Bioinspired Multilayer Silicone Composites: Autonomous Healing and Rate-Dependent Mechanics via Dynamic Boron Coordination Networks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
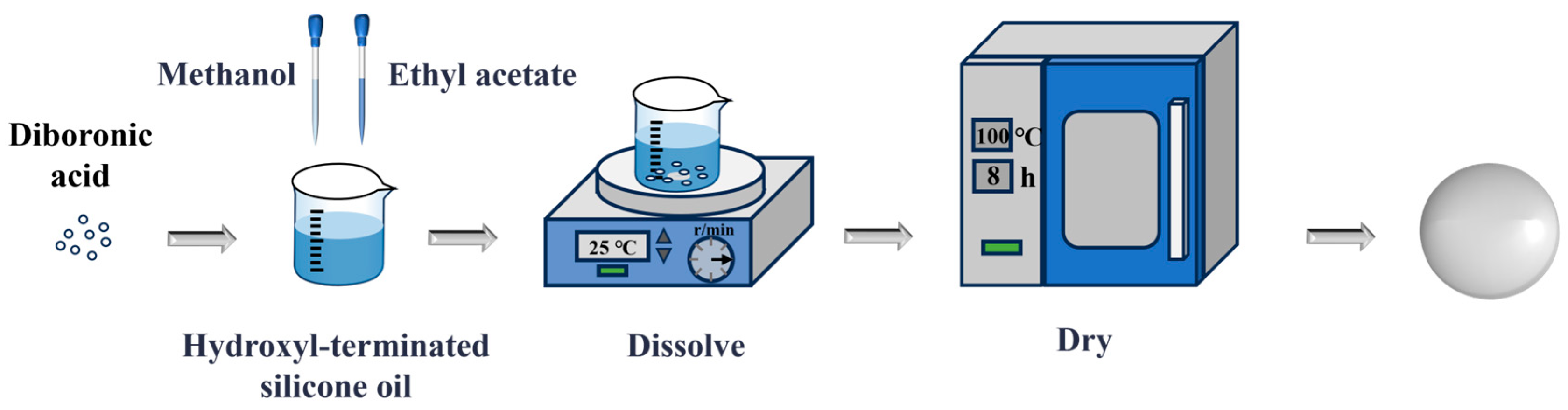
2.2. Preparation Process of PDBS
2.3. Preparation Process of Multilayer Silicone Rubber
2.4. Characterization
3. Results and Discussion
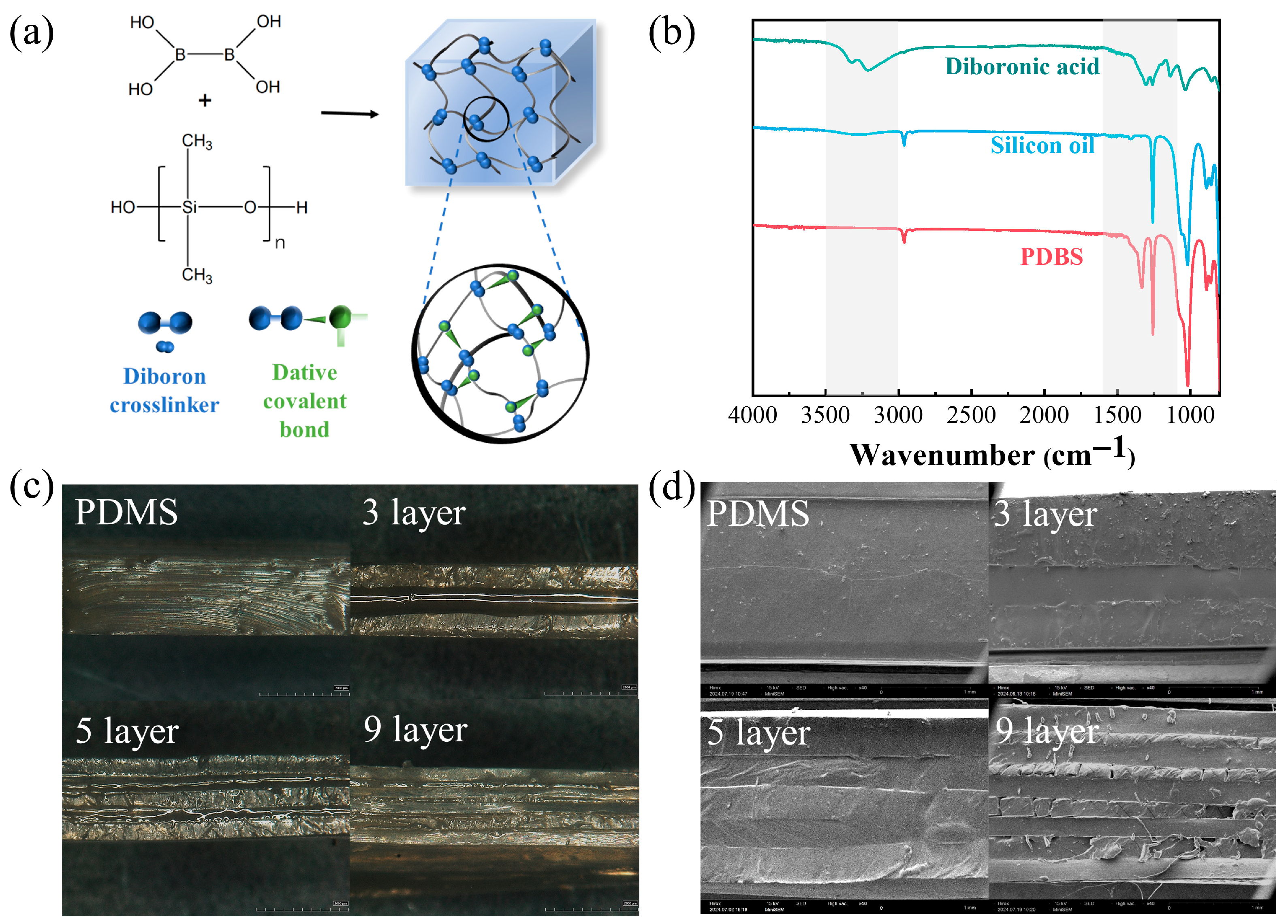
3.1. Synthesis and Characterization of Samples
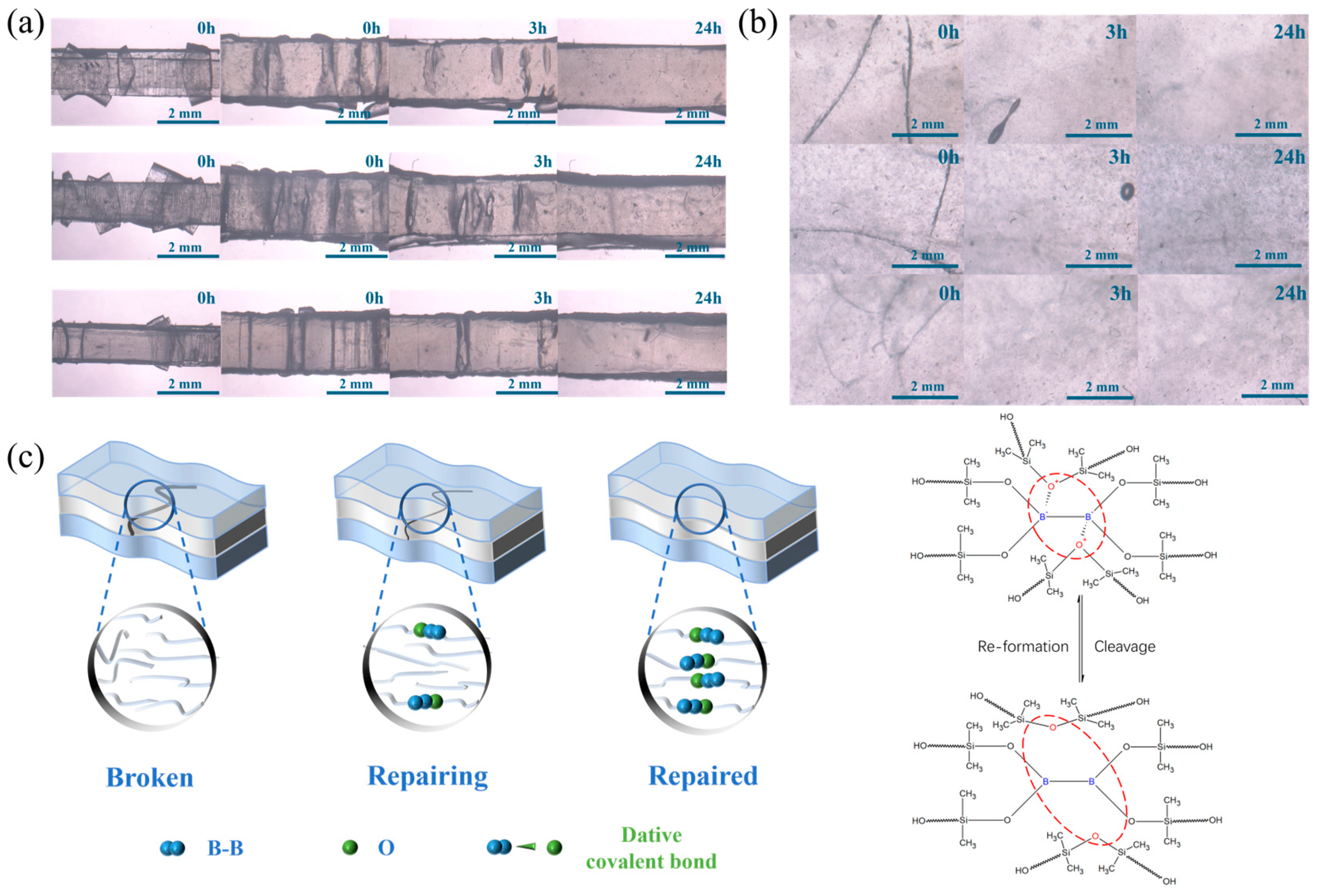
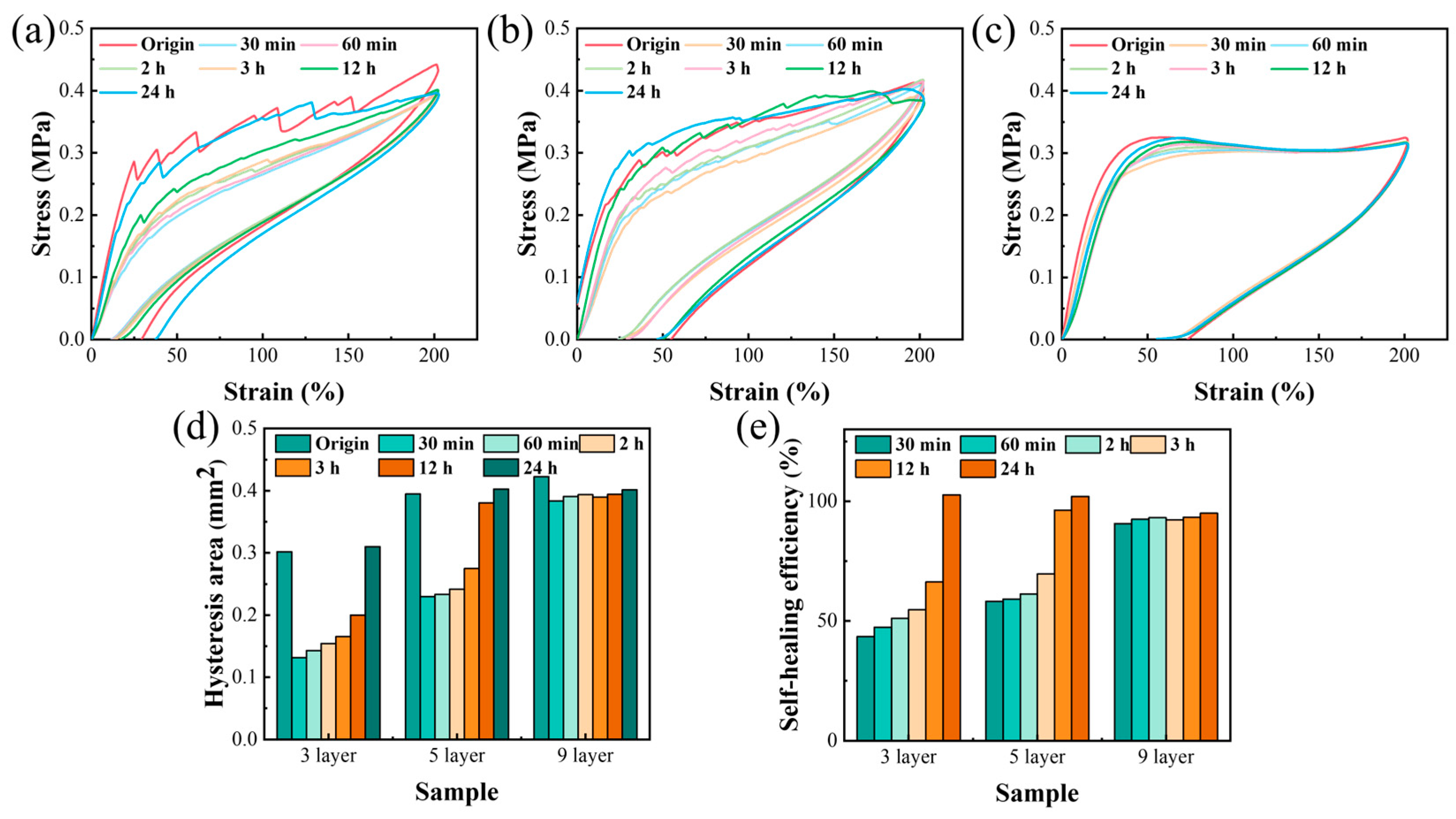
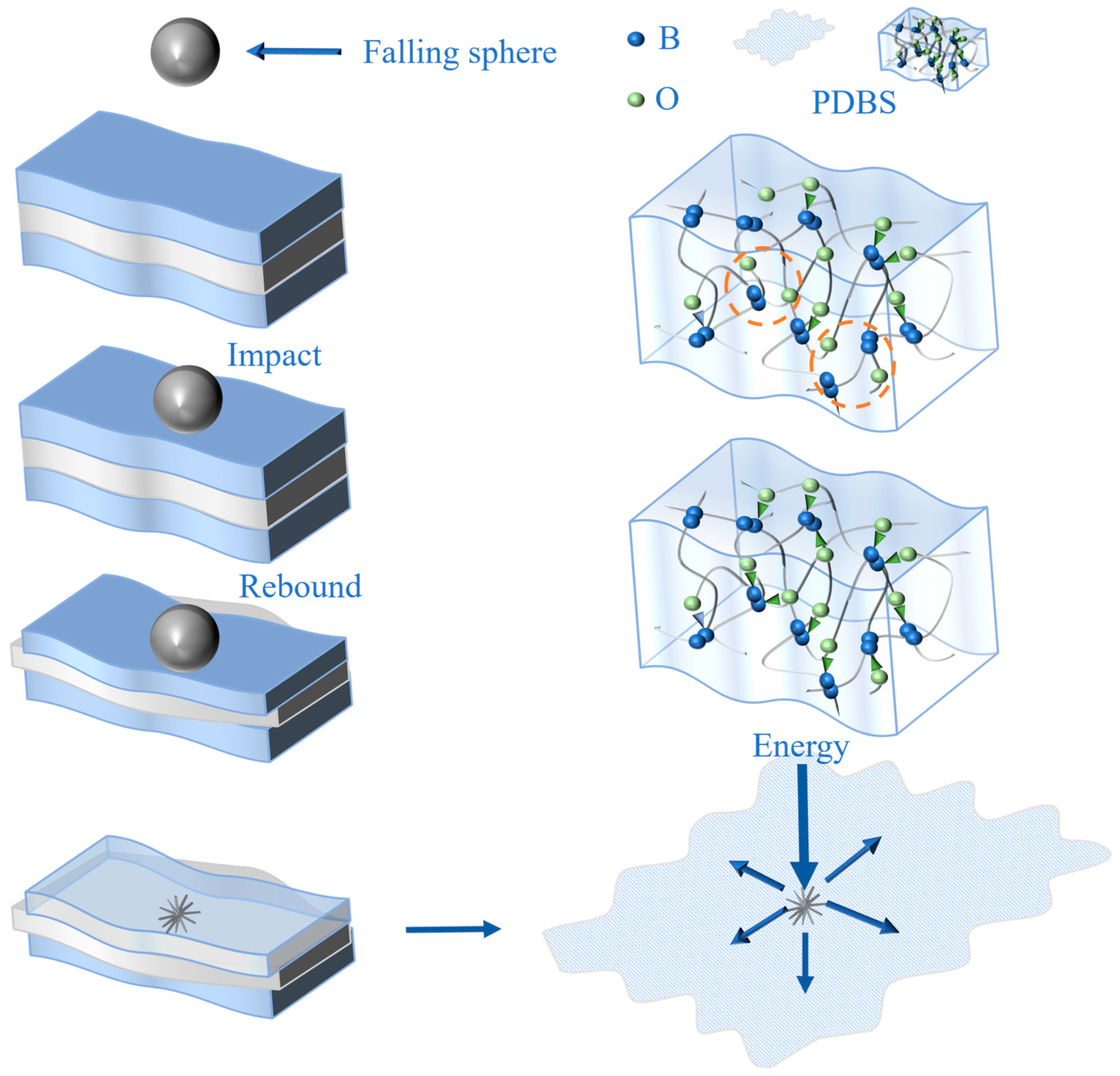
3.2. Self-Repairing Behavior and Mechanism Diagram
3.3. Tensile Rate Response Characteristics
3.4. Compression Rate Response Characteristic
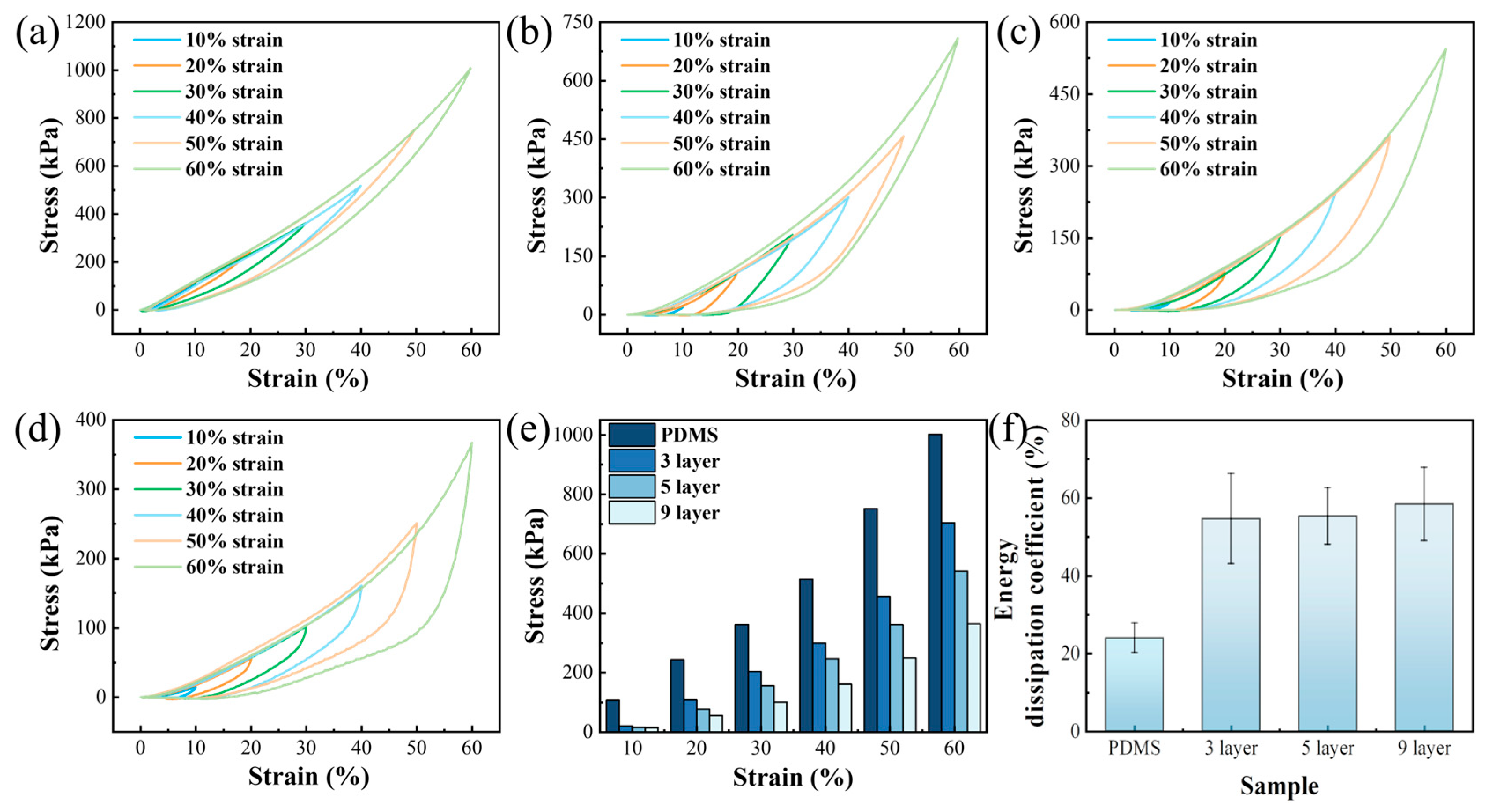
3.5. Cyclic Compression Characteristics
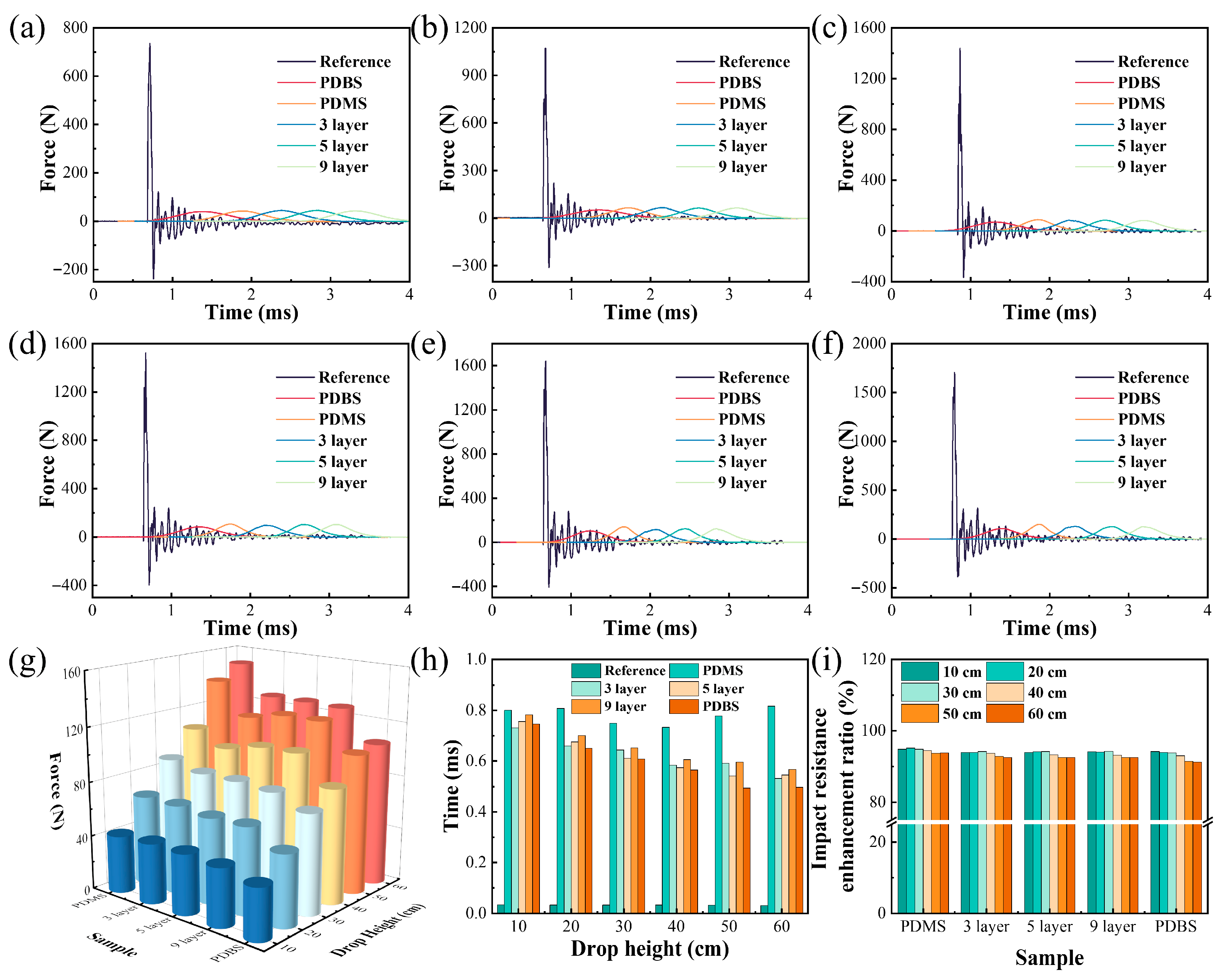
3.6. Impact Resistance Performance and Mechanism Diagram
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, Z.; Xu, B.; Su, Z.; Wang, X.; Lyu, Y.; Liu, S.; Wu, T.; Wang, X. Advanced vat photopolymerization 3D printing of silicone rubber with high precision and superior stability. Int. J. Extrem. Manuf. 2024, 7, 025001. [Google Scholar] [CrossRef]
- Kumar, V.; Parvin, N.; Park, S.-S.; Joo, S.W.; Mandal, T.K. Review on cutting-edge innovations in carbon nanomaterials reinforced silicone rubber composites for flexible electronics and healthcare devices. ACS Appl. Polym. Mater. 2024, 6, 14235–14259. [Google Scholar] [CrossRef]
