The Concept of a 3D-Printed Microfluidic Device on Oxyfluorinated PDMS Substrates
Abstract
1. Introduction
2. Materials and Methods
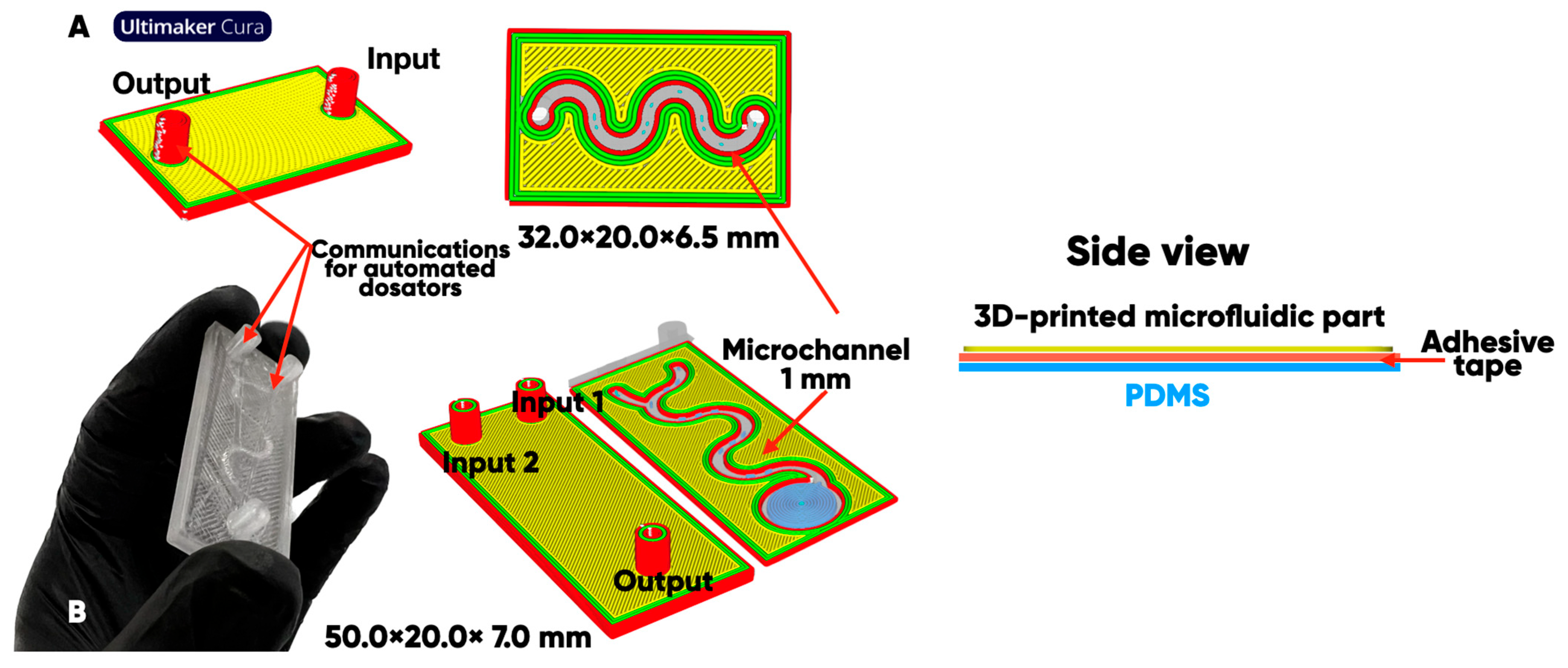
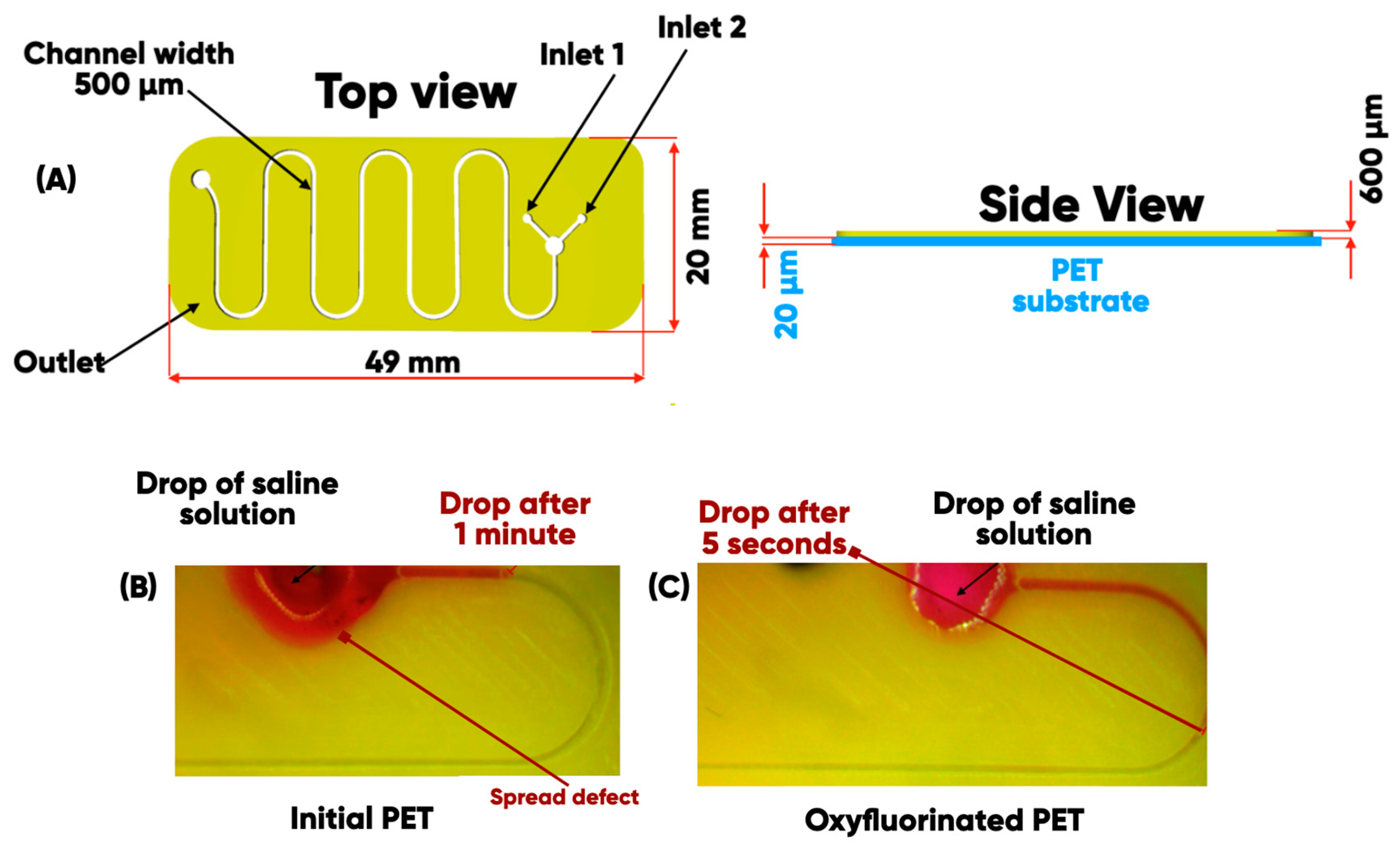
2.1. The Digital Design and Additive Manufacturing of Microfluidic Bioreactor
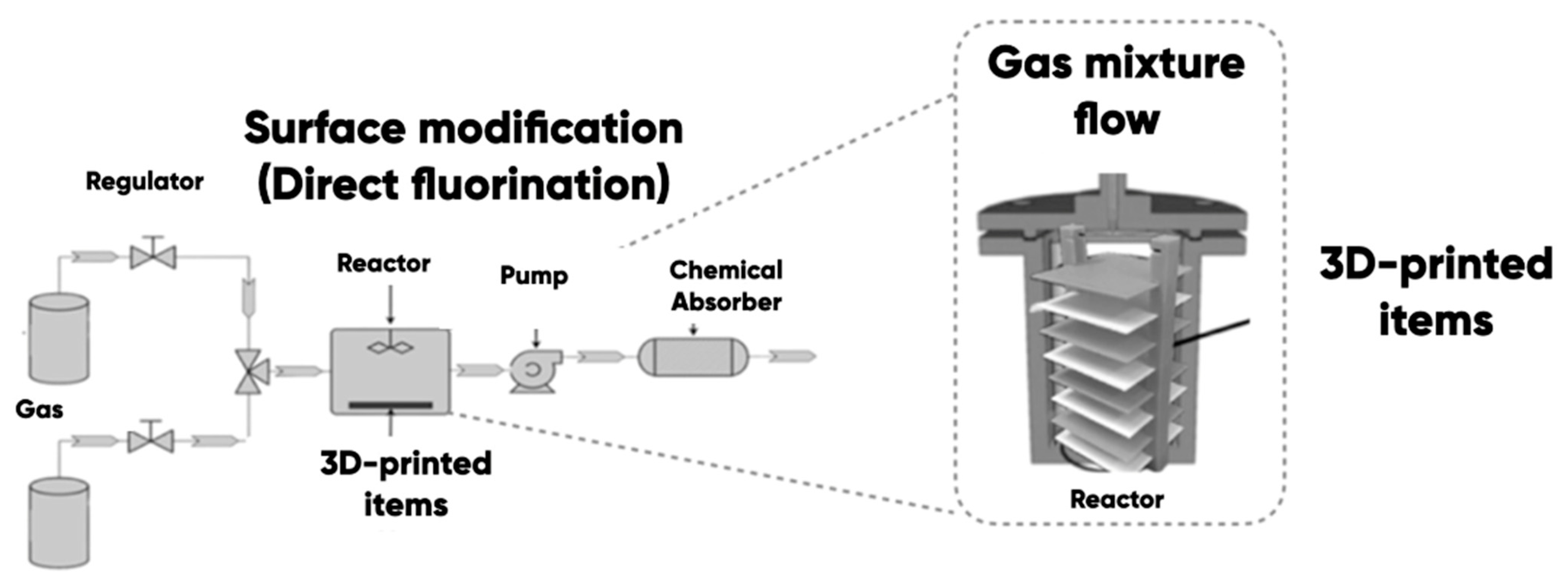
2.2. Direct Oxyfluorination of 3D-Printed Items
