Synergistic Impact of Copper Nanoparticles Functionalized with Magnetic Chitosan on the Enhanced Adsorptive Sequestration of Metformin Diabetic Drug from Environmental Samples
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Instrumentation
2.3. Preparation of Cu@MCS Nanoparticles
2.4. Batch Experiments for the Adsorption Removal of Metformin by Cu@MCS Nanocomposite
3. Results and Discussion
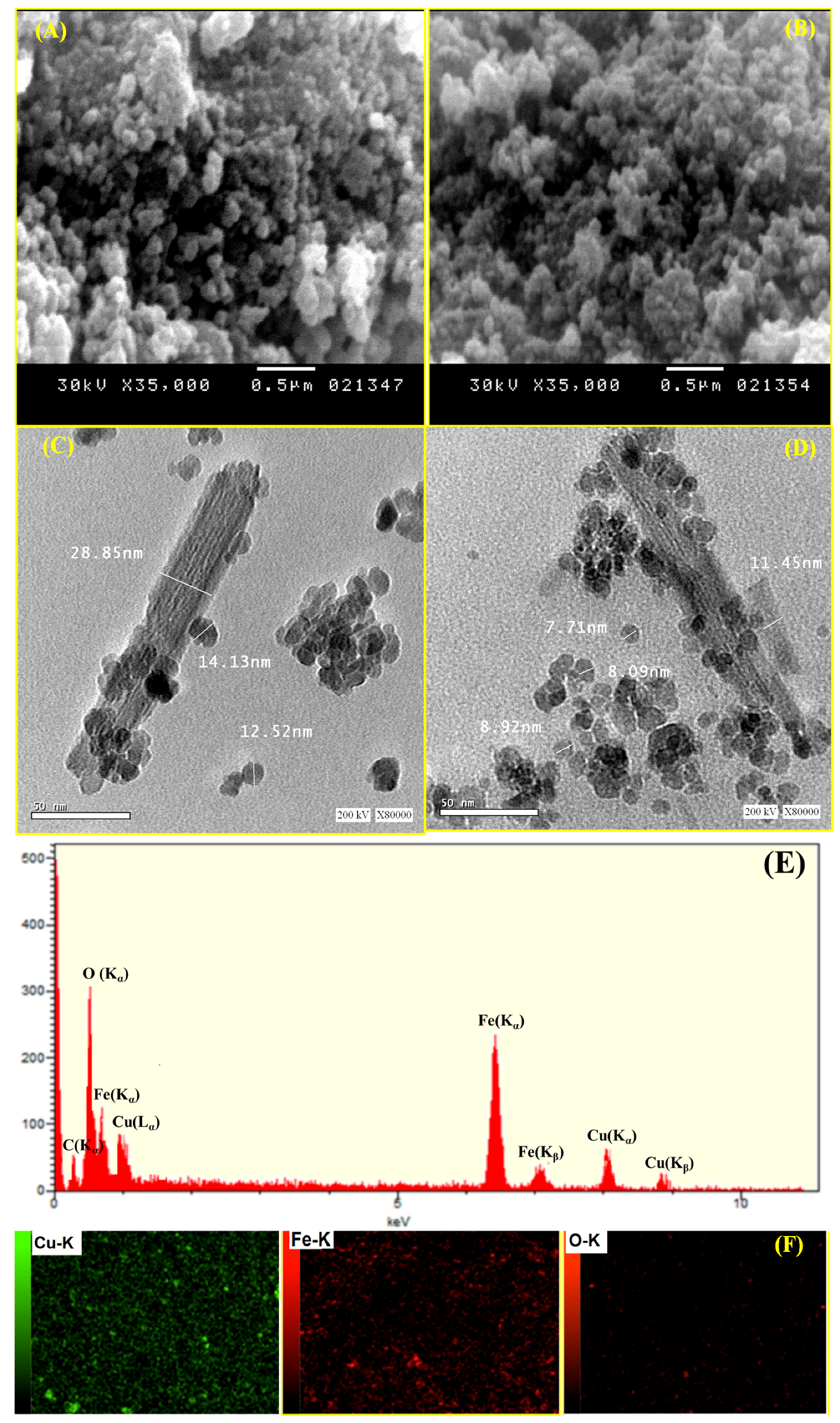
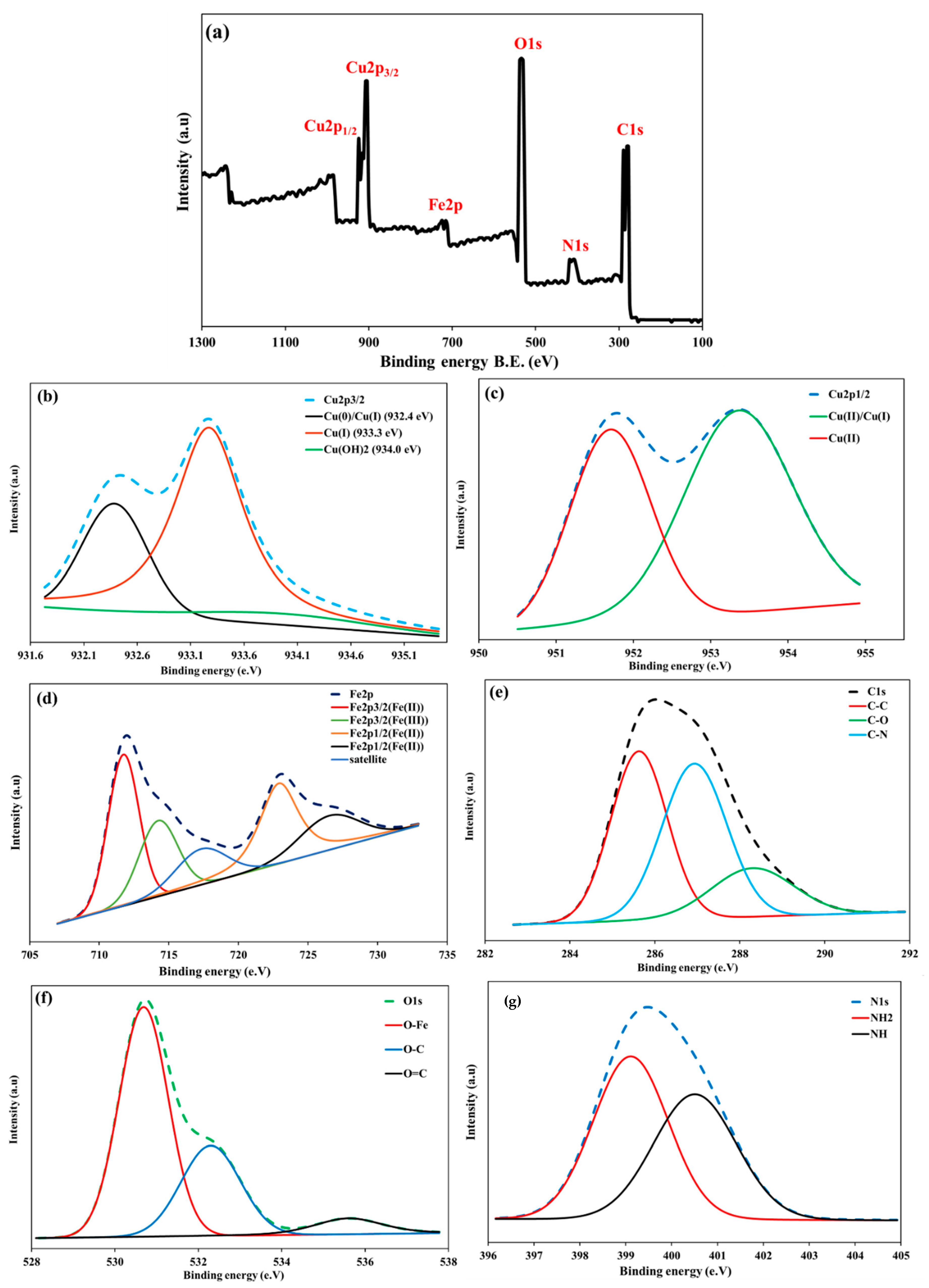
3.1. Surface Morphology and Characterization
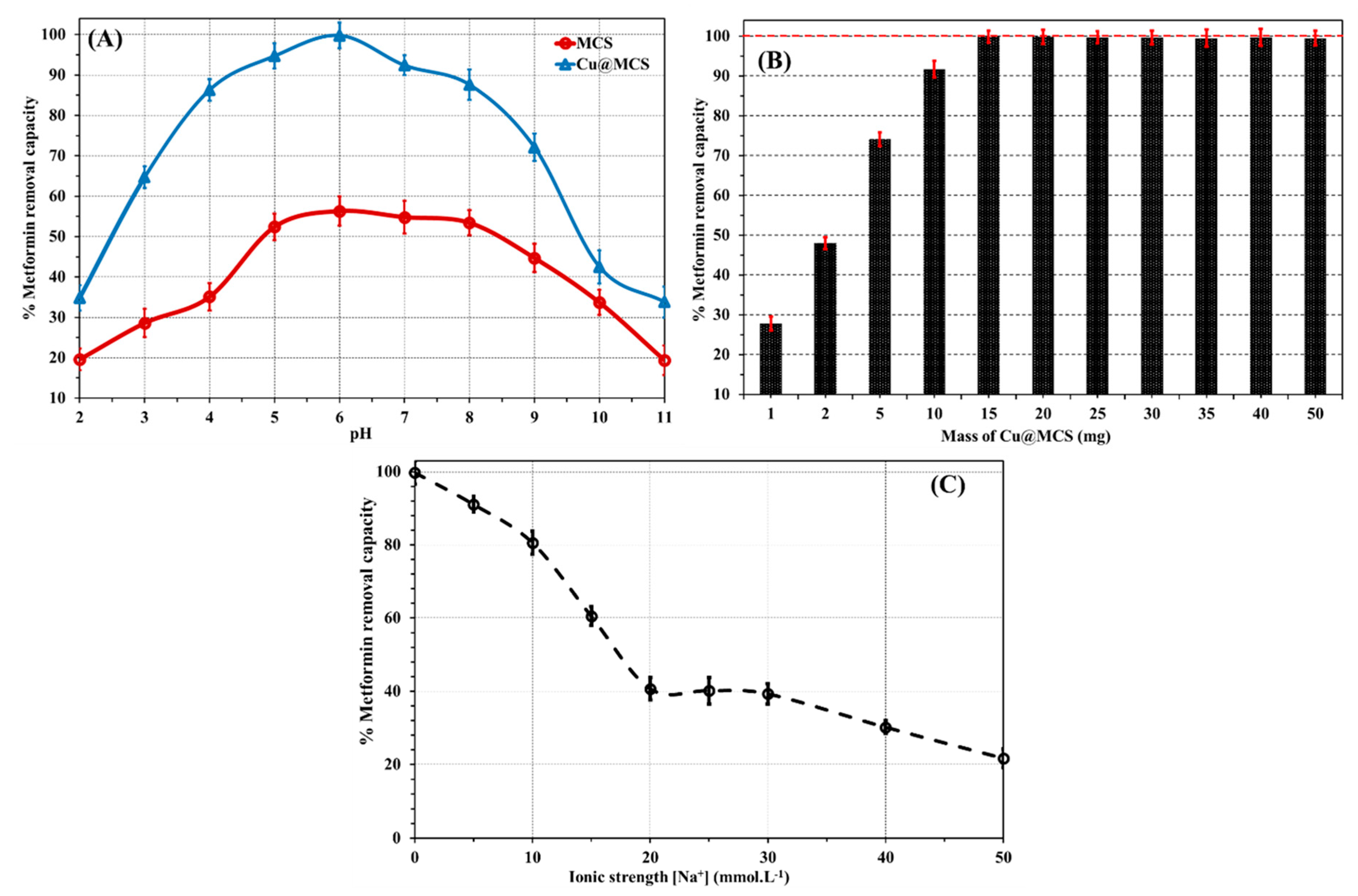
3.2. Impact of pH
3.3. Impact of Mass and Ionic Strength
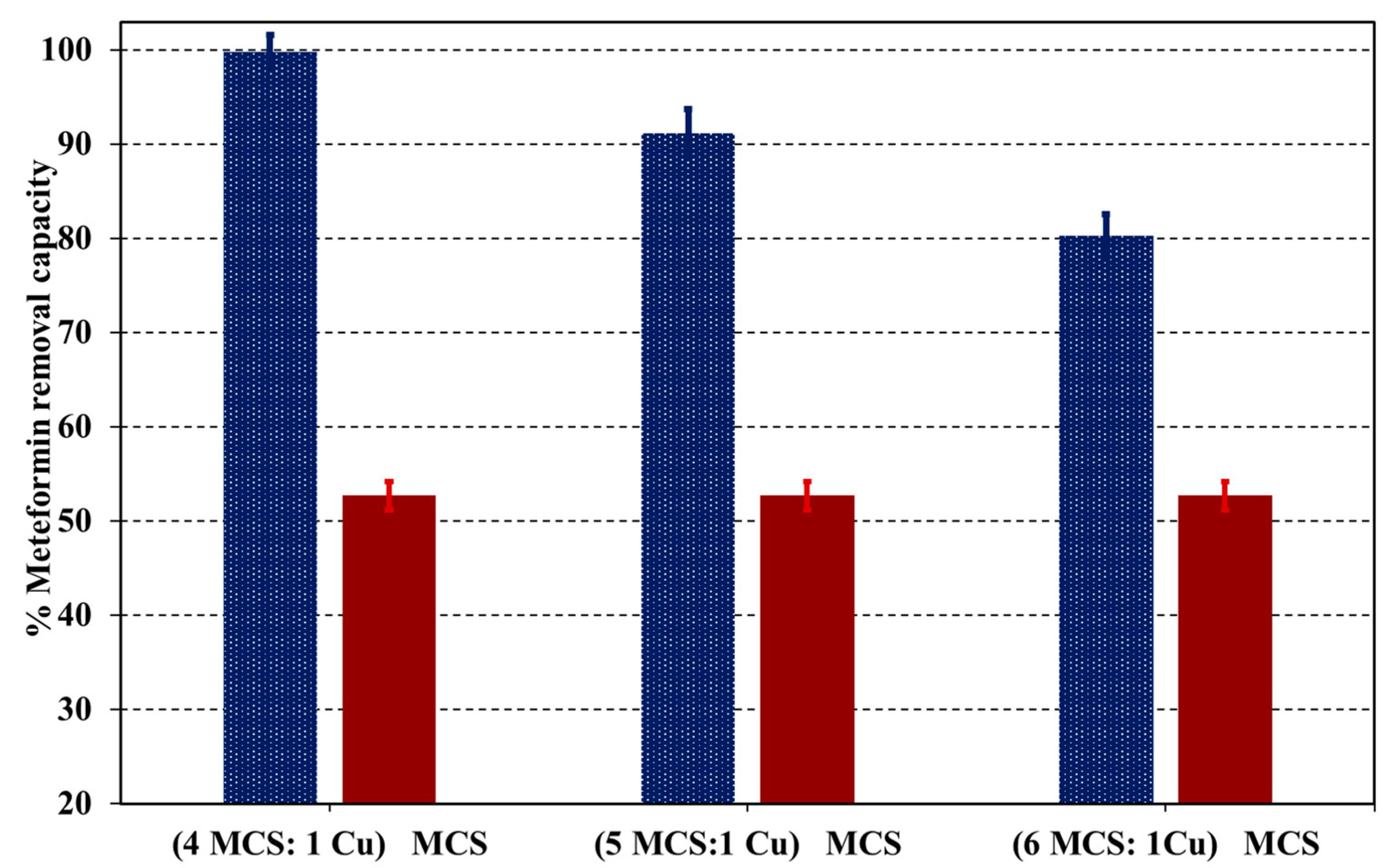
3.4. Cu-Supported Nanoparticles’ Impact on Metformin Uptake by the Cu@MCS
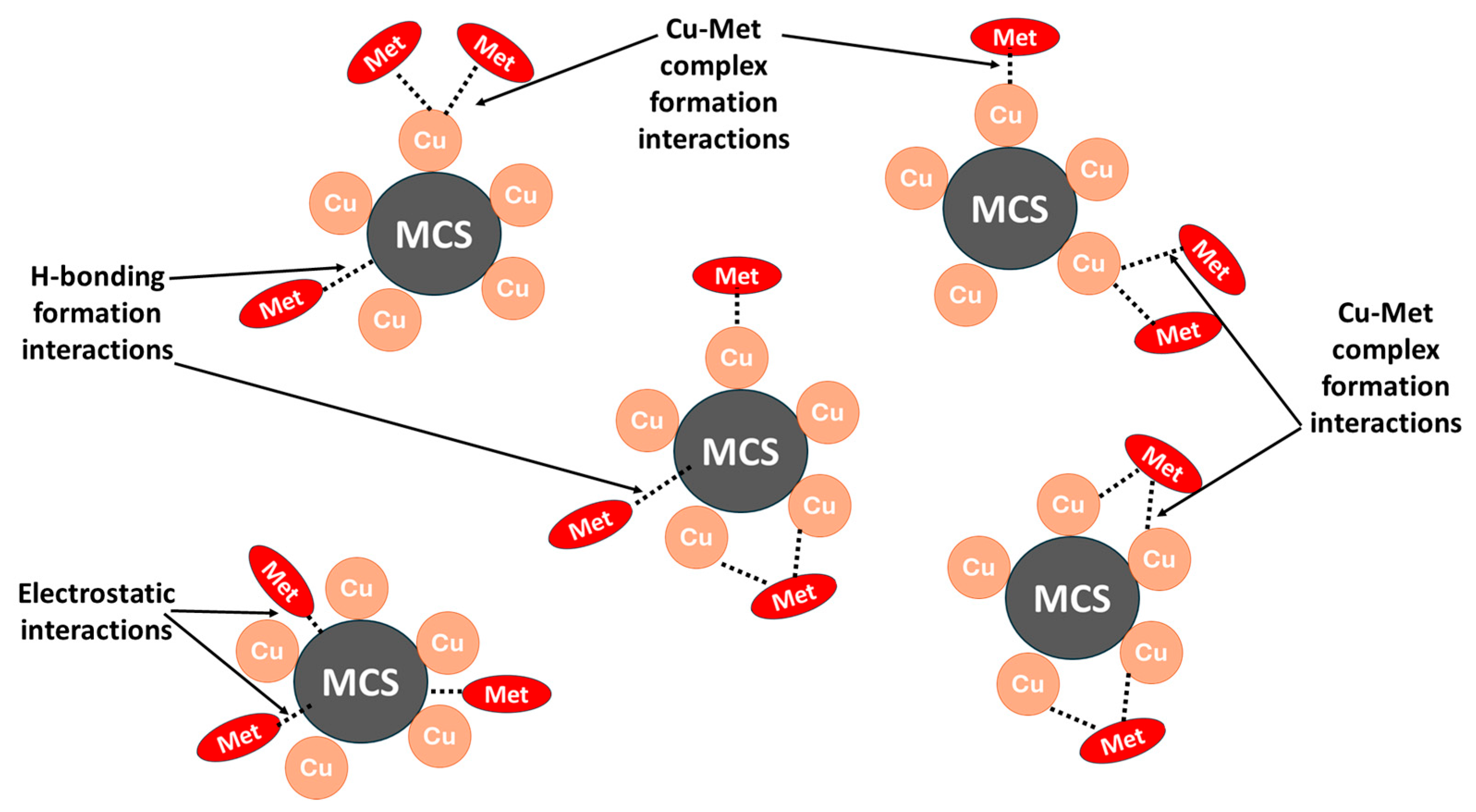
3.5. Sorption Mechanisms
3.6. Sorption Kinetics
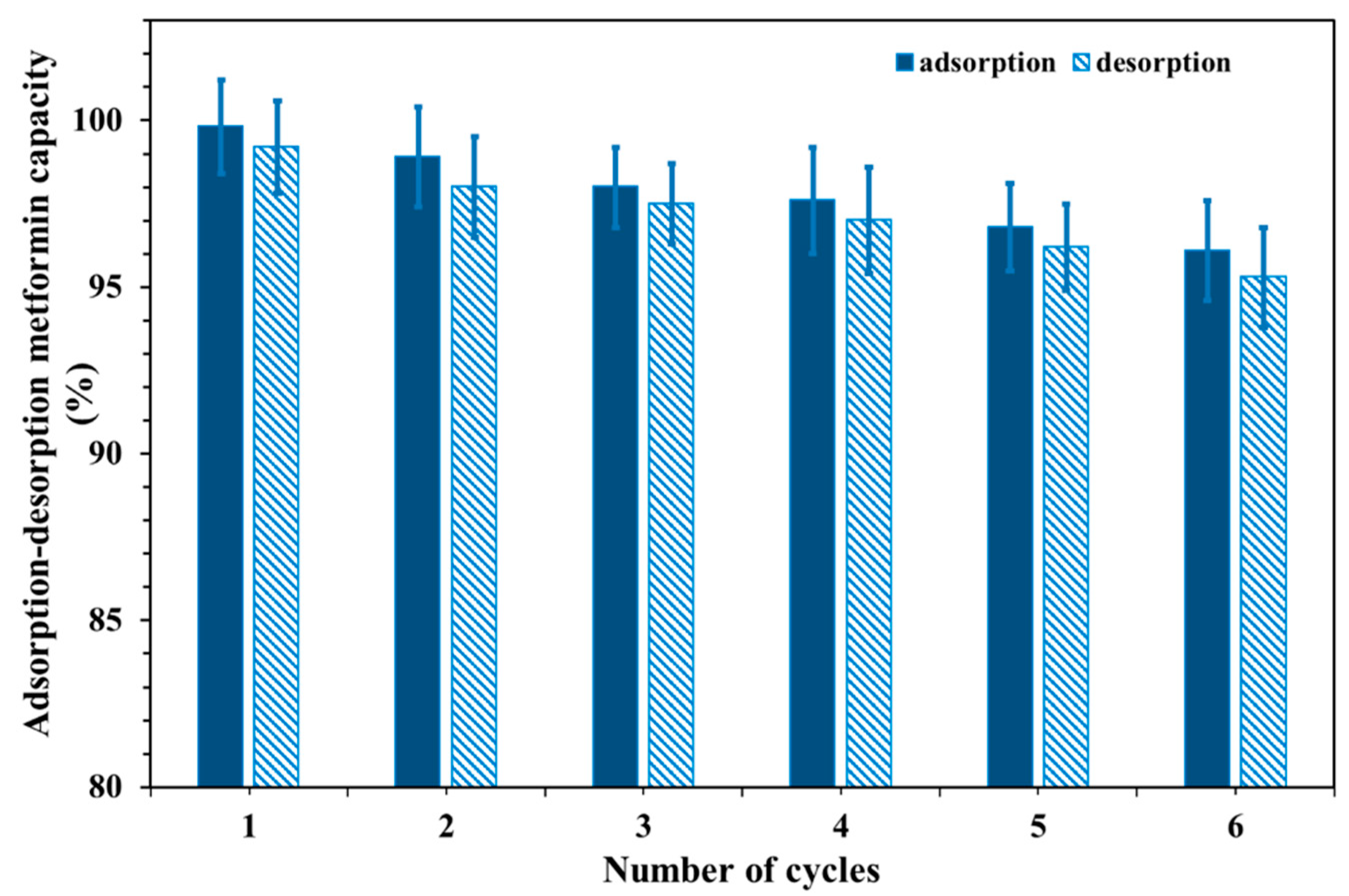
3.7. Reusability of Cu@MCS Nanocomposite
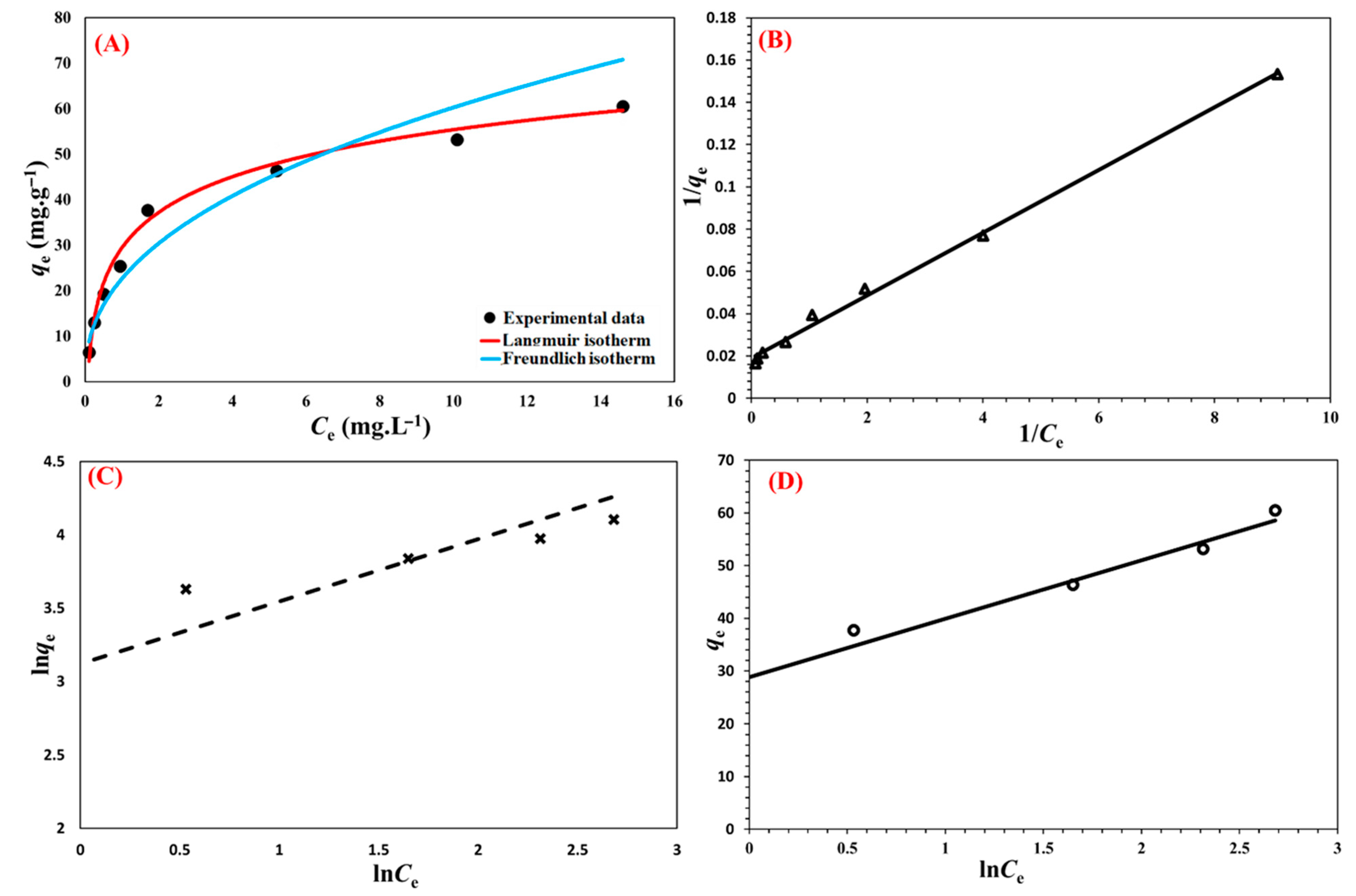
3.8. Sorption Isotherms
3.9. Analytical Performance of Cu@MCS Nanocomposite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumirska, J. Special issue “Pharmaceutical Residues in the Environment”. Molecules 2020, 25, 2941. [Google Scholar] [CrossRef]
- González-González, R.B.; Martínez-Zamudio, L.Y.; Hernández, J.A.R.; González-Meza, G.M.; Parra-Saldívar, R.; Iqbal, H.M.N. Pharmaceutical pollution fingerprinting and waterbodies remediation using waste-derived carbon dots as sustainable advanced nanomaterials. Environ. Res. 2023, 238, 117180. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P. Occurrence and removal of pharmaceuticals in wastewater treatment plants. Process Saf. Environ. Prot. 2021, 150, 532–556. [Google Scholar] [CrossRef]
- Al Falahi, O.A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ewadh, H.M.; Kurniawan, S.B.; Imron, M.F. Occurrence of pharmaceuticals and personal care products in domestic wastewater, available treatment technologies, and potential treatment using constructed wetland: A review. Process Saf. Environ. Prot. 2022, 168, 1067–1088. [Google Scholar] [CrossRef]
- Caban, M.; Stepnowski, P. How to decrease pharmaceuticals in the environment? A review. Environ. Chem. Lett. 2021, 19, 3115–3138. [Google Scholar] [CrossRef]
- Fork, M.L.; Fick, J.B.; Reisinger, A.J.; Rosi, E.J. Dosing the coast: Leaking sewage infrastructure delivers large annual doses and dynamic mixtures of pharmaceuticals to urban rivers. Environ. Sci. Technol. 2021, 55, 11637–11645. [Google Scholar] [CrossRef]
- Langford, K.H.; Thomas, K.V. Determination of pharmaceutical compounds in hospital effluents and their contribution to wastewater treatment works. Environ. Int. 2009, 35, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio-Albuquerque, E.P.; Cusioli, L.F.; Bergamasco, R.; Gigliolli, A.A.S.; Lupepsa, L.; Paupitz, B.R.; Barbieri, P.A.; Borin-Carvalho, L.A.; De Brito Portela-Castro, A.L. Metformin environmental exposure: A systematic review. Environ. Toxicol. Pharmacol. 2021, 83, 103588. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Sillanpää, M.; Jacob, M.M.; Vo, D.N. Metformin as an emerging concern in wastewater: Occurrence, analysis and treatment methods. Environ. Res. 2022, 213, 113613. [Google Scholar] [CrossRef]
- Zheng, Y.; Shao, Y.; Zhang, Y.; Liu, Z.; Zhao, Z.; Xu, R.; Ding, J.; Li, W.; Wang, B.; Zhang, H. Metformin as an emerging pollutant in the aquatic environment: Occurrence, analysis, and toxicity. Toxics 2024, 12, 483. [Google Scholar] [CrossRef]
