Essential Oils as Green Antibacterial Modifiers of Polymeric Materials
Abstract
1. Introduction
2. Interactions of Essential Oils with Gram-Positive and Gram-Negative Bacteria
2.1. Bacterial Biofilm and Quorum Signaling
2.2. The Influence of the Cell Wall Structure of G+ and G- Bacteria on Their Interactions with Essential Oils
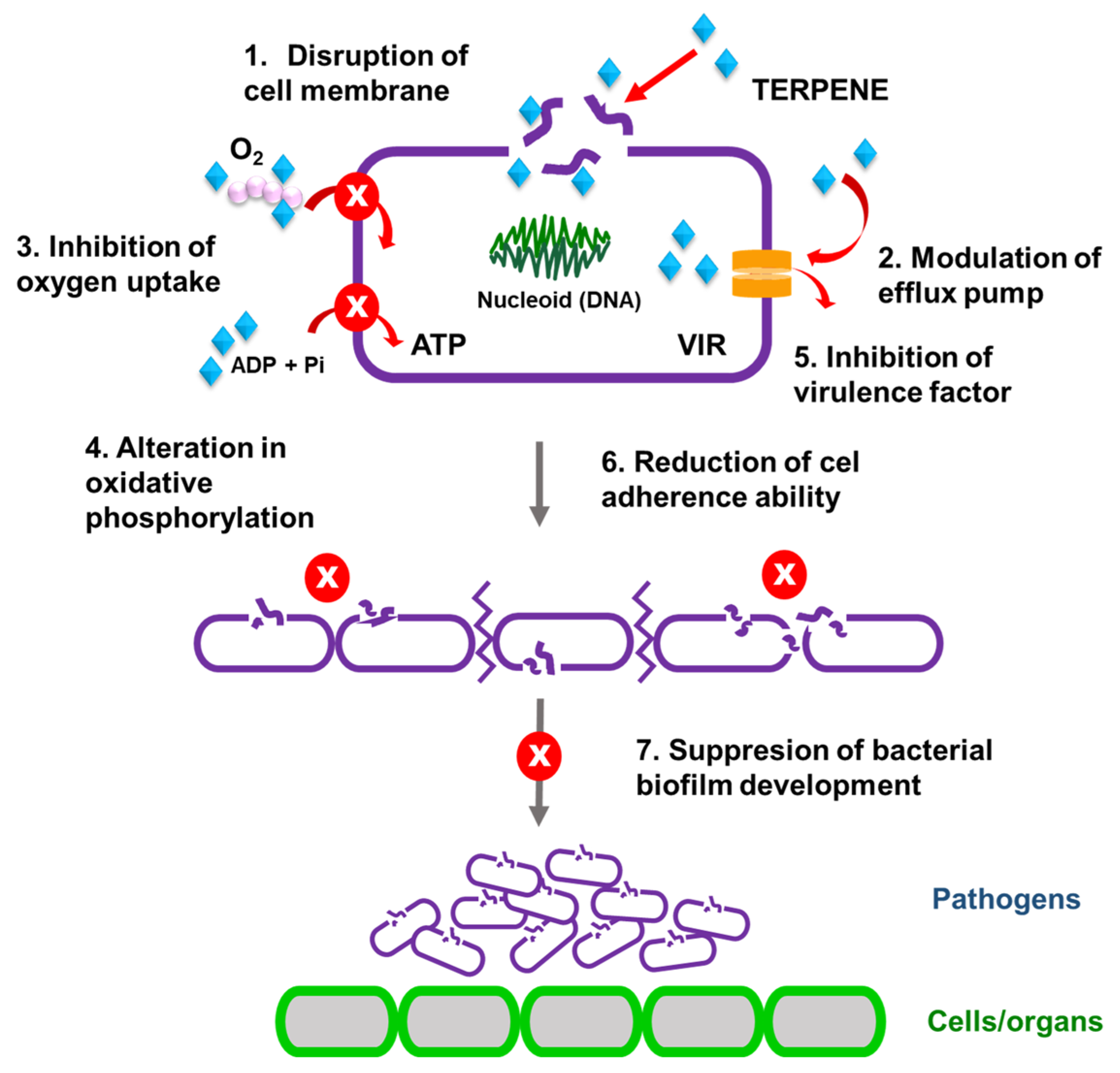
3. Antibacterial and Antibiofilm Activity of the Main Components of Essential Oils—The Influence of the Chemical Structure on Their Metabolism and Effectiveness in Quorum Quenching
3.1. Monoterpenoid Phenols
3.1.1. Eugenol
3.1.2. Carvacrol
3.2. Acyclic Terpenes and Monoterpenoid Alcohols
3.2.1. Linalool
3.2.2. Geraniol
3.2.3. Citronellol
3.2.4. Farnesol
3.2.5. Phytol
4. Polymeric Materials with Antibacterial Properties Resulting from Their Modification with Essential Oils
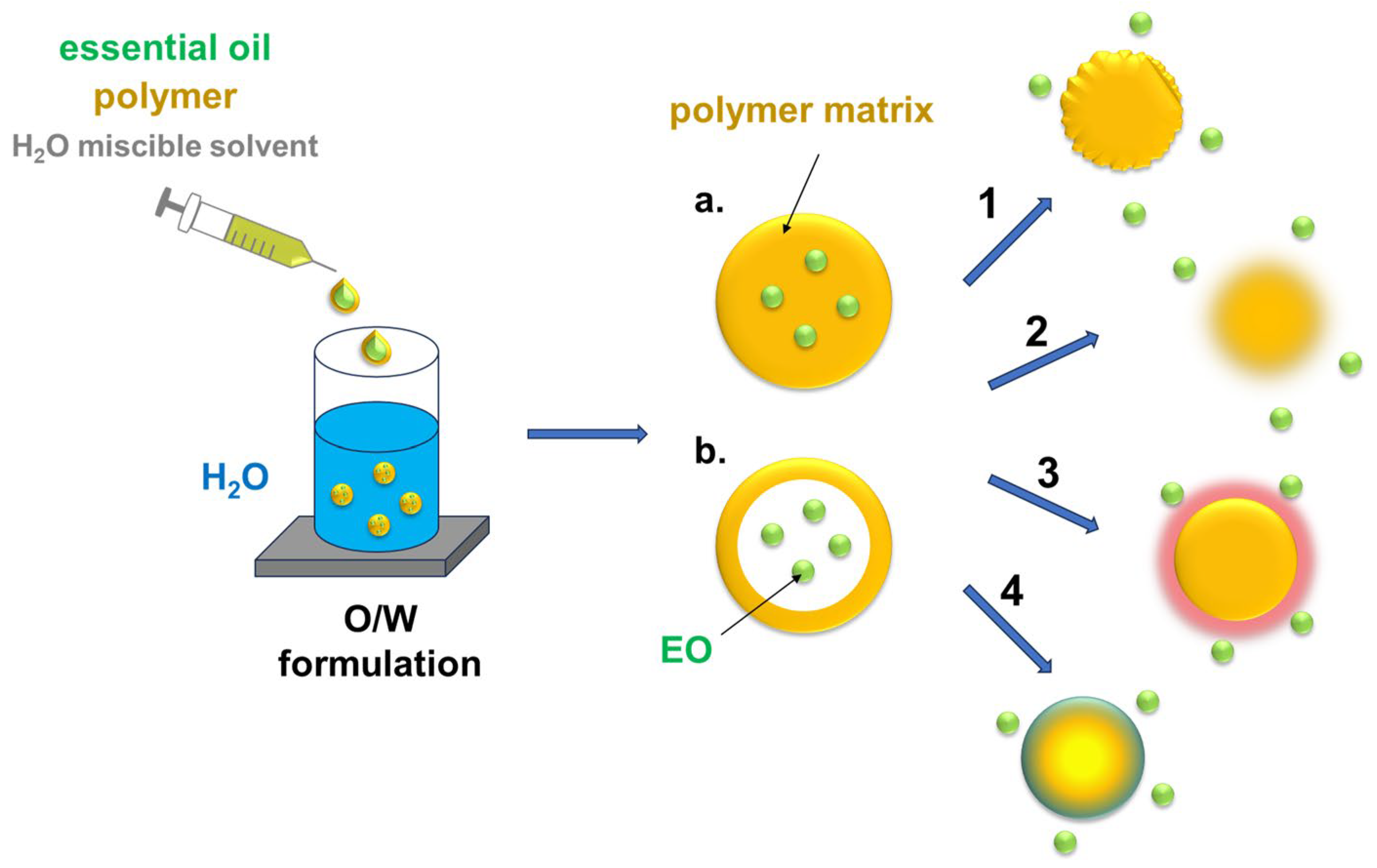
4.1. Nanoencapsulation
4.2. Nanofibers
4.3. Nanocomposites and Blends
4.3.1. Physical Blends
4.3.2. Covalent Grafting
4.4. Active Packaging Materials
4.4.1. Synthetic Biocompatible Polymers
4.4.2. Biopolymers
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EO | Essential oil |
| G+ | Gram positive |
| G- | Gram negative |
| EPSs | Extracellular polymeric substances |
| QS | Quorum sensing |
| Quorum quenching | |
| AHL | Acylated homoserine lactone |
| EP | Efflux pumps |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| MRSA | Methicilin-resistant Staphylococcus aureus |
| VRSA | Vanomycin-resistant Staphylococcus aureus |
| EUG | Eugenol |
| GAR | Carvacrol |
| LIN | Linalool |
| GER | Geraniol |
| CIT | Citronellol |
| FAR | Farnesol |
| PHY | Phytol |
| LNCs | Lipid nanocapsules |
| CD | Cyclodextrin |
| IC | Inclusion complex |
| NF | Nanofiber |
| NP | Nanoparticle |
References
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial Resistance to Antimicrobial Agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Rowaiye, A.; Ibeanu, G.C.; Bur, D.; Nnadi, S.; Morikwe, U.; Ogugua, A.J.; Chukwudi, C.U. Phyto-molecules show potentials to combat drug-resistance in bacterial cell membranes. Microb. Pathog. 2025, 205, 107723. [Google Scholar] [CrossRef]
- Burts, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- da Cruz, E.P.; Souza, E.J.D.; Pail, G.L.; Siebeneichler, T.J.; Fonseca, L.M.; Rombaldi, C.V.; Zavareze, E.D.R.; Dias, A.R.G. Sweet Orange and Sour Orange Essential Oils: A Review of Extraction Methods, Chemical Composition, Antioxidant and Antimicrobial Activities, and Applications in Innovative Food Technologies. Food Biophys. 2025, 20, 101. [Google Scholar] [CrossRef]
- Kowalewska, A.; Majewska-Smolarek, K. Eugenol-Based Polymeric Materials—Antibacterial Activity and Applications. Antibiotics 2023, 12, 1570. [Google Scholar] [CrossRef]
- Nowacka, M.; Kowalewska, A.; Kręgiel, D. Farnesol-Containing Macromolecular Systems for Antibiofilm Strategies. Surfaces 2020, 3, 197–210. [Google Scholar] [CrossRef]
- Hajiagha, M.N.; Kafil, H.S. Efflux pumps and microbial biofilm formation. Infect. Genet. Evol. 2023, 112, 105459. [Google Scholar] [CrossRef]
- Vani, S.; Vadakkan, K.; Mani, B. A narrative review on bacterial biofilm: Its formation, clinical aspects and inhibition strategies. Futur. J. Pharm. Sci. 2023, 9, 50. [Google Scholar] [CrossRef]
- Iaconis, A.; De Plano, L.M.; Caccamo, A.; Franco, D.; Conoci, S. Anti-biofilm strategies: A focused review on innovative approaches. Microorganisms 2024, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Jayaprakashvel, M. Bacterial quorum sensing: Biofilm formation, survival behaviour and antibiotic resistance. In Implication of Quorum Sensing and Biofilm Formation in Medicine, Agriculture and Food Industry; Bramhachari, P., Ed.; Springer: Singapore, 2019; Part I, Chapter 3; pp. 21–37. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory Mechanisms and Promising Applications of Quorum Sensing-Inhibiting Agents in Control of Bacterial Biofilm Formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef]
- Hawver, L.A.; Jung, S.A.; Ng, W.L. Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol. Rev. 2016, 40, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Biarnes-Carrera, M.; Breitling, R.; Takano, E. Butyrolactone signalling circuits for synthetic biology. Curr. Opin. Chem. Biol. 2015, 28, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Cen, Z.; Chen, Y.; Shang, K.; Zhai, J.; Han, M.; Wang, J.; Chen, Z.; Wei, T.; Han, Z. α-Pyrone mediates quorum sensing through the conservon system in Nocardiopsis sp. Microbiol. Res. 2024, 285, 127767. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Alum, E.U.; Gulumbe, B.H.; Izah, S.C.; Uti, D.E.; Aja, P.M.; Igwenyi, I.O.; Offor, C.E. Natural product-based inhibitors of quorum sensing: A novel approach to combat antibiotic resistance. Biochem. Biophys. Rep. 2025, 43, 102111. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; Coppola, R.; Ayala-Zavala, F.J.; da Cruz, A.G.; De Feo, V. Essential Oils and Microbial Communication. In Essential Oils—Oils of Nature; El-Shemy, H., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Maggio, F.; Rossi, C.; Serio, A.; Chaves-Lopez, C.; Casaccia, M.; Paparella, A. Anti-biofilm mechanisms of action of essential oils by targeting genes involved in quorum sensing, motility, adhesion, and virulence: A review. Int. J. Food Microbiol. 2025, 426, 110874. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Antibiofilm potential of natural essential oils. Appl. Sci. 2025, 15, 5847. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Lotfalizadeh, M.; Badpeyma, M.; Shakeri, A.; Soheili, V. From plants to antimicrobials: Natural products against bacterial membranes. Phytother. Res. 2022, 36, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science 1994, 264, 382–388. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I.; Asif, M.; Tahseen, Q. Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Biosci. 2013, 38, 835–844. [Google Scholar] [CrossRef]
- Schindler, B.D.; Kaatz, G.W. Multidrug efflux pumps of Gram-positive bacteria. Drug Res. Updates 2016, 27, 1–13. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Ahn, J. Advances in the discovery of efflux pump inhibitors as novel potentiators to Control antimicrobial-resistant pathogens. Antibiotics 2023, 12, 1417. [Google Scholar] [CrossRef]
- Nikaido, H.; Pagès, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of gram-negative bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef]
- Lamut, A.; Peterlin Mašič, L.; Kikelj, D.; Tomašič, T. Efflux pump inhibitors of clinically relevant multidrug resistant bacteria. Med. Res. Rev. 2019, 39, 2460–2504. [Google Scholar] [CrossRef]
