Enhancing Fire Resistance and Mechanical Properties of Wood Strand Boards by Impregnation with Sodium Bicarbonate and Sodium Borate
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Treating Solution
2.2. Impregnation Procedure
2.3. Manufacturing Parameters
2.4. Characterization of Physical and Mechanical Properties
2.5. Fire Performance and Thermal Stability
2.6. Statistical Data Analysis
3. Results and Discussions
3.1. Mechanical and Physical Properties
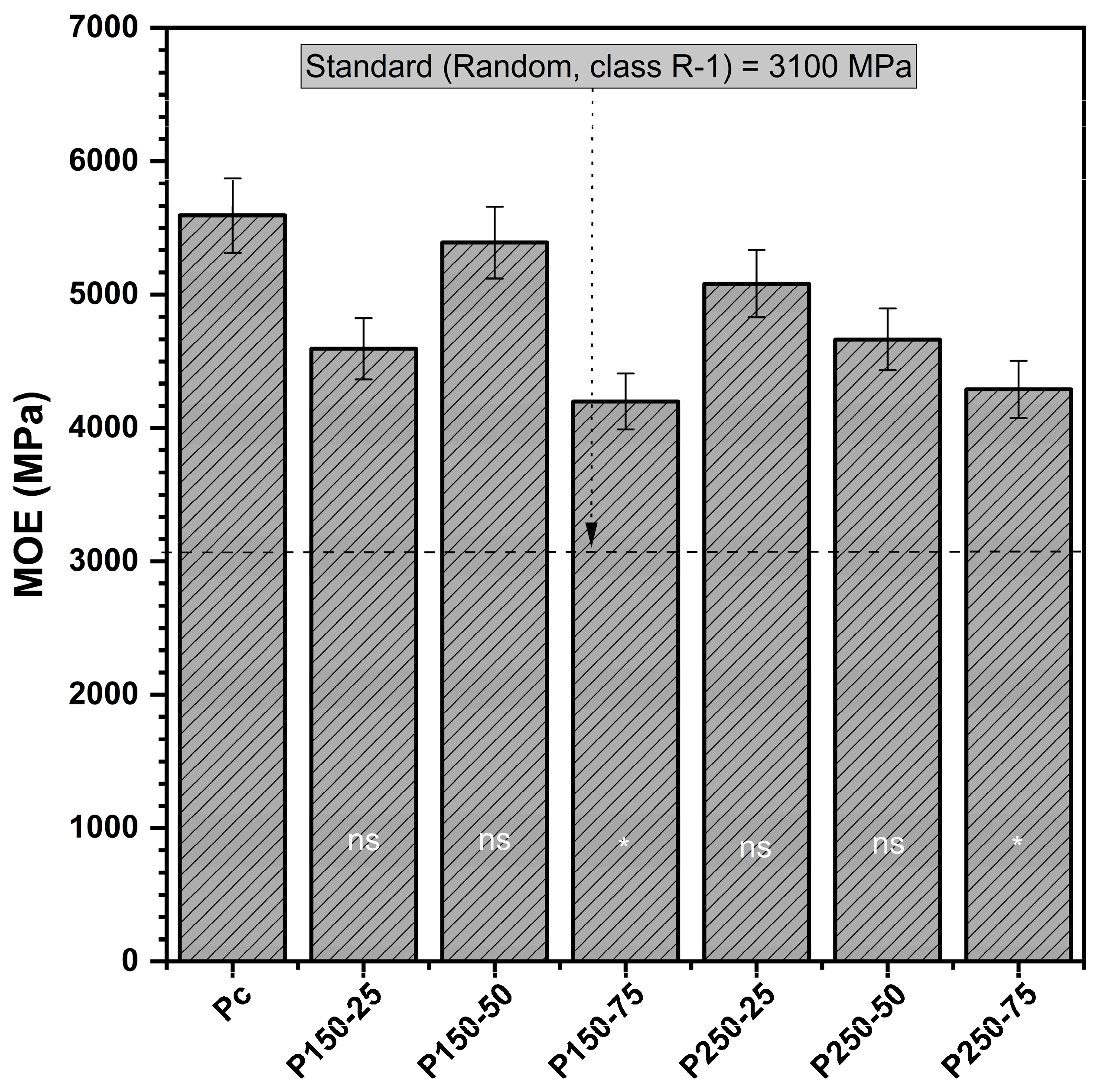
3.1.1. Modulus of Elasticity (MOE)
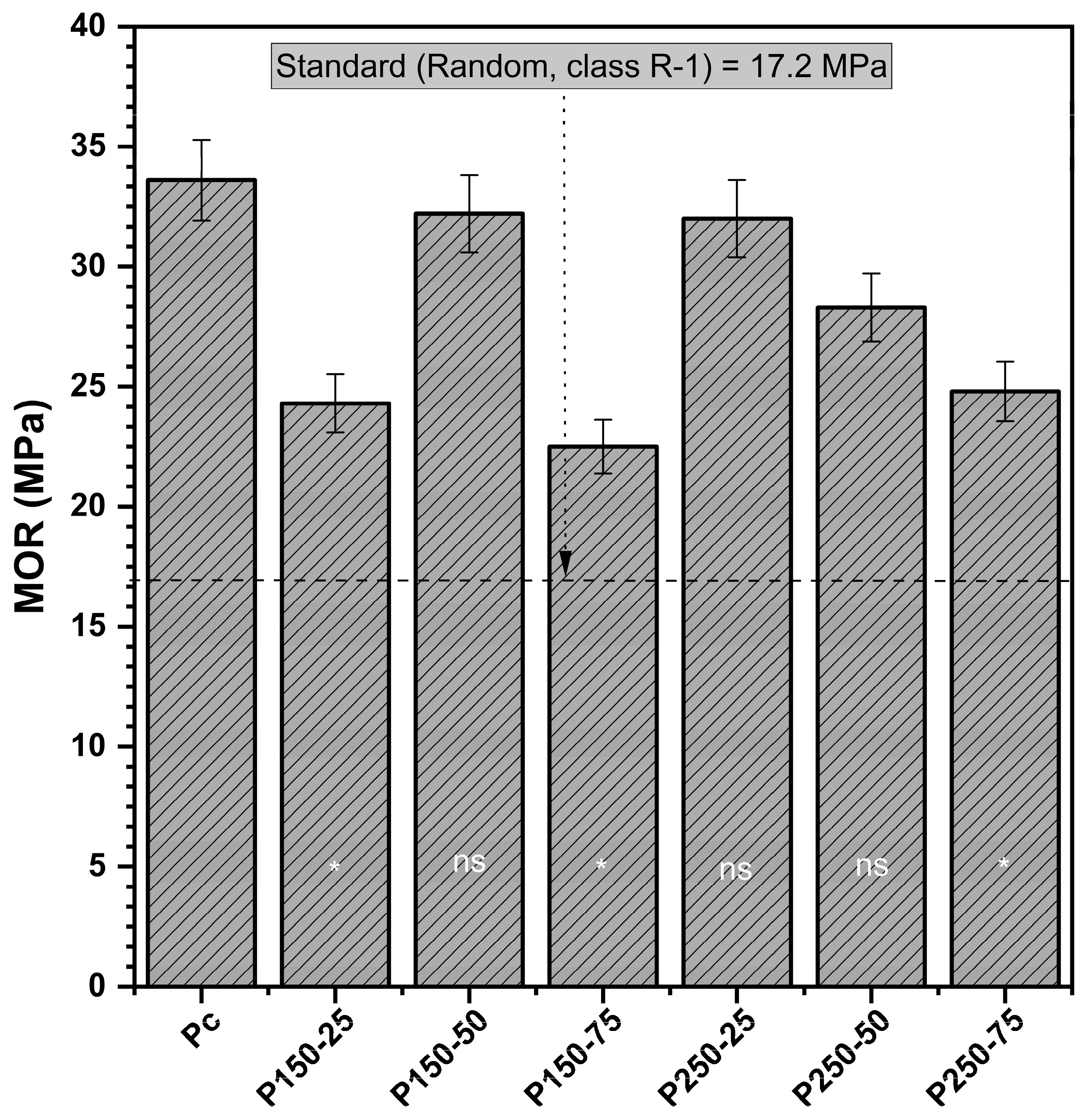
3.1.2. Modulus of Rupture (MOR)
3.1.3. Internal Bond (IB)
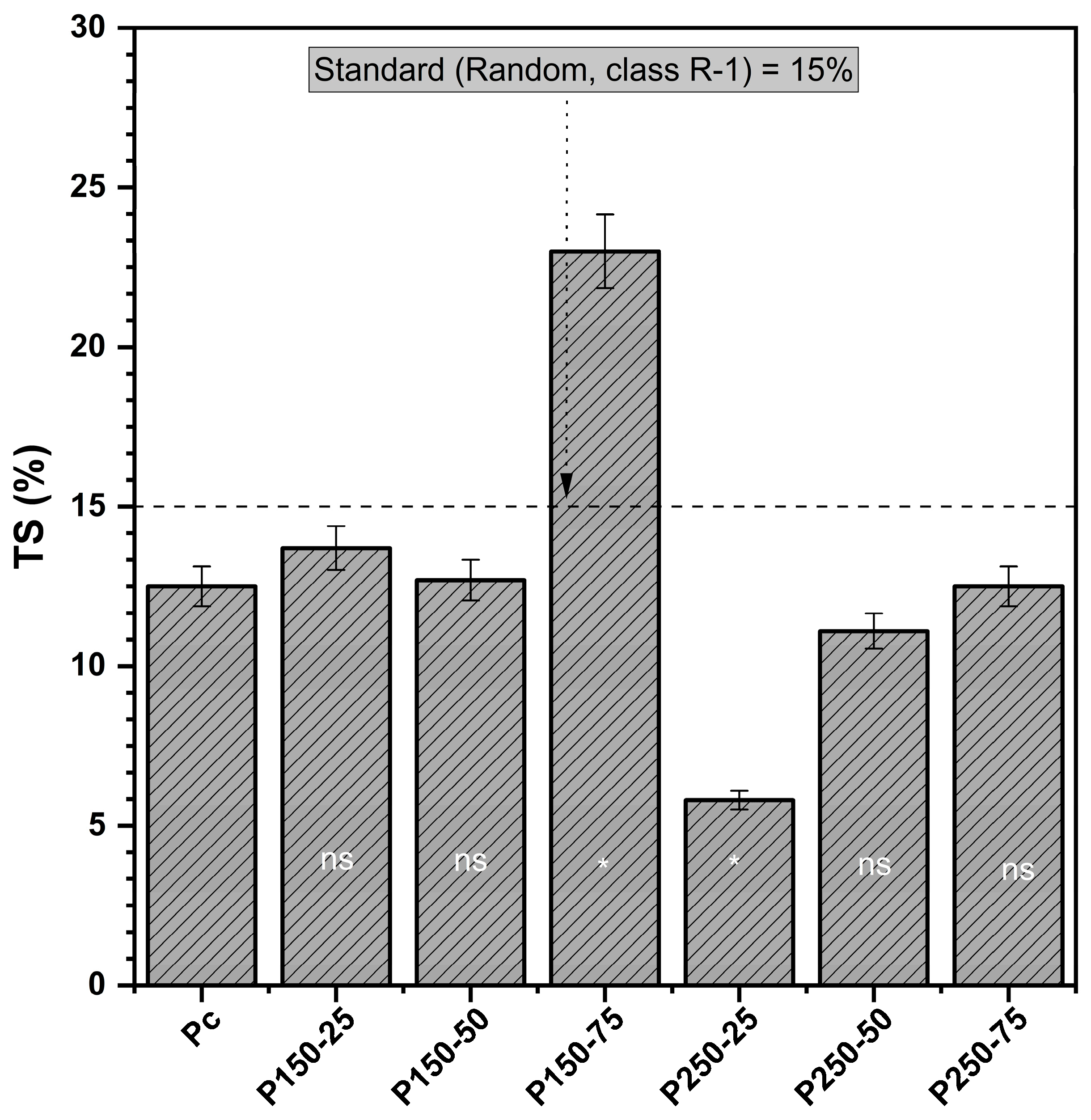
3.1.4. Thickness Swelling (TS)
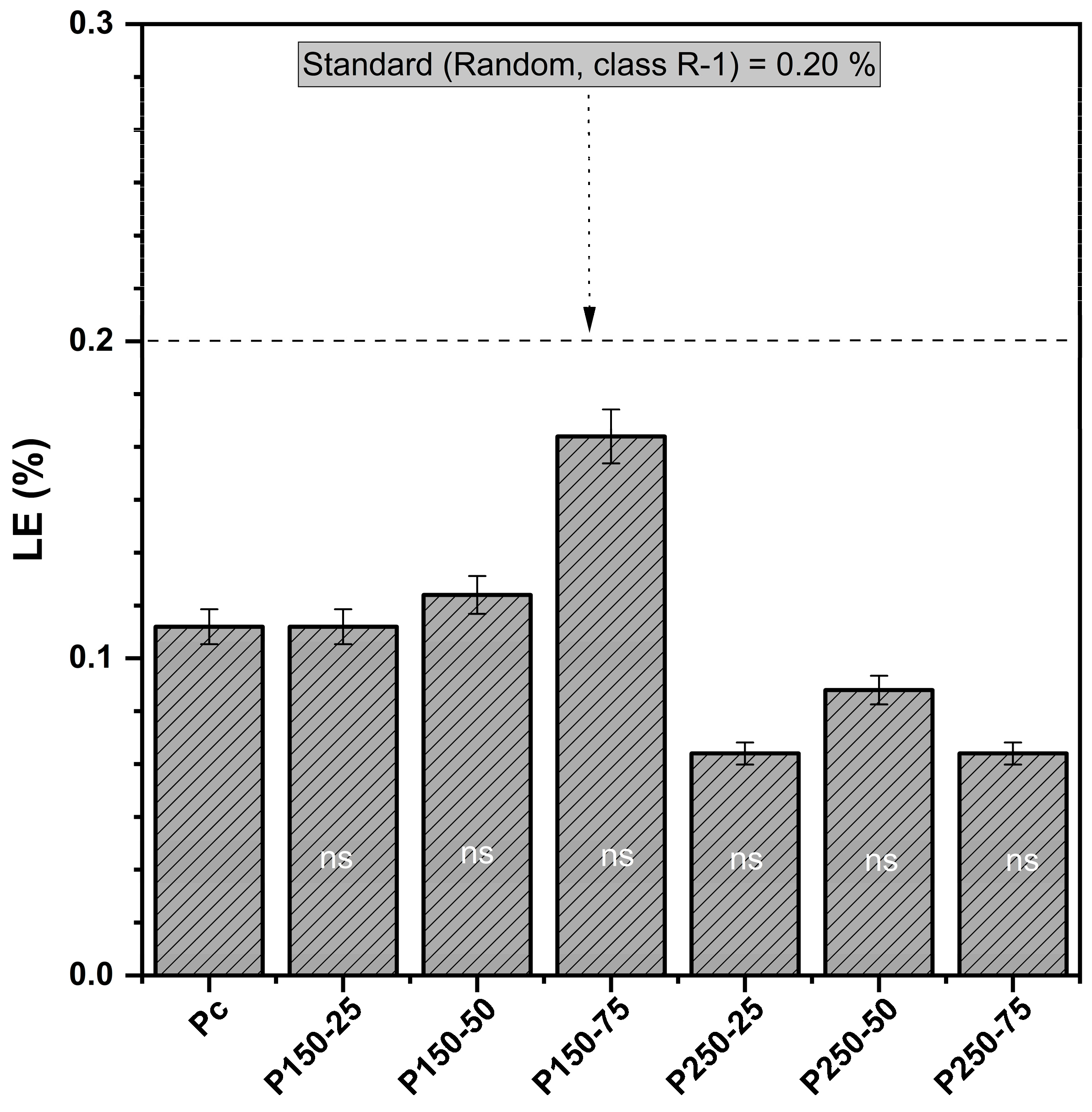
3.1.5. Linear Expansion (LE)
3.1.6. Comparative Analysis of Mechanical and Physical Performance with Literature Data
3.2. Flammability and Thermal Properties
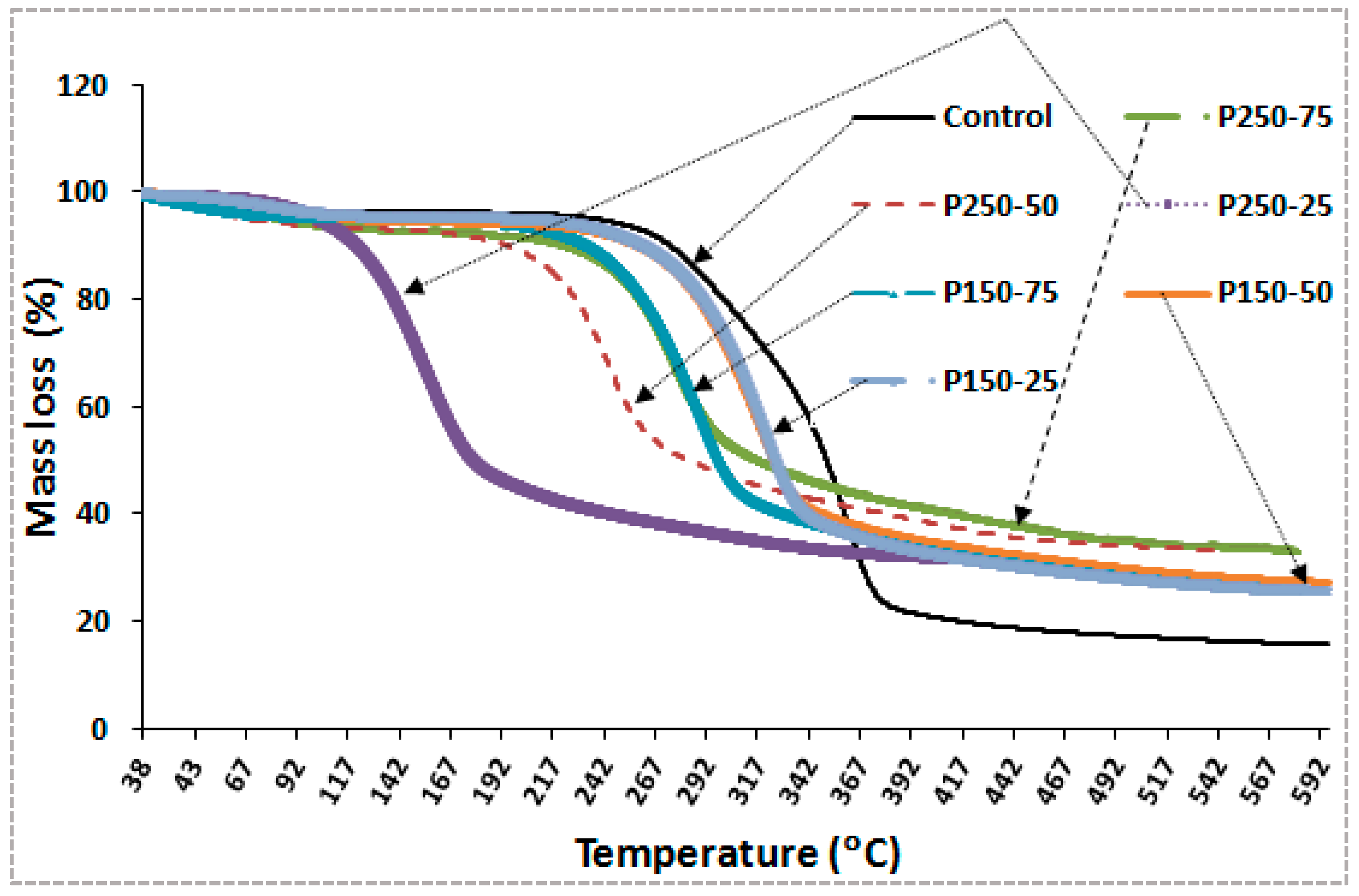
3.2.1. Thermogravimetric Analysis (TGA)
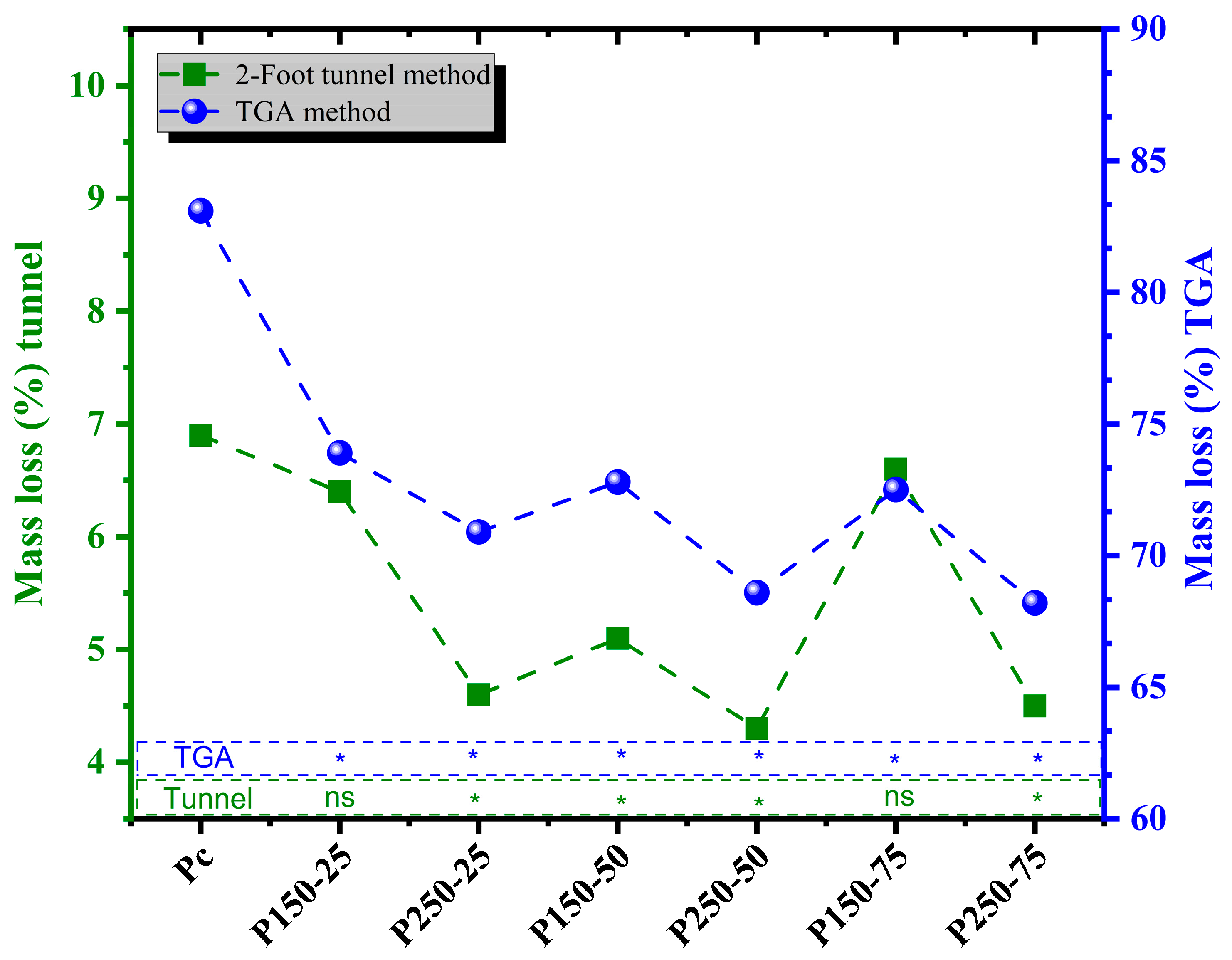
3.2.2. Mass Loss (ML): Tunnel and TGA Methods
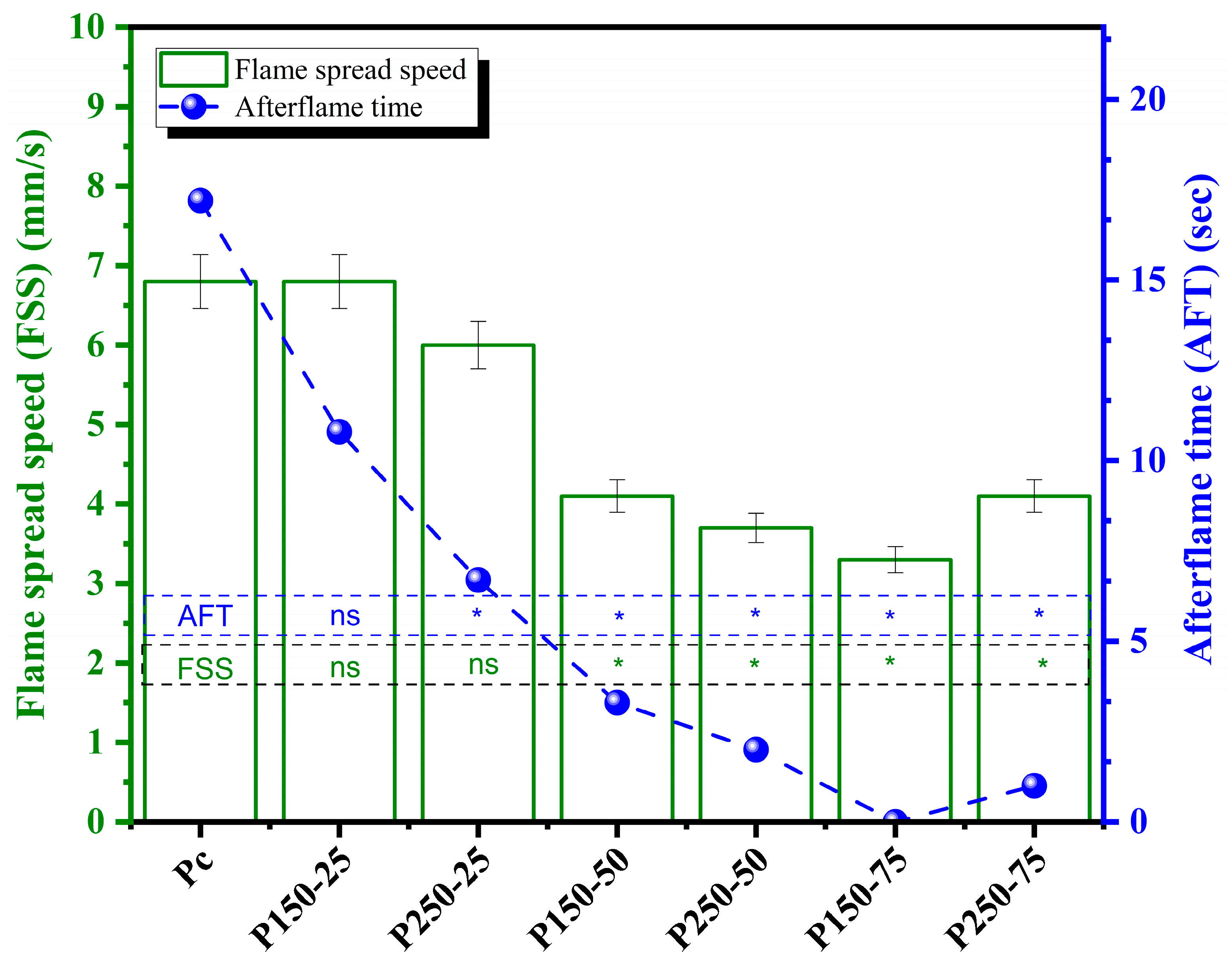
3.2.3. Flame Spread Speed (FSS)
3.2.4. Mechanistic Insights into the Flame Retardancy of SBo and SBi in OSB
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Sun, Y.; Dong, D.; Gao, G.; Zhang, S.; Wang, Y.; Xiang, J.; Hu, S.; Mortaza, G.; Hu, X. Co-Pyrolysis of Cellulose/Lignin and Sawdust: Influence of Secondary Condensation of the Volatiles on Characteristics of Biochar. Energy 2021, 226, 120442. [Google Scholar] [CrossRef]
- Ramage, M.H.; Burridge, H.; Busse-Wicher, M.; Fereday, G.; Reynolds, T.; Shah, D.U.; Wu, G.; Yu, L.; Fleming, P.; Densley-Tingley, D.; et al. The Wood from the Trees: The Use of Timber in Construction. Renew. Sustain. Energy Rev. 2017, 68, 333–359. [Google Scholar] [CrossRef]