- Yan, J.-H.; Xiao, Y.; Tan, D.-Q.; Shao, X.-T.; Wang, Z.; Wang, D.-G. Wastewater analysis reveals spatial pattern in consumption of anti-diabetes drug metformin in China. Chemosphere 2019, 222, 688–695. [Google Scholar] [CrossRef]
- Oosterhuis, M.; Sacher, F.; Ter Laak, T.L. Prediction of concentration levels of metformin and other high consumption pharmaceuticals in wastewater and regional surface water based on sales data. Sci. Total Environ. 2013, 442, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.D.; Crago, J.P.; Hedman, C.J.; Treguer, R.J.F.; Magruder, C.; Royer, L.S.; Klaper, R.D. Evaluation of a model for the removal of pharmaceuticals, personal care products, and hormones from wastewater. Sci. Total Environ. 2013, 444, 515–521. [Google Scholar] [CrossRef]
- Scheurer, M.; Michel, A.; Brauch, H.-J.; Ruck, W.; Sacher, F. Occurrence and fate of the antidiabetic drug metformin and its metabolite guanylurea in the environment and during drinking water treatment. Water Res. 2012, 46, 4790–4802. [Google Scholar] [CrossRef]
- Gaffney, V.J.; Cardoso, V.V.; Cardoso, E.; Teixeira, A.P.; Martins, J.; Benoliel, J.M.; Almeida, C.M.M. Occurrence and behaviour of pharmaceutical compounds in a Portuguese wastewater treatment plant: Removal efficiency through conventional treatment processes. Environ. Sci. Pollut. Res. 2017, 24, 14717–14734. [Google Scholar] [CrossRef]
- Al-Odaini, N.A.; Zakaria, M.P.; Yaziz, M.I.; Surif, S.; Abdulghani, M. The occurrence of human pharmaceuticals in wastewater effluents and surface water of Langat River and its tributaries, Malaysia. Int. J. Environ. Anal. Chem. 2013, 93, 245–264. [Google Scholar] [CrossRef]
- Zheng, Q.; Dewapriya, P.; Eaglesham, G.; Reeks, T.; Thompson, J.; Ahmed, F.; Prasad, P.; Thomas, K.V.; Mueller, J.F.; Thai, P.K. Direct injection analysis of oxypurinol and metformin in wastewater by hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry. Drug Test. Anal. 2022, 14, 1519–1524. [Google Scholar] [CrossRef]
- Ramzan, N.U.H.; Shahjahan, K.; Dhillon, R.A.; Khan, N.T.A.; Hashmat, M.B.; Anwer, M.U.; Ahmed, D.; Afzal, F.; Tahir, M.M.; Muzaffar, A. Vitamin B12 deficiency in patients taking metformin: Pathogenesis and recommendations. Cureus 2024, 16, e68550. [Google Scholar] [CrossRef]
- Sayedali, E.; Yalin, A.E.; Yalin, S. Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes. World J. Diabetes 2023, 14, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Koojiman, G.; De-Kreuk, M.K.; Houtman, C.; Van-Lier, J.B. Perspectives of coagulation/flocculation for the removal of pharmaceuticals from domestic wastewater: A critical view at experimental procedures. J. Water Process Eng. 2020, 34, 101161. [Google Scholar] [CrossRef]
- Alamo, A.C.; Pariente, M.I.; Martinez, F.; Molina, R. Trametes versicolor immobilized on rotating biological contactors as alternative biological treatment for the removal of emerging concern micropollutants. Water Res. 2020, 170, 115313. [Google Scholar] [CrossRef]
- Awafa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Photocatalytic degradation of organic micropollutants: Inhibition mechanisms by different fractions of natural organic matter. Water Res. 2020, 174, 115643. [Google Scholar] [CrossRef] [PubMed]