- Liu, L.; Guo, C.; Xiang, Y.; Tu, Y.; Mei, H.; Wang, L.; Xuan, F.-Z. Health monitoring of RTV silicone rubber coating on insulators based on multimode features of active infrared thermography. IEEE Trans. Instrum. Meas. 2022, 71, 4502609. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, X.; Shi, J.; Shan, H.; Liu, M.; Liu, J.; Wang, J.; Jia, B.; Zheng, Y.; Chen, Q.; et al. Preparation of cleaning and repairing agent and its double cleaning effect on surface contamination of silicone rubber external insulation. Mater. Res. Express 2021, 8, 065101. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.Á.; Santana, M.H. The final frontier of sustainable materials: Current developments in self-healing elastomers. Int. J. Mol. Sci. 2022, 23, 4757. [Google Scholar] [CrossRef]
- An, S.; Yoon, S.S.; Lee, M.W. Self-healing structural materials. Polymers 2021, 13, 2297. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, X.-Y.; Wang, S.; Shao, R.-Y.; Su, J.-F. Investigation of asphalt self-healing capability using microvasculars containing rejuvenator: Effects of microvascular content, self-healing time and temperature. Materials 2023, 16, 4746. [Google Scholar] [CrossRef]
- Li, M.-Z.; Cao, B.; Yin, F.; Mei, H.; Wang, L. Preparation and performance improvement of a dual-component microcapsule self-healing system for silicone rubber insulating material. High Volt. 2024, 9, 453–465. [Google Scholar] [CrossRef]