- Dias, K.J.S.D.O.; Miranda, G.M.; Bessa, J.R.; Araújo, A.C.J.D.; Freitas, P.R.; Almeida, R.S.D.; Paulo, C.L.R.; Neto, J.B.D.A.; Coutinho, H.D.M.; Ribeiro-Filho, J. Terpenes as bacterial efflux pump inhibitors: A systematic review. Front. Pharmacol. 2022, 13, 953982. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Khan, S.S.; Khan, A.; Rani, M.; Ahmad, V.U.; Choudhary, M.I. Biotransformation of monoterpenoids and their antimicrobial activities. Phytomedicine 2014, 21, 1597–1626. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Bhattacharjee, M.; Unni, B.; Kashyap, R.S.; Malik, A.; Akhtar, S.; Fatima, S. In silico testing to identify compounds that inhibit ClfA and ClfB binding to the host for the formulation of future drugs against Staphylococcus aureus colonization and infection. Front. Cell. Infect. Microbiol. 2024, 14, 1422500. [Google Scholar] [CrossRef]
- Ullah, N.; Hasnain, S.Z.U.; Baloch, R.; Amin, A.; Nasibova, A.; Selakovic, D.; Rosic, G.L.; Islamov, S.; Naraliyeva, N.; Jaradat, N.; et al. Exploring essential oil-based bio-composites: Molecular docking and in vitro analysis for oral bacterial biofilm inhibition. Front. Chem. 2024, 12, 1383620. [Google Scholar] [CrossRef]
- Atteya, M.R.; Romeilah, R.M.; Ramadan, K.M.A.; El-Beltagi, H.S.; Gaber, A.M.; Al Hashedi, S.A.; AboZaid, N.A.; Mahmoud, M.A.A.; Youssef, R.; Eslam, M.; et al. Clove and thyme essential oils: From molecular docking to food application—A study of their preservative properties in buttermilk. ACS Omega 2025, 10, 5119–5137. [Google Scholar] [CrossRef]
- Ahmad, M.U.; Sharif, S.; Ali, S.; Ahmad, I.; Khan, Q.F.; Liaqat, I.; William, K.; Shah, T.A.; Alamri, A.S.; galanakis, C.M.; et al. Exploring in vitro and in silico potential inhibitory effects of eugenol and its analogues for broad range development of antibacterial drugs. Discov. Life 2025, 55, 5. [Google Scholar] [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Barbosa-Filho, J.; Lima, E. Antibacterial and antibiofilm activity of myrtenol against Staphylococcus aureus. Pharmaceuticals 2020, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Aderibigbe, B.A. Antibacterial activity of selected essential oil components and their derivatives: A Review. Antibiotics 2025, 14, 68. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial activity of naturally occurring phenols and derivatives against biofilm and planktonic bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Ahmad, I.; Khan, M.S.; Ahmad, E.; Tahseen, Q.; Khan, M.S.; Alshabib, N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of gram-negative bacteria. Front. Microbiol. 2015, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Abdou, A.; Elmakssoudi, A.; El Amrani, A.; JamalEddine, J.; Dakir, M. Recent advances in chemical reactivity and biological activities of eugenol derivatives. Med. Chem. Rev. 2021, 30, 1011–1030. [Google Scholar] [CrossRef]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of bactericidal action of Eugenol against Escherichia coli. J. Herbal. Med. 2021, 26, 100406. [Google Scholar] [CrossRef]
- Abdou, A.; Elshafei, A.M.; Salama, A.; Ali, F.; Abdelkader, A. Phytochemical study: Molecular docking of eugenol derivatives as antioxidant and antimicrobial agents. Lett. Org. Chem. 2022, 19, 774–783. [Google Scholar] [CrossRef]
- da Silva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; Do Nascimento, P.G.G.; de Medeiros Costa, A.K.; de Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef]
- Martins, R.M.; Farias, M.D.A.; Nedel, F.; Pereira, C.M.P.D.; Lencina, C.; Lund, R.G. Antimicrobial and cytotoxic evaluation of eugenol derivatives. Med. Chem. Res. 2016, 25, 2360–2367. [Google Scholar] [CrossRef]
- Dhara, D.; Tripathi, A. Antimicrobial activity of eugenol and cinnamaldehyde against extended spectrum beta lactamase producing enterobacteriaceae by in vitro and molecular docking analysis. Eur. J. Integr. Med. 2013, 5, 527–536. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, H.; Tang, Y.; Yu, W.; Gong, Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol. Lett. 2013, 35, 631–637. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, I.; Baig, M.H. Eugenol inhibits quorum sensing and biofilm of toxigenic MRSA strains isolated from food handlers employed in Saudi Arabia. Biotechnol. Biotechnol. Equip. 2017, 31, 387–396. [Google Scholar] [CrossRef]
- Chang, J.; Chen, B.; Du, Z.; Zhao, B.; Li, J.; Li, Z.; Arunachalam, K.; Shi, T.; Wei, D.; Shi, C. Eugenol targeting CrtM inhibits the biosynthesis of staphyloxanthin in Staphylococcus aureus. Food Sci. Hum. Wellness 2024, 13, 1368–1377. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Chen, Z.; Bai, X.; Yang, Z.; Wang, Z.; Yang, Y. Antibacterial activity and mechanism of clove essential oil against foodborne pathogens. LWT 2023, 173, 114249. [Google Scholar] [CrossRef]
- Muniz, D.F.; dos Santos Barbosa, C.R.; de Menezes, I.R.A.; de Sousa, E.O.; Pereira, R.L.S.; Júnior, J.T.C.; Pereira, P.S.; de Matos, Y.M.; da Costa, R.H.; de Morais Oliveira-Tintino, C.D.; et al. In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem. 2021, 337, 127776. [Google Scholar] [CrossRef]
- Ashtiani, E.E.; Gholizadeh Siahmazgi, Z.; Mirpour, M.; Soltani, B.M. RND pump inhibition: In-silico and in-vitro study by eugenol on clinical strain of E. coli and P. aeruginosa. Silico Pharmacol. 2023, 11, 22. [Google Scholar] [CrossRef]
- Ni, K.H.; Cai, D.; Lu, J.; Tian, J. Eugenol—Mediated inhibition of biofilm formed by S. aureus: A potent organism for pediatric digestive system diseases. Appl. Biochem. Biotechnol. 2022, 194, 1340–1358. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Shrivastava, R.; Vashistt, J. Eugenol and geraniol impede Csu-pilus assembly and evades multidrug-resistant Acinetobacter baumannii biofilms: In-vitro and in-silico evidence. Biochem. Biophys. Res. Commun. 2022, 636, 10–17. [Google Scholar] [CrossRef]
- Pakharukova, N.; Tuittila, M.; Paavilainen, S.; Malmi, H.; Parilova, O.; Teneberg, S.; Knight, S.D.; Zavialov, A.V. Structural basis for Acinetobacter baumannii biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Benaissa, A.; Bouali, W.; Ngenge Tamfu, A.; Ammara, B.; Kucukaydin, S.; Latti, N.; Khadir, A.; Bendahou, M.; Anouar, E.H.; Ceylan, O. Inhibition of clinical multidrug—Resistant Pseudomonas aeruginosa biofilms by cinnamaldehyde and eugenol from essential oils: In vitro and in silico analysis. Chem. Biodivers. 2025, 22, e202402693. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- de Souza, G.H.D.A.; dos Santos Radai, J.A.; Mattos Vaz, M.S.; Esther da Silva, K.; Fraga, T.L.; Barbosa, L.S.; Simionatto, S. In vitro and in vivo antibacterial activity assays of carvacrol: A candidate for development of innovative treatments against KPC—Producing Klebsiella pneumoniae. PLoS ONE 2021, 16, e0246003. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G. Carvacrol—Rich oregano oil and thymol—Rich thyme red oil inhibit biofilm formation and the virulence of uropathogenic Escherichia coli. J. Appl. Microbiol. 2017, 123, 1420–1428. [Google Scholar] [CrossRef]