- Švajlenka, J.; Pošiváková, T. Innovation Potential of Wood Constructions in the Context of Sustainability and Efficiency of the Construction Industry. J. Clean. Prod. 2023, 411, 137209. [Google Scholar] [CrossRef]
- Pedieu, R.; Koubaa, A.; Riedl, B.; Wang, X.-M.; Deng, J. Fire-Retardant Properties of Wood Particleboards Treated with Boric Acid. Eur. J. Wood Prod. 2012, 70, 191–197. [Google Scholar] [CrossRef]
- Lowden, L.A.; Hull, T.R. Flammability Behaviour of Wood and a Review of the Methods for Its Reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef]
- Zang, X.; Liu, W.; Wu, D.; Pan, X.; Zhang, W.; Bian, H.; Shen, R. Contemporary Fire Safety Engineering in Timber Structures: Challenges and Solutions. Fire 2024, 7, 2. [Google Scholar] [CrossRef]
- Albert, C.M.; Liew, K.C. Recent Development and Challenges in Enhancing Fire Performance on Wood and Wood-Based Composites: A 10-Year Review from 2012 to 2021. J. Bioresour. Bioprod. 2024, 9, 27–42. [Google Scholar] [CrossRef]
- Lee, Y.X.; Wang, W.; Lei, Y.; Xu, L.; Agarwal, V.; Wang, C.; Yeoh, G.H. Flame-Retardant Coatings for Wooden Structures. Prog. Org. Coat. 2025, 198, 108903. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Pfriem, A. Treatments and Modification to Improve the Reaction to Fire of Wood and Wood Based Products—An Overview. Fire Mater. 2020, 44, 100–111. [Google Scholar] [CrossRef]
- Marino, S.; Gaff, M.; Sethy, A.K.; Kamboj, G.; Rezaei, F.; Kačík, F.; Hosseini, S.B.; Li, H.; Hui, D. Enhancing the Fire Resistance Properties of Thermally Modified Robinia Pseudoacacia Wood with Natural and Synthetic Flame Retardants: Chemical Characterisation and Fire Behaviour. Eur. J. Wood Prod. 2024, 82, 1145–1157. [Google Scholar] [CrossRef]
- Jia, H.; Chen, Z.; Huang, Y.; Döring, M.; Pan, F.; Ren, S.; Jiang, P. Enhancing Flame Retardant Wood’s Versatility and Adjustable Properties through Multi-Scale Micro-Coating Strategy. Chem. Eng. J. 2024, 495, 153293. [Google Scholar] [CrossRef]