- Xu, G.-W.; Liang, Z.-P.; Huang, Q.-N.; Wang, Y.-M.; Yang, J.; Nie, Y.-J. Tough self-healing polyurethane elastomers based on interpenetrating networks containing multiple hydrogen bond networks, flexible blocks, metal coordination and covalent Cross-Linking. Prog. Org. Coat. 2023, 175, 107391. [Google Scholar] [CrossRef]
- Wang, P.; Yang, L.; Dai, B.; Yang, Z.-H.; Guo, S.; Gao, G.; Xu, L.-G.; Sun, M.-Q.; Yao, K.-L.; Zhu, J.-Q. A self-healing transparent polydimethylsiloxane elastomer based on imine bonds. Eur. Polym. J. 2020, 123, 109382. [Google Scholar] [CrossRef]
- Nam, J.; Jang, W.; Rajeev, K.K.; Lee, J.-H.; Kim, Y.; Kim, T.-H. Ion-conductive self-healing polymer network based on reversible imine bonding for Si electrodes. J. Power Sources 2021, 499, 229968. [Google Scholar] [CrossRef]
- Liu, S.-H.; Liu, X.-Y.; He, Z.-K.; Liu, L.-Y.; Niu, H. Thermoreversible cross-linking of ethylene/propylene copolymers based on Diels–Alder chemistry: The cross-linking reaction kinetics. Polym. Chem. 2020, 11, 5851–5860. [Google Scholar] [CrossRef]
- Chen, X.-X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Dang, H.; Zhang, Z.; Sun, R.; Li, Y.; Lin, M.; Yang, S.; He, M.; Xu, Z.; Bian, X. Construction of a transparent, robust, shape-memory and self-healing MDI-based polyurethane elastomer. Polymers 2025, 17, 1243. [Google Scholar] [CrossRef]