- Bnyan, I.A.; Abid, A.T.; Obied, H.N. Antibacterial Activity of Carvacrol against Different Types of Bacteria. J. Nat. Sci. Res. 2014, 4, 2224–3186. [Google Scholar]
- Datta, S.; Singh, V.; Nag, S.; Roy, D.N. Carvacrol, a monoterpenoid, binds quorum sensing proteins (LasI and LasR) and swarming motility protein BswR. of Pseudomonas aeruginosa, resulting in loss of pathogenicity: An in silico approach. Can. J. Microbiol. 2025, 7, 1. [Google Scholar] [CrossRef]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuize, E.J.A. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules 2016, 12, 1709. [Google Scholar] [CrossRef]
- Fajdek-Bieda, A.; Pawlińska, J.; Wróblewska, A.; Łuś, A. Evaluation of the antimicrobial activity of geraniol and selected geraniol transformation products against gram-positive bacteria. Molecules 2024, 29, 950. [Google Scholar] [CrossRef]
- Ahmad, A.; Viljoen, A.M.; Chenia, H.Y. The impact of plant volatiles on bacterial quorum sensing. Lett. Appl. Microbiol. 2015, 60, 8–19. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Grabarczyk, M.; Wińska, K. Natural Compounds in the Battle against Microorganisms—Linalool. Molecules 2022, 27, 6928. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Tang, P.; Liu, T.; Zhao, T.; Guo, J.; Gao, Z. Linalool nanoemulsion preparation, characterization and antimicrobial activity against Aeromonas hydrophila. Int. J. Mol. Sci. 2021, 22, 11003. [Google Scholar] [CrossRef] [PubMed]
- Uttu, A.J.; Sallau, M.S.; Iyun, O.R.A.; Ibrahim, H. Isolation, Characterization and in silico molecular docking studies of two terpenoids from Strychnos innocua (Delile) root bark for antibacterial properties. Adv. J. Chem. A 2022, 5, 241–252. [Google Scholar] [CrossRef]
- Alves, S.; Duarte, A.; Sousa, S.; Domingues, F.C. Study of the major essential oil compounds of Coriandrum sativum against Acinetobacter baumannii and the effect of linalool on adhesion, biofilms and quorum sensing. Biofouling 2016, 32, 155–165. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Zhang, K.; Zhang, J.; Cui, D.; Wang, J.; Ji, P.; Wei, Y.; Li, J. Linalool as a potential agent for inhibiting Escherichia coli biofilm formation and exopolysaccharide production. BMC Vet. Res. 2025, 21, 235. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, S.; Mukherjee, D.; Joshi, S.J.; Ray, R.R. Antibiofilm and anti-quorum sensing activities of eugenol and linalool from Ocimum tenuiflorum against Pseudomonas aeruginosa biofilm. J. Appl. Microbiol. 2021, 131, 2821–2837. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; El-Tarabili, R.M.; Bahnass, M.M.; Alshahrani, M.A.; Saif, A.; Alwutayd, K.M.; Safhi, F.A.; Mansour, A.T.; Alblwi, N.A.N.; Ghoneim, M.M.; et al. Partnering essential oils with antibiotics: Proven therapies against bovine Staphylococcus aureus mastitis. Front. Cell. Infect. Microbiol. 2023, 13, 1265027. [Google Scholar] [CrossRef]
- Long, N.; Qiu, M.; Zuo, Y.; Deng, H. Antimicrobial Activity and Metabolomic Analysis of Linalool Against Pathogenic Bacteria Methicillin-Resistant Staphylococcus aureus. Infect. Drug Resist. 2025, 18, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Yang, L.; Lv, X.; Zhang, Y.; Hou, X.; Li, M.; Zhou, M.; Pan, L.; Chen, A.; Zhang, Z. Antibiofilm Activity and Mechanism of Linalool against Food Spoilage Bacillus amyloliquefaciens. Int. J. Mol. Sci. 2023, 24, 10980. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One hundred faces of geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Viljoen, A.M. Geraniol—A review update. South Afr. J. Bot. 2022, 150, 1205–1219. [Google Scholar] [CrossRef]
- Nemoda, M.; Veljković, F.; Nikolić, B.; Brkić, S.; Marković, D.; Momčilović, M.; Lal, M.; Živković, L.; Marinković, J. Geraniol in vitro and geraniol-based emulsion ex vivo potential against four-species Streptococcus spp. biofilm relevant for dentistry. Ind. Crops Prod. 2024, 222, 119827. [Google Scholar] [CrossRef]
- Kannappan, A.; Sivaranjani, M.; Srinivasan, R.; Rathna, J.; Pandian, S.K.; Ravi, A.V. Inhibitory efficacy of geraniol on biofilm formation and development of adaptive resistance in Staphylococcus epidermidis RP62A. J. Med. Microbiol. 2017, 66, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Pontes, E.K.U.; Melo, H.M.; Nogueira, J.W.A.; Firmino, N.C.S.; de Carvalho, M.G.; Catunda Júnior, F.E.A.; Cavalcante, T.T.A. Antibiofilm activity of the essential oil of citronella (Cymbopogon nardus) and its major component, geraniol, on the bacterial biofilms of Staphylococcus aureus. Food Sci. Biotechnol. 2019, 28, 633–639. [Google Scholar] [CrossRef]
- Lira, M.H.P.D.; Andrade Júnior, F.P.D.; Moraes, G.F.Q.; Macena, G.D.S.; Pereira, F.D.O.; Lima, I.O. Antimicrobial activity of geraniol: An integrative review. J. Essent. Oil Res. 2020, 32, 187–197. [Google Scholar] [CrossRef]
- Gu, K.; Ouyang, P.; Hong, Y.; Dai, Y.; Tang, T.; He, C.; Shu, G.; Liang, X.; Tang, H.; Zhu, L.; et al. Geraniol inhibits biofilm formation of methicillin-resistant Staphylococcus aureus and increase the therapeutic effect of vancomycin in vivo. Front. Microbiol. 2022, 13, 960728. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Yan, V.L.Y.; Lee, C.K.; Kuean, C.H.; Candasamy, M.; Liew, Y.K.; Sahu, P.S. Protectiveactivity of geraniol against acetic acid and Helicobacter pylori-induced gastric ulcers in rats. J. Tradit. Complement. Med. 2019, 9, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Yao, W. Geraniol as a Quorum Sensing inhibitor of Erwinia carotovora and Pseudomonas fluorescens isolated from vegetable and their dual-species biofilm production on stainless steel. J. Food Sci. 2021, 45, e16042. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Yang, F.; Xie, Y.; Guo, Y.; Cheng, Y.; Yao, W. The combination of hexanal and geraniol in sublethal concentrations synergistically inhibits quorum sensing in Pseudomonas fluorescens—In vitro and in silico approaches. J. Appl. Microbiol. 2022, 133, 2122–2136. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Gędas, A.; Simões, M. The Effects of Eugenol, Trans-Cinnamaldehyde, Citronellol, and Terpineol on Escherichia coli Biofilm Control as Assessed by Culture-Dependent and -Independent Methods. Molecules 2020, 25, 2641. [Google Scholar] [CrossRef]
