, S. Epoxy resin cured with poly(4-vinylpyridine). J. Mater. Sci. 2005, 40, 6367–6373. [Google Scholar] [CrossRef]
- Elgoud, A.E.; Abd-Elhamid, A.I.; Aly, H.F. Adsorption behavior of Mo(VI) from aqueous solutions using tungstate-modified magnetic nanoparticle. Environ. Sci. Pollut. Res. 2024, 31, 18900–18915. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, P.; Huang, R.; Kou, J. Adsorption Behavior of U(VI) and Re(VII) on Iron Sulfide Nanoparticles Modified with Titanate Nanotubes: Mechanism and Performance Insights. Eur. J. Inorg. Chem. 2023, 26, e202300121. [Google Scholar] [CrossRef]









| Model | R2 | RMSE | χ2 | AIC | qm (mg·g−1) | Kₗ (L·mg−1) |
|---|---|---|---|---|---|---|
| Langmuir | 0.998 | 0.012 | 0.003 | 24.5 | 48.6 | 0.045 |
| Freundlich | 0.976 | 0.034 | 0.009 | 31.2 | — | — |
| Sorbent System | Target Ion | qe/qmax (mg g−1) | pH/Conditions | Equilibrium Time (min) | Model/Mechanism | Reference |
|---|---|---|---|---|---|---|
| PMAA–P4VP (2:4) | Re(VII) | 48.6 | pH 5.2, 298 K | 2880 | PSO, Langmuir (monolayer) | This work |
| PMAA–P4VP (3:3) | Mo(VI) | 42.7 | pH 5.2, 298 K | 2880 | PSO, Langmuir (monolayer) | This work |
| NH4HCO3–modified nano-Al2O3 | Re(VII) | 1.94 | pH 2–3, 298 K | 120 | PSO, Langmuir (monolayer) | [47] |
| Purolite A170 resin (weak-base) | Re(VII) | 166.7 | pH 5.0, 298 K | 60 | PSO, Freundlich + D-R, ion Exchange | [48] |
| Dowex 21 K resin (strong-base) | Re(VII) | 142.9 | pH 5.0, 298 K | 60 | PSO, Freundlich + D-R | [48] |
| γ-Al2O3 | Mo(VI) | 31.0 | pH 4.0 | 120 | PFO | [49] |
| Magnetic Cr-ferrite | Mo(VI) | 26.8 | pH 4.0 | 180 | PSO | [50] |
| Ion-imprinted polymer | Mo(VI) | 126.1 | pH 3–4 | 10 | Langmuir (monolayer) | [51] |
| Chitosan sorbent | Mo(VI) | 124.3 | pH 5.0 | 15 | PSO (chemisorption) | [52] |
| Modified water-treatment residue | Mo(VI) | 39.5 | pH 6.0 | — | Freundlich (heterogeneous surface) | [53] |
| Activated carbon | Mo(VI) | 16.5 | pH 4.0 | 30 | PFO (physical adsorption) | [54] |
| Functional Group | Assignment | ν (Before), cm−1 (± 2) | ν (After Re(VII)), cm−1 | Δν (cm−1) | ν (After Mo(VI)), cm−1 | Δν (cm−1) | ReferenceBandrange (lit.), cm−1 | Interpretation |
|---|---|---|---|---|---|---|---|---|
| –COOH/–COO− | ν(C=O) stretch (PMAA) | 1715/1707 | 1685 | −25 | 1688 | −22 | 1695–1715 [55,56] | Coordination via carboxyl oxygen |
| –C=N (pyridyl) | ν(C=N) stretch (P4VP) | ~1594 | ~1582 | −12 | ~1583 | −11 | 1585–1605 [55,56] | Donor–acceptor interaction with metal center |
| –C–O (acidic) | ν(C–O) stretch | 1263/1174 | ~1190 | −10 −15 | ~1195 | −10 | 1170–1270 [57] | Deprotonation and participation in binding |
| Re–O | ν3(F2) ReO4− | — | 908 | +908 | — | — | 910–930 [56,57,58] | Formation of perrhenate complex |
| Mo–O | ν4(F2) MoO42− | — | — | — | 363 | +363 | 850–900 [57,58,59] | Formation of molybdate complex |