2.3. Investigation of the 3D-Printed Items and Microfluidic Devices Properties
3. Results and Discussion
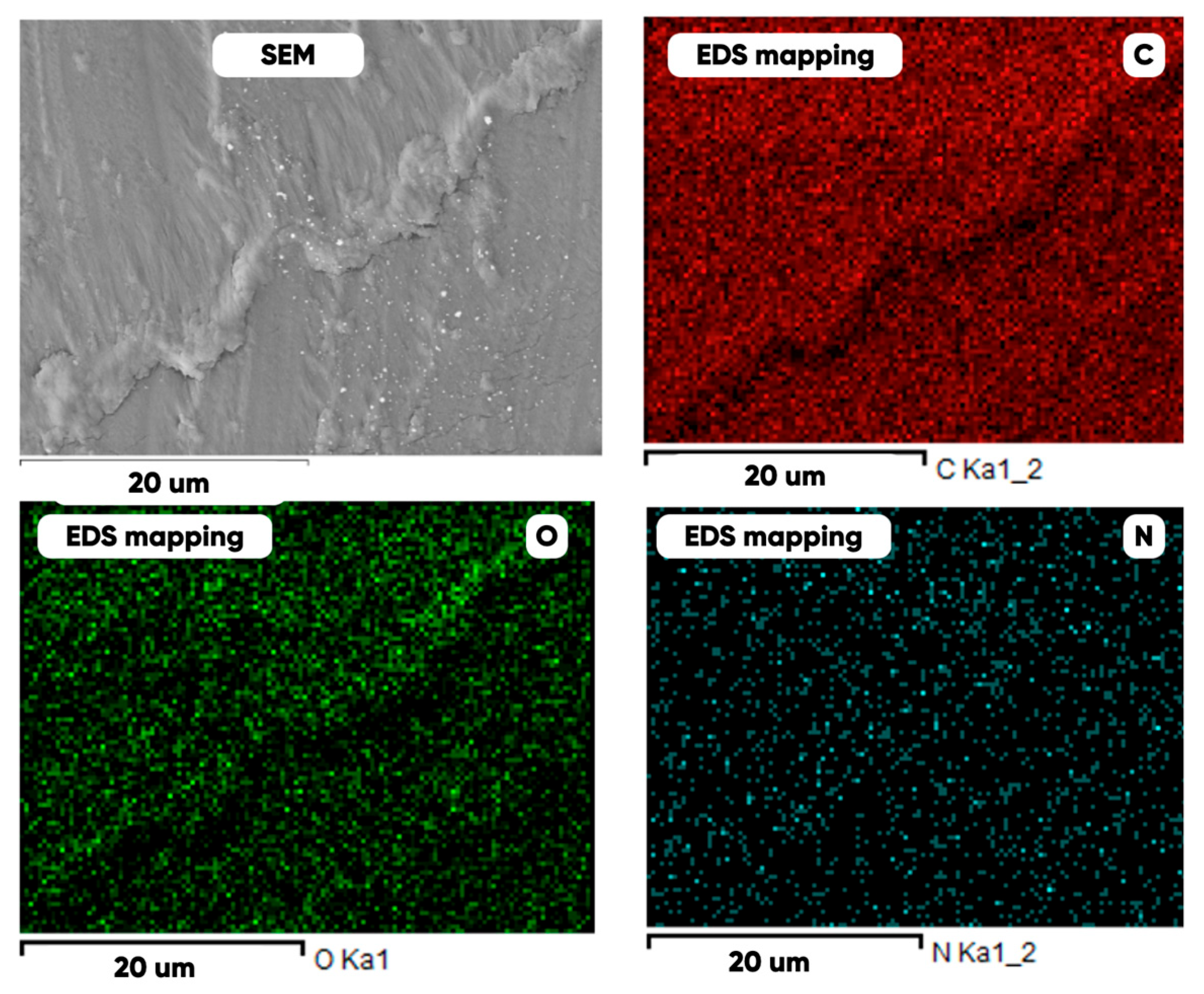
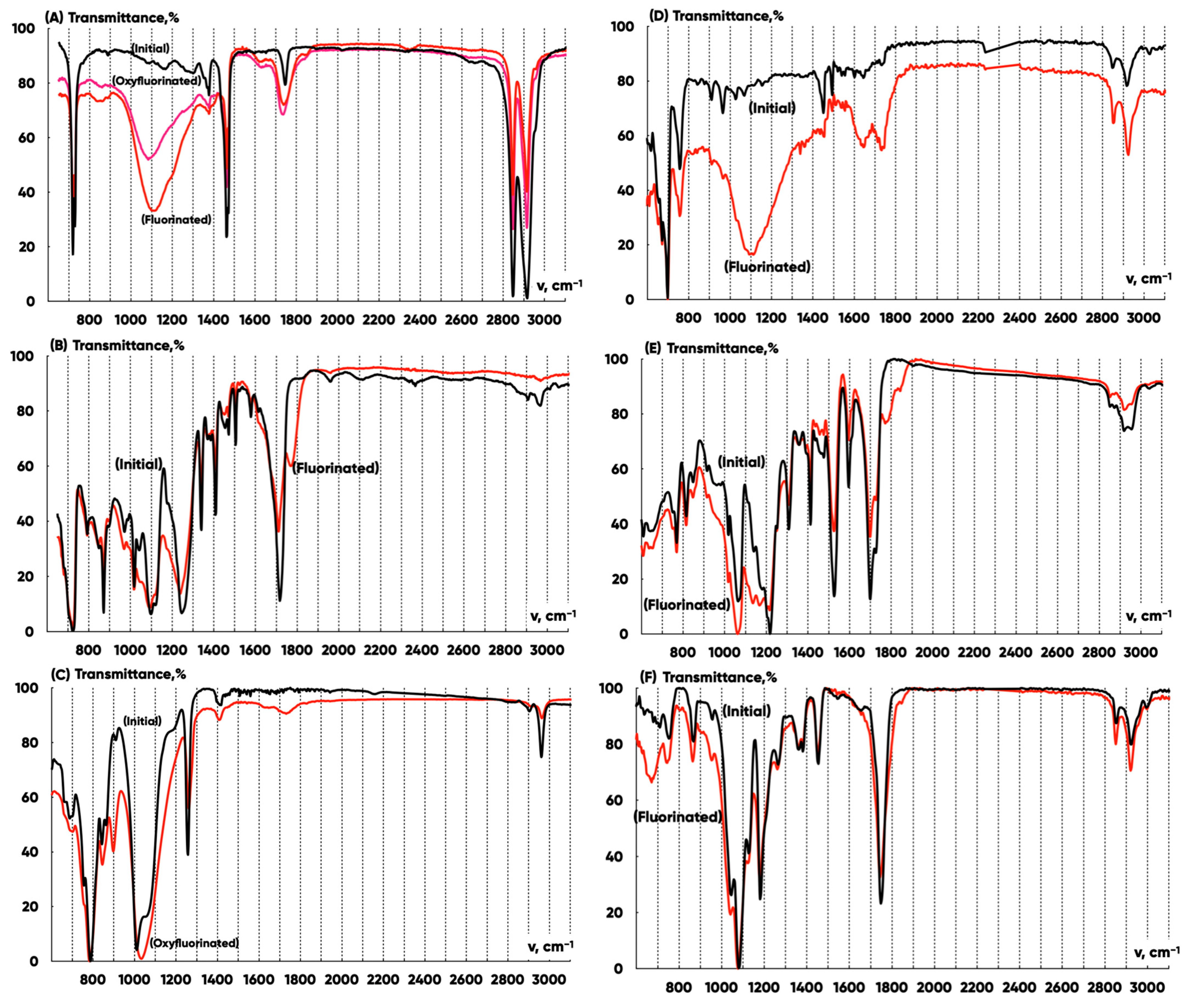
3.1. Experimental Samples Characterization
- -
- Fluorine atoms replace hydrogen ones effectively in and macromolecules’ groups. The absorption