Functional Hydrogels in Food Applications: A Review of Crosslinking Technologies, Encapsulation Trends, and Emerging Challenges
Abstract
1. Introduction
2. Classification According to Origin
2.1. Natural Hydrogels
2.2. Synthetic Hydrogels
2.3. Hybrid Hydrogels
3. Chemical Crosslinking
3.1. Chemical Crosslinking by Radiation
3.2. Chemical Crosslinking by Photo-Crosslinking
3.3. Crosslinking by Click Chemistry
4. Physical Crosslinking
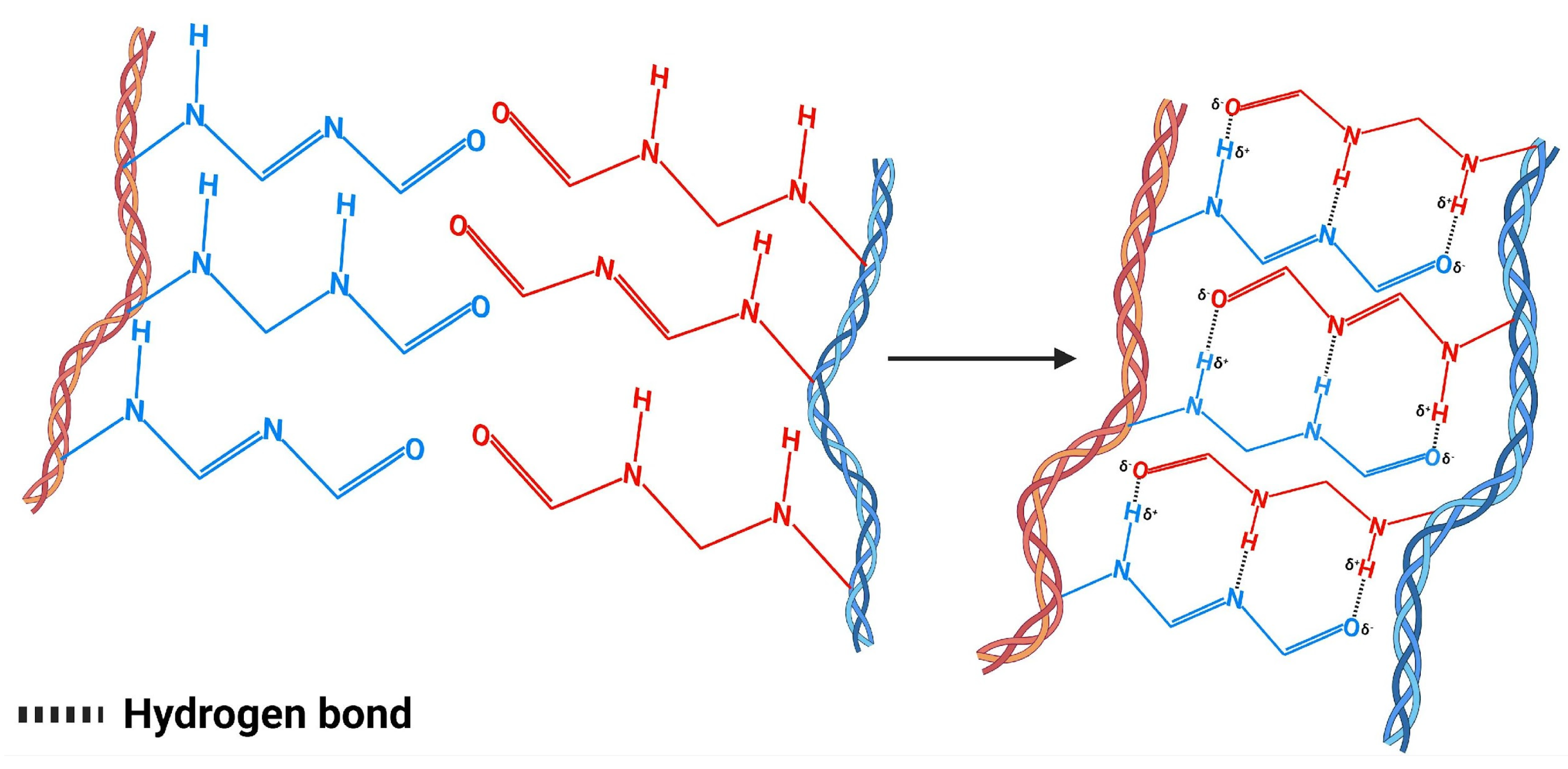
4.1. Hydrogen Bonds
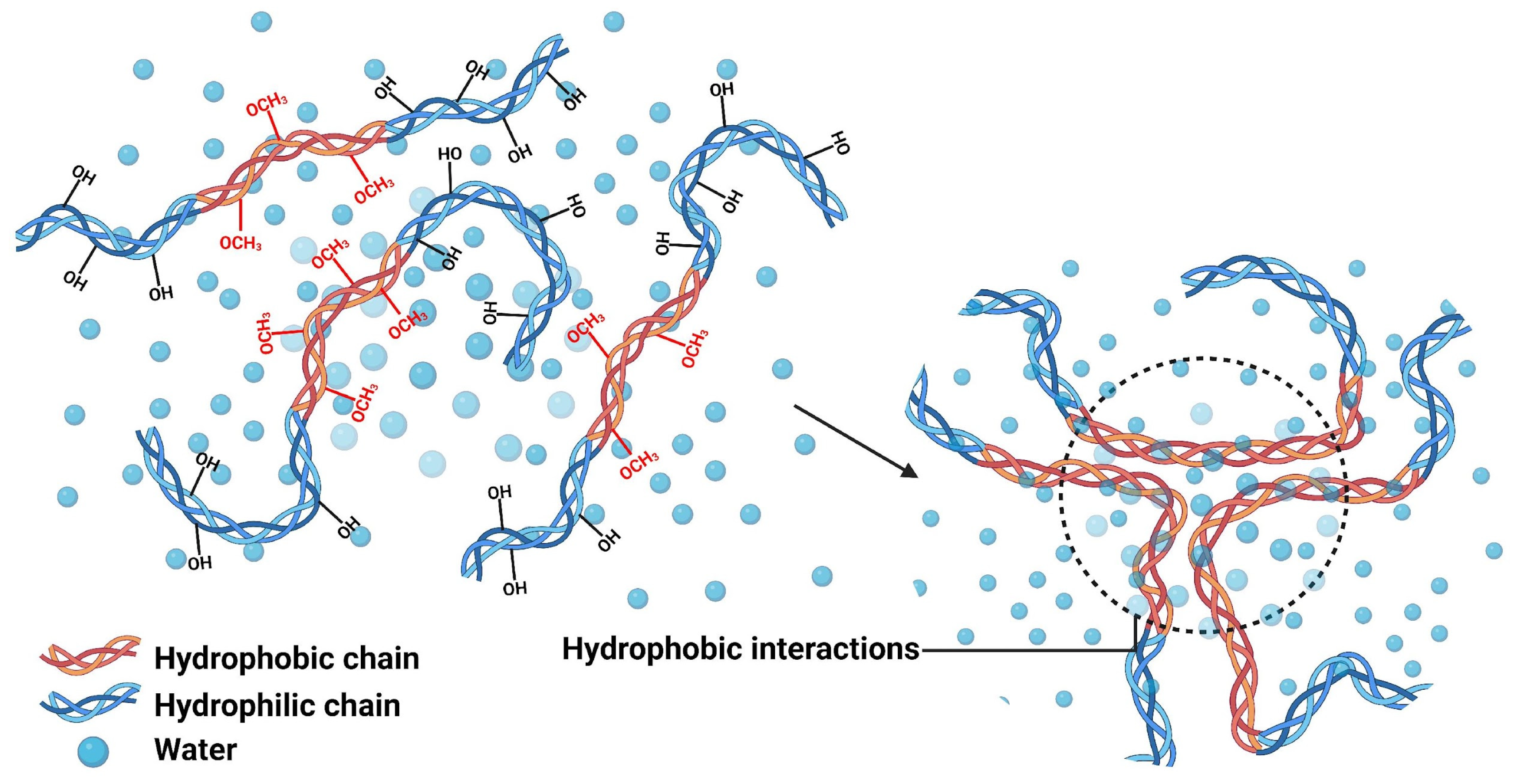
4.2. Hydrophobic Interactions
4.3. Enzymatic Crosslinking
4.3.1. Transglutaminases