- Khani, L.; Martin, L.; Pułaski, Ł. Cellular and Physiological Mechanisms of Halogenated and Organophosphorus Flame Retardant Toxicity. Sci. Total Environ. 2023, 897, 165272. [Google Scholar] [CrossRef]
- Mensah, R.A.; Shanmugam, V.; Narayanan, S.; Renner, J.S.; Babu, K.; Neisiany, R.E.; Försth, M.; Sas, G.; Das, O. A Review of Sustainable and Environment-Friendly Flame Retardants Used in Plastics. Polym. Test. 2022, 108, 107511. [Google Scholar] [CrossRef]
- Elvira-León, J.C.; Chimenos, J.M.; Isábal, C.; Monton, J.; Formosa, J.; Haurie, L. Epsomite as Flame Retardant Treatment for Wood: Preliminary Study. Constr. Build. Mater. 2016, 126, 936–942. [Google Scholar] [CrossRef]
- Zhang, J.; Koubaa, A.; Xing, D.; Godard, F.; Li, P.; Tao, Y.; Wang, X.-M.; Wang, H. Fire Retardancy, Water Absorption, and Viscoelasticity of Borated Wood—Polycarbonate Biocomposites. Polymers 2021, 13, 2234. [Google Scholar] [CrossRef] [PubMed]
- Bentis, A.; Boukhriss, A.; Grancaric, A.M.; El Bouchti, M.; El Achaby, M.; Gmouh, S. Flammability and Combustion Behavior of Cotton Fabrics Treated by the Sol Gel Method Using Ionic Liquids Combined with Different Anions. Cellulose 2019, 26, 2139–2153. [Google Scholar] [CrossRef]
- Depczynska, E.; Burawska, I. Overview and Evaluation of Chemicals and Methods for Flame Retardancy in Glued Laminated Wood Systems. Polymers 2025, 17, 1459. [Google Scholar] [CrossRef]
- Bentis, A.; Boukhriss, A.; Boyer, D.; Gmouh, S. Development of Flame Retardant Cotton Fabric Based on Ionic Liquids via Sol-Gel Technique; IOP Publishing: Bristol, UK, 2017; Volume 254, p. 122001. [Google Scholar]
- Pries, M.; Mai, C. Fire Resistance of Wood Treated with a Cationic Silica Sol. Eur. J. Wood Prod. 2013, 71, 237–244. [Google Scholar] [CrossRef]