- Pelalak, R.; Alizadeh, R.; Ghareshabani, E. Enhanced heterogeneous catalytic ozonation of pharmaceutical pollutants using a novel nanostructure of iron-based mineral prepared via plasma technology: A comparative study. J. Hazard Mater. 2020, 392, 122269. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, B.; Venkateshwaran, U.; Durairaj, N.; Divyapriya, G.; Nambi, I.M.; Josep, A. Comprehensive treatment of urban wastewaters using electrochemical advanced oxidation process. J. Environ. Manag. 2020, 266, 110469. [Google Scholar] [CrossRef] [PubMed]
- Azmi, S.N.H.; Al-Lawati, W.M.; Al Hoqani, U.H.A.; Al Aufi, E.; Al Hatmi, K.; Al Zadjali, J.S.; Rahman, N.; Nasir, M.; Rahman, H.; Khan, S.A. Development of a citric acid-modified cellulose adsorbent derived from Moringa peregrina leaf for adsorptive removal of citalopram HBr in aqueous solutions. Pharmaceuticals 2022, 15, 760. [Google Scholar] [CrossRef]
- Rahman, N.; Varshney, P. Effective removal of doxycycline from aqueous solution using CuO nanoparticles decorated poly(2-acrylamido-2-methyl-1-propanesulfonic acid)/chitosan. Environ. Sci. Pollut. Res. 2021, 28, 43599–43617. [Google Scholar] [CrossRef]
- Rahman, N.; Varshney, P. Facile synthesis and characterization of Zn(II)- Impregnated chitosan/graphene oxide: Evaluation of its efficiency for removal of ciprofloxacin from aqueous solution. J. Inorg. Organomet. Polym. 2021, 31, 3595–3612. [Google Scholar] [CrossRef]
- Rahman, N.; Varshney, P. Assessment of ampicillin removal efficiency from aqueous solution by polydopamine/zirconium (IV) iodate: Optimization by response surface methodology. RSC Adv. 2020, 10, 20322–20337. [Google Scholar] [CrossRef]
- Mehdimoghadam, H.; Alijani, H.; Pourreza, N. Hydrophilic magnetic chitosan/gelatin hydrogel with enhanced fuel dehydration characteristics: Modeling, kinetic, isotherm and thermodynamic study. Int. J. Biol. Macromol. 2025, 289, 138812. [Google Scholar] [CrossRef]
- Mohammad, A.H.; Radovic, I.; Ivanović, M.; Kijevčanin, M. Adsorption of metformin on activated carbon produced from the water hyacinth biowaste using H3PO4 as a chemical activator. Sustainability 2022, 14, 11144. [Google Scholar] [CrossRef]
- Pap, S.; Shearer, L.; Gibb, S.W. Effective removal of metformin from water using an iron-biochar composite: Mechanistic studies and performance optimisation. J. Environ. Chem. Eng. 2023, 11, 110360. [Google Scholar] [CrossRef]
- Çavuşoğlu, F.C.; Bayazit, Ş.S.; Secula, M.S.; Cagnon, B. Magnetic carbon composites as regenerable and fully recoverable adsorbents: Performance on the removal of antidiabetic agent metformin hydrochloride. Chem. Eng. Res. Des. 2021, 168, 443–452. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Quesada, H.B.; De Brito Portela Castro, A.L.; Gomes, R.G.; Bergamasco, R. Development of a new low-cost adsorbent functionalized with iron nanoparticles for removal of metformin from contaminated water. Chemosphere 2020, 247, 125852. [Google Scholar] [CrossRef] [PubMed]
- Alnajjar, M.; Hethnawi, A.; Nafie, G.; Hassan, A.; Vitale, G.; Nassar, N.N. Silica alumina composite as an effective adsorbent for the removal of metformin from water. J. Environ. Chem. Eng. 2019, 7, 102994. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Briones, R.M.; Thiele-Bruhn, S.; Sen, R.; Sarmah, A.K. Adsorptive removal of metformin on specially designed algae-lignocellulosic biochar mix and techno-economic feasibility assessment. Environ. Pollut. 2021, 292, 118256. [Google Scholar] [CrossRef]
