Fabrication of Crosslinked Poly(L-lactic acid) with Enhanced Shape Memory Performance via γ-Ray Irradiation
Abstract
1. Introduction
2. Materials and Methods
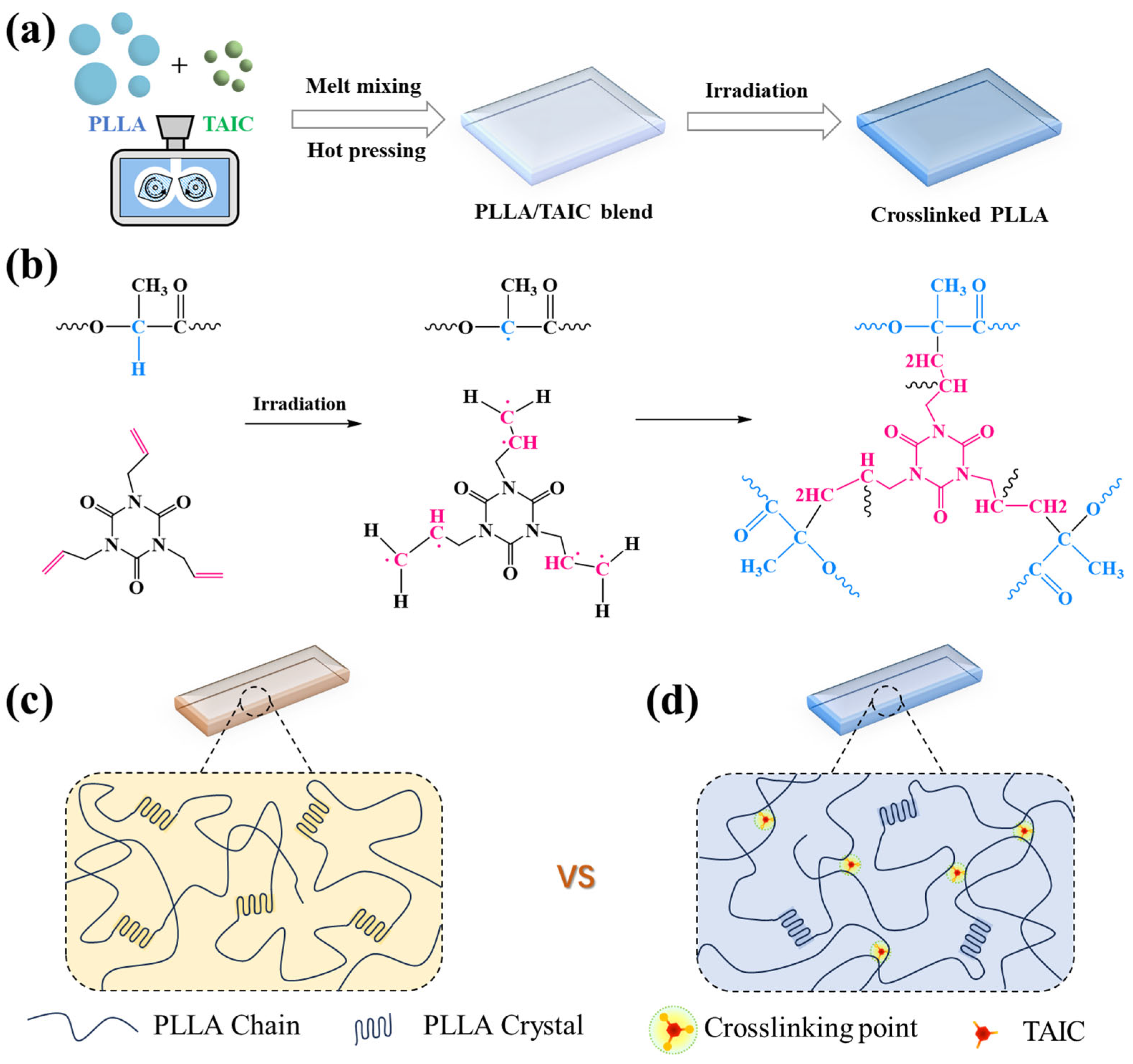
2.1. Materials and Sample Preparation
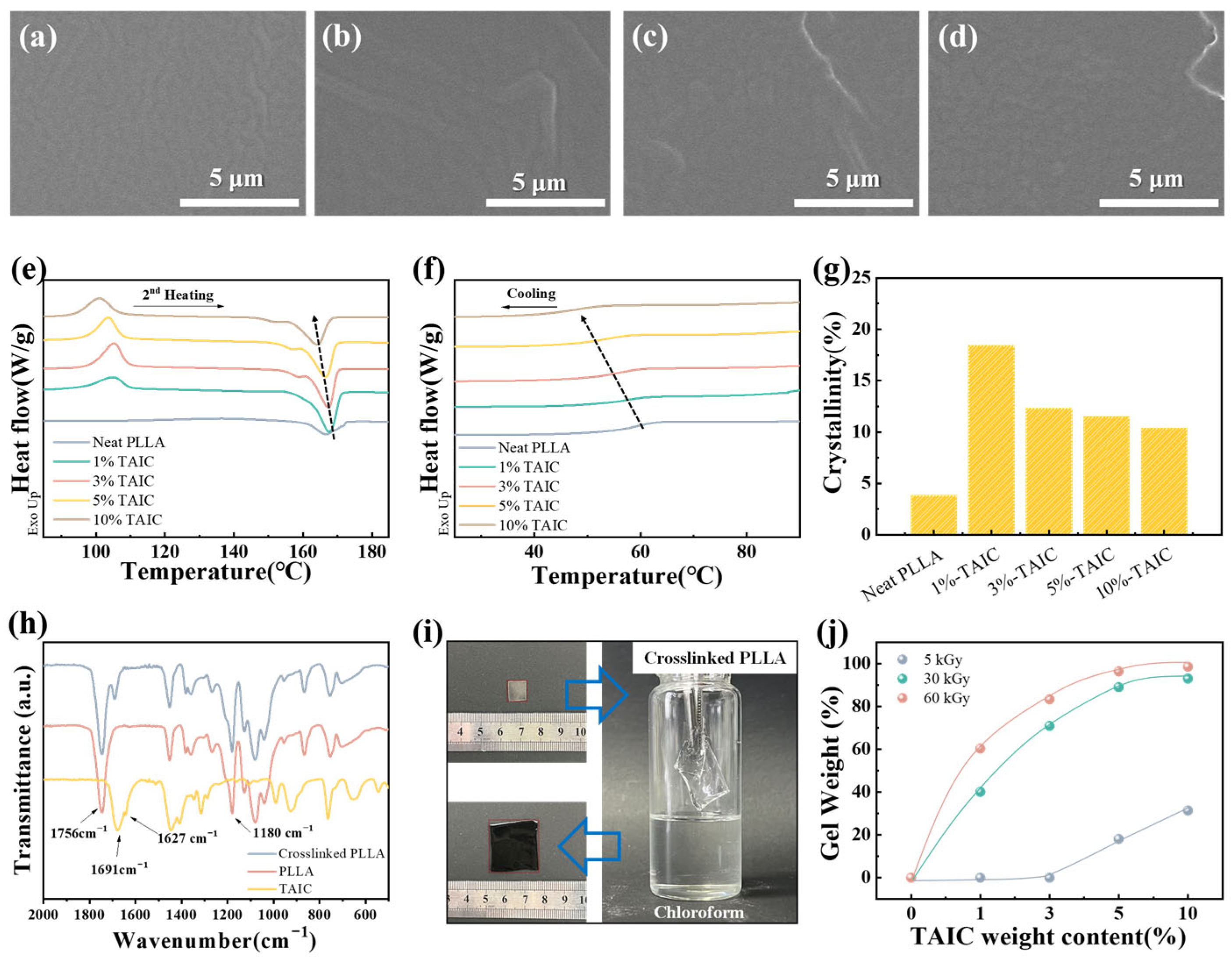
2.2. Characterizations
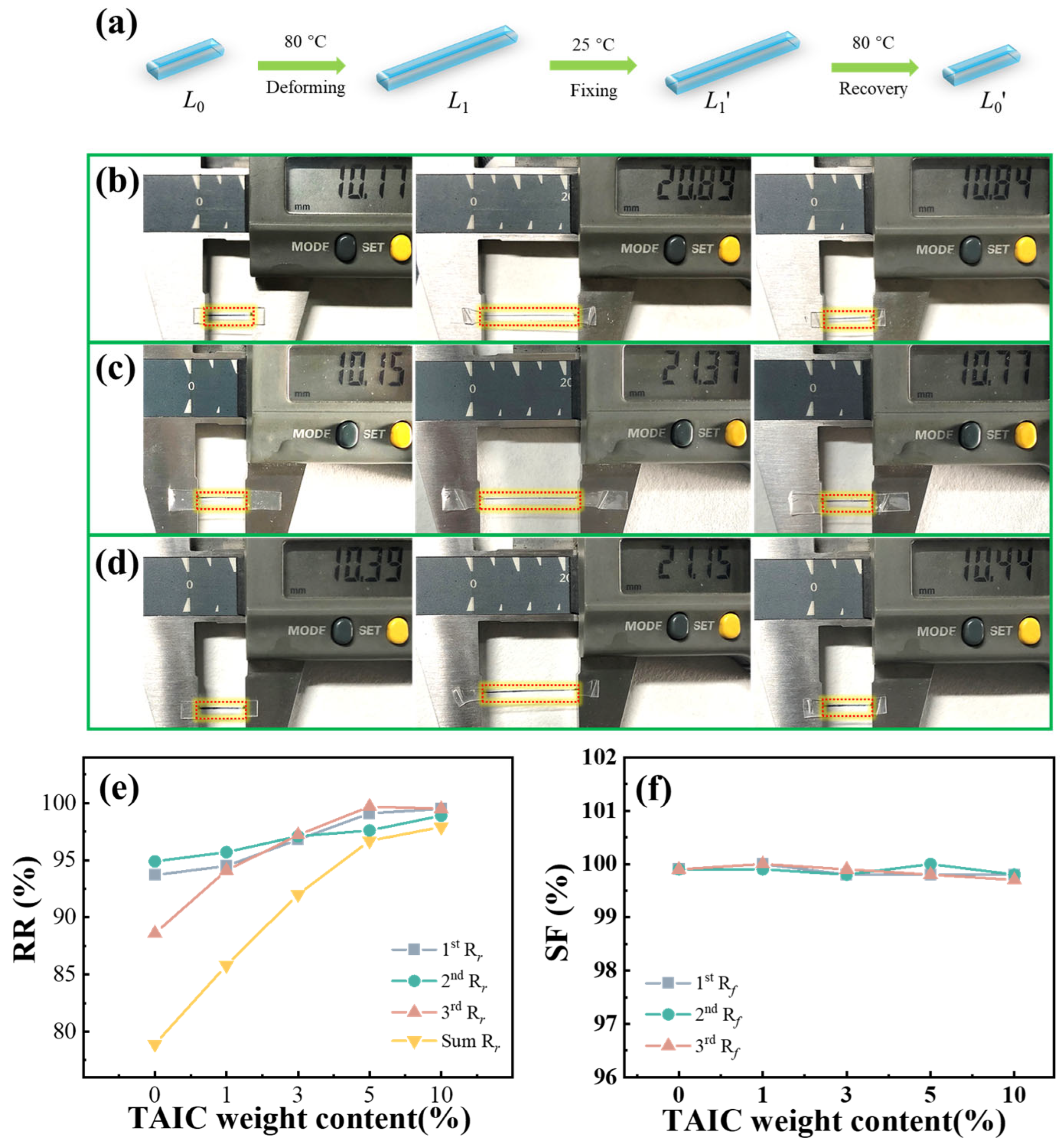
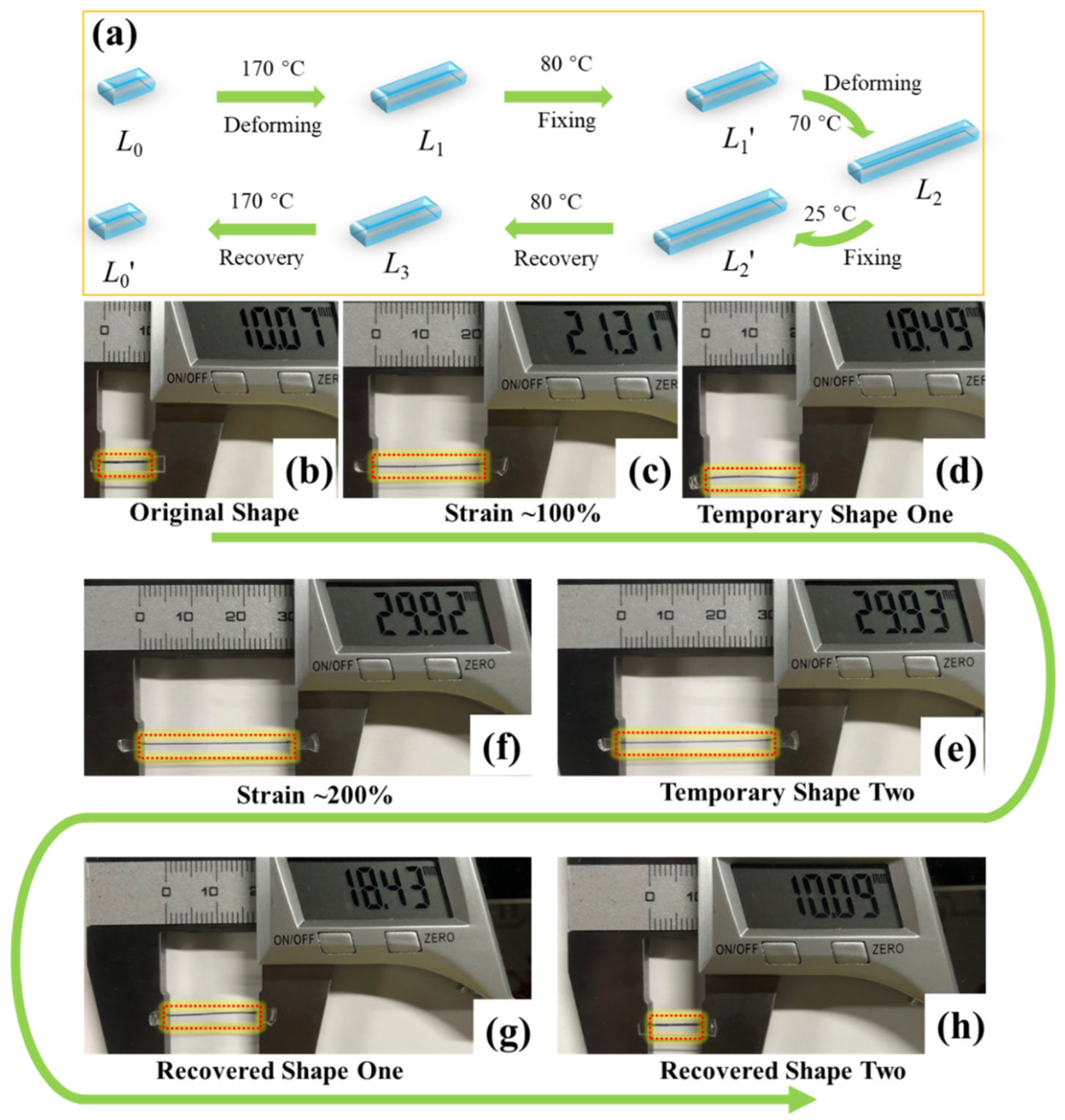
2.3. Shape Memory Polymer Evaluation
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lendlein, A.; Kelch, S. Shape-Memory Polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-Memory Polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Schmidt, A.M. Electromagnetic Activation of Shape Memory Polymer Networks Containing Magnetic Nanoparticles. Macromol. Rapid Commun. 2006, 27, 1168–1172. [Google Scholar] [CrossRef]
- Leng, J.; Lan, X.; Liu, Y.; Du, S. Shape-Memory Polymers and Their Composites: Stimulus Methods and Applications. Prog. Mater. Sci. 2011, 56, 1077–1135. [Google Scholar] [CrossRef]
- Xiao, X.; Kong, D.; Qiu, X.; Zhang, W.; Zhang, F.; Liu, L.; Liu, Y.; Zhang, S.; Hu, Y.; Leng, J. Shape-Memory Polymers with Adjustable High Glass Transition Temperatures. Macromolecules 2015, 48, 3582–3589. [Google Scholar] [CrossRef]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape Memory Polymers: Past, Present and Future Developments. Prog. Polym. Sci. 2015, 49, 3–33. [Google Scholar] [CrossRef]