decreases in ~2950–3050 and grows in the ~800–1300 wave number ranges for all the samples;
- -
- (–C=O) groups are formed. The absorption increases for all samples in the ~1750–1810 wave number range;
- -
- and groups appear. The absorption increases for all the samples in the ~1000–1200 wave number range.
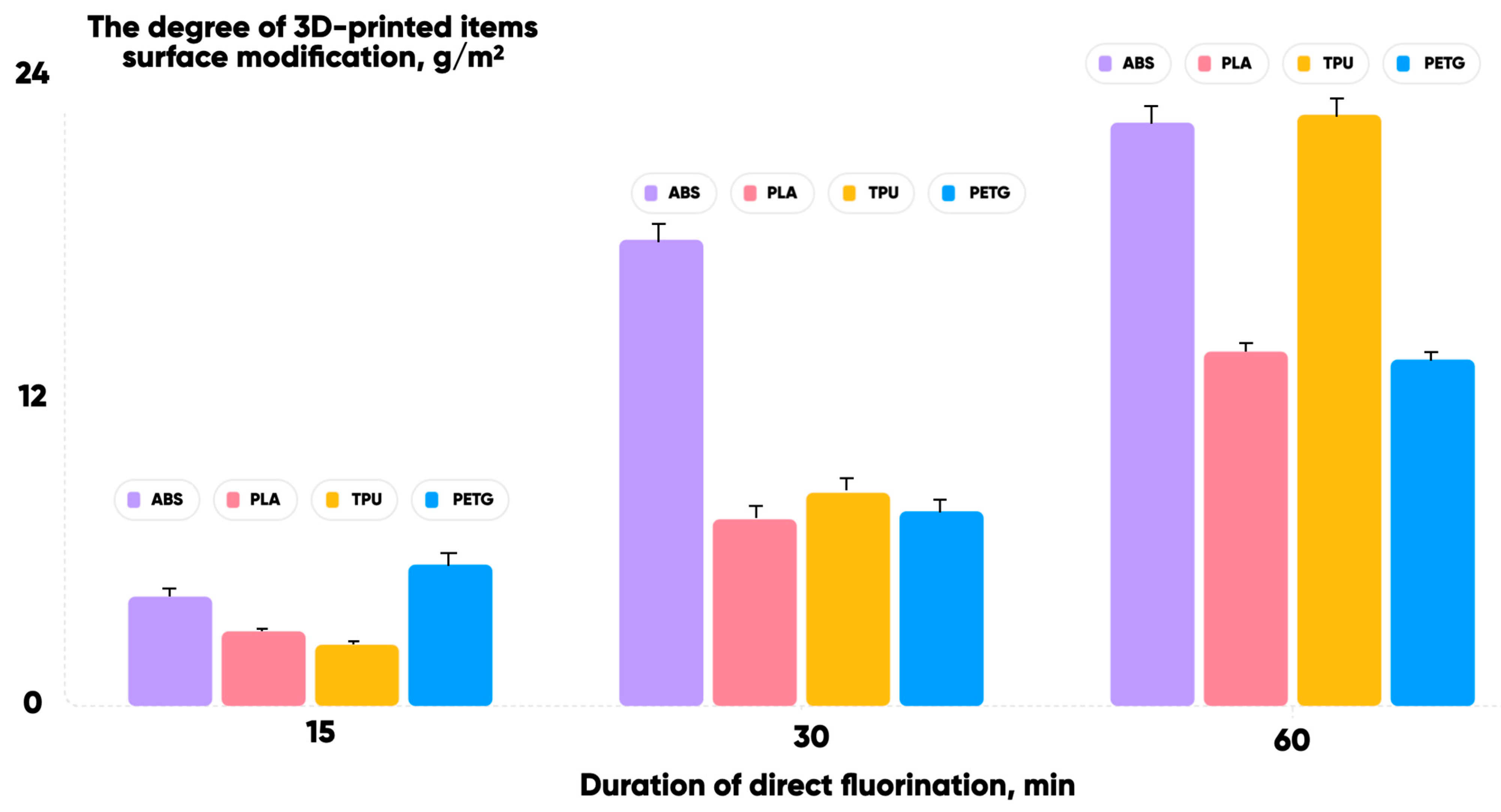