- Neha, S.; Rajput, P.; Remya, N. Biochar from microwave co-pyrolysis of food waste and polyethylene using different microwave susceptors—Production, modification and application for metformin removal. Environ. Res. 2022, 210, 112922. [Google Scholar] [CrossRef]
- Rebitski, E.P.; Aranda, P.; Darder, M.; Carraro, R.; Ruiz-Hitzky, E. Intercalation of metformin into montmorillonite. Dalton Trans. 2018, 47, 3185–3192. [Google Scholar] [CrossRef]
- Biswas, U.K.; Bose, A.; Ghosh, B.; Sharma, S. An insight into chemically modified chitosan and their biological, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 2025, 303, 140612. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.H.; Hussain, N.; Muneer, M.A.; Arif, I.; Ali, M.R. Chitosan-based nanomaterials for pharmaceutical waste remediation. In Advances in Chemical Pollution, Environmental Management and Protection; Elsevier: Amsterdam, The Netherlands, 2023; pp. 83–116. [Google Scholar] [CrossRef]
- Tamer, T.M.; Hassan, M.A.; Valachová, K.; Omer, A.M.; El-Shafeey, M.E.; Eldin, M.S.M.; Šoltés, L. Enhancement of wound healing by chitosan/hyaluronan polyelectrolyte membrane loaded with glutathione: In vitro and in vivo evaluations. J. Biotechnol. 2020, 310, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Ahmed, M.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. pH-sensitive alginate/carboxymethyl chitosan/aminated chitosan microcapsules for efficient encapsulation and delivery of diclofenac sodium. Pharmaceutics 2021, 13, 338. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Xie, D.; Wang, X. Multi-enzyme-immobilized robust self-floating covalent organic framework/chitosan aerogels for the efficient remediation of emerging pollutants in water. J. Environ. Chem. Eng. 2024, 12, 114791. [Google Scholar] [CrossRef]
- Al-Harthy, E.; Shaker, M.A.; Yakout, A.A. Synergetic Enhancement of Copper and Manganese Dioxide Nanoparticles With the biobased Chitosan-crosslinked-graphene Nanocomposite for Potential Adsorptive Remediation of Tetracycline Antibiotics From Food Samples. Mater. Today Commun. 2025, 1, 112930. [Google Scholar] [CrossRef]
- Yuwei, C.; Jianlong, W. Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu (II) removal. Chem. Eng. J. 2011, 168, 286–292. [Google Scholar] [CrossRef]
- Islam, M.N.; Khan, M.N.; Mallik, A.K.; Rahman, M.M. Preparation of bio-inspired trimethoxysilyl group terminated poly (1-vinylimidazole)-modified-chitosan composite for adsorption of chromium (VI) ions. J. Hazard. Mater. 2019, 379, 120792. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.A.; Rios, R.V.R.A.; Fabris, J.D.; Sapag, K.; Garg, V.K.; Lago, R.M. Clay–iron oxide magnetic composites for the biosorption of contaminants in water. Appl. Clay Sci. 2003, 22, 169–177. [Google Scholar] [CrossRef]
- Graves, D.J. Bioseparations in the magnetically stabilized fluidized bed. Chromatogr. Sci. 1993, 61, 187–207. [Google Scholar]
- Huang, G.L.; Yang, C.; Zhang, K.; Shi, J. Adsorption removal of copper ions from aqueous solution using cross-linked magnetic chitosan bead. Chin. J. Chem. Eng. 2009, 17, 960–966. [Google Scholar] [CrossRef]