- Nami Kartal, S.; Hwang, W.-J.; Yamamoto, A.; Tanaka, M.; Matsumura, K.; Imamura, Y. Wood Modification with a Commercial Silicon Emulsion: Effects on Boron Release and Decay and Termite Resistance. Int. Biodeterior. Biodegrad. 2007, 60, 189–196. [Google Scholar] [CrossRef]
- Bentis, A.; Boukhriss, A.; Gmouh, S. Flame-Retardant and Water-Repellent Coating on Cotton Fabric by Titania–Boron Sol–Gel Method. J. Sol-Gel Sci. Technol. 2020, 94, 719–730. [Google Scholar] [CrossRef]
- Kocaman, S.; Temiz, M.; Işık, M.; Ahmetli, G.; Ceyhan, A.A.; Karakaya, Ş. Halogen-free Boron-based Hybrid System for Enhancing Flame Retardancy, Mechanical and Thermal Properties of Epoxy. J. Appl. Polym. Sci. 2024, 141, e55424. [Google Scholar] [CrossRef]
- Adeniran, A.T.; Englund, K.; Li, H.; Kim, J.-W. Influence of Borate Chemical Treatment on Adhesive Bond Performance of Douglas Fir and Grand Fir Laminates. Eur. J. Wood Prod. 2025, 83, 58. [Google Scholar] [CrossRef]
- Khademibami, L.; Bobadilha, G.S. Recent Developments Studies on Wood Protection Research in Academia: A Review. Front. For. Glob. Change 2022, 5, 793177. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, J.; Sun, X.; Li, M.; Yu, C.; Han, Z.; Wei, P.; Liu, T. Enhanced Fire-Retardant, Smoke-Suppressing, and Ultra-Strong Mechanical Properties of Non-Adhesive Laminated Wood through Borate Ion Crosslinking. Ind. Crops Prod. 2025, 224, 120412. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Synergistic Flame-Retardant and Smoke Suppression Effects of Zinc Borate in Transparent Intumescent Fire-Retardant Coatings Applied on Wood Substrates. J. Therm. Anal. Calorim. 2019, 136, 1563–1574. [Google Scholar] [CrossRef]
- Terzi, E. Thermal Degradation of Particleboards Incorporated with Colemanite and Common Boron-Based Fire Retardants. BioResources 2018, 13, 4239–4251. [Google Scholar] [CrossRef]
- He, Y.; Jin, X.; Li, J.; Qin, D. Mechanical and Fire Properties of Flame-Retardant Laminated Bamboo Lumber Glued with Phenol Formaldehyde and Melamine Urea Formaldehyde Adhesives. Polymers 2024, 16, 781. [Google Scholar] [CrossRef]