| O–H (H-bonded) | ν(O–H) broad | 3300–2500 | broad | — | broad | — | 2500–3500 [60] | Hydrogen-bond reorganization |
| Ion | System (PMAA:P4VP) | qe (48 h), mg·g−1 | qmax (Langmuir), mg·g−1 | b (L·mg−1) | Kd, mL·g−1 | βRe/Mo | R2 (Langmuir) | R2 (Freundlich) | KDR (mol2∙kJ−2) | E (kJ∙mol−1) | R2 (Dubinin–Radushkevich) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Re(VII) | 2:4 | 45.8 ± 1.3 | 48.6 ± 1.1 | 0.052 | 4450 | — | 0.999 | 0.982 | 1.2 × 10−6 | 20.4 | 0.961 |
| Re(VII) | 3:3 | 41.5 ± 1.1 | 43.2 ± 0.9 | 0.047 | 4170 | — | 0.993 | 0.978 | 1.4 × 10−6 | 18,9 | 0.957 |
| Mo(VI) | 2:4 | 38.1 ± 1.4 | 42.7 ± 1.0 | 0.039 | 3890 | 1.15 | 0.991 | 0.987 | 1.6 × 10−6 | 17.7 | 0.948 |
| Mo(VI) | 3:3 | 36.3 ± 1.2 | 40.2 ± 0.9 | 0.035 | 3560 | 1.17 | 0.985 | 0.982 | 1.9 × 10−6 | 16.2 | 0.942 |
| System (PMAA:P4VP) | PFO Model | PSO Model | Intraparticle Diffusion (Weber–Morris) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ion | qe (mg·g−1) | k1 (h−1) | R2 | qe (mg·g−1) | k2 (g·mg−1·h−1) | R2 | kid (mg∙g−1∙h−1/2) | C (mg∙g−1) | R2 | |
| ReO4− | 2:4 | 51.180 | 0.0472 | 0.365 | 45.830 | 3.36 × 10−3 | 0.469 | 5.100 | 8.380 | 0.807 |
| ReO4− | 3:3 | 48.720 | 0.0545 | 0.849 | 44.250 | 2.95 × 10−3 | 0.849 | 6.170 | −2.180 | 0.817 |
| MoO42− | 2:4 | 34.180 | 0.197 | 0.725 | 38.650 | 6.24 × 10−3 | 0.797 | 4.450 | 8.360 | 0.958 |
| MoO42− | 3:3 | 54.900 | 0.0614 | 0.962 | 73.180 | 7.28 × 10−4 | 0.962 | 7.740 | −0.022 | 0.964 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baishibekov, A.; Fischer, D.; Jumadilov, T.; Temirova, S.; Yulusov, S.; Altaibayev, B.; Karim, D. Structural, Sorption, and Regeneration Properties of Poly(methacrylic acid): Poly(4-vinylpyridine) Interpolymer Systems for the Recovery of Rhenium and Molybdenum. Polymers 2025, 17, 3054. https://doi.org/10.3390/polym17223054
Baishibekov A, Fischer D, Jumadilov T, Temirova S, Yulusov S, Altaibayev B, Karim D. Structural, Sorption, and Regeneration Properties of Poly(methacrylic acid): Poly(4-vinylpyridine) Interpolymer Systems for the Recovery of Rhenium and Molybdenum. Polymers. 2025; 17(22):3054. https://doi.org/10.3390/polym17223054
Chicago/Turabian StyleBaishibekov, Arman, Dametken Fischer, Talkybek Jumadilov, Saniya Temirova, Sultan Yulusov, Bagdat Altaibayev, and Diana Karim. 2025. "Structural, Sorption, and Regeneration Properties of Poly(methacrylic acid): Poly(4-vinylpyridine) Interpolymer Systems for the Recovery of Rhenium and Molybdenum" Polymers 17, no. 22: 3054. https://doi.org/10.3390/polym17223054
APA StyleBaishibekov, A., Fischer, D., Jumadilov, T., Temirova, S., Yulusov, S., Altaibayev, B., & Karim, D. (2025). Structural, Sorption, and Regeneration Properties of Poly(methacrylic acid): Poly(4-vinylpyridine) Interpolymer Systems for the Recovery of Rhenium and Molybdenum. Polymers, 17(22), 3054. https://doi.org/10.3390/polym17223054