- Yu, S.-J.; Li, F.-Z.; Fang, S.-Z.; Yin, X.-C.; Wu, S.-W.; Tang, Z.-H.; Zhang, L.-Q.; Guo, B.-C. Extrudable vitrimeric rubbers enabled via heterogeneous network design. Macromolecules 2022, 55, 3236–3248. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Z.-H.; Cen, W.-T.; Zhang, C.-F.; Guo, B.-C. Creating molecular bridges across the interfaces in segregated composites toward improved conductive and mechanical properties. Compos. Sci. Technol. 2022, 222, 109377. [Google Scholar] [CrossRef]
- Huang, Q.-Y.; Tang, Z.-H.; Wang, D.; Wu, S.-W.; Guo, B.-G. Engineering segregated structures in a cross-linked elastomeric network enabled by dynamic cross-link reshuffling. ACS Macro Lett. 2021, 10, 231–236. [Google Scholar] [CrossRef]
- Jin, B.; Wu, W.; Wu, H. Mechanical robust, self-healable polyurethane elastomer enabled by hierarchical hydrogen bonds and disulfide bonds. Polymers 2023, 15, 4020. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-T.; Jin, B.-Q.; Wang, H.; Wu, W.-Q.; Cao, Z.-X.; Wu, J.-R.; Huang, G.-S. A degradable and self-healable vitrimer based on non-isocyanate polyurethane. Front. Chem. 2020, 8, 585569. [Google Scholar] [CrossRef]
- Xu, J.-H.; Chen, J.-Y.; Zhang, Y.-N.; Liu, T.; Fu, J.-J. A fast room-temperature self-healing glassy polyurethane. Angew. Chem. Int. Ed. 2021, 60, 7947–7955. [Google Scholar] [CrossRef]