- Pfau, M.R.; Grunlan, M.A. Smart Scaffolds: Shape Memory Polymers (SMPs) in Tissue Engineering. J. Mater. Chem. B 2021, 9, 4287–4297. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, F.; Wang, L.; Liu, Y.; Leng, J. Recent Advances in Shape Memory Polymers: Multifunctional Materials, Multiscale Structures, and Applications. Adv. Funct. Mater. 2024, 34, 2312036. [Google Scholar] [CrossRef]
- Zhao, W.; Yue, C.; Liu, L.; Liu, Y.; Leng, J. Research Progress of Shape Memory Polymer and 4D Printing in Biomedical Application. Adv. Healthc. Mater. 2023, 12, 2201975. [Google Scholar] [CrossRef]
- Chen, H.M.; Wang, L.; Zhou, S.B. Recent Progress in Shape Memory Polymers for Biomedical Applications. Chin. J. Polym. Sci. 2018, 36, 905–917. [Google Scholar] [CrossRef]
- Chan, B.Q.; Low, Z.W.; Heng, S.J.; Chan, S.Y.; Owh, C.; Loh, X.J. Recent Advances in Shape Memory Soft Materials for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 10070–10087. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Liu, Y.; Leng, J. Shape Memory Polymer Fibers: Materials, Structures, and Applications. Adv. Fiber Mat. 2022, 4, 5–23. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, B.; Pan, T.; Peng, W. Thermal Responsive Shape Memory Textiles for Self-adaptive Applications: A Review. Sens. Actuators A Phys. 2025, 394, 116935. [Google Scholar] [CrossRef]
- Spiegel, C.A.; Hackner, M.; Bothe, V.P.; Spatz, J.P.; Blasco, E. 4D Printing of Shape Memory Polymers: From Macro to Micro. Adv. Funct. Mater. 2022, 32, 2110580. [Google Scholar] [CrossRef]
- Alshebly, Y.S.; Nafea, M.; Mohamed Ali, M.S.; Almurib, H.A.F. Review on Recent Advances in 4D Printing of Shape Memory Polymers. Eur. Polym. J. 2021, 159, 110708. [Google Scholar] [CrossRef]