4.3.2. Laccases
4.3.3. Peroxidases
5. Ultrasound-Assisted Crosslinking
6. Structural Configurations
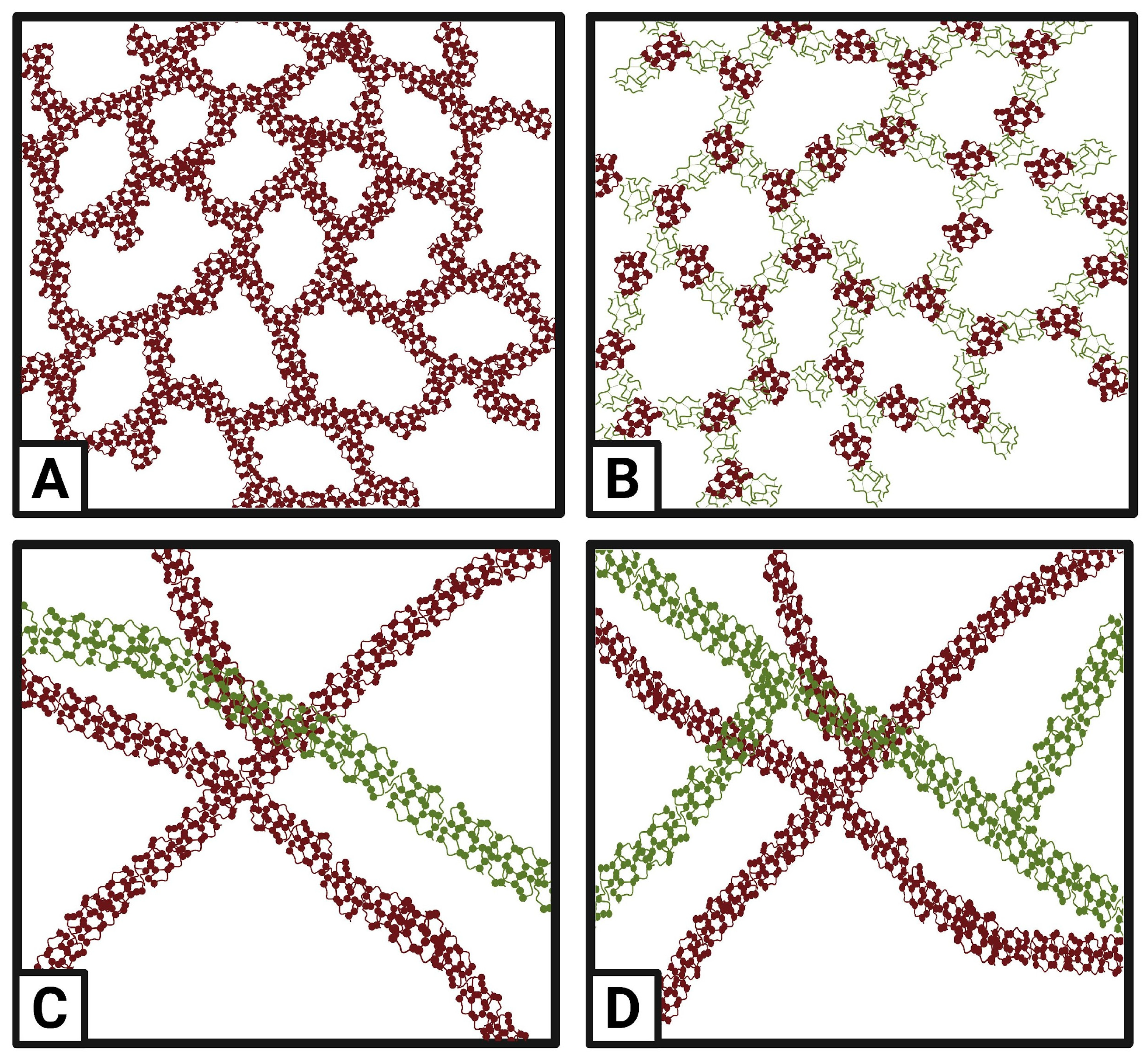
7. Applications in Foods
7.1. Encapsulation and Delivery of Nutrients for Functional Foods
7.2. Texture Modification and Functional Foods
7.3. Hydrogels in Food Active Packaging
7.4. Hydrogels in Cultured Meat
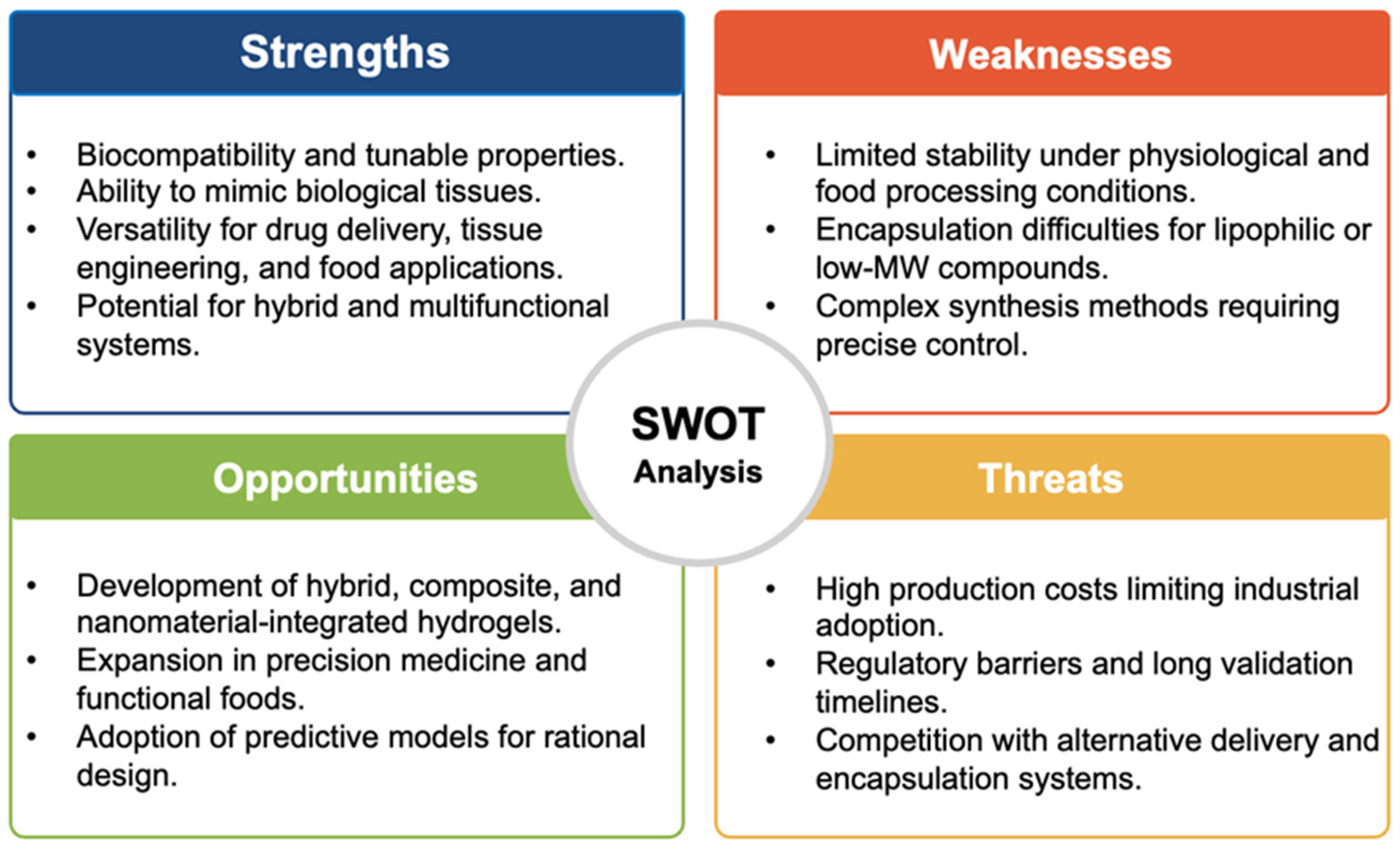
8. Emerging Trends and Current Challenges
9. Conclusions
- −
- Smart and responsive hydrogels that adapt their behavior to pH, temperature, or enzymatic activity, enabling precision delivery of nutrients or bioactives.