- Dyal, A.; Loos, K.; Noto, M.; Chang, S.W.; Spagnoli, C.; Shafi, K.V.P.M.; Ulman, A.; Cowman, M.; Gross, R.A. Activity of Candida rugosa lipase immobilized on -Fe2O3 magnetic nanoparticles. J. Am. Chem. Soc. 2003, 125, 1664–1665. [Google Scholar] [CrossRef]
- Liao, M.H.; Chen, D.H. Preparation characterization of a novel magnetic nano-adsorbent. J. Mater. Chem. 2002, 12, 3654–3659. [Google Scholar] [CrossRef]
- Bulin, C.; Guo, T.; Ma, Y. Spectroscopic and statistical physics elucidation for Cu(II) remediation using magnetic bio adsorbent based on Fe3O4-chitosan-graphene oxide. Int. J. Biol. Macromol. 2024, 276 Pt 1, 133895. [Google Scholar] [CrossRef]
- Saberi-Zare, M.; Bodaghifard, M.A. A Schiff base-functionalized chitosan magnetic bio-nanocomposite for efficient removal of Pb (II) and Cd (II) ions from aqueous solutions. Int. J. Biol. Macromol. 2025, 296, 39794. [Google Scholar] [CrossRef]
- Billah, R.E.; Islam, M.A.; Khan, M.A.; Nazal, M.K.; Bahsis, L.; Achak, M.; Mahmoud, A.E.; Abdul Aziz, M.; Jeon, B.-H. Adsorption of lead(II) onto magnetic chitosan@calcium phosphate rock biocomposite. Mater. Chem. Phys. 2025, 332, 130249. [Google Scholar] [CrossRef]
- Dang, M.; Yin, J.; Wu, M.; Liu, C. Study on the preparation of a novel acrylic modified magnetic chitosan adsorbent and the adsorption behavior on Ga (III). Sep. Purif. Technol. 2025, 358, 130245. [Google Scholar] [CrossRef]
- Bulin, C.; Guo, T.; Zheng, R. Preparation of ion imprinted EDTA modified chitosan-magnetic graphene oxide for selective recovery and adsorption mechanism of Ce(III). Sci. Total Environ. 2025, 962, 178468. [Google Scholar] [CrossRef]
- Alyasi, H.; Wahib, S.; Tong, Y.; Gomez, T.; Mahmoud, K.A. Magnetic MXene chitosan-lignosulfonate composite (Fe3O4@ MCLS) for the reductive removal of Cr(VI) and other heavy metals from water. J. Hazard. Mater. Adv. 2025, 17, 100536. [Google Scholar] [CrossRef]
- Sadat, Z.; Eivazzadeh-Keihan, R.; Kashtiara, A.; Maleki, A. Magnetic gelatin-chitosan hydrogel containing zinc chromite nanoparticles as novel and antibacterial nanostructure for efficient removal of methylene blue and crystal violet from aqueous solutions. J. Environ. Chem. Eng. 2024, 120–126, 114875. [Google Scholar] [CrossRef]
- Jawad, A.H.; Maharani, R.A.; Hapiz, A.; Khadiran, T.; Jani, N.A.; ALOthman, Z.A.; Wilson, L.D. Freeze-drying synthesis of mesoporous magnetic grafted chitosan/calcium oxide nanoparticle for remazol brilliant blue dye removal: A statistical optimization. Int. J. Biol. Macromol. 2025, 286, 138373. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizade-Shirvan, A.; Ghasemi, A.; Mazaheri, Y.; Shokri, S.; Fallahizadeh, S.; Sani, M.A.; Mohtashami, M.; Mahmoudzadeh, M.; Sarafraz, M.; Darroudi, M.; et al. Removal of aflatoxin M1 in milk using magnetic laccase/MoS2/chitosan nanocomposite as an efficient sorbent. Chemosphere 2024, 365, 143334. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, F.; Liu, X.; Zheng, W.; Wang, Z.; Yang, S.; Tang, X. Magnetic chitosan supported copper particles as a heterogeneous catalyst for benzaldehyde glycol acetal reaction. Int. J. Biol. Macromol. 2024, 281, 136269. [Google Scholar] [CrossRef]
- Hassanzadeh, N.; Dekamin, M.G.; Valiey, E. A supramolecular magnetic and multifunctional Titriplex V-grafted chitosan organocatalyst for the synthesis of acridine-1,8-diones and 2-amino-3-cyano-4H-pyran derivatives. Nanoscale Adv. 2024, 7, 99–123. [Google Scholar] [CrossRef]