- Subash, A.; Kandasubramanian, B. 4D Printing of Shape Memory Polymers. Eur. Polym. J. 2020, 134, 109771. [Google Scholar] [CrossRef]
- Qiao, T.; Song, P.; Guo, H.; Song, X.; Zhang, B.; Chen, X. Reinforced Electrospun PLLA Fiber Membrane via Chemical Crosslinking. Eur. Polym. J. 2016, 74, 101–108. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Q.; Wang, T.; Wang, L.; You, J.; Li, Y. Micropore Geometry Manipulation by Macroscopic Deformation Based on Shape Memory Effect in Porous PLLA Membrane and its Enhanced Separation Performance. ACS Appl. Mater. Interfaces 2017, 9, 43415–43419. [Google Scholar] [CrossRef]
- Balk, M.; Behl, M.; Wischke, C.; Zotzmann, J.; Lendlein, A. Recent Advances in Degradable Lactide-Based Shape-Memory Polymers. Adv. Drug Delivery Rev. 2016, 107, 136–152. [Google Scholar] [CrossRef]
- Chafran, L.; Campos, J.; Santos, J.; Sales, M.; Dias, S.; Dias, J. Synthesis of Poly(lactic acid) by Heterogeneous Acid Catalysis from D,L-lactic Acid. J. Polym. Res. 2016, 23, 107. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Y.; Pang, X.; Chen, X. Poly(Lactic Acid): Recent Stereochemical Advances and New Materials Engineering. Adv. Mater. 2025, 37, 2412185. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Progress in Polymer Science Poly (Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Yang, W.G.; Lu, H.; Huang, W.M.; Qi, H.J.; Wu, X.L.; Sun, K.Y. Advanced Shape Memory Technology to Reshape Product Design, Manufacturing and Recycling. Polymers 2014, 6, 2287–2308. [Google Scholar] [CrossRef]