- Patil, D.M.; Phalak, G.A.; Mhakse, S.T. Boron-Containing UV-Curable Oligomer-Based Linseed Oil as Flame-Retardant Coatings: Synthesis and Characterization. Iran. Polym. J. 2018, 27, 795–806. [Google Scholar] [CrossRef]
- Ai, L.; Yang, L.; Hu, J.; Chen, S.; Zeng, J.; Liu, P. Synergistic Flame Retardant Effect of Organic Phosphorus–Nitrogen and Inorganic Boron Flame Retardant on Polyethylene. Polym. Eng. Sci. 2020, 60, 414–422. [Google Scholar] [CrossRef]
- Ai, L.; Chen, S.; Zeng, J.; Yang, L.; Liu, P. Synergistic Flame Retardant Effect of an Intumescent Flame Retardant Containing Boron and Magnesium Hydroxide. ACS Omega 2019, 4, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Bakirtzis, D.; Delichatsios, M.; Liodakis, S.; Ahmed, W. Fire Retardancy Impact of Sodium Bicarbonate on Ligno-Cellulosic Materials. Thermochim. Acta 2009, 486, 11–19. [Google Scholar] [CrossRef]
- Farooq, M.; Sipponen, M.H.; Seppälä, A.; Österberg, M. Eco-Friendly Flame-Retardant Cellulose Nanofibril Aerogels by Incorporating Sodium Bicarbonate. ACS Appl. Mater. Interfaces 2018, 10, 27407–27415. [Google Scholar] [CrossRef]
- Tian, F.; Xu, D.; Xu, X. Synergistic Effect of APP and TBC Fire-Retardants on the Physico-Mechanical Properties of Strandboard. Materials 2022, 15, 435. [Google Scholar] [CrossRef]
- ASTM D1413; Standard Test Method for Wood Preservatives by Laboratory Soil-Block Cultures. ASTM International: West Conshohocken, PA, USA, 2005.
- ASTM D1037-99; Standard Test Methods for Evaluating Properties of Wood-Base Fiber and Particle Panel Materials. ASTM International: West Conshohocken, PA, USA, 1999.
- ASTM D3806-77a; Standard Test Method of Small-Scale Evaluation of Fire-Retardant Paints (2-Foot Tunnel Method). ASTM International: West Conshohocken, PA, USA, 1977.