- Wu, Q.; Xiong, H.; Zhu, Y.; Ren, X.; Chu, L.-L.; Yao, Y.-F.; Huang, G.; Wu, J. Self-healing amorphous polymers with room-temperature phosphorescence enabled by boron-based dative bonds. ACS Appl. Polym. Mater. 2020, 2, 699–705. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Cao, W.; Zhu, J. Facile Preparation of a transparent, self-healing, and recyclable polysiloxane elastomer based on a dynamic imine and boroxine bond. Polymers 2024, 16, 1262. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Matsuno, T.; Shimojima, A. Multilayered organosiloxane films with self-healing ability converted from block copolymer nanocomposites. Chem. Commun. 2025, 61, 3319–3322. [Google Scholar] [CrossRef]
- Li, X.; Yu, R.; Zhao, T.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. A self-healing polysiloxane elastomer based on siloxane equilibration synthesized through amino-ene Michael addition Reaction. Eur. Polym. J. 2018, 108, 399–405. [Google Scholar] [CrossRef]
- Wang, X.; Liang, D.; Cheng, B. Preparation and research of intrinsic self-healing elastomers based on hydrogen and ionic bond. Compos. Sci. Technol. 2020, 193, 108127. [Google Scholar] [CrossRef]
- Zibafar, M.; Eslami-Farsani, R. Reversible ionic bond mechanism in self-healing of natural rubber: A review. Polym. Rev. 2025, 65, 453–482. [Google Scholar] [CrossRef]
- Cui, L.-F.; Zeng, G.-Y.; Li, X.; Bian, F.; Xiong, Y.-Z. Enhanced design of dual dynamic cross-linked rubber composites: Achieving self-healing and recyclability through imine and metal-ligand bonding. Compos. Sci. Technol. 2024, 246, 110382. [Google Scholar] [CrossRef]