- Tan, Z.; Shi, H.; Zheng, Y.; He, W.; Xu, W.; Cao, Y.; Liao, J.; Dan, Z.; Huang, S. Application of microreactor constructed with micro-mixing channel featuring 3D lateral secondary flow structure for ultra-small particle size preparation of magnetic chitosan nanodrug carriers. Chem. Eng. J. 2024, 499, 156040. [Google Scholar] [CrossRef]
- Yousefi, Q.; Nezamzadeh-Ejhieh, A. A chitosan-based magnetic system for response surface methodology (RSM) optimization of the influencing variables in ciprofloxacin loading/releasing. Int. J. Biol. Macromol. 2024, 283, 137717. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, M.; Bajpai, S.K. Chitosan–magnetite nanocomposites (magnetic chitosan nanoparticles) as magnetic carrier particles for removal of Fe(III) from aqueous solutions. Colloids Surf. A Physicochem. Eng. 2008, 320, 161–168. [Google Scholar] [CrossRef]
- Li, J.; Su, J.; Yang, Q.; Yang, Z. Hydrothermal synthesis of Zr-doped chitosan carbon-shell protected magnetic composites (Zr–Fe3O4@C) for stable removal of Cr(VI) from water: Enhanced adsorption and pH adaptability. Mater. Chem. Phys. 2023, 306, 128057. [Google Scholar] [CrossRef]
- Fedushchak, T.A.; Petrenko, T.V.; Vosmerikov, A.V.; Velichkina, L.M. Inorganic reagents for testing the properties of copper nanopowders. J. Anal. Chem. 2009, 64, 566–570. [Google Scholar] [CrossRef]
- Denkbas, E.; Kilicay, E.; Ozturk, E. Magnetic chitosanmicrospheres: Preparation and characterization. Reac. Func. Polym. 2002, 50, 225–232. [Google Scholar] [CrossRef]
- Zhou, L.M.; Wang, Y.P.; Liu, Z.R.; Huang, Q.W. Characteristics of equilibrium kinetic studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea modified magnetic chitosan microspheres. J. Hazard. Mater. 2009, 161, 995–1002. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer: Shelton, CT, USA, 1992. [Google Scholar]
- Tang, X.Q.; Zhang, Y.D.; Jiang, Z.W.; Wang, D.M.; Huang, C.Z.; Li, Y.F. Fe3O4 and metal–organic framework MIL-101 (Fe) composites catalyze luminol chemiluminescence for sensitively sensing hydrogen peroxide and glucose. Talanta 2018, 179, 43–50. [Google Scholar] [CrossRef]
- Gao, W.; Zhong, D.; Xu, Y.; Luo, H.; Zeng, S. Nano zero-valent iron supported by macroporous styrene ion exchange resin for enhanced Cr (VI) removal from aqueous solution. J. Dispers. Sci. Technol. 2020, 2, 1197–1207. [Google Scholar] [CrossRef]
- Al-Janabi, N.K.W.S.; Mahmood, N.A.K.; Luaibi, N.H.M. Determination of the Dissociation Constants of Metformin from a Second Derivative UV Spectrum. Int. J. Res. Pharm. Sci. 2020, 11, 790–796. [Google Scholar] [CrossRef]
- Yakout, A.A.; Alshutairi, A.M.; Albishri, H.M.; Alshitari, W.H.; Basha, M.T. Cu-nanoparticles graphene nanocomposite: A robust and efficient nanocomposite for micro-solid phase extraction of trace aflatoxins in different foodstuffs. Food Chem. 2024, 440, 138239. [Google Scholar] [CrossRef]
- Shaker, M.A.; Alshitari, W.H.; Basha, M.T.; Asim, M.; Albishri, H.M.; Bhawani, S.A.; Yakout, A.A. Synergetic impact of copper nanoparticles and polyaniline reinforced graphene oxide nanocomposite on the sequestration of tetracycline antibiotic from milk and wastewaters samples. Mater. Today Commun. 2024, 38, 107869. [Google Scholar] [CrossRef]