- Ayrilmis, N. Combined Effects of Boron and Compatibilizer on Dimensional Stability and Mechanical Properties of Wood/HDPE Composites. Compos. Part B Eng. 2013, 44, 745–749. [Google Scholar] [CrossRef]
- Toker, H.; Baysal, E.; Simsek, H.; Senel, A.; Sonmez, A.; Altinok, M.; Ozcifci, A.; Yapici, F. Effects of Some Environmentally-Friendly Fire-Retardant Boron Compounds on Modulus of Rupture and Modulus of Elasticity of Wood. Wood Res. 2009, 54, 77–88. [Google Scholar]
- Simsek, H.; Baysal, E.; Peker, H. Some Mechanical Properties and Decay Resistance of Wood Impregnated with Environmentally-Friendly Borates. Constr. Build. Mater. 2010, 24, 2279–2284. [Google Scholar] [CrossRef]
- Doruk, Ş.; Yildirim, M.N.; Özcan, S. Effect of Sodium Borate on Adhesion Resistance of Varnished Surfaces. Drew. Pr. Nauk. Doniesienia Komun. 2025, 68, 216. [Google Scholar] [CrossRef]
- Candan, Z.; Ayrilmis, N.; Akbulut, T. Dimensional Stability Performance of Fire Retardant Treated Veneer-Oriented Strandboard Composites. BioResources 2011, 6, 308–316. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Candan, Z.; White, R. Physical, Mechanical, and Fire Properties of Oriented Strandboard with Fire Retardant Treated Veneers. Holz Als Roh-Und Werkst. 2007, 65, 449–458. [Google Scholar] [CrossRef]
- Shen, K.K. Boron-Based Flame Retardants. In Fire Retardancy of Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-003-38068-9. [Google Scholar]
- El Messoudi, M.; Boukhriss, A.; Bentis, A.; El Bouchti, M.; Ait Chaoui, M.; El Kouali, M.; Gmouh, S. Flame Retardant Finishing of Cotton Fabric Based on Ionic Liquid Compounds Containing Boron Prepared with the Sol-Gel Method. J. Coat. Technol. Res. 2022, 19, 1609–1619. [Google Scholar] [CrossRef]
- Nazarenko, O.B.; Amelkovich, Y.A.; Bannov, A.G.; Berdyugina, I.S.; Maniyan, V.P. Thermal Stability and Flammability of Epoxy Composites Filled with Multi-Walled Carbon Nanotubes, Boric Acid, and Sodium Bicarbonate. Polymers 2021, 13, 638. [Google Scholar] [CrossRef] [PubMed]