- −
- Green and sustainable synthesis routes, employing renewable biopolymers and mild processing conditions to minimize environmental impact.
- −
- Integration with advanced processing technologies (e.g., 3D printing, microfluidics, and membrane-based systems) to design customizable food structures and functional biomaterials.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Mehta, P.; Sharma, M.; Devi, M. Hydrogels: An overview of its classifications, properties, and applications. J. Mech. Behav. Biomed. Mater. 2023, 147, 106145. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Bio-medicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, H.; Liu, S.; Pu, L.; Hu, X.; Ding, J.; Xu, G.; Xu, W.; Xiang, S.; Yuan, Z. A review of protein hydrogels: Protein assembly mechanisms, properties, and bio-logical applications. Colloids Surf. B Biointerfaces 2022, 220, 112973. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, L. Progress, challenge and perspective of hydrogels application in food: A review. npj Sci. Food 2025, 9, 155. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Li, M.; He, X.; Zhao, R.; Shi, Q.; Nian, Y.; Hu, B. Hydrogels as promising carriers for the delivery of food bioactive ingredients. Front. Nutr. 2022, 9, 1006520. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Micro-structure, and Its Applications. Gels 2023, 9, 1. [Google Scholar] [CrossRef]
- Kaur, N.; Hamid Choudhary, P.; Jaiswal, A.K. Recent progress in bioactive loaded hydrogels for food applications. J. Agric. Food Res. 2025, 20, 101756. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Miao, W.; Lin, Q.; Ji, H.; Li, X.; McClements, D.J.; Jin, Z.; Qiu, C. Recent advances in bio-based co-delivery systems for food bioactive compounds: A review. Food Biosci. 2025, 63, 105758. [Google Scholar] [CrossRef]
- Dodda, J.M.; Deshmukh, K.; Bezuidenhout, D.; Yeh, Y.-C. Hydrogels: Definition, History, Classifications, Formation, Constitutive Characteristics, and Applications. In Multicomponent Hydrogels; Royal Society of Chemistry: Cambridge, UK, 2023; pp. 1–25. [Google Scholar] [CrossRef]
- Islam, M.R.; Oyen, M.L. Mechanical characterization of hydrogels. In The Mechanics of Hydrogels: Mechanical Properties, Testing, and Applications; Li, E.H., Silberschmidt, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–24. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Hydrogels: Characteristics and Application as Delivery Systems of Phenolic and Aroma Compounds. Foods 2021, 10, 1252. [Google Scholar] [CrossRef]
- Ghorpade, V.S. Preparation of hydrogels based on natural polymers via chemical reaction and cross-Linking. In Hydrogels Based on Natural Polymers; Chen, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–118. [Google Scholar] [CrossRef]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S. Cross-Linking Agents in Three-Component Materials Dedicated to Biomedical Applications: A Review. Polymers 2024, 16, 2679. [Google Scholar] [CrossRef]
- Yánez-Vega, H.; Yánez-Vega, K.; Bustamante-Torres, M. Synthesis of Hydrogels: Physical and Chemical Cross-Linking. In Multifunctional Hydrogels: From Basic Concepts to Advanced Applications; Taylor & Francis Group: Abingdon, UK, 2024; pp. 70–87. [Google Scholar] [CrossRef]
- Mhaske, S.T.; Mestry, S.U.; Patil, D.A. Cross-linking of polymers by various radiations: Mechanisms and parameters. In Radiation Technologies and Applications in Materials Science; Taylor & Francis Group: Abingdon, UK, 2022; pp. 1–28. [Google Scholar] [CrossRef]
- Yang, J.; Rao, L.; Wang, Y.; Zhao, Y.; Liu, D.; Wang, Z.; Fu, L.; Wang, Y.; Yang, X.; Li, Y.; et al. Recent Advances in Smart Hydrogels Prepared by Ionizing Radiation Technology for Biomedical Applications. Polymers 2022, 14, 4377. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Kumar, V. Synthesis of Hydrogels by Modification of Natural Polysaccharides Through Radiation Cross-Linking Polymerization for Use in Drug Delivery. In Radiation Effects in Polymeric Materials; Springer: Cham, Switzerland, 2019; pp. 269–292. [Google Scholar] [CrossRef]
- Raza, M.A.; Jeong, J.O.; Park, S.H. State-of-the-Art Irradiation Technology for Polymeric Hydrogel Fabrication and Application in Drug Release System. Front. Mater. 2021, 8, 769436. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Hasan, M.K.; Nam, K.W. A Comprehensive Re-view of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Wang, J. Photo-crosslinkable hydrogel and its biological applications. Chin. Chem. Lett. 2021, 32, 1603–1614. [Google Scholar] [CrossRef]
- Liu, J.; Su, C.; Chen, Y.; Tian, S.; Lu, C.; Huang, W.; Lv, Q. Current Under-standing of the Applications of Photocrosslinked Hydrogels in Biomedical Engineering. Gels 2022, 8, 216. [Google Scholar] [CrossRef]
- Ma, H.; Peng, Y.; Zhang, S.; Zhang, Y.; Min, P. Effects and Progress of Pho-to-Crosslinking Hydrogels in Wound Healing Improvement. Gels 2022, 8, 609. [Google Scholar] [CrossRef]