- Yakout, A.A.; Mahmoud, M.E. Fabrication of magnetite-functionalized-graphene oxide and hexadecyltrimethyl ammonium bromide nanocomposite for efficient nanosorption of sunset yellow. Mater. Sci. Eng. C 2018, 92, 287–296. [Google Scholar] [CrossRef]
- Yakout, A.A.; Alshitari, W.; Akhdhar, A. Synergistic effect of Cu-nanoparticles and β-cyclodextrin functionalized reduced graphene oxide nanocomposite on the adsorptive remediation of tetracycline antibiotics. Carbohydr. Polym. 2021, 273, 118528. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, X.; He, Y.; Chen, Y.; Luo, X.; Shang, R. Study on adsorption of tetracycline by Cu-immobilized alginate adsorbent from water environment. Int. J. Biol. Macromol. 2019, 12, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Yu, F.; Ma, J.; Chen, J.H. Enhanced adsorption removal of antibiotics from aqueous solutions by modified alginate/graphene double network porous hydrogel. J. Colloid Interface Sci. 2017, 507, 250–259. [Google Scholar] [CrossRef]
- Han, X.L.; Wang, W.; Ma, X.J. Adsorption characteristics of methylene blue onto low-cost biomass material lotus leaf. Chem. Eng. J. 2011, 171, 1–8. [Google Scholar] [CrossRef]
- Ma, Q.; Song, T.-Y.; Yuan, P.; Wang, C.; Su, X.-G. QDs-labeled microspheres for the adsorption of rabbit immunoglobulin G and fluoroimmuno-assay. Colloids Surf. B 2008, 64, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Quesada, H.B.; De Araújo, T.P.; Cusioli, L.F.; De Barros, M.A.S.D.; Gomes, R.G.; Bergamasco, R. Evaluation of novel activated carbons from chichá-do-cerrado (Sterculia striata St. Hil. et Naud) fruit shells on metformin adsorption and treatment of a synthetic mixture. J. Environ. Chem. Eng. 2020, 9, 104914. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.G.; Liu, S.B.; Zeng, G.M.; Jiang, L.H.; Tan, X.F.; Zhou, L.; Zeng, W.; Li, T.T.; Yang, C.P. Adsorption of emerging contaminant metformin using graphene oxide. Chemosphere 2017, 179, 20–28. [Google Scholar] [CrossRef]
- Niaei, H.A.; Rostamizadeh, M. Adsorption of metformin from an aqueous solution by Fe-ZSM-5 nano-adsorbent: Isotherm, kinetic and thermodynamic studies. J. Chem. Thermodyn. 2020, 142, 106003. [Google Scholar] [CrossRef]
- Oluwatimileyin, S.J.; Asiata Omotayo, I.; Olugbenga, S.B. Metformin adsorption onto activated carbon prepared by acid activation and carbonization of orange peel. Int. J. Phytoremediat. 2022, 25, 125–136. [Google Scholar] [CrossRef]
- Kalumpha, M.; Guyo, U.; Zinyama, N.P.; Vakira, F.M.; Nyamunda, B.C. Adsorptive potential of Zea mays tassel activated carbon towards the removal of metformin hydrochloride from pharmaceutical effluent. Int. J. Phytoremed. 2020, 22, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.R.; Abdulkhair, B.Y.; Algethami, F.K.; Khezami, L. Linear and nonlinear investigations for the adsorption of paracetamol and metformin from water on acid-treated clay. Sci. Rep. 2021, 11, 13606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J. Colloid Interface Sci. 2011, 361, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Yakout, A.A.; Basha, M.T.; Al-Raimi, D.S.; Aljadaani, A.H.; Zainy, F.M.A. Synergistic impact of Cu and MnO2 nanoparticles in Cu/MnO2/chitosan/graphene oxide hybrid nanocomposite for improved antibacterial activity and fast adsorption of environmentally relevant anionic and cationic dyes from industrial wastewater. Int. J. Biol. Macromol. 2025, 1, 144839. [Google Scholar] [CrossRef]