- Wei, T.; Regeard, C.; Barroca-Aubry, N.; Roger, P.; Aymes-Chodur, C. Chemoenzymatic oxidation of citronellol and geraniol: Synthesis and antibacterial activity assessment. Colloids Surf. B Biointerf. 2025, 253, 114723. [Google Scholar] [CrossRef]
- da Silva, A.J.A.; de Sousa Silveira, Z.; Macêdo, N.S.; dos Santos Barbosa, C.R.; da Silva Sousa, Â.E.; da Silva, T.F.; de Sousa, J.T.; de Morais Oliveira-Tintino, C.D.; Tintino, S.R.; Marinho, E.S.; et al. Evaluation of the antibacterial and inhibitory activity of the NorA efflux pump in Staphylococcus aureus by citronellol. Biologia 2025, 80, 2503–2517. [Google Scholar] [CrossRef]
- Yang, Z.; He, S.; Wei, Y.; Li, X.; Shan, A.; Wang, J. Antimicrobial peptides in combination with citronellal efficiently kills multidrug resistance bacteria. Phytomedicine 2023, 120, 155070. [Google Scholar] [CrossRef]
- Caixin, Y.; Hao, X. Antibacterial mechanism of action and in silico molecular docking studies of Cupressus funebris essential oil against drug resistant bacterial strains. Heliyon 2023, 9, e18742. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Yang, L.; Bai, Y.; Yong, J.; Li, Y. Exploring the Potential of Farnesol as a Novel Antifungal Drug and Related Challenges. Curr. Infect. Dis. Rep. 2024, 26, 123–125. [Google Scholar] [CrossRef]
- Shchepin, R.; Hornby, J.M.; Burger, E.; Niessen, T.; Dussault, P.; Nickerson, K.W. Quorum sensing in Candida albicans: Probing farnesol’s mode of action with 40 natural and synthetic farnesol analogs. Chem. Biol. 2003, 10, 743–750. [Google Scholar] [CrossRef]
- Lopes, A.P.; de Oliveira Castelo Branco, R.R.; de Alcântara Oliveira, F.A.; Campos, M.A.S.; de Carvalho Sousa, B.; Agostinho, Í.R.C.; Gonzalez, A.G.M.; Rocha, J.A.; Pinheiro, R.E.E.; Araújo, A.R.; et al. Antimicrobial, modulatory, and antibiofilm activity of tt-farnesol on bacterial and fungal strains of importance to human health. Bioorg. Med. Chem. Lett. 2021, 47, 128192. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E.; Shirtliff, M.E. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Shiraishi, A.; Hada, T.; Hirose, K.; Hamashima, H.; Shimada, J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 2004, 237, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ma, R.; Reeves, T.; Katz, A.J.; Levi, N. Repurposing Farnesol for Combating Drug-Resistant and Persistent Single and Polymicrobial Biofilms. Antibiotics 2024, 13, 350. [Google Scholar] [CrossRef]
- Gomes, F.; Teixeira, P.; Cerca, N.; Azeredo, J.; Oliveira, R. Effect of Farnesol on Structure and Composition of Staphylococcus epidermidis Biofilm Matrix. Curr. Microbiol. 2011, 63, 354–359. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Van Dijck, P.; Jabra-Rizk, M.A. Modulation of Staphylococcus aureus response to antimicrobials by the Candida albicans quorum sensing molecule farnesol. Antimicrob. Agents Chemother. 2017, 61, e01573-17. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Carvalho, A.M.S.; Heimfarth, L.; Menezes Pereira, E.W.; Santana Oliveira, F.; Menezes, I.R.A.; Coutinho, H.D.M.; Picot, L.; Antoniolli, A.R.; Quintans, J.S.S.; Quintans-Júnior, L.J. Phytol, a Chlorophyll Component, Produces Antihyperalgesic, Anti-inflammatory, and Antiarthritic Effects: Possible NFκB Pathway Involvement and Reduced Levels of the Proinflammatory Cytokines TNF-α and IL-6. J. Nat. Prod. 2020, 83, 1107–1117. [Google Scholar] [CrossRef]
- Gamal El-Din, M.I.; Youssef, F.S.; Altyar, A.E.; Ashour, M.L. GC/MS Analyses of the Essential Oils Obtained from Different Jatropha Species, Their Discrimination Using Chemometric Analysis and Assessment of Their Antibacterial and Anti-Biofilm Activities. Plants 2022, 11, 1268. [Google Scholar] [CrossRef]
- Alshwyeh, H.A. Phenolic profiling and antibacterial potential of Saudi Arabian native date palm (Phoenix dactylifera) cultivars. Int. J. Food Prop. 2020, 23, 627–638. [Google Scholar] [CrossRef]
- Šovljanski, O.; Aćimović, M.; Sikora, V.; Koren, A.; Saveljić, A.; Tomić, A.; Tešević, V. Exploring (Un)Covered Potentials of Industrial Hemp (Cannabis sativa L.) Essential Oil and Hydrolate: From Chemical Characterization to Biological Activities. Nat. Prod. Commun. 2024, 19, 1934578X241264712. [Google Scholar] [CrossRef]
- Hawas, U.W.; Shaher, F.; Ghandourah, M.; El-Kassem, L.T.A.; Satheesh, S.; Al-Sofyani, A.M.A. Lipids and free fatty acids of red sea Avrainvillea amadelpha, Holothuria atra, and Sarcocornia fruticosa inhibit marine bacterial biofilms. Lett. Org. Chem. 2020, 17, 466–471. [Google Scholar] [CrossRef]
- Abo-Elghiet, F.; Rushdi, A.; Ibrahim, M.H.; Mahmoud, S.H.; Rabeh, M.A.; Alshehri, S.A.; El Menofy, N.G. Chemical Profile, Antibacterial, Antibiofilm, and Antiviral Activities of Pulicaria crispa Most Potent Fraction: An In Vitro and In Silico Study. Molecules 2023, 28, 4184. [Google Scholar] [CrossRef]
- Lee, W.; Woo, E.-R.; Lee, D.G. Phytol has antibacterial property by inducing oxidative stress response in Pseudomonas aeruginosa. Free Radic. Res. 2016, 50, 1–26. [Google Scholar] [CrossRef]
- Almeida-Bezerra, J.W.; Menezes, S.A.; Silva, J.T.d.C.; de Sousa, S.G.; Alves, D.S.; Alencar, G.G.; Araújo, I.M.; Rodrigues, E.Y.d.S.; Oliveira-Tintino, C.D.d.M.; da Cruz, R.P.; et al. Analysis of the Antibiotic-Potentiating Activity, Absorption, Distribution, Metabolism, and Excretion (ADME) and the Molecular Docking Properties of Phytol Against Multi-Drug-Resistant (MDR) Strains. Antibiotics 2024, 13, 1171. [Google Scholar] [CrossRef]
- Vui, N.V.; Linh, N.T.; Quyen, N.T.K.; Nang, K.; Trinh, L.T.T. The Interaction of Ocimum basilicum, Perilla frutescens and Mentha spicata Essential Oils with Norfloxacin Against Antibiotic-Resistant Salmonella spp. That Cause Disease in Chickens. Vet. Med. Sci. 2025, 11, e70316. [Google Scholar] [CrossRef]
- Tao, R.; Wang, C.-Z.; Kong, Z.-W. Antibacterial/Antifungal Activity and Synergistic Interactions between Polyprenols and Other Lipids Isolated from Ginkgo biloba L. Leaves. Molecules 2013, 18, 2166–2182. [Google Scholar] [CrossRef]
- Adeosun, I.J.; Baloyi, I.T.; Cosa, S. Anti-Biofilm and Associated Anti-Virulence Activities of Selected Phytochemical Compounds against Klebsiella pneumoniae. Plants 2022, 11, 1429. [Google Scholar] [CrossRef]
- Zhan, H.; Zhou, X.; Li, J.; Zhang, L.; Qu, G.; Li, Y.; Sun, Y.; Ju, W.; Ye, M.; Deng, Y.; et al. Phytol inhibits quorum sensing and biogenic amine production in Pseudomonas fluorescens PF12. Food Biosci. 2025, 71, 107290. [Google Scholar] [CrossRef]