- Ghobadi, E.; Heuchel, M.; Kratz, K.; Lendlein, A. Influence of the Addition of Water to Amorphous Switching Domains on the Simulated Shape-Memory Properties of Poly (L-Lactide). Polymer 2013, 54, 4204–4211. [Google Scholar] [CrossRef]
- Lai, S.M.; Lan, Y.C. Shape Memory Properties of Melt-Blended Polylactic Acid (PLA)/Thermoplastic Polyurethane (TPU) Bio-Based Blends. J. Polym. Res. 2013, 20, 140. [Google Scholar] [CrossRef]
- Li, M.; Song, F.; Chen, L.; Wang, X.; Wang, Y. Flexible Material Based on Poly(Lactic Acid) and Liquid Crystal with Multishape Memory Effects. ACS Sustain. Chem. Eng. 2016, 4, 3820–3829. [Google Scholar] [CrossRef]
- Odent, J.; Raquez, J.M.; Samuel, C.; Barrau, S.; Enotiadis, A.; Dubois, P.; Giannelis, E.P. Shape-Memory Behavior of Polylactide/Silica Ionic Hybrids. Macromolecules 2017, 50, 2896–2905. [Google Scholar] [CrossRef]
- Huang, J.R.; Fan, J.F.; Cao, L.M.; Xu, C.H.; Chen, Y.K. A Novel Strategy to Construct Co-Continuous PLA/NBR Thermoplastic Vulcanizates: Metal-Ligand Coordination-Induced Dynamic Vulcanization, Balanced Stiffness-Toughness and Shape Memory Effect. Chem. Eng. J. 2020, 385, 123828. [Google Scholar] [CrossRef]
- Nonkrathok, W.; Trongsatitkul, T.; Suppakarn, N. Role of Maleic Anhydride-Grafted Poly(lactic acid) in Improving Shape Memory Properties of Thermoresponsive Poly(ethylene glycol) and Poly(lactic acid) Blends. Polymers 2022, 14, 3923. [Google Scholar] [CrossRef]