| Kinetic Model/Linear Equation | Kinetic Parameters | 10.0 mg∙L−1 | 20.0 mg∙L−1 | 40.0 mg∙L−1 |
|---|---|---|---|---|
| qe, exp. (mg g−1) | 18.20 | 31.42 | 60.15 | |
| Pseudo-second order | ||||
| qe, calc. (mg g−1) | 18.59 | 30.86 | 59.17 | |
| K2 (g mg −1 min −1) | 7.0 × 10−3 | 0.011 | 0.008 | |
| R2 | 0.9952 | 0.9953 | 0.9980 | |
| Pseudo-first order | ||||
| qe, calc. (mg g−1) | 19.63 | 35.74 | 143.5 | |
| K1 (min−1) | 0.252 | 0.329 | 0.768 | |
| R2 | 0.9541 | 0.9691 | 0.9349 | |
| Intraparticle diffusion | ||||
| Kp (mg·g−1·min−1/2) | 7.345 | 3.307 | 9.206 | |
| C | 3.643 | 0.343 | 17.09 | |
| R2 | 0.9909 | 0.9688 | 0.8868 | |
| Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | KF (L mg−1) | n | R2 | Kr | f | R2 |
| 52.91 | 1.268 | 0.9963 | 3.0 × 1012 | 0.0900 | 0.9395 | 11.10 | 13.40 | 0.9881 |
| Adsorbent | Adsorption Parameters | qmax | Reference |
|---|---|---|---|
| Multi-walled carbon nanotubes, commercial | pH = 8.20, T = 25 °C | 79.94 | [81] |
| Graphene oxide | pH = 6; contact time 160 min T = 15, 30, and 45 °C. | 96.748, 89.099, 88.517 | [82] |
| Fe-ZSM-5 nano-adsorbent | Contact time 20 min. T = 25 °C | 14.992 | [83] |
| Activated carbon from orange peel | pH = 7, contact time 240 min, T = 50 °C | 50.99 | [84] |
| Activated carbon from agricultural waste | pH = 7, contact time 125 min, T = 20 °C | 44.84 | [85] |
| Water-treated clay | pH = 6, contact time 30 min, T = 25 °C | 25.268 | [86] |
| Cu@MCS | pH = 6, contact time 20 min, T = 25 °C | 52.91 | The present study |
| Water Sample | Sample Parameters | Conc. Added (mg L−1) | Conc. Found (mg L−1) | Recovery (%) | |
|---|---|---|---|---|---|
| pH | Conductivity (μs cm−1) | ||||
| River water | 7.83 | 324.0 | 5.0 | 4.98 | 99.7 ± 4.2 |
| 10.6 | 10.59 | 99.9 ± 3.7 | |||
| 20.0 | 19.96 | 99.8 ± 4.5 | |||
| Wastewater | 9.05 | 304.7 | 5.3 | 5.29 | 99.8 ± 4.1 |
| 10.2 | 10.18 | 99.8 ± 3.5 | |||
| 20.0 | 19.92 | 99.6 ± 3.7 | |||
| Tap water | 7.13 | 81.3 | 5.0 | 4.98 | 99.7 ± 2.7 |
| 10.4 | 10.36 | 99.6 ± 3.0 | |||
| 20.3 | 20.20 | 99.5 ± 2.5 | |||
| Bottled water | 8.62 | 24.15 | 10.2 | 10.17 | 99.7 ± 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ahmary, K.M.; Aljadaani, A.H.; Yakout, A.A. Synergistic Impact of Copper Nanoparticles Functionalized with Magnetic Chitosan on the Enhanced Adsorptive Sequestration of Metformin Diabetic Drug from Environmental Samples. Polymers 2025, 17, 3046. https://doi.org/10.3390/polym17223046
Al-Ahmary KM, Aljadaani AH, Yakout AA. Synergistic Impact of Copper Nanoparticles Functionalized with Magnetic Chitosan on the Enhanced Adsorptive Sequestration of Metformin Diabetic Drug from Environmental Samples. Polymers. 2025; 17(22):3046. https://doi.org/10.3390/polym17223046
Chicago/Turabian StyleAl-Ahmary, Khairia M., Abeer H. Aljadaani, and Amr A. Yakout. 2025. "Synergistic Impact of Copper Nanoparticles Functionalized with Magnetic Chitosan on the Enhanced Adsorptive Sequestration of Metformin Diabetic Drug from Environmental Samples" Polymers 17, no. 22: 3046. https://doi.org/10.3390/polym17223046
APA StyleAl-Ahmary, K. M., Aljadaani, A. H., & Yakout, A. A. (2025). Synergistic Impact of Copper Nanoparticles Functionalized with Magnetic Chitosan on the Enhanced Adsorptive Sequestration of Metformin Diabetic Drug from Environmental Samples. Polymers, 17(22), 3046. https://doi.org/10.3390/polym17223046