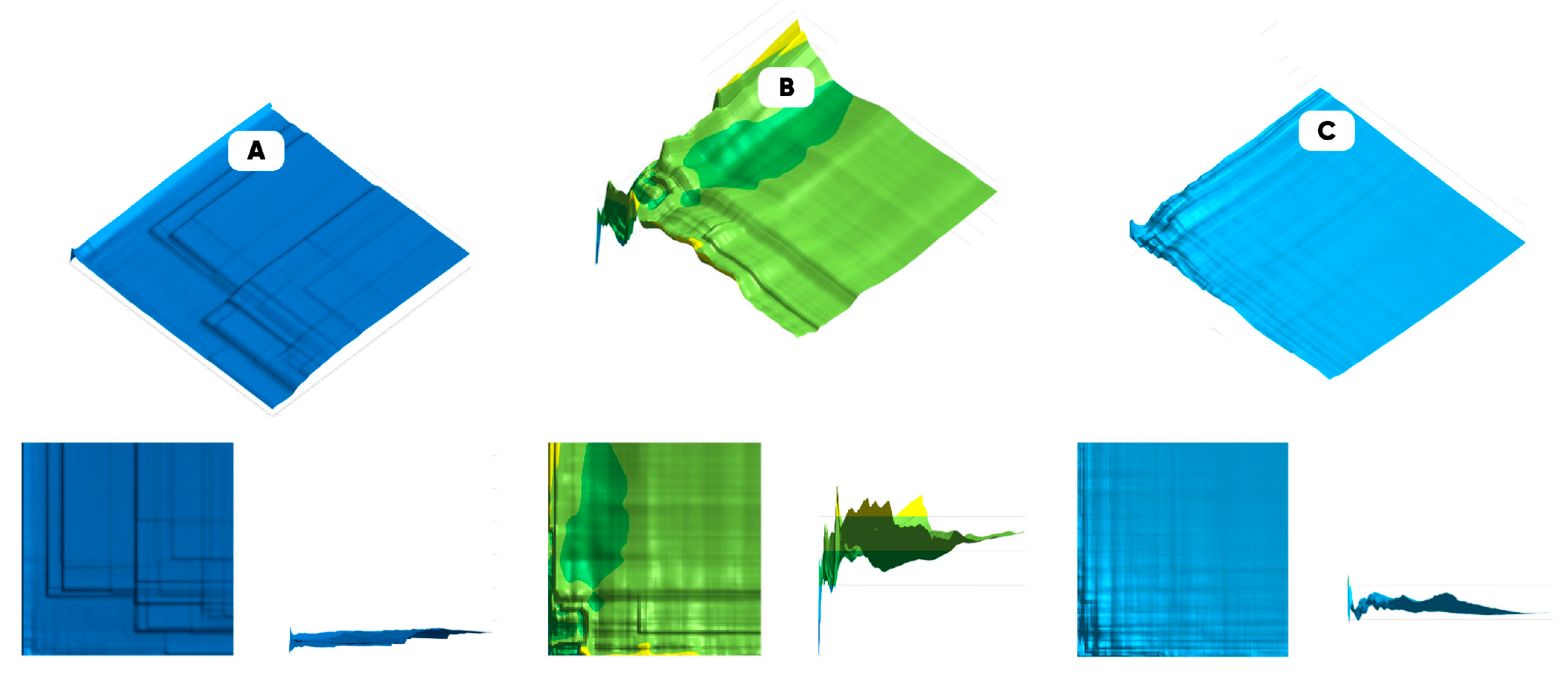
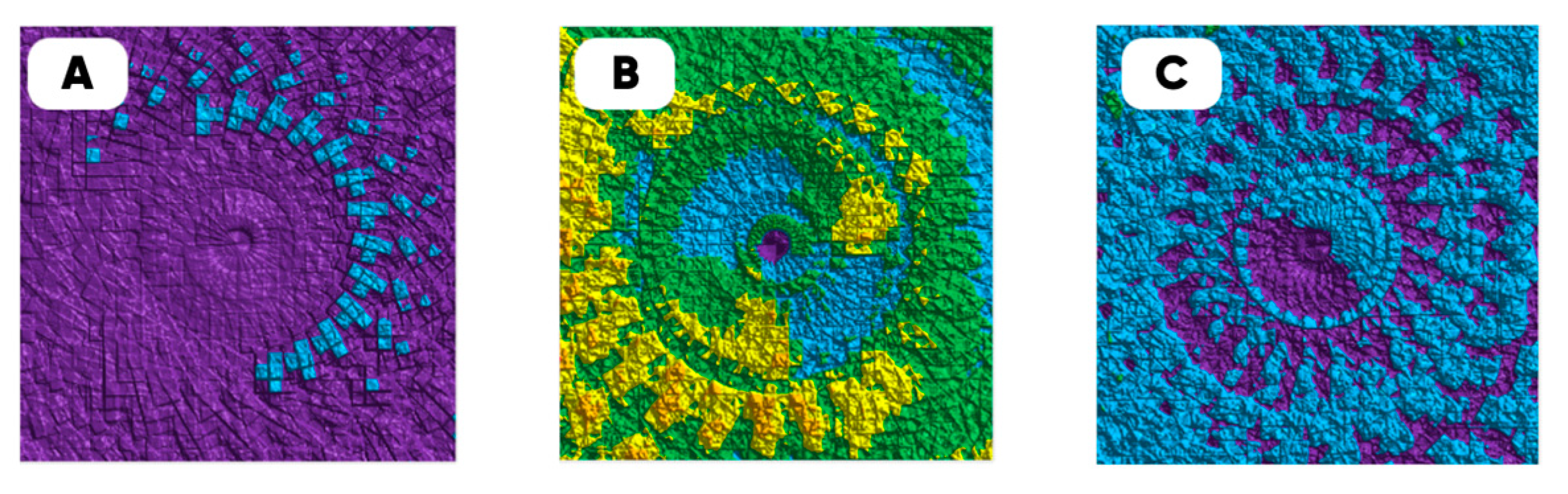
3.2. Oxyfluorination Effect on the 3D-Printed Samples’ Properties
- (1)
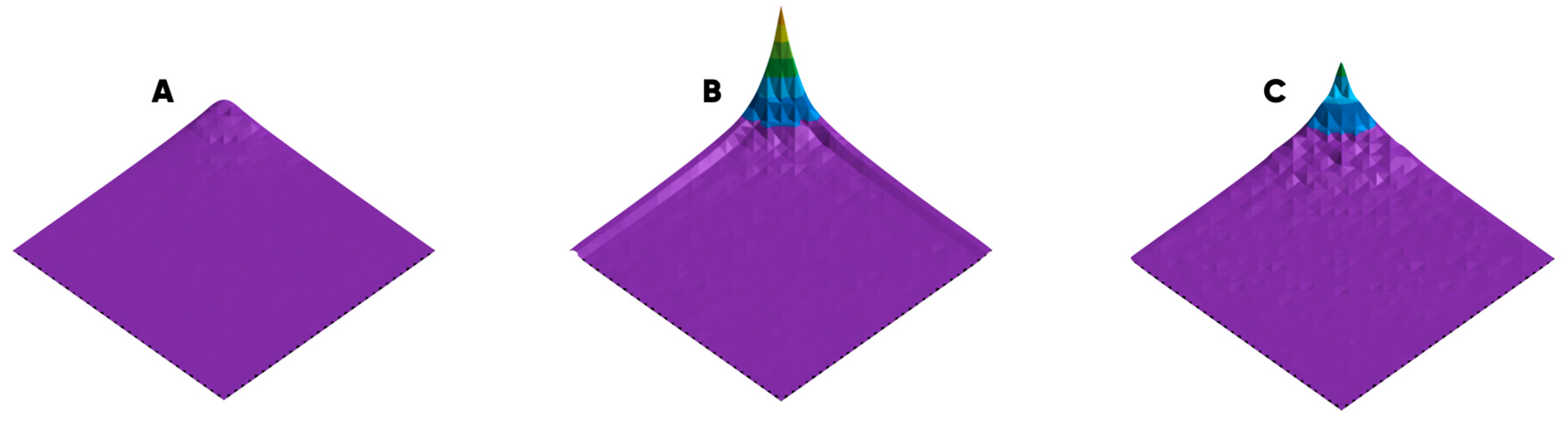
- For type A images, in which cells can still be seen separately, the number of pixels representing one cell was determined (values between 40 and 70 pixels were obtained).