- Yan, H.; Dai, S.-P.; Chen, Y.-W.; Ding, J.-N.; Yuan, N.-Y. A high stretchable and self–healing silicone rubber with double reversible bonds. Chem. Sel. 2019, 4, 10719–10725. [Google Scholar] [CrossRef]
- Wu, T.; Chen, B. Synthesis of multiwalled carbon nanotube-reinforced polyborosiloxane nanocomposites with mechanically adaptive and self-healing capabilities for flexible conductors. ACS Appl. Mater. Iterfaces 2016, 8, 24071–24078. [Google Scholar] [CrossRef]
- Drozdov, F.V.; Manokhina, E.A.; Vu, T.D.; Muzafarov, A.M. Polyborosiloxanes (PBS): Evolution of approaches to the synthesis and the prospects of their application. Polymers 2022, 14, 4824. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, N.; Zhang, D.; He, B.; Chen, X. Study on optimization of damping performance and damping temperature range of silicone rubber by polyborosiloxane gel. Polymers 2020, 12, 1196. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xiong, H.; Peng, Y.; Yang, Y.; Kang, J.; Huang, G.; Ren, X.; Wu, J. Highly stretchable and self-healing “solid–liquid” elastomer with strain-rate sensing capability. ACS Appl. Mater. Interfaces 2019, 11, 19534–19540. [Google Scholar] [CrossRef]
- Tang, M.; Zheng, P.; Wang, K.-Q.; Qin, Y.-J.; Jiang, Y.-Z.; Cheng, Y.-R.; Li, Z.; Wu, L.-M. Autonomous self-healing, self-adhesive, highly conductive composites based on a silver-filled polyborosiloxane/polydimethylsiloxane double-network elastomer. J. Mater. Chem. A 2019, 7, 27278–27288. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, J.; Shan, Z.; Guo, C.; Tao, L.; Zhang, Y.; Wang, T.; Wang, Q. NIR-triggered rapid self-healing polyimide with on-demand tunable friction properties. Chem. Eng. J. 2025, 518, 164571. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Shi, Z.; Wang, D.; Yang, H.; Zhang, Y.; Qin, H.; Lu, W.; Chen, J.; Li, Y.; et al. Stable boroxine structure with dynamic covalent bonds. Nat. Commun. 2024, 15, 1207. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Huang, Y.; Yuan, Y.; Wang, Y.-L.; Yang, B.; Zhao, X.-L.; Liu, L.-L. Development of a strong, recyclable poly(dimethylsiloxane) elastomer with autonomic self-healing capabilities and fluorescence response properties at room temperature. Macromol. Mater. Eng. 2021, 306, 2100132. [Google Scholar] [CrossRef]
- Wu, Q.; Peng, Y.; Xiong, H.; Hou, Y.-J.; Cai, M.-J.; Wang, Y.; Zhao, L.-J.; Wu, J.-R. A novel shear-stiffening supramolecular material derived from diboron structure. Sci. China Mater. 2023, 66, 4489–4498. [Google Scholar] [CrossRef]
- Ma, X.; Luo, C.; Zeng, H.; Peng, Y.; Zhao, L.; Zhang, F. Effect of polydiborosiloxane on the mechanical properties of silicone rubber foam with dual network structure. Polym. Eng. Sci. 2024, 64, 1971–1980. [Google Scholar] [CrossRef]