- Li, C.; Sheng, L.; Sun, G.; Wang, L. The application of ultraviolet-induced photo-crosslinking in edible film preparation and its implication in food safety. LWT 2020, 131, 109791. [Google Scholar] [CrossRef]
- Echalier, C.; Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical cross-linking methods for cell encapsulation in hydrogels. Mater. Today Commun. 2019, 20, 100536. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of “Click” Chemistry in Biomedical Hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B. Cycloaddition Reactions. In Essentials of Pericyclic and Photochemical Reactions; Springer: Cham, Switzerland, 2017; pp. 37–106. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Michniak-Kohn, B.B.; Deol, P.K.; Morozova, S.M. Recent Advances in Hydrogels via Diels–Alder Crosslinking: Design and Applications. Gels 2023, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Aflak, N.; Bahsis, L.; Julve, M.; Stiriba, S.E. Copper-catalyzed azide-alkyne cycloaddition click reaction: Mechanistic and synthetic aspects. Click Chem. 2024, 1, 105–122. [Google Scholar] [CrossRef]
- Mehak, N.; Singh, G.; Singh, R.; Singh, G.; Stanzin, J.; Singh, H.; Kaur, G.; Singh, J. Clicking in harmony: Exploring the bio-orthogonal overlap in click chemistry. RSC Adv. 2024, 14, 7383–7413. [Google Scholar] [CrossRef]
- Singh, G.; Majeed, A.; Singh, R.; George, N.; Singh, G.; Sophia, N.; Singh, H.; Kaur, G.; Singh, J. CuAAC ensembled 1,2,3-triazole linked nanogels for targeted drug delivery: A review. RSC Adv. 2023, 13, 2912–2936. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. Development and Applications of the Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) as a Bioorthogonal Reaction. Molecules 2016, 21, 1393. [Google Scholar] [CrossRef]
- Pirsa, S.; Khodaei, S.M.; Sani, I.K.; Ghasemi, Y.; Jawhar, Z.H.; Eghbaljoo, H. Hydrogels and biohydrogels: Investigation of origin of production, production methods, and application. Polym. Bull. 2023, 80, 10593–10632. [Google Scholar] [CrossRef]
- Rizwan, M.; Gilani, S.R.; Durani, A.I.; Naseem, S. Materials diversity of hydrogel: Synthesis, polymerization process and soil conditioning properties in agricultural field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhao, L.; Yuan, J.; Chen, Y.; Leng, Y. Physical hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–89. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Q.; Liu, H.; Zhao, Q.; Niu, Y.; Zhao, D. Adhesion mechanism and application progress of hydrogels. Eur. Polym. J. 2022, 173, 111277. [Google Scholar] [CrossRef]
- Yin, H.; Liu, F.; Abdiryim, T.; Liu, X. Self-Healing Hydrogels: From Synthesis to Multiple Applications. ACS Mater. Lett. 2023, 5, 1787–1830. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; Yin, L. Hydrogel: Diversity of Structures and Applications in Food Science. Food Rev. Int. 2021, 37, 313–372. [Google Scholar] [CrossRef]
- Quan, L.; Xin, Y.; Wu, X.; Ao, Q. Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polymers 2022, 14, 2184. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.X.; Xu, W.; Yang, Z.; Wu, Y.; Pi, F. An Overview on Recent Progress of the Hydrogels: From Material Resources, Properties, to Functional Applications. Macromol. Rapid Commun. 2022, 43, 2100785. [Google Scholar] [CrossRef]
- Liu, B.; Yang, H.; Zhu, C.; Xiao, J.; Cao, H.; Simal-Gandara, J.; Li, Y.; Fan, D.; Deng, J. A comprehensive review of food gels: Formation mechanisms, functions, applications, and challenges. Crit. Rev. Food Sci. Nutr. 2024, 64, 760–782. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A. Developing tough terpolymer hydrogel with out-standing swelling ability by hydrophobic association cross-linking. Polymer 2022, 254, 125037. [Google Scholar] [CrossRef]
- Gosecka, M.; Gosecki, M.; Jaworska-Krych, D. Hydrophobized Hydrogels: Construction Strategies, Properties, and Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2212302. [Google Scholar] [CrossRef]
- Sun Han Chang, R.; Lee, J.C.W.; Pedron, S.; Harley, B.A.C.; Rogers, S.A. Rheological Analysis of the Gelation Kinetics of an Enzyme Cross-linked PEG Hydrogel. Biomacromolecules 2019, 20, 2198–2206. [Google Scholar] [CrossRef]
- Naranjo-Alcazar, R.; Bendix, S.; Groth, T.; Gallego Ferrer, G. Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering. Gels 2023, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, C.; Hu, X. Enzymatic synthesis, characterization and properties of the protein-polysaccharide conjugate: A review. Food Chem. 2022, 372, 131332. [Google Scholar] [CrossRef]
- Elham Badali Hosseini, M.; Mohajer, M.; Hassanzadeh, S.; Saghati, S.; Hilborn, J.; Khanmohammadi, M. Enzymatic Crosslinked Hydrogels for Biomedical Application. Polym. Sci. Ser. A 2021, 63, S1–S22. [Google Scholar] [CrossRef]
- Li, Z.; Lu, F.; Liu, Y. A Review of the Mechanism, Properties, and Applications of Hydrogels Prepared by Enzymatic Cross-linking. J. Agric. Food Chem. 2023, 71, 10238–10249. [Google Scholar] [CrossRef]