- Ramanathan, S.; Arunachalam, K.; Chandran, S.; Selvaraj, R.; Shunmugiah, K.P.; Arumugam, V.R. Biofilm inhibitory efficiency of phytol in combination with cefotaxime against nosocomial pathogen Acinetobacter baumannii. J. Appl. Microbiol. 2018, 125, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Ciric, A.; Glamoclija, J.; Nikolic, M.; Sokovic, M. In vitro anti-quorum sensing activity of phytol. Nat. Prod. Res. 2015, 29, 374–377. [Google Scholar] [CrossRef]
- Chatterjee, B.; Vittal, R.R. Quorum sensing modulatory and biofilm inhibitory activity of Plectranthus barbatus essential oil: A novel intervention strategy. Arch. Microbiol. 2021, 203, 1767–1778. [Google Scholar] [CrossRef]
- Srinivasan, R.; Mohankumar, R.; Kannappan, A.; Raja, V.K.; Archunan, G.; Pandian, S.K.; Ruckmani, K.; Ravi, A.V. Exploring the anti-quorum sensing and antibiofilm efficacy of phytol against Serratia marcescens associated acute pyelonephritis infection in wistar rats. Front. Cell. Infect. Microbiol. 2017, 7, 498. [Google Scholar] [CrossRef]
- Pérez-Recalde, M.; Ruiz Arias, I.E.; Hermida, É.B. Could essential oils enhance biopolymers performance for wound healing? A systematic review. Phytomedicine 2018, 38, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.; Mazumder, A. A critical overview of challenging roles of medicinal plants in improvement of wound healing technology. DARU J. Pharm. Sci. 2024, 32, 379–419. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Chen, X.; Landis, R.F.; Geng, Y.; Makabenta, J.M.; Lemnios, W.; Gupta, A.; Rotello, V.M. Phytochemical-Based Nanocomposites for the Treatment of Bacterial Biofilms. ACS Infect. Dis. 2019, 5, 1590–1596. [Google Scholar] [CrossRef]
- Kaspute, G.; Ivaskiene, T.; Ramanavicius, A.; Ramanavicius, S.; Prentice, U. Terpenes and Essential Oils in Pharmaceutics: Applications as Therapeutic Agents and Penetration Enhancers with Advanced Delivery Systems for Improved Stability and Bioavailability. Pharmaceutics 2025, 17, 793. [Google Scholar] [CrossRef]
- Maurya, A.; Singh, V.K.; Das, S.; Prasad, J.; Kedia, A.; Upadhyay, N.; Dubey, N.K.; Dwivedy, A.K. Essential Oil Nanoemulsion as Eco-Friendly and Safe Preservative: Bioefficacy Against Microbial Food Deterioration and Toxin Secretion, Mode of Action, and Future Opportunities. Front. Microbiol. 2021, 29, 751062. [Google Scholar] [CrossRef]
- Lelis, C.A.; de Carvalho, A.P.A.; Junior, C.A.C. A systematic review on nanoencapsulation natural antimicrobials in foods: In vitro versus in situ evaluation, mechanisms of action and implications on physical-chemical quality. Int. J. Mol. Sci. 2021, 22, 12055. [Google Scholar] [CrossRef]
- Mohamadi, N.; Adeli-Sardou, M.; Ansari, M.; Pakdel, A.; Kosar, M.; Sharififar, F. Nanoencapsulation of Zataria multiflora Essential Oil Containing Linalool Reduced Antibiofilm Resistance against Multidrug-resistant Clinical Strains. Curr. Nanosci. 2025, 21, 111–118. [Google Scholar] [CrossRef]
- Hanan, E.; Dar, A.H.; Sghams, R.; Goksen, G. New insights into essential oil nano emulsions loaded natural biopolymers recent development, formulation, characterization and packaging applications: A comprehensive review. Int. J. Biol. Macromol. 2024, 280, 135751. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.H.; Dinh, K.B.; Luu, T.; Chapman, J.; Baji, A.; Truong, V.K. Nanoengineered sustainable antimicrobial packaging: Integrating essential oils into polymer matrices to combat food waste. Food Sci. Technol. 2024, 59, 5887–5901. [Google Scholar] [CrossRef]
- Naz, S.; Javaid, S.; Rehman, S.U.; Razzaq, H. Recent advances in polymer nanoencapsulation of essential oils for multi-functional textile finishing. Mater. Adv. 2025, 6, 2460–2476. [Google Scholar] [CrossRef]
- Abbas, H.S.; Mahmoud, A.M.; Wahed, R.A.; Elsantawy, M.A.A.; Hamdy, N.M.; Ismail, S.E.S.; Nabil, M.A. Prospects of using bioactive compounds in nanomaterials surface decoration and their biomedical purposes. Int. Nano Lett. 2022, 12, 125–138. [Google Scholar] [CrossRef]
- Silva, R.C.S.D.; de Souza Arruda, I.R.; Malafaia, C.B.; de Moraes, M.M.; Beck, T.S.; Gomes da Camara, C.A.; Henrique da Silva, N.; Vanusa da Silva, M.; dos Santos Correia, M.T.; Frizzo, C.P.; et al. Synthesis, characterization and antibiofilm/antimicrobial activity of nanoemulsions containing Tetragastris catuaba (Burseraceae) essential oil against disease-causing pathogens. J. Drug Deliv. Sci. Technol. 2022, 67, 102795. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Natural plant-derived chemical compounds as listeria monocytogenes inhibitors in vitro and in food model systems. Pathogens 2021, 10, 1–36. [Google Scholar] [CrossRef]
- Mehdipour, E.; Iranbakhsh, A.; Mirzaie, A. Formulation, characterization and co-delivery of curcumin-rosemary loaded niosomes to enhance antimicrobial activity against staphylococcus aureus strains. Nanomed. J. 2025, 12, 313–323. [Google Scholar] [CrossRef]
- Demirci Kayiran, S.; Guven Bolgen, U.M.; Cevikelli, T.; Kızılyıldırım, S.; Yıldır, B.; Ferahoglu, E.; Kırıcı, S.; Ozogul, F. Chemical composition and antibacterial properties of microemulsion and microemulgel formulations containing Lavandula angustifolia Mill. essential oils. Ind. Crops Prod. 2025, 226, 120654. [Google Scholar] [CrossRef]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerf. 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A. Linalool: Therapeutic Indication And Their Multifaceted Biomedical Applications. Drug Res. 2024, 74, 255–268. [Google Scholar] [CrossRef]
- Makhlouf, Z.; Ali, A.A.; Al-Sayah, M.H. Liposomes-Based Drug Delivery Systems of Anti-Biofilm Agents to Combat Bacterial Biofilm Formation. Antibiotics 2023, 12, 875. [Google Scholar] [CrossRef]
- Mun, H.; Townley, H.E. Nanoencapsulation of Plant Volatile Organic Compounds to Improve Their Biological Activities. Planta Med. 2021, 87, 236–251. [Google Scholar] [CrossRef]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N.; Hussain, T. Nano-technology platforms to increase the antibacterial drug suitability of essential oils: A drug prospective assessment. OpenNano 2023, 9, 100115. [Google Scholar] [CrossRef]
- Shamsuddin, N.A.M.; Zulfakar, M.H. Nanostructured Lipid Carriers for the Delivery of Natural Bioactive Compounds. Curr. Drug Deliv. 2023, 20, 127–143. [Google Scholar] [CrossRef]
- Silva, C.G.; Yudice, E.D.C.; Campini, P.A.L.; Rosa, D.S. The performance evaluation of Eugenol and Linalool microencapsulated by PLA on their activities against pathogenic bacteria. Mater. Today Commun. 2021, 21, 100493. [Google Scholar] [CrossRef]