- (2)
- A representative image region was chosen for which the average pixel brightness and its standard deviation were calculated.
- (3)
- To estimate the number of observed cells, the total number of pixels whose brightness exceeds the sum of the average value and standard deviation was divided by the number of pixels used to display one cell.
- (4)
- Paragraphs 2 and 3 were repeated for the following representative image fragments in the working cycle of the computing script.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuhr, G.; Glasser, H.; Müller, T.; Schnelle, T. Cell manipulation and cultivation under a.c. electric field influence in highly conductive culture media. Biochim. Biophys. Acta (BBA)/Gen. Subj. 1994, 1201, 353–360. [Google Scholar] [CrossRef]
- Qarri, A.; Rinkevich, Y.; Rinkevich, B. Employing marine invertebrate cell culture media for isolation and cultivation of thraustochytrids. Bot. Mar. 2021, 64, 447–454. [Google Scholar] [CrossRef]
- Rohde, F.; Danz, K.; Jung, N.; Wagner, S.; Windbergs, M. Electrospun scaffolds as cell culture substrates for the cultivation of an in vitro blood–brain barrier model using human induced pluripotent stem cells. Pharmaceutics 2022, 14, 1308. [Google Scholar] [CrossRef]
- Butenko, R.G. Cultivation of isolated protoplasts and hybridization of somatic plant cells. Int. Rev. Cytol. 1979, 59, 323–373. [Google Scholar]
- Patel, J.; Vries, R.G.J.; Flynn, A.; Wang, X.; Proud, C.G.; McLeod, L.E. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur. J. Biochem. 2002, 269, 3076–3085. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.-J.; Cheng, F.-F.; Zheng, T.-T.; Zhu, J.-J.; Wang, C. Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery. J. Mater. Chem. 2011, 21, 12034–12040. [Google Scholar] [CrossRef]
- Wang, X.-W.; Zhang, W.-B. Cellular synthesis of protein catenanes. Angew. Chem. Int. Ed. 2016, 55, 3442–3446. [Google Scholar] [CrossRef]
- Coulibaly, L.; Naveau, H.; Agathos, S.N. A tanks-in-series bioreactor to simulate macromolecule-laden wastewater pretreatment under sewer conditions by aspergillus niger. Water Res. 2002, 36, 3941–3948. [Google Scholar] [CrossRef]
- Procházková, G.; Brányiková, I.; Brányik, T.; Zachleder, V. Effect of nutrient supply status on biomass composition of eukaryotic green microalgae. J. Appl. Phycol. 2014, 26, 1359–1377. [Google Scholar] [CrossRef]
- Guo, J.; Liu, W.; Zhu, C.; Luo, G.; Kong, Y.; Ling, N.; Wang, M.; Shen, Q.; Guo, S.; Dai, J. Bacterial rather than fungal community composition is associated with microbial activities and nutrient-use efficiencies in a paddy soil with short-term organic amendments. Plant Soil 2018, 424, 335–349. [Google Scholar] [CrossRef]
- Benckiser, G. Plastics, micro- and nanomaterials, and virus-soil microbe-plant interactions in the environment. In Plant Nanobionics: Volume 1, Advances in the Understanding of Nanomaterials Research and Applications; Springer International Publishing: Cham, Switzerland, 2019; pp. 83–101. [Google Scholar]
- Achinas, S.; Heins, J.-I.; Krooneman, J.; Euverink, G.J.W. Miniaturization and 3d printing of bioreactors: A technological mini review. Micromachines 2020, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Tang YuSh Tsai YuCh Chen, T.W.; Li, S.Y. Artificial kidney engineering: The development of dialysis membranes for blood purification. Membranes 2022, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, H.; Hyeon, T.; Kim, D.-H.; Ghaffari, R. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of vital signs with flexible and wearable medical devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Rim, Y.S.; Bae, S.-H.; Chen, H.; De Marco, N.; Yang, Y. Recent progress in materials and devices toward printable and flexible sensors. Adv. Mater. 2016, 28, 4415–4440. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Tran, P.; Do, T. FDM-based 3D printing of polymer and associated composite: A review on mechanical properties, defects and treatments. Polymers 2020, 12, 1529. [Google Scholar] [CrossRef]
- Belgiu, G.; Turc, C.G.; Carausu, C. Selection of subtractive manufacturing technology versus additive manufacturing technology for rapid prototyping of a polymeric product. Mater. Plast. 2021, 57, 343–352. [Google Scholar] [CrossRef]
- Neuenfeldt, A., Jr.; Cheiram, M.; Eckhardt, M.; Scheuer, C.; Siluk, J.; Francescatto, M. Additive and subtractive rapid prototyping techniques: A comparative analysis of FDM & CNC processes. Int. J. Ind. Eng. Manag. 2021, 12, 262–273. [Google Scholar]
- Gates, B.D.; Xu, Q.; Ryan, D.; Whitesides, G.M.; Stewart, M.; Willson, C.G. New approaches to nanofabrication: Molding, printing, and other techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef]
- Wang, X.; Gou, J.; Jiang, M.; Zhou, Z.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, J.; Chua, C.K. Fundamentals and applications of 3d printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Gusarov, E.E.; Malkov, Y.P.; Stepanov, S.G.; Troshchinenko, G.A.; Zasypkin, I.M. Plasma-chemical technology of treatment of halogen-containing waste including polychlorinated biphenyls. Thermophys. Aeromechanics 2010, 17, 605–612. [Google Scholar] [CrossRef]
- Skiba, M.I.; Vorobyova, V. The plasma-chemical formation of polysorbate 80-coated silver nanoparticles and composite materials for water treatment. Pigment. Resin Technol. 2019, 48, 431–438. [Google Scholar] [CrossRef]
- Doronin, F.; Rytikov, G.; Evdokimov, A.; Rudyak, Y.; Taranets, I.; Nazarov, V. The effect of electro-induced multi-gas modification on polymer substrates’ surface structure for additive manufacturing. Processes 2023, 11, 774. [Google Scholar] [CrossRef]