- Arkenberg, M.R.; Nguyen, H.D.; Lin, C.C. Recent advances in bio-orthogonal and dynamic crosslinking of biomimetic hydrogels. J. Mater. Chem. B 2020, 8, 7835–7855. [Google Scholar] [CrossRef]
- Maddock, R.M.A.; Pollard, G.J.; Moreau, N.G.; Perry, J.J.; Race, P.R. Enzyme-catalysed polymer cross-linking: Biocatalytic tools for chemical biology, materials science and beyond. Biopolymers 2020, 111, e23390. [Google Scholar] [CrossRef]
- Santoso, T.; Al-Shaikhli, Y.; Ho, T.M.; Rajapakse, M.; Le, T.T. Optimising Enzymatic Cross-Linking: Impact on Physicochemical and Functional Properties of Lupin Flour and Soy Protein Isolate. Foods 2025, 14, 1976. [Google Scholar] [CrossRef]
- McKenzie, T.G.; Karimi, F.; Ashokkumar, M.; Qiao, G.G. Ultrasound and Sonochemistry for Radical Polymerization: Sound Synthesis. Chem.—A Eur. J. 2019, 25, 5372–5388. [Google Scholar] [CrossRef]
- Rokita, B.; Rosiak, J.M.; Ulanski, P. Ultrasound-induced cross-linking and formation of macroscopic covalent hydrogels in aqueous polymer and monomer solutions. Macromolecules 2009, 42, 3269–3274. [Google Scholar] [CrossRef]
- Santha Kumar, A.R.S.; Padmakumar, A.; Kalita, U.; Samanta, S.; Baral, A.; Singha, N.K.; Ashokkumar, M.; Qiao, G.G. Ultrasonics in polymer science: Applications and challenges. Prog. Mater. Sci. 2023, 136, 101113. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, C.; Guo, M. Effects of high intensity ultrasound on ac-id-induced gelation properties of whey protein gel. Ultrason. Sonochemistry 2017, 39, 810–815. [Google Scholar] [CrossRef]
- Zhao, Y.; Xue, S.; Zhang, X.; Zhang, T.; Shen, X. Improved Gel Properties of Whey Protein-Stabilized Emulsions by Ultrasound and Enzymatic Cross-Linking. Gels 2021, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Muhaimin, M.; Chaerunisaa, A.Y.; Bodmeier, R. Sonication-assisted emulsification: Analyzing different polymers in aqueous systems for microparticle preparation by the double emulsion technique. Ultrason. Sonochem. 2025, 121, 107555. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Sotiropoulos, K. Current Advances of Polysaccharide-Based Nanogels and Microgels in Food and Biomedical Sciences. Polymers 2022, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, B.; Madadlou, A.; Salami, M. Functional and in vitro gastric digestibility of the whey protein hydrogel loaded with nanostructured lipid carriers and gelled via citric acid-mediated crosslinking. Food Chem. 2017, 237, 23–29. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Sun, S.; Yu, L.; Teng, J.; Gu, Y.; Pang, Y.; Xu, X.; Wang, W.; Li, Y. Eutectic gels: Presentation and prospect. Appl. Mater. Today 2024, 39, 102342. [Google Scholar] [CrossRef]
- Yuan, R.; Lv, L.; Wang, F.; Yu, Y.; Zhang, S.; Yin, J.; Huang, R. Multiple hydrogen bond-crosslinked hydrogels engineered from natural microalgae framework and deep eutectic solvents for flexible freeze-resistant strain sensors. Colloids Surf. A Physicochem. Eng. Asp. 2025, 727, 138249. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kang, H.W. Natural deep eutectic solvents (NADES)-based chitosan hydrogel for enhanced photoimmunomodulation treatment on various wounds. Int. J. Biol. Macromol. 2025, 321, 146591. [Google Scholar] [CrossRef]
- Alpaslan, D.; Erşen Dudu, T.; Aktas, N. Development of onion oil-based organo-hydrogel for drug delivery material. J. Dispers. Sci. Technol. 2023, 44, 750–762. [Google Scholar] [CrossRef]
- Alpaslan, D.; Dudu, T.E.; Aktaş, N. Synthesis and characterization of novel organo-hydrogel based agar, glycerol and peppermint oil as a natural drug carrier/release material. Mater. Sci. Eng. C 2021, 118, 111534. [Google Scholar] [CrossRef]
- Hashim, A.F.; El-Sayed, S.M.; El-Sayed, H.S. Bigel formulations based on sesame oleogel with probiotics alginate hydrogel: A novel structure for nutritious spread-able butter. Int. J. Biol. Macromol. 2023, 242, 124782. [Google Scholar] [CrossRef]
- Zhou, M.; Li, B.; Wu, A.; Hu, Z.; Liu, J.; Wang, Y.; Liu, H. Preparation of a bigel system based on k-carrageenan hydrogel and beeswax oleogel and the effect of starch on the bigel properties. LWT 2024, 205, 116516. [Google Scholar] [CrossRef]
- Zampouni, K.; Dimakopoulou-Papazoglou, D.; Katsanidis, E. Food-Grade Bigel Systems: Formulation, Characterization, and Applications for Novel Food Product Development. Gels 2024, 10, 712. [Google Scholar] [CrossRef]
- Fasolin, L.H.; Martins, A.J.; Cerqueira, M.A.; Vicente, A.A. Modulating process parameters to change physical properties of bigels for food applications. Food Struct. 2021, 28, 100173. [Google Scholar] [CrossRef]
- Lu, Y.; Zhong, Y.; Guo, X.; Zhang, J.; Gao, Y.; Mao, L. Structural Modification of O/W Bigels by Glycerol Monostearate for Improved Co-Delivery of Curcumin and Epigallocatechin Gallate. ACS Food Sci. Technol. 2022, 2, 975–983. [Google Scholar] [CrossRef]
- Ghiasi, F.; Golmakani, M.T. Fabrication and characterization of a novel biphasic system based on starch and ethylcellulose as an alternative fat replacer in a model food system. Innov. Food Sci. Emerg. Technol. 2022, 78, 103028. [Google Scholar] [CrossRef]