- Siavoshani, A.Y.; Fan, Z.; Yang, M.; Liu, S.; Wang, M.-C.; Liu, J.; Xu, W.; Wang, J.; Lin, S.; Wang, S.-Q. How do stretch rate, temperature, and solvent exchange affect elastic network rupture? Soft Matter 2024, 20, 7657–7667. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Fan, Z.-H.; Gupta, C.; Siavoshani, A.; Smith, T. Fracture behavior of polymers in plastic and elastomeric states. Macromolecules 2024, 57, 3875–3900. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Peng, Y.; Liu, T.; Zhao, L.; Zhang, F. Bioinspired Multilayer Silicone Composites: Autonomous Healing and Rate-Dependent Mechanics via Dynamic Boron Coordination Networks. Polymers 2025, 17, 3040. https://doi.org/10.3390/polym17223040
Zeng H, Peng Y, Liu T, Zhao L, Zhang F. Bioinspired Multilayer Silicone Composites: Autonomous Healing and Rate-Dependent Mechanics via Dynamic Boron Coordination Networks. Polymers. 2025; 17(22):3040. https://doi.org/10.3390/polym17223040
Chicago/Turabian StyleZeng, Hongwen, Yan Peng, Tao Liu, Lijuan Zhao, and Fengshun Zhang. 2025. "Bioinspired Multilayer Silicone Composites: Autonomous Healing and Rate-Dependent Mechanics via Dynamic Boron Coordination Networks" Polymers 17, no. 22: 3040. https://doi.org/10.3390/polym17223040
APA StyleZeng, H., Peng, Y., Liu, T., Zhao, L., & Zhang, F. (2025). Bioinspired Multilayer Silicone Composites: Autonomous Healing and Rate-Dependent Mechanics via Dynamic Boron Coordination Networks. Polymers, 17(22), 3040. https://doi.org/10.3390/polym17223040