- Park, S.J.; Jeong, H.J.; Nah, C. A study of oxyfluorination of multi-walled carbon nanotubes on mechanical interfacial properties of epoxy matrix nanocomposites. Mater. Sci. Eng. A 2004, 385, 13–16. [Google Scholar] [CrossRef]
- Yun, J.; Im, J.S.; Lee, Y.-S.; Kim, H.-I. Effect of oxyfluorination on electromagnetic interference shielding behavior of MWCNT/PVA/PAAC composite microcapsules. Eur. Polym. J. 2010, 46, 900–909. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Doronin, F.A.; Evdokimov, A.G.; Rytikov, G.O.; Stolyarov, V.P. Oxyfluorination-controlled variations in the wettability of polymer film surfaces. Colloid J. 2019, 81, 146–157. [Google Scholar] [CrossRef]
- McCaig, M.S.; Paul, D.R. Effect of film thickness on the changes in gas permeability of a glassy polyarylate due to physical aging: Part I. Experimental observations. Polymer 2000, 41, 629–637. [Google Scholar] [CrossRef]
- Lin, W.H.; Chung, T.S. Gas permeability, diffusivity, solubility, and aging characteristics of 6fda-durene polyimide membranes. J. Membr. Sci. 2001, 186, 183–193. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl. Phys. A Mater. Sci. Process. 2004, 78, 547–552. [Google Scholar] [CrossRef]
- Vadukumpully, S.; Paul, J.; Mahanta, N.; Valiyaveettil, S. Flexible conductive graphene/poly(vinyl chloride) composite thin films with high mechanical strength and thermal stability. Carbon 2011, 49, 198–205. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.I.; Newman, P.; Zreiqat, H. Design and fabrication of 3d printed scaffolds with a mechanical strength comparable to cortical bone to repair large bone defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.J.; Poole Warren, L.A.; Gunatillake, P.A.; McCarthy, S.J.; Meijs, G.F.; Schindhelm, K. Polydimethylsiloxane/polyether-mixed macrodiol-based polyurethane elastomers: Biostability. Biomaterials 2000, 21, 1021–1029. [Google Scholar] [CrossRef]
- Seifalian, A.M.; Salacinski, H.J.; Tiwari, A.; Edwards, A.; Bowald, S.; Hamilton, G. In vivo biostability of a poly(carbonate-urea)urethane graft. Biomaterials 2003, 24, 2549–2557. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. Physico-chemical stability of colloidal lipid particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Sokhanvar, S.; Kaboli, A.; Khorsand, S. Science and engineering of superhydrophobic surfaces: Review of corrosion resistance, chemical and mechanical stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Geoghegan, M.; Krausch, G. Wetting at polymer surfaces and interfaces. Prog. Polym. Sci. 2003, 28, 261–302. [Google Scholar] [CrossRef]
- Barbieri, L.; Wagner, E.; Hoffmann, P. Water wetting transition parameters of perfluorinated substrates with periodically distributed flat-top microscale obstacles. Langmuir 2007, 23, 1723–1734. [Google Scholar] [CrossRef]
- Kovalchuk, N.M.; Trybala, A.; Starov, V.; Matar, O.; Ivanova, N. Fluoro- vs hydrocarbon surfactants: Why do they differ in wetting performance? Adv. Colloid Interface Sci. 2014, 210, 65–71. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Myshkin, N.K.; Petrokovets, M.I.; Kovalev, A.V. Tribology of polymers: Adhesion, friction, wear, and mass-transfer. Tribol. Int. 2005, 38, 910–921. [Google Scholar] [CrossRef]
- Persson, B.N.J.; Tartaglino, U.; Volokitin, A.I.; Tosatti, E.; Albohr, O. On the nature of surface roughness with application to contact mechanics, sealing, rubber friction and adhesion. J. Phys. Condens. Matter 2005, 17, R1–R62. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Liang, M.N.; Meluleni, G.; Pier, G.; Ingber, D.E.; Whitesides, G.M. Self-assembled monolayers that resist the adsorption of proteins and the adhesion of bacterial and mammalian cells. Langmuir 2001, 17, 6336–6343. [Google Scholar] [CrossRef]
- Schneider, A.; Francius, G.; Voegel, J.-C.; Picart, C.; Jedrzejwska, J.; Frisch, B.; Schaaf, P.; Bolcato-Bellemin, A.-L. Glycated polyelectrolyte multilayer films: Differential adhesion of primary vesus tumor cells. Biomacromolecules 2006, 7, 2882–2889. [Google Scholar] [CrossRef]
- Hsu, L.C.; Worobo, R.W.; Moraru, C.I.; Fang, J.; Borca-Tasciuc, D.A. Effect of micro- and nanoscale topography on the adhesion of bacterial cells to solid surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Zinger, O.; Anselme, K.; Denzer, A.; Habersetzer, P.; Wieland, M.; Jeanfils, J.; Hardouin, P.; Landolt, D. Time-dependent morphology and adhesion of osteoblastic cells on titanium model surfaces featuring scale-resolved topography. Biomaterials 2004, 25, 2695–2711. [Google Scholar] [CrossRef]
- Tugulu, S.; Silacci, P.; Stergiopulos, N.; Klok, H.A. RGD-functionalized polymer brushes as substrates for the integrin specific adhesion of human umbilical vein endothelial cells. Biomaterials 2007, 28, 2536–2546. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Yao, X.; Ding, J. Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion. Biomaterials 2011, 32, 8048–8057. [Google Scholar] [CrossRef]
- Rudyak, Y.V.; Doronin, F.A.; Rytikov, G.O.; Filyugina, E.K.; Nazarov, V.G. Nanotexture effect of the fiber surface on the sorption capacity of nonwoven fabrics. Nanosyst. Phys. Chem. Math. 2020, 11, 553–564. [Google Scholar] [CrossRef]