- He, M.; Hsu, Y.-I.; Uyama, H. Design of novel poly(L-lactide)-based shape memory multiblock copolymers for biodegradable esophageal stent application. Appl. Mater. Today 2024, 36, 102057. [Google Scholar] [CrossRef]
- Xie, M.; Wang, L.; Ge, J.; Guo, B.; Ma, P.X. Strong Electroactive Biodegradable Shape Memory Polymer Networks Based on Star-Shaped Polylactide and Aniline Trimer for Bone Tissue Engineering ACS Appl. Mater. Interfaces 2015, 7, 6772–6781. [Google Scholar] [CrossRef]
- Naddeo, M.; Sorrentino, A.; Pappalardo, D. Thermo-Rheological and Shape Memory Properties of Block and Random Copolymers of Lactide and ε-Caprolactone. Polymers 2021, 13, 627. [Google Scholar] [CrossRef]
- Zhao, J.X.; Li, J.Y.; Li, Y.J.; You, J.C. Thermoplastic Shape Memory Composites with Enhanced Recovery Stress and Recovery Ratio Based on Double Roles of PVAc-g-GO. Compos. Commun. 2019, 13, 52–56. [Google Scholar] [CrossRef]
- Baratta, M.; Olivito, F.; Simari, C.; Wan-Mohtar, W.A.A.Q.; Nicotera, I.I.; Nicoletta, F.P.; De Filpo, G.; Golemme, G. New Dispersible and Low-melting Cellulose Ester Produced with Molten Adipic Acid as A Solvent, Reagent and Catalyst, and Its Application to Improve the Mechanical Properties of PLA. React. Chem. Eng. 2025, 10, 1615. [Google Scholar] [CrossRef]
- Rusa, C.; Tonelli, A. Polymer/Polymer Inclusion Compounds as a Novel Approach to Obtaining a PLLA/PCL Intimately Compatible Blend. Macromolecules 2000, 33, 5321–5324. [Google Scholar] [CrossRef]