- Han, L.; Chen, F.; Qiu, Y.; Gao, J.; Zhu, Q.; Wu, T.; Wang, P.; Zhang, M. Development and characterization of hydrogel-in-oleogel (bigel) systems and their application as a butter replacer for bread making. J. Sci. Food Agric. 2024, 104, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Composite hydrogels assembled from food-grade biopolymers: Fabrication, properties, and applications. Adv. Colloid Interface Sci. 2024, 332, 103278. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Nardin, C.; Zhang, Y. Novel soft food gels using beta-lactoglobulin via enzymatic crosslinking as agar gel alternatives. Food Hydrocoll. 2024, 155, 110213. [Google Scholar] [CrossRef]
- Delanne-Cuménal, A.; Lainé, E.; Hoffart, V.; Verney, V.; Garrait, G.; Beyssac, E. Effect of Molecules’ Physicochemical Properties on Whey Protein/Alginate Hydro-gel Rheology, Microstructure and Release Profile. Pharmaceutics 2024, 16, 258. [Google Scholar] [CrossRef]
- Maire Du Poset, A.; Börjesson, M.; Rameau, C.; Madeleine-Perdrillat, C.; Lerbret, A.; Loupiac, C.; Cousin, F.; Assifaoui, A. Controlled Loading and Release of Be-ta-Lactoglobulin in Calcium-Polygalacturonate Hydrogels. Biomacromolecules 2020, 21, 1417–1426. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, Y.; Chen, B.; Qiu, D.; Sun, P.; Shao, P.; Feng, S. Fabrication of high strength cold-set sodium alginate/whey protein nanofiber double network hydrogels and their interaction with curcumin. Food Res. Int. 2023, 165, 112490. [Google Scholar] [CrossRef]
- Sasikumar, R.; Sharma, P.; Jaiswal, A.K. Alginate and β-lactoglobulin matrix as wall materials for encapsulation of polyphenols to improve efficiency and stability. Int. J. Food Eng. 2023, 19, 1–13. [Google Scholar] [CrossRef]
- Madsen, M.; Khan, S.; Kunstmann, S.; Aachmann, F.L.; Ipsen, R.; Westh, P.; Emanuelsson, C.; Svensson, B. Unaided efficient transglutaminase cross-linking of whey proteins strongly impacts the formation and structure of protein alginate particles. Food Chem. Mol. Sci. 2022, 5, 100137. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Miao, Y.; Zhang, X.; Fan, Z.; Singh, G.; Zhang, X.; Xu, K.; Li, B.; Hu, Z.; et al. Hydrogen bonds autonomously powered gelatin methacrylate hydrogels with super-elasticity, self-heal and underwater self-adhesion for sutureless skin and stomach surgery and E-skin. Biomaterials 2018, 171, 83–96. [Google Scholar] [CrossRef]
- Su, J.; Cai, Y.; Zhi, Z.; Guo, Q.; Mao, L.; Gao, Y.; Yuan, F.; Van der Meeren, P. Assembly of propylene glycol alginate/β-lactoglobulin composite hydrogels induced by ethanol for co-delivery of probiotics and curcumin. Carbohydr. Polym. 2021, 254, 117446. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Park, S.; Rho, S.J.; Kim, Y.R. Rice flour-based filled hydrogel: An effective vitamin D encapsulation system as influenced by rice flour variety. Food Sci. Biotechnol. 2025, 34, 1617–1629. [Google Scholar] [CrossRef]
- Camacho, D.H.; Uy, S.J.Y.; Cabrera, M.J.F.; Lobregas, M.O.S.; Fajardo, T.J.M.C. Encapsulation of folic acid in copper-alginate hydrogels and it’s slow in vitro re-lease in physiological pH condition. Food Res. Int. 2019, 119, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Dafe, A.; Etemadi, H.; Dilmaghani, A.; Mahdavinia, G.R. Investigation of pectin/starch hydrogel as a carrier for oral delivery of probiotic bacteria. Int. J. Biol. Macromol. 2017, 97, 536–543. [Google Scholar] [CrossRef] [PubMed]
- How, S.C.; Lin, T.H.; Chang, C.C.; Wang, S.S.S. Examining the effect of bovine serum albumin on the properties and drug release behavior of β-lactoglobulin-derived amyloid fibril-based hydrogels. Int. J. Biol. Macromol. 2021, 184, 79–91. [Google Scholar] [CrossRef]
- Neeland, I.J.; de Gregório, L.H.; Zagury, R.; Ahrén, B.; Neutel, J.; Darimont, C.; Corthesy, J.; Grzywinski, Y.; Perrin, E.; von Eynatten, M.; et al. A Randomized, Placebo-Controlled, Single-Center, Crossover Study to Evaluate the Effects of Pre-Meal Whey Protein Microgel on Post-Prandial Glucometabolic and Amino Acid Response in People with Type 2 Diabetes and Overweight or Obesity. Metabolites 2025, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Seçmeler, Ö.; Gözet, T.; Azbaz, S.; Dülger, M.M.; Yavuz Düzgün, M.; Argin, S. Functional food development using olive pomace phenolics and hydrogels. Int. J. Food Sci. Technol. 2024, 59, 4451–4460. [Google Scholar] [CrossRef]
- Mu, Y.; Gong, L.; Peng, T.; Yao, J.; Lin, Z. Advances in pH-responsive drug delivery systems. OpenNano 2021, 5, 100031. [Google Scholar] [CrossRef]
- Foggiaro, D.; Domínguez, R.; Pateiro, M.; Cittadini, A.; Munekata, P.E.S.; Campagnol, P.C.B.; Fraqueza, M.J.; De Palo, P.; Lorenzo, J.M. Use of Healthy Emulsion Hydrogels to Improve the Quality of Pork Burgers. Foods 2022, 11, 596. [Google Scholar] [CrossRef]