- Doronin, F.A.; Saveliev, M.A.; Taranets, I.P.; Rytikov, G.O.; Nazarov, V.G. Direct Structuring of Polymers Used in Additive Manufacturing. Russ. J. Gen. Chem 2024, 94, 1550–1557. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities-properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Miranda, I.; Sousa, P.; Minas, G.; Souza, A.; Lima, R.; Ribeiro, J.; Castanheira, E.M.S. Properties and applications of PDMS for biomedical engineering: A review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Jain, V.; Raj, T.P.; Deshmukh, R.; Patrikar, R. Design, fabrication and characterization of low cost printed circuit board based ewod device for digital microfluidics applications. Microsyst. Technol. 2017, 23, 389–397. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Leng, K.; Li, B.; Feng, C.; Huo, X. Can supramolecular polymers become another material choice for polymer flooding to enhance oil recovery? Polymers 2022, 14, 4405. [Google Scholar] [CrossRef] [PubMed]
- Naresh Kumar, M.; Ravikumar, R.; Thenmozhi, S.; Ranjith Kumar, M.; Kirupa Shankar, M. Choice of pretreatment technology for sustainable production of bioethanol from lignocellulosic biomass: Bottle necks and recommendations. Waste Biomass Valorization 2019, 10, 1693–1709. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 5737. [Google Scholar] [CrossRef]
- Xu, S.; Li, M.; Hong, M.; Yang, L.; Sun, Q.; Sun, S.; Lyu, W.; Dargusch, M.; Zou, J.; Chen, Z.G. Optimal array alignment to deliver high performance in flexible conducting polymer-based thermoelectric devices. J. Mater. Sci. Technol. 2022, 124, 252–259. [Google Scholar] [CrossRef]
- Nazarov, V.; Doronin, F.; Dedov, A.; Evdokimov, A.; Rytikov, G.; Savel’ev, M. Design of a Hybrid 3D-Printed Composite Material Based on Non-Woven Needle-Punched Fabrics with Radio-Absorbing Properties. Polymers 2025, 17, 2324. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Kim, S.J.; Sohn, J.u.H.; Kim, I.G.; Kim, S.W.; Sohn, D.W.; Kim, J.H.; Choi, B. Biocompatibility of a PDMS-coated micro-device: Bladder volume monitoring sensor. Chin. J. Polym. Sci. 2012, 30, 242–249. [Google Scholar] [CrossRef]
- Sreekantan, S.; Hassan, M.; Sundera Murthe, S.; Seeni, A. Biocompatibility and cytotoxicity study of polydimethylsiloxane (PDMS) and palm oil fuel ash (POFA) sustainable super-hydrophobic coating for biomedical applications. Polymers 2020, 12, 3034. [Google Scholar] [CrossRef]
- Chen Ya Zhou, X.; Huang Sh Lan Yu Yan, R.; Shi, X.; Li, X.; Zhang, Y.; Lei, Z.; Fan, D. Effect of microgroove structure in PDMS-based silicone implants on biocompatibility. Front. Bioeng. Biotechnol. 2022, 9, 793778. [Google Scholar] [CrossRef]
- Leroux, F.; Campagne, C.; Perwuelz, A.; Gengembre, L. Polypropylene film chemical and physical modifications by dielectric barrier discharge plasma treatment at atmospheric pressure. J. Colloid Interface Sci. 2008, 328, 412–420. [Google Scholar] [CrossRef]
- Lopez, L.C.; Iacoviello, G.; D’Agostino, R.; Favia, P.; Buonomenna, M.G.; Fontananova, E.; Drioli, E. A new generation of catalytic poly(vinylidene fluoride) membranes: Coupling plasma treatment with chemical immobilization of tungsten-based catalysts. Adv. Funct. Mater. 2006, 16, 1417–1424. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-Malek, C. Hydrophilization and hydrophobic recovery of pdms by oxygen plasma and chemical treatment-an sem investigation. Sens. Actuators B Chem. 2007, 123, 368–373. [Google Scholar] [CrossRef]
- Ilchenko, N.O.; Hedberg, M.; Szabó, K.J. Fluorinative ring-opening of cyclopropanes by hypervalent iodine reagents. An efficient method for 1,3-oxyfluorination and 1,3-difluorination. Chem. Sci. 2017, 8, 1056–1061. [Google Scholar] [CrossRef]
- Taege, R.; Ferrier, G.G. Reliable, robust and safe: How surface oxyfluorination improves adhesion of coatings to plastics. Eur. Coat. J. 2006, 5, 36–41. [Google Scholar]
- Nazarov, V.G.; Stolyarov, V.P.; Doronin, F.A.; Evdokimov, A.G.; Rytikov, G.O.; Brevnov, P.N.; Zabolotnov, A.S.; Novokshonova, L.A.; Berlin, A.A. Comparison of the effects of some modification methods on the characteristics of ultrahigh-molecular-weight polyethylene and composites on its basis. Polym. Sci. Ser. A 2019, 61, 325–333. [Google Scholar] [CrossRef]
- Doronin, F.A.; Rudyak, Y.V.; Rytikov, G.O.; Evdokimov, A.G.; Nazarov, V.G. 3D-printed planar microfluidic device on oxyfluorinated PET-substrate. Polym. Test. 2021, 99, 107209. [Google Scholar] [CrossRef]
- De Lange, P.J.; Akker, P.G. Adhesion activation of twaron aramid fibers for application in rubber: Plasma versus chemical treatment. J. Adhes. Sci. Technol. 2012, 26, 827–839. [Google Scholar] [CrossRef]
- Nandi, A.; Kumar Biswal, A.; Nguyen, A.; Nordyke, L.; Behling, E.; Foulds, T.; Schultz, K.; Vashisth, A. Comparative study of surface preparation for paint adhesion on cf-pekk composites: Plasma, chemical, and flame treatment. Appl. Surf. Sci. 2024, 669, 160533. [Google Scholar] [CrossRef]
- Adekunle, F.; Genzer Ja Seyam, A.F.M. Enhancing interfacial adhesion in kevlar and ultra-high molecular weight polyethylene fiber-reinforced laminates: A comparative study of surface roughening, plasma treatment, and chemical functionalization using graphene nanoparticles. Fibers 2025, 13, 19. [Google Scholar] [CrossRef]
- Doronin, F.A.; Rytikov, G.O.; Evdokimov, A.G.; Ruduak, Y.V.; Nazarov, V.G. The synergistic effect of bulk-surface modification onto the wear resistance of the ultrahigh molecular weight polyethylene. Polym. Polym. Compos. 2023, 31, 09673911221150132. [Google Scholar] [CrossRef]
- ISO 527-2:2012; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. ISO: Geneva, Switzerland, 2012.
- GOST 11262-2017; Plastics. Tensile Test Method. Rosstandart: Moscow, Russia, 2018.