| Code Sample | Solution Content of Impregnation | ||
|---|---|---|---|
| SBi (g) | SBo (g) | Water (L) | |
| Pc 1 | 0 | 0 | 0 |
| P150-25 | 150 | 25 | 4 |
| P150-50 | 150 | 50 | 4 |
| P150-75 | 150 | 75 | 4 |
| P250-25 | 250 | 25 | 4 |
| P250-50 | 250 | 50 | 4 |
| P250-75 | 250 | 75 | 4 |
| Specimens | Fire Retardant Additive | MOR Change (%) | MOE Change (%) | IB Change (%) | TS Change (%) | Reference |
|---|---|---|---|---|---|---|
| OSB-APP | Ammonium polyphosphate (APP) | −39.5 | −12.2 | −27.4 | −22.6 | [34] |
| OSB-TBC | 1,3,5-tris(2,3-dibromopropyl)-1,3,5-triazinane-2,4,6-trione (TBC) | −29.1 | +14.5 | +0.9 | −73.9 | [34] |
| OSB-(APP + TBC) | (APP + TBC) | −36.0 | −7.3 | −20.5 | −29.2 | [34] |
| OSB-(BX/BA) | Borax + boric acid- (BX/BA) | −5.6 | −4.4 | −4.9 | −24.4 | [43] |
| OSB-DAP | Diammonium phosphate (DAP) | −9.0 | −13.2 | −11.3 | −5.3 | [43] |
| OSB-MAP | Monoammonium phosphate- (MAP) | −13.5 | −18.0 | −7.4 | −8.4 | [43] |
| OSB-(BA/BP) P16-20 | 16% boric acid + 20% bark particles | −20.4 | −19.7 | 34.6 | −61 | [4] |
| OSB-(BA/BP) P16-30 | 16% boric acid + 30% bark particles | −32.9 | −27.1 | 30.9 | −85.4 | [4] |
| OSB-(SBi/SBo) P150-50 | 150 g sodium bicarbonate + 50 g sodium borate | −4.2 | −3.6 | −11.4 | 1.6 | This work |
| OSB-(SBi/SBo) P250-50 | 250 g sodium bicarbonate + 50 g sodium borate | −15.8 | −16.6 | −6.82 | −11.2 | This work |
| Source of Variation | Physical and Mechanical Properties (F Values) | Fire-Retardant Performance (F Values) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Df | MOE | MOR | IB | TS | LE | FSS | MLtu | MLTGA | |
| Block | 2 | 3.85 ns | 1.18 ns | 0.16 ns | 0.30 ns | 4.31 * | 0.89 ns | 0.54 ns | 0.23 ns |
| MBi | 1 | 0.08 ns | 1.91 ns | 33.55 ** | 69.88 ** | 18.30 ** | 0.03 ns | 29.09 ** | 19.27 ** |
| MBo | 2 | 7.09 * | 6.82 * | 64.60 ** | 36.45 ** | 1.98 ns | 10.53 ** | 3.95 ns | 2.20 ns |