- Zhou, X.W.; Huang, J.; Zhang, X.H.; Li, T.; Wang, Y.; Wang, S.B.; Xia, B.H.; Dong, W.F. Design of Tough, yet Strong, Heat-resistant PLA/PBAT Blends with Reconfigurable Shape Memory Behavior by Engineering Exchangeable Covalent Crosslinks. Chin. J. Polym. Sci. 2023, 41, 1868–1878. [Google Scholar] [CrossRef]
- Fan, X.; Tan, B.; Li, Z.; Loh, X. Control of PLA Stereoisomers-Based Polyurethane Elastomers as Highly Efficient Shape Memory Materials. ACS Sustain. Chem. Eng. 2017, 5, 1217–1227. [Google Scholar] [CrossRef]
- Quynh, T.; Mitomo, H.; Nagasawa, N.; Wada, Y.; Yoshii, F.; Tamada, M. Properties of crosslinked polylactides (PLLA&PDLA) by radiation and its biodegradability. Eur. Polym. J. 2007, 43, 1779–1785. [Google Scholar]
- Yang, S.; Wu, Z.; Yang, W.; Yang, M. Thermal and mechanical properties of chemical crosslinked polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Yin, L.; Wang, J.; Lin, T.; You, J. Inclusion/Exclusion Behaviors of Small Molecules during Crystallization of Polymers in Miscible PLLA/TAIC Blend. Polymers 2022, 14, 2737. [Google Scholar] [CrossRef]
- Kodal, M.; Wis, A.A.; Ozkoc, G. The mechanical, thermal and morphological properties of γ-irradiated PLA/TAIC and PLA/OvPOSS. Radiat. Phys. Chem. 2018, 153, 214–225. [Google Scholar] [CrossRef]
- Rangari, D.; Vasanthan, N. Study of Strain-Induced Crystallization and Enzymatic Degradation of Drawn Poly(l-lactic acid) (PLLA) Films. Macromolecules 2012, 45, 7397–7403. [Google Scholar] [CrossRef]
- Nishi, T.; Wang, T. Melting-Point Depression and Kinetic Effects of Cooling on Crystallization in Poly (vinylidene fluoride) Poly(Methyl methacrylate) Mixtures. Macromolecules 1975, 8, 909–915. [Google Scholar] [CrossRef]