- Dreher, J.; König, M.; Herrmann, K.; Terjung, N.; Gibis, M.; Weiss, J. Varying the amount of solid fat in animal fat mimetics for plant-based salami analogues influences texture, appearance and sensory characteristics. LWT 2021, 143, 111140. [Google Scholar] [CrossRef]
- Barragán, V.; Triviño, E.C.; Tenorio-Alfonso, A.; Niemczyk-Soczynska, B.; Sajkiewicz, P.Ł. Hydrogel-Based Systems as Smart Food Packaging: A Review. Polymers 2025, 17, 1005. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, X.; Du, Z.; Zhao, G.; Zang, J. Construction of protein hydro-gels with antibacterial activity by interaction of β-lactoglobulin amyloid fibrils with epi-gallocatechin-3-gallate. Food Biosci. 2024, 58, 103632. [Google Scholar] [CrossRef]
- Salmas, C.E.; Kollia, E.; Avdylaj, L.; Kopsacheili, A.; Zaharioudakis, K.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Kehayias, G.; Karakassides, A.; et al. Thymol@Natural Zeolite Nanohybrids for Chi-tosan/Polyvinyl-Alcohol-Based Hydrogels Applied as Active Pads. Gels 2023, 9, 570. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, J.; Sun, N.; Jiang, Y.; Yu, Y.; Lai, G.; Yang, X. UV–Curable antibacterial and pH–sensitive eugenol functionalized chitosan–polyurethane hydrogels for shelf–life extension of chicken. Food Control 2025, 168, 110918. [Google Scholar] [CrossRef]
- Tahir, I.; Foley, C.; Floreani, R. Whey Protein Isolate and β-Lactoglobulin-Modified Alginate Hydrogel Scaffolds Enhance Cell Proliferation for Cultivated Meat Applications. Foods 2025, 14, 2534. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Zhao, J.; Zhu, Q.; Yi, W.; Hau, E.; Ren, D. Design of edible whey protein isolate hydrogels with cell adhesion via a two-step crosslinking method for cultured meat scaffolds. Food Hydrocoll. 2025, 168, 111562. [Google Scholar] [CrossRef]
- Seo, J.W.; Jung, W.K.; Park, Y.H.; Bae, H. Development of cultivable alginate fibers for an ideal cell-cultivated meat scaffold and production of hybrid cultured meat. Carbohydr. Polym. 2023, 321, 121287. [Google Scholar] [CrossRef]
- Chen, Y.; Bassey, A.P.; Zhu, H.; Zhou, G. Fabrication of cell cultured meat by hydrogel with topographic microstructures. Food Biosci. 2023, 55, 102910. [Google Scholar] [CrossRef]
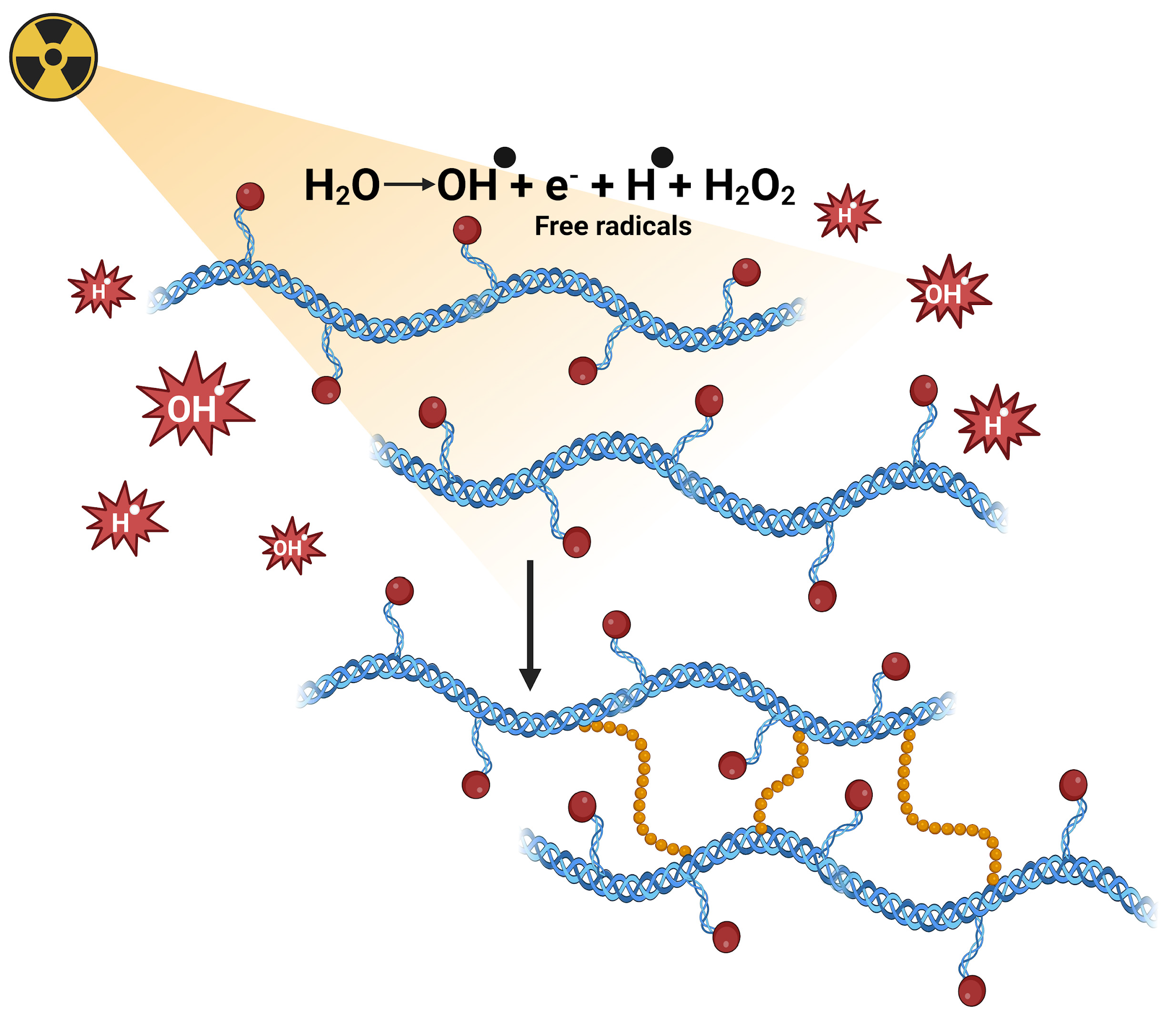
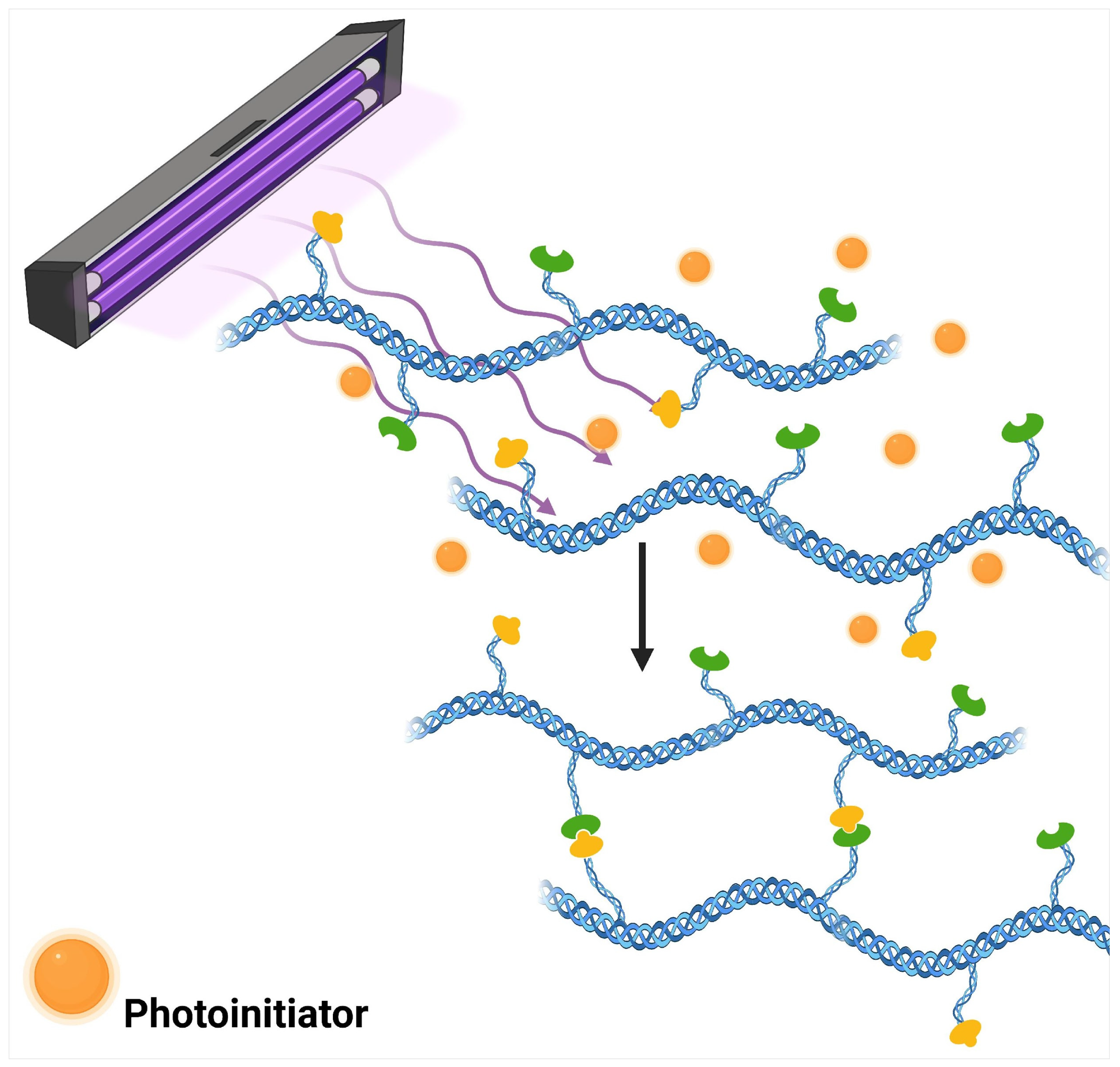
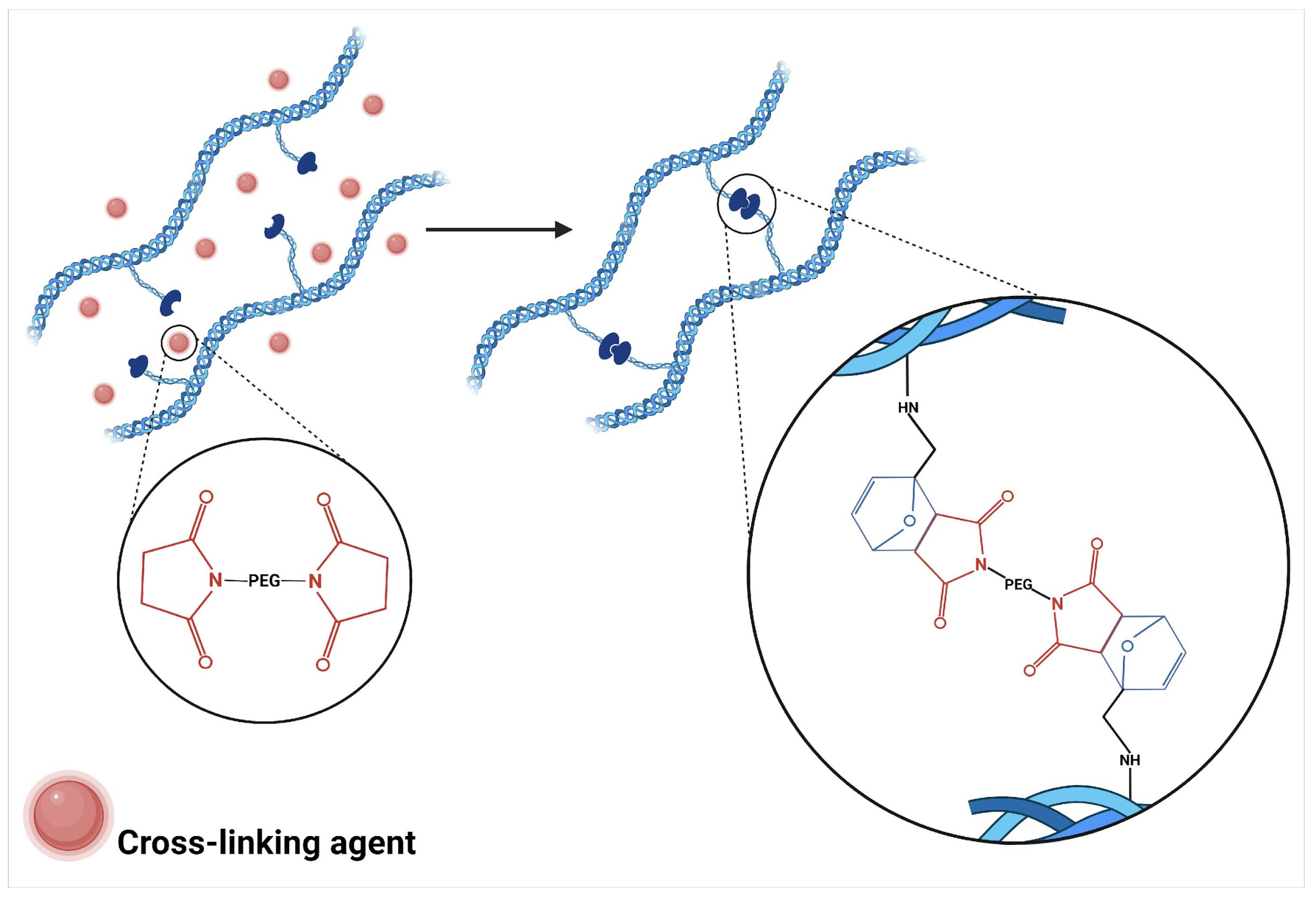


| Crosslinking Mechanism | Reaction Conditions | Gelation Speed | Mechanical Strength | Biocompatibility/Regulation | Encapsulation of Lipophilic Compounds | Industrial Scalability | References |
|---|---|---|---|---|---|---|---|
| Chemical (e.g., CuAAC, UV, aldehydes) | May require catalysts or harsh conditions (pH, temperature, radiation) | Fast (seconds to minutes) | High (covalent bonding) | Variable (may require purification of toxic residues) | Limited (weak interactions with nonpolar molecules) | High, but process-dependent (e.g., photochemical or thermal methods) | [14,16,17,49] |
| Physical (e.g., H-bonds, ionic, hydrophobic) | Mild, no need for toxic reagents | Moderate to fast | Low to medium (reversible interactions) | High (generally good biocompatibility) | Low to moderate (rapid diffusion and poor stability) | High (simple and reproducible processes) | [37,39,50] |
| Enzymatic (e.g., TGase, laccase, peroxidase) | Mild, aqueous, physiologically relevant conditions | Moderate (minutes to hours) | Medium to high (specific covalent bonding) | Very high (GRAS enzymes, no toxic residues) | Limited (polar environments restrict lipophilic interactions) | Medium (cost and enzyme availability can be limiting factors) | [50,52] |
| Ultrasonic (acoustic cavitation-induced) | Generally mild, but requires specific equipment | Very fast (seconds) | Variable (depends on polymer system) | High (no external reagents required) | Promising (enables direct emulsification of lipophilic compounds) | Medium to low (emerging technology, needs validation) | [57,58,59,61] |
| Substrate/ Base Material | Reported Limitations | Identified Advantages | Main Results | Operating Specifications | Crosslinking Mechanism | References |
|---|---|---|---|---|---|---|
| Beta-lactoglobulin (β-LG) | Concentrations < 5% do not gel; Two-stage process with extended times; Lower mechanical resistance vs. conventional gels | Low protein concentration required (5%); Green process without hazardous chemicals; Superior texture: softer and less brittle than agar | G’: <103 Pa; Rupture force: 0.40 ± 0.08 N; Rupture penetration: 3.7 ± 0.3 mm; Morphology: aggregates 50–150 × 10 nm; Thermal stability: 329.76 °C | pH 7.5 (Tris-HCl 50 mM), 80 °C × 30 min (Heat treatment), 37 °C, 300 rpm, ON (Incubation), 80 °C × 30 min (Inactivation), 4 °C (Storage) | Thermal + Enzymatic | [79] |
| Whey protein 8.8% + Sodium alginate 2.4% (80/20 w/w ratio) | Rapid release, pH dependence, coating loss, ionic sensitivity | Biocompatible, gastro-resistant, controlled release, simple process | EE: 23–100%, size: ~1.5 mm, coating: 68–146 µm, release: 5–80%/h | pH 7.0, denaturation 80 °C/40 min, extrusion 23G needle, coating 5 min | Thermal + Ionic | [80] |