- Venkateswaran, S.; Henrique Dos Santos, O.D.; Scholefield, E.; Lilienkampf, A.; Gwynne, P.J.; Swann, D.G.; Dhaliwal, K.; Gallagher, M.P.; Bradley, M. Fortified interpenetrating polymers-bacteria resistant coatings for medical devices. J. Mater. Chem. B 2016, 4, 5405–5411. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Arribas, N.; Guzmán, E.; Lucia, A.; Toloza, A.C.; Velarde, M.G.; Ortega, F.; Rubio, R.G. Environmentally friendly platforms for encapsulation of an essential oil: Fabrication, characterization and application in pests control. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 473–481. [Google Scholar] [CrossRef]
- Dogheim, G.M.; Shehat, M.G.; Mahdy, D.M.; Barakat, H.S.; Abouelfetouh, A.; Ramadan, A.A. Antibacterial and anti-virulence activity of eco-friendly resveratrol-loaded lipid nanocapsules against methicillin-resistant staphylococcus aureus. Sci. Rep. 2025, 15, 14677. [Google Scholar] [CrossRef]
- Yegin, Y.; Perez-Lewis, K.L.; Zhang, M.; Akbulut, M.; Taylor, T.M. Development and characterization of geraniol-loaded polymeric nanoparticles with antimicrobial activity against foodborne bacterial pathogens. J. Food Eng. 2015, 170, 64–71. [Google Scholar] [CrossRef]
- Carniel, T.K.; Fagundes, P.; Vivan, A.C.; Silva, L.L.; Zanetti, M.; Dalcanton, F.; De Mello, J.M.M.; Fiori, M.A. Application of the polycaprolactone polymer for the encapsulation of geraniol: Evaluation of the efficiency and stability. J. Polym. Eng. 2021, 41, 480–489. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan nanoparticles encapsulating lemongrass (Cymbopogon commutatus) essential oil: Physicochemical, structural, antimicrobial and in-vitro release properties. Int. J. Biol. Macromol. 2021, 192, 1084–1097. [Google Scholar] [CrossRef]
- Costa, A.F.; Silva, L.D.C.; Amaral, A.C. Farnesol: An approach on biofilms and nanotechnology. Med. Mycol. 2021, 59, 958–969. [Google Scholar] [CrossRef]
- Ali, A.; Obireddy, S.R.; Lai, W.-F. Farnesol as a multifunctional candidate for treatment development. Biocell 2024, 48, 163–171. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Fiandra, L.; Mantecca, P.; Catelani, T.; Natan, M.; Banin, E.; Jacobi, G.; Tzanov, T. Antibacterial, Antibiofilm, and Antiviral Farnesol-Containing Nanoparticles Prevent Staphylococcus aureus from Drug Resistance Development. Int. J. Mol. Sci. 2022, 23, 7527. [Google Scholar] [CrossRef]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S.W. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Horev, B.; Hwang, G.; Klein, M.I.; Koo, H.; Benoit, D.S.W. Characterization and optimization of pH responsive polymer nanoparticles for drug delivery to oral biofilms. J. Mater. Chem. B 2016, 4, 3075–3085. [Google Scholar] [CrossRef] [PubMed]
- Bükay, Y.G. Potential antibiofilm activity of farnesol-loaded poly(DL-lactide-co-glycolide) (PLGA) nanoparticles against Candida albicans. J. Anal. Sci. Technol. 2020, 11, 43. [Google Scholar] [CrossRef]
- Santana, J.E.G.; Oliveira-Tintino, C.D.d.M.; Gonçalves Alencar, G.; Siqueira, G.M.; Sampaio Alves, D.; Moura, T.F.; Tintino, S.R.; de Menezes, I.R.A.; Rodrigues, J.P.V.; Gonçalves, V.B.P.; et al. Comparative Antibacterial and Efflux Pump Inhibitory Activity of Isolated Nerolidol, Farnesol, and α-Bisabolol Sesquiterpenes and Their Liposomal Nanoformulations. Molecules 2023, 28, 7649. [Google Scholar] [CrossRef]
- Bandara, H.M.H.N.; Herpin, M.J.; Kolacny, D., Jr.; Harb, A.; Romanovicz, D.; Smyth, H.D.C. Incorporation of farnesol significantly increases the efficacy of liposomal Ciprofloxacin against Pseudomonas aeruginosa biofilms in vitro. Mol. Pharm. 2016, 13, 2760–2770. [Google Scholar] [CrossRef]
- Valcourt, C.; Buyck, J.M.; Grégoire, N.; Couet, W.; Marchand, S.; Tewes, F. Lipid Nanoparticles Loaded with Farnesol or Geraniol to Enhance the Susceptibility of E. coli MCR-1 to Colistin. Pharmaceutics 2021, 13, 1849. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.L.; Horta, S.; Santos, M.; Rocha, S.M.; Trindade, T. Release behavior of trans,trans-farnesol entrapped in amorphous silica capsules. Results Pharma Sci. 2012, 2, 52–56. [Google Scholar] [CrossRef]
- Sousa, F.L.; Santos, M.; Rocha, S.M.; Trindade, T. Encapsulation of essential oils in SiO2 microcapsules and release behaviour of volatile compounds. J. Microencapsul. 2014, 7, 627–635. [Google Scholar] [CrossRef]
- Ke, Q.; Ma, K.; Zhang, Y.; Meng, Q.; Huang, X.; Kou, X. Antibacterial aroma compounds as property modifiers for electrospun biopolymer nanofibers of proteins and polysaccharides: A review. Int. J. Biol. Macromol. 2023, 253, 126563. [Google Scholar] [CrossRef]
- Souza, M.A.; Oliveira, J.E.; Medeiros, E.S.; Glenn, G.M.; Mattoso, L.H.C. Controlled release of linalool using nanofibrous membranes of poly(lactic acid) obtained by electrospinning and solution blow spinning: A comparative study. J. Nanosci. Nanotechnol. 2015, 15, 5628–5636. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Saini, D.; Mishra, P.; Pandav, K.; Prasad, R. Essential oil active constituents loaded PVA nanofibers enhance antibiofilm activity against Candida albicans and Candida tropicalis. J. Drug Deliv. Sci. Technol. 2024, 98, 105871. [Google Scholar] [CrossRef]
- Hamzekhani, E.S.; Najafi, M.A.; Miri, M.A.; Ghaghelestani, S.N. Evaluation of antimicrobial activity and properties of gelatin nanofibers containing lavender essential oil. J. Food. Sci. Technol. 2024, 21, 82–91. [Google Scholar] [CrossRef]
- Bartošová, L.; Sedlaříková, J.; Peer, P.; Janalíková, M.; Pleva, P. Antibacterial and Antifouling Efficiency of Essential Oils-Loaded Electrospun Polyvinylidene Difluoride Membranes. Int. J. Mol. Sci. 2023, 24, 423. [Google Scholar] [CrossRef]
- Wang, M.-L.; Yu, D.-G.; Annie Bligh, S.W. Side-by-Side Electrospun PCL-Ag NPs/CA-Lavender Oil Janus Nanobelt as a Potential Dressing. In Proceedings of the IEEE International Conference Nanomaterials: Applications & Properties (NAP), Bratislava, Slovakia, 10–15 September 2023; pp. NRA181–NRA184. [Google Scholar] [CrossRef]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; San Keskin, N.O.; Tekinay, T.; Uyar, T. Electrospinning of polymer-free cyclodextrin/geraniol-inclusion complex nanofibers: Enhanced shelf-life of geraniol with antibacterial and antioxidant properties. RSC Adv. 2016, 6, 46089–46099. [Google Scholar] [CrossRef]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Tekinay, T.; Uyar, T. Electrospinning of cyclodextrin/linalool-inclusion complex nanofibers: Fast-dissolving nanofibrous web with prolonged release and antibacterial activity. Food Chem. 2017, 231, 192–201. [Google Scholar] [CrossRef]
- Pereira, R.L.S.; Campina, F.F.; do Socorro Costa, M.; Pereira da Cruz, R.; de Freitas, T.S.; dos Santos, T.L.; Cruz, B.G.; de Sena Junior, D.M.; Lima, I.K.C.; Xavier, M.R.; et al. Antibacterial and modulatory activities of β-cyclodextrin complexed with (+)-β-citronellol against multidrug-resistant strains. Microb. Pathogen. 2021, 156, 104928. [Google Scholar] [CrossRef]