| MBi × MBo | 2 | 4.06 ns | 5.06 * | 11.04 ** | 10.95 ** | 2.55 ns | 0.89 ns | 2.04 ns | 0.24 ns |
| Contrasts | |||||||||
| MBi | 1 | 0.08 ns | 1.91 ns | 33.55 ** | 69.88 ** | 18.30 ** | 0.03 ns | 29.09 ** | 19.27 ** |
| MBo-L | 1 | 7.47 * | 6.13 * | 114.36 ** | 68.15 ** | 3.96 ns | 16.87 ** | 0.04 ns | 3.83 ns |
| MBo-Q | 1 | 6.70 * | 7.50 * | 14.83 ** | 4.76 ns | 0.00 ns | 4.20 ns | 7.86 * | 0.56 ns |
| MBi × MBo-L | 1 | 0.83 ns | 2.22 ns | 10.77 ** | 1.73 ns | 3.17 ns | 1.61 ns | 0.24 ns | 0.38 ns |
| MBi × MBo-Q | 1 | 7.30 * | 7.91 * | 11.30 ** | 20.16 ** | 1.94 ns | 0.17 ns | 3.85 ns | 0.09 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedieu, R.; Bentis, A.; Riedl, B.; Wang, X.-M.; Deng, J.; Braghiroli, F.L.; Koubaa, A. Enhancing Fire Resistance and Mechanical Properties of Wood Strand Boards by Impregnation with Sodium Bicarbonate and Sodium Borate. Polymers 2025, 17, 2943. https://doi.org/10.3390/polym17212943
Pedieu R, Bentis A, Riedl B, Wang X-M, Deng J, Braghiroli FL, Koubaa A. Enhancing Fire Resistance and Mechanical Properties of Wood Strand Boards by Impregnation with Sodium Bicarbonate and Sodium Borate. Polymers. 2025; 17(21):2943. https://doi.org/10.3390/polym17212943
Chicago/Turabian StylePedieu, Roger, Aziz Bentis, Bernard Riedl, Xiang-Ming Wang, James Deng, Flavia Lega Braghiroli, and Ahmed Koubaa. 2025. "Enhancing Fire Resistance and Mechanical Properties of Wood Strand Boards by Impregnation with Sodium Bicarbonate and Sodium Borate" Polymers 17, no. 21: 2943. https://doi.org/10.3390/polym17212943
APA StylePedieu, R., Bentis, A., Riedl, B., Wang, X.-M., Deng, J., Braghiroli, F. L., & Koubaa, A. (2025). Enhancing Fire Resistance and Mechanical Properties of Wood Strand Boards by Impregnation with Sodium Bicarbonate and Sodium Borate. Polymers, 17(21), 2943. https://doi.org/10.3390/polym17212943