- Quynh, T.; Mitomo, H.; Zhao, L.; Tamada, M. Properties of a poly (L-lactic acid)/poly (D-lactic acid) stereocomplex and the stereocomplex crosslinked with triallyl isocyanurate by irradiation. J. Appl. Polym. Sci. 2008, 110, 2358–2365. [Google Scholar] [CrossRef]



| PLLA Specimens | (%) | (%) | (%) | |
|---|---|---|---|---|
| 1 wt% 30 kGy | 99.9 | 99.9 | 94.5 | 98.4 |
| 3 wt% 30 kGy | 74.9 | 99.9 | 100 | 99.8 |
| 5 wt% 30 kGy | 52.7 | 99.9 | 100 | 100 |
| 10 wt% 30 kGy | 42.2 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, J.; Yang, D.; You, J.; Yu, G. Fabrication of Crosslinked Poly(L-lactic acid) with Enhanced Shape Memory Performance via γ-Ray Irradiation. Polymers 2025, 17, 3041. https://doi.org/10.3390/polym17223041
Wang J, Zhao J, Yang D, You J, Yu G. Fabrication of Crosslinked Poly(L-lactic acid) with Enhanced Shape Memory Performance via γ-Ray Irradiation. Polymers. 2025; 17(22):3041. https://doi.org/10.3390/polym17223041
Chicago/Turabian StyleWang, Jiayao, Jingxin Zhao, Dong Yang, Jichun You, and Guipeng Yu. 2025. "Fabrication of Crosslinked Poly(L-lactic acid) with Enhanced Shape Memory Performance via γ-Ray Irradiation" Polymers 17, no. 22: 3041. https://doi.org/10.3390/polym17223041
APA StyleWang, J., Zhao, J., Yang, D., You, J., & Yu, G. (2025). Fabrication of Crosslinked Poly(L-lactic acid) with Enhanced Shape Memory Performance via γ-Ray Irradiation. Polymers, 17(22), 3041. https://doi.org/10.3390/polym17223041