- Gupta, P.; Mishra, P.; Verma, N.; Alhariry, J.; Kumar, C.; Prasad, R.; Poluri, K.M. Assessing the eradication potential of fungal biofilms using acacia gum/PVA nanofibers functionalized with geraniol-β cyclodextrin inclusion complex. J. Drug Deliv. Sci. Technol. 2024, 91, 105186. [Google Scholar] [CrossRef]
- Wang, C.; Wu, L.; Zhang, Y.; Xu, M.; Saldaña, M.D.A.; Fan, X.; Sun, W. A Water-absorbent Mat Incorporating β-cyclodextrin/eugenol Inclusion Complex for Preservation of Cold Fresh Mutton. Food Biophys. 2022, 17, 437–447. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Karim, A.A.; Ariffin, F. Fabrication and Characterization of Electrospun Fish Gelatin Mats Doped with Essential Oils and β-Cyclodextrins for Food Packaging Applications. Food Biophys. 2023, 18, 186–197. [Google Scholar] [CrossRef]
- Celebioglu, A.; Hsiung, E.; Aboelkheir, M.; Chowdhury, R.; Altier, C.; Uyar, T. Encapsulation of Essential Oil-Cyclodextrin Inclusion Complexes in Electrospun Pullulan Nanofibers: Enhanced Storage Stability and Antibacterial Property for Geraniol and Linalool. Food Bioprocess Technol. 2025, 18, 1296–1310. [Google Scholar] [CrossRef]
- Kumar, B.; Agumba, D.O.; Pham, D.H.; Kim, H.C.; Kim, J. Recent progress in bio-based eugenol resins: From synthetic strategies to structural properties and coating applications. J. Appl. Polym. Sci. 2022, 139, 51532. [Google Scholar] [CrossRef]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eugenol-containing essential oils loaded onto chitosan/polyvinyl alcohol blended films and their ability to eradicate Staphylococcus aureus or Pseudomonas aeruginosa from infected microenvironments. Pharmaceutics 2021, 13, 195. [Google Scholar] [CrossRef]
- Maslii, Y.; Herbina, N.; Dene, L.; Ivanauskas, L.; Matulis, G.; Bernatoniene, J. Mucoadhesive polymeric film with plant-based compounds for dental applications: Formulation, characterization and evaluation. Pharm. Dev. Technol. 2025, 30, 505–520. [Google Scholar] [CrossRef]
- Sathianarayanan, M.P.; Bhat, N.V.; Kokate, S.S.; Walunj, V.E. Antibacterial finish for cotton fabric from herbal products. Indian J. Fibre Text. Res. 2010, 35, 50–58. [Google Scholar]
- Gressier, P.; De Smet, D.; Behary, N.; Campagne, C.; Vanneste, M. Antibacterial polyester fabrics via diffusion process using active bio-based agents from essential oils. Ind. Crops Prod. 2019, 136, 11–20. [Google Scholar] [CrossRef]
- Nostro, A.; Scaffaro, R.; D’Arrigo, M.; Botta, L.; Filocamo, A.; Marino, A.; Bisignano, G. Development and characterization of essential oil component-based polymer films: A potential approach to reduce bacterial biofilm. Appl. Microbiol. Biotechnol. 2013, 97, 9515–9523. [Google Scholar] [CrossRef]
- Akgün, M.; Başaran, İ.; Suner, S.C.; Oral, A. Geraniol and cinnamaldehyde as natural antibacterial additives for poly(lactic acid) and their plasticizing effects. J. Polym. Eng. 2019, 40, 38–48. [Google Scholar] [CrossRef]
- Piłat, E.; Gnatowski, P.; Kurdyn, A.; Cieśliński, H.; Augustin, E.; Kucińska-Lipka, J. Investigation of bioprintable modified agar-based hydrogels with antimicrobial properties. Int. J. Biol. Macromol. 2025, 289, 138707. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Bonvin, D.; Todic, J.; Zivkovic, R.; Randjelovic, M.; Arsenijevic, V.A.; Ebersold, M.M.; Otasevic, S. Surface modification of poly(methyl-methacrylate) with farnesol to prevent Candida biofilm formation. Lett. Appl. Microbiol. 2022, 75, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Išljamović, M.; Bonvin, D.; Milojević, M.; Stojanović, S.; Spasić, M.; Stojković, B.; Janošević, P.; Otašević, S.; Ebersold, M.M. Antifungal Effect of Poly(methyl methacrylate) with Farnesol and Undecylenic Acid against Candida albicans Biofilm Formation. Materials 2024, 17, 3936. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Agrawal, A.; Knabe, C.; Ducheyne, P. Sol gel silica controlled release thin films for the inhibition of methicillin-resistant Staphylococcus aureus. Biomaterials 2014, 35, 509–517. [Google Scholar] [CrossRef]
- Gomathi, M.; Deepa, N.; Muraleedharan, A.; Maheswari, S.U.; Thirumalaisamy, R.; Selvankumar, T.; Chinnathambi, A.; Alharbi, S.A. Novel drug delivery materials: Chitosan polymers conjugated with Spondias pinnata phytocompounds for enhanced anti-microbial and anti-cancer properties. Polym. Adv. Technol. 2024, 35, e6561. [Google Scholar] [CrossRef]
- Durgadevi, R.; Kaleeshwari, R.; Swetha, T.K.; Alexpandi, R.; Karutha Pandian, S.; Veera Ravi, A. Attenuation of Proteus mirabilis colonization and swarming motility on indwelling urinary catheter by antibiofilm impregnation: An in vitro study. Colloids Surf. B Biointerf. 2020, 194, 111207. [Google Scholar] [CrossRef] [PubMed]
- Urbankova, M.; Hrabalikova, M.; Poljansek, I.; Miskolczi, N.; Sedlarik, V. Antibacterial polymer composites based on low-density polyethylene and essential oils immobilized on various solid carriers. J. Appl. Polym. Sci. 2015, 132, 42816. [Google Scholar] [CrossRef]
- Morales-Cerrada, R.; Molina-Gutierrez, S.; Lacroix-Desmazes, P.; Caillol, S. Eugenol, a Promising Building Block for Biobased Polymers with Cutting-Edge Properties. Biomacromolecules 2021, 22, 3625–3648. [Google Scholar] [CrossRef]
- Kerosenewala, J.; Vaidya, P.; Ozarkar, V.; Shirapure, Y.; More, A.P. Eugenol: Extraction, properties and its applications on incorporation with polymers and resins—A review. Polym. Bull. 2023, 80, 7047–7099. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, X.; Chen, X.; Li, J.; Huang, Q.; Hu, Z.; Tu, Y. Self-healing polymers based on eugenol via combination of thiol-ene and thiol oxidation reactions. J. Polym. Res. 2016, 23, 110. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Cai, X.; Yang, G.; Sun, X.S. Eugenol-Derived Molecular Glass: A Promising Biobased Material in the Design of Self-Healing Polymeric Materials. ACS Sustain. Chem. Eng. 2020, 8, 3553–3560. [Google Scholar] [CrossRef]
- Nowacka, M.; Kowalewska, A.; Rygala, A.; Kregiel, D.; Kaczorowski, W. Hybrid Bio-Based Silicone Coatings with Anti-adhesive Properties. Materials 2023, 16, 1381. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Ge, X.; Liang, W.; Liang, R.; Pang, X.; Liu, R.; Wen, S.; Sun, J.; Chen, X.; Ge, J. A Simple Preparation Route for Bio-Phenol MQ Silicone Resin via the Hydrosilylation Method and its Autonomic Antibacterial Property. Polymers 2019, 11, 1389. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.; Li, J. Antibacterial Nanoparticles with Universal Adhesion Function Based on Dopamine and Eugenol. J. Biores. Bioprod. 2019, 4, 177–182. [Google Scholar] [CrossRef]