- Doronin, F.; Rudakova, A.; Rytikov, G.; Nazarov, V. A novel determination of the melt flow index of composite filaments used in extrusion additive manufacturing. Polym. Test. 2024, 133, 108376. [Google Scholar] [CrossRef]
- Doronin, F.; Savel’ev, M.; Rytikov, G.; Evdokimov, A.; Nazarov, V. A new approach to carbon nanotube filament nanostructuring for additive manufacturing. Polymers 2024, 16, 1423. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Stolyarov, V.P.; Evlampieva, L.A.; Fokin, A.V. Heterophase fluorination of polymers. Dokl. Phys. Chem. 1996, 350, 268–270. [Google Scholar]
- Vega-Cantú, Y.; Hauge, R.; Billups, W.E.; Norman, L. Enhancement of the chemical resistance of nitrile rubber by direct fluorination. J. Appl. Polym. Sci. 2003, 89, 971–979. [Google Scholar] [CrossRef]
- Wirti, M.; Biondo, G.R.R.; Zattera, A.J.; Romanzini, D.; Amico, S.C. The effect of fluorination of aramid fibers on vinyl ester composites. Polym. Compos. 2019, 40, 2095–2102. [Google Scholar] [CrossRef]
- Doronin, F.; Rytikov, G.; Evdokimov, A.; Rudyak, Y.; Savel’ev, M.; Nazarov, V. Biostable fluorine-containing coatings on the surface of polymers. Coatings 2023, 13, 424. [Google Scholar] [CrossRef]
- Drozdov, S.A.; Nazarov, V.G.; Nozdrachev, S.A.; Rudyak, Y.V.; Rytikov, G.O. The polymer composites’ morphological structure simulation. Nanosyst. Phys. Chem. Math. 2017, 8, 137–145. [Google Scholar] [CrossRef]



| Filaments | Manufacturer | Technological Parameter of FFF 3D-Printing | ||
|---|---|---|---|---|
| Temperature of Extrusion, °C | Build Platform Temperature, °C | Printing Speed, mm/min | ||
| Acrylonitrile, butadiene, and styrene copolymer (ABS) | Shenzhen Esun Industrial Co., Shenzhen China | 250 | 80 | 40 |
| Polylactide (PLA) | 210 | 50 | 40 | |
| Polyethylene terephthalate glycol (PETG) | 235 | 60 | 40 | |
| Thermoplastic polyurethane (TPU) | U3print, Moscow, Russia | 210 | 60 | 30 |
| Polyethylene terephthalate glycol (PETG) containing 1 mass.% of thermochromic microcapsules | Moscow Polytechnic University, Moscow, Russia [66] | 240 | 60 | 40 |
| Duration of Oxyfluorination, min | PET | PP | PVC | LDPE |
|---|---|---|---|---|
| 30 | 0.40 ± 0.07 | 0.36 ± 0.04 | 0.19 ± 0.03 | 0.23 ± 0.03 |
| 60 | 1.3 ± 0.2 | 0.63 ± 0.07 | 0.37 ± 0.05 | 0.35 ± 0.04 |
| 90 | 1.4 ± 0.2 | 0.69 ± 0.08 | 0.46 ± 0.06 | 0.43 ± 0.04 |
| 180 | 1.6 ± 0.4 | 0.73 ± 0.08 | 0.48 ± 0.06 | 0.49 ± 0.06 |
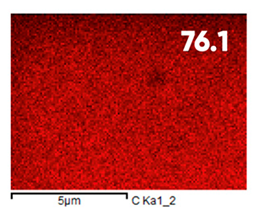
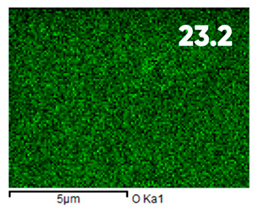
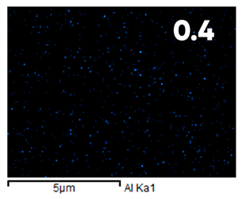
| Elemental Composition, mass. % | ||||
|---|---|---|---|---|
| C | O | F | Al | Si |
| Initial | ||||
 |  | - |  | 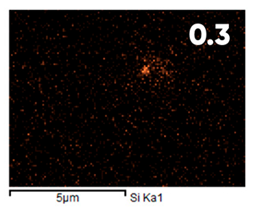 |
| Fluorinated | ||||
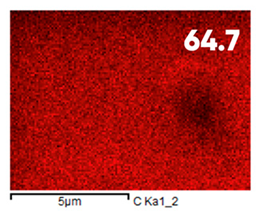 |  |  | 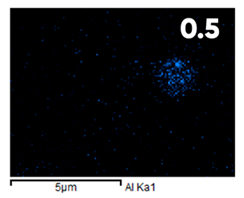 | 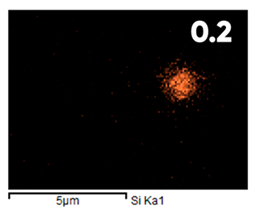 |
| 3D-Printed Items | ABS | PLA | TPU | PETG |
|---|---|---|---|---|
| Parameter | Initial/Oxyfluorinated | |||
| 67 ± 7/45 ± 5 | 74 ± 7/52 ± 5 | 85 ± 9/48 ± 5 | 79 ± 8/43 ± 4 | |
| 62 ± 6/18 ± 2 | 46 ± 5/23 ± 2 | 48 ± 5/20 ± 2 | 51 ± 5/17 ± 2 | |
| 6.8 ± 0.7/9.8 ± 0.9 | 5.9 ± 0.6/4.9 ± 0.5 | 4.9 ± 0.5/4.6 ± 0.5 | 12 ± 1/8.0 ± 0.8 | |
| 1.2 ± 0.1/0.22 ± 0.02 | 0.5 ± 0.05/0.21 ± 0.02 | 0.85 ± 0.08/0.41 ± 0.04 | 1.1 ± 0.1/0.43 ± 0.04 | |
| 3D-Printed 1BB Blades | Duration of Direct Oxyfluorination, min | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | 45 | 60 | 90 | |
| TPU | 36 ± 4 | 38 ± 4 | 37 ± 4 | 39 ± 5 | 40 ± 4 | 41 ± 4 | 40 ± 4 | 40 ± 5 | 41 ± 4 | 40 ± 5 |
| ABS | 41 ± 4 | 43 ± 5 | 42 ± 4 | 44 ± 5 | 43 ± 6 | 43 ± 6 | 41 ± 4 | 42 ± 6 | 43 ± 4 | 42 ± 5 |
| PETG | 52 ± 5 | 51 ± 6 | 53 ± 5 | 52 ± 5 | 53 ± 5 | 51 ± 6 | 52 ± 5 | 52 ± 5 | 52 ± 6 | 51 ± 6 |
| PLA | 64 ± 6 | 66 ± 7 | 67 ± 5 | 68 ± 7 | 67 ± 6 | 68 ± 7 | 67 ± 5 | 67 ± 6 | 65 ± 7 | 66 ± 7 |
| Material | Oxyfluorination Duration (minutes) | Wetting Edge Angle, ° | ||||
|---|---|---|---|---|---|---|
| PDMS | 0 | 96 ± 9 | 70 ± 7 | 25.7 ± 0.8 | 23.8 ± 0.6 | 1.9 ± 0.2 |
| 15 | 94 ± 8 | 65 ± 7 | 30.1 ± 0.8 | 28.5 ± 0.7 | 1.6 ± 0.1 | |
| 30 | 89 ± 8 | 60 ± 6 | 31.6 ± 0.9 | 29.0 ± 0.7 | 2.6 ± 0.2 | |
| 60 | 74 ± 7 | 46 ± 6 | 34.9 ± 0.9 | 24.1 ± 0.5 | 10.8 ± 0.4 | |
| PDMS | Duration of Direct Oxyfluorination, min | ||
|---|---|---|---|
| Parameter | 0 | 15 | 60 |
| 20 | 25 | 57 | |
| 2 | 10 | 35 | |
| 0.23 | 1.32 | 0.57 | |
| 0.15 | 0.33 | 0.14 | |
| 1.6 | 19 | 6.8 | |
| 11 | 5.9 | 12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doronin, F.; Rytikov, G.; Evdokimov, A.; Savel’ev, M.; Rudakova, A.; Rudyak, Y.; Nazarov, V. The Concept of a 3D-Printed Microfluidic Device on Oxyfluorinated PDMS Substrates. Polymers 2025, 17, 3044. https://doi.org/10.3390/polym17223044
Doronin F, Rytikov G, Evdokimov A, Savel’ev M, Rudakova A, Rudyak Y, Nazarov V. The Concept of a 3D-Printed Microfluidic Device on Oxyfluorinated PDMS Substrates. Polymers. 2025; 17(22):3044. https://doi.org/10.3390/polym17223044
Chicago/Turabian StyleDoronin, Fedor, Georgy Rytikov, Andrey Evdokimov, Mikhail Savel’ev, Anna Rudakova, Yuriy Rudyak, and Victor Nazarov. 2025. "The Concept of a 3D-Printed Microfluidic Device on Oxyfluorinated PDMS Substrates" Polymers 17, no. 22: 3044. https://doi.org/10.3390/polym17223044
APA StyleDoronin, F., Rytikov, G., Evdokimov, A., Savel’ev, M., Rudakova, A., Rudyak, Y., & Nazarov, V. (2025). The Concept of a 3D-Printed Microfluidic Device on Oxyfluorinated PDMS Substrates. Polymers, 17(22), 3044. https://doi.org/10.3390/polym17223044