| Polygalacturonic acid (polyGalA)–anionic polysaccharide derived from pectin | Slower release in complete hydrogels vs. sectioned; Limited diffusion by mesh size and electrostatic repulsions | Dual control of dosage and release rate; Ideal candidates for oral release systems; Maintenance of native protein form | Release by Fickean diffusion (n ≈ 0.5); Mesh size: 75–95 Å; Nearly complete recovery of nominal BLG/polyGalA ratio | pH: 7.0 ± 0.3; [NaCl]: 10 mM; Gelation temperature: ambient; Gelation time: 24 h; [polyGalA]: 10–20 g/L; [BLG]: 10–16 g/L; Release temperature: 37 °C | Ionic | [81] |
| Sodium alginate + Whey protein nanofibers | Decrease in properties >50 mg/mL WPN, Requires more in vivo release studies | Cold processing preserves thermosensitive compounds, Superior mechanical properties, Excellent bioactive encapsulation | G’ = 768.2 Pa (3.75× greater than pure SA), Hardness = 273.3 g (2.26× greater), Curcumin encapsulation = 91.6% | 25 °C, pH 6.5, 12 h of gelation | Ionic with Ca2+ + electrostatic and hydrogen interactions | [82] |
| Sodium alginate + β-lactoglobulin | Process sensitivity: tail formation under suboptimal conditions. Critical concentrations | Improved thermal stability. UV protection: 90% reduction in photodegradation. Preferential targeted release in intestinal environment. Preservation of antioxidant activity (DPPH, ABTS, FRAP) | Encapsulation efficiency: 84.58%. Average size: 1053.73 nm. Zeta potential: −25.44 mV. Intestinal release: 84% vs. 36% (free polyphenols). Bioaccessibility: 3× superior to free polyphenols | Vibratory frequency: 953.14 Hz. Voltage: 1893.76 V. Temperature: 45 °C. pH: 4.6 | Ionic with Ca2+ | [83] |
| β-lactoglobulin + sodium alginate | Secondary structure loss (20%), conformational changes | No pretreatment, mild conditions, high efficiency | >70% crosslinking, polymers 18–240 kDa, hydrophobic interaction with alginate | pH 8.5, 37 °C, 24 h, 300 rpm | Enzymatic + Ionic | [84] |
| β-lactoglobulin | Intramolecular crosslinking, lower polymerization | Rapid reaction, structural preservation | Polymers 18–120 kDa, 37 crosslinks, preserved native structure | pH 8.5, ambient temperature, 30 min | Covalent bonds | [84] |
| Thiolated tetrafunctional polyethylene glycol (PEG-SH) | Cell viability: Only 28.8–30.8% post-degradation with L-cysteine; Toxicity: Risk of cell reduction by H2O2; Technical: Difficulty applying standard rheological criteria for rapid reactions | Mild conditions compatible with biological materials; No UV light exposure required; Ambient temperature operation; Physiological pH (7.3) | Crosslinking time (tcross): 2–12 min, Equilibrium storage modulus (G’eq): 1–16 kPa | pH 7.3 (DPBS), 25 °C × 1 h (Gelation) | Enzymatic | [49] |
| Gelatin methacrylate (GelMA) derived from type A collagen | Decreased strength with prolonged exposure, lower swelling vs. pure GelMA, brittleness at high concentrations | Superelasticity, spontaneous self-healing, water-resistant adhesion, excellent biocompatibility, surgical application without sutures, sensory capability with CNTs | Maximum strength: +4.3×, Compressive modulus: +2.5×, Elongation: +6× (277%), Adhesion: 81 kPa, Recovery: 90% | GelMA: 10–20% (w/v), Temperature: 37 °C, Time TA: 24 h optimal, Concentration TA: 100% optimal. | Dual: chemical covalent (primary) and physical by hydrogen bonds (secondary) | [85] |
| Propylene glycol alginate (PGA) + β-lactoglobulin nanoparticles | Use of ethanol, loss in intestinal fluid, pH dependence, not evaluated in vivo | Green method, biocompatible, dual protection, controlled release, excellent stability | LGG encapsulation > 98%, curcumin retention 91.3% (4 weeks), LGG survival 9.72 log CFU mL−1 | pH 4.0, ambient temperature, 12 h of gelation | Physical non-covalent induced by solvent | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briones, S.C.; Mussagy, C.U.; Farias, F.O.; Córdova, A. Functional Hydrogels in Food Applications: A Review of Crosslinking Technologies, Encapsulation Trends, and Emerging Challenges. Polymers 2025, 17, 2955. https://doi.org/10.3390/polym17212955
Briones SC, Mussagy CU, Farias FO, Córdova A. Functional Hydrogels in Food Applications: A Review of Crosslinking Technologies, Encapsulation Trends, and Emerging Challenges. Polymers. 2025; 17(21):2955. https://doi.org/10.3390/polym17212955
Chicago/Turabian StyleBriones, Sebastián Catalán, Cassamo U. Mussagy, Fabiane O. Farias, and Andrés Córdova. 2025. "Functional Hydrogels in Food Applications: A Review of Crosslinking Technologies, Encapsulation Trends, and Emerging Challenges" Polymers 17, no. 21: 2955. https://doi.org/10.3390/polym17212955
APA StyleBriones, S. C., Mussagy, C. U., Farias, F. O., & Córdova, A. (2025). Functional Hydrogels in Food Applications: A Review of Crosslinking Technologies, Encapsulation Trends, and Emerging Challenges. Polymers, 17(21), 2955. https://doi.org/10.3390/polym17212955