- Di Consiglio, M.; Sturabotti, E.; Brugnoli, B.; Piozzi, A.; Migneco, L.M.; Francolini, I. Synthesis of sustainable eugenol/hydroxyethylmethacrylate-based polymers with antioxidant and antimicrobial properties. Polym. Chem. 2023, 14, 432–442. [Google Scholar] [CrossRef]
- Gazzotti, S.; Todisco, S.A.; Picozzi, C.; Ortenzi, M.A.; Farina, H.; Lesma, G.; Silvani, A. Eugenol-grafted aliphatic polyesters: Towards inherently antimicrobial PLA-based materials exploiting OCAs chemistry. Eur. Polym. J. 2019, 114, 369–379. [Google Scholar] [CrossRef]
- Naddeo, M.; Vigliotta, G.; Pellecchia, C.; Pappalardo, D. Synthesis of bio-based polymacrolactones with pendant eugenol moieties as novel antimicrobial thermoplastic materials. React. Funct. Polym. 2020, 155, 104714. [Google Scholar] [CrossRef]
- Xu, H.; Ma, H.; Guo, G.; Wei, C.; Zhang, X.; Guo, M.; Li, Y. Research progress on chitosan-based sutures: A review. Carbohydr. Polym. 2025, 366, 123868. [Google Scholar] [CrossRef]
- Yue, L.; Li, J.; Chen, W.; Liu, X.; Jiang, Q.; Xia, W. Geraniol grafted chitosan oligosaccharide as a potential antibacterial agent. Carbohydr. Polym. 2017, 176, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Pang, C.; Wei, H.; Hong, L. Citronellol-Based Long-Lasting Antibacterial Cotton Fabrics without Bacterial Resistance. Macromol. Biosci. 2023, 23, 2300169. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.-H. Synergistic antioxidant and antibacterial advantages of essential oils for food packaging applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Sarkar, T.; Chakraborty, R. Critical Review on Nutritional, Bioactive, and Medicinal Potential of Spices and Herbs and Their Application in Food Fortification and Nanotechnology. Appl. Biochem. Biotechnol. 2023, 195, 1319–1513. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Oliveira Ribeiro, S.; Fontaine, V.; Mathieu, V.; Zhiri, A.; Baudoux, D.; Stévigny, C.; Souard, F. Antibacterial and Cytotoxic Activities of Ten Commercially Available Essential Oils. Antibiotics 2020, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Lee, J.; Park, M.; Kim, J.; Yang, J.; Yoo, Y.; Jeung, E. Cytostatic effects of plant essential oils on human skin and lung cells. Exp. Ther. Med. 2020, 19, 2008–2018. [Google Scholar] [CrossRef]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef]
- Moshe Dvir, I.; Weizman, O.; Weintraub, S.; Ophir, A.; Dotan, A. Antimicrobial active packaging combining essential oils mixture: Migration and odor control study. Polym. Adv. Technol. 2019, 30, 2558–2566. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. Npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Dallaev, R.; Papež, N.; Allaham, M.M.; Holcman, V. Biodegradable Polymers: Properties, Applications, and Environmental Impact. Polymers 2025, 17, 1981. [Google Scholar] [CrossRef] [PubMed]
- Petchwattana, N.; Naknaen, P.; Cha-Aim, K.; Sanetuntikul, J. Application of antimicrobial plates in food packaging as an alternative way for food waste minimisation. Int. J. Sustain. Eng. 2021, 14, 600–608. [Google Scholar] [CrossRef]
- Petchwattana, N.; Naknaen, P.; Cha-aim, K.; Suksri, C.; Sanetuntikul, J. Controlled release antimicrobial sachet prepared from poly(butylene succinate)/geraniol and ethylene vinyl alcohol coated paper for bread shelf-life extension application. Int. J. Biol. Macromol. 2021, 189, 251–261. [Google Scholar] [CrossRef]
- Pleva, P.; Bartošová, L.; Máčalová, D.; Zálešáková, L.; Sedlaříková, J.; Janalíková, M. Biofilm Formation Reduction by Eugenol and Thymol on Biodegradable Food Packaging Material. Foods 2022, 11, 2. [Google Scholar] [CrossRef]
- Wongphan, P.; Nampanya, P.; Chakpha, W.; Promhuad, K.; Laorenza, Y.; Leelaphiwat, P.; Bumbudsanpharoke, N.; Sodsai, J.; Lorenzo, J.M.; Harnkarnsujarit, N. Lesser galangal (Alpinia officinarum Hance) essential oil incorporated biodegradable PLA/PBS films as shelf-life extension packaging of cooked rice. Food Packag. Shelf Life 2023, 37, 101077. [Google Scholar] [CrossRef]
- Ahmed, J.; Bher, A.; Auras, R. Microstructural, mechanical, thermo-rheological, barrier, and antimicrobial properties of coextruded tri-layer polylactide/encapsulated geraniol/polylactide-graphene nanoplatelets films. Polym. Adv. Technol. 2024, 35, e6488. [Google Scholar] [CrossRef]
- Krebs de Souza, C.; Ghosh, T.; Lukhmana, N.; Tahiliani, S.; Priyadarshi, R.; Hoffman, T.G.; Purohit, S.D.; Han, S.S. Pullulan as a sustainable biopolymer for versatile applications: A review. Mater. Today Commun. 2023, 36, 106477. [Google Scholar] [CrossRef]
- Simões, A.; Ramos, A.; Domingues, F.; Luís, Â. Pullulan-Tween 40 emulsified films containing geraniol: Production and characterization as potential food packaging materials. Eur. Food Res. Technol. 2024, 250, 1721–1732. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Antimicrobial Activity of Natural Agents Coated on Starch-Based Films against Staphylococcus aureus. J. Food. Sci. 2011, 76, M531–M537. [Google Scholar] [CrossRef]
- Yu, H.; Huang, X.; Zhou, L.; Wang, Y. Incorporation of cinnamaldehyde, carvacrol, and eugenol into zein films for active food packaging: Enhanced mechanical properties, antimicrobial activity, and controlled release. J. Food Sci. Technol. 2023, 60, 2846–2857. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ge, X.; Zhou, L.; Wang, Y. Eugenol embedded zein and poly(lactic acid) film as active food packaging: Formation, characterization, and antimicrobial effects. Food Chem. 2022, 384, 132482. [Google Scholar] [CrossRef]
- Reyes Méndez, L.M.; Méndez Morales, P.A.; López-Córdoba, A.; Ortega-Toro, R.; Gutiérrez, T.J. Active chitosan/gelatin-based films and coatings containing eugenol and oregano essential oil for fresh cheese preservation. J. Food Process Eng. 2023, 46, e14396. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; El-Nouby, M.A.M.; Ismail, R.I.A.; Taktak, N.E.M. Strawberry Shelf Life, Composition, and Enzymes Activity in Response to Edible Chitosan Coatings. Int. J. Fruit Sci. 2017, 17, 117–136. [Google Scholar] [CrossRef]
- Lv, N.; Zhao, M.; Hao, L.; Zhou, X.; Chen, H.; Zhou, H. Eugenol and carboxymethyl cellulose derived nanocoating with insect repellent and long-term antibacterial activity. Ind. Crops Prod. 2022, 190, 115902. [Google Scholar] [CrossRef]
- Thivya, P.; Bhanu Prakash Reddy, N.; Sinija, V.R. Studies on unlocking the potential of onion waste extracts in edible coating for shelf-life extension of strawberry. J. Food Sci. Technol. 2025, 62, 667–679. [Google Scholar] [CrossRef]
- Jayakumar, G.C.; Usharani, N.; Kawakami, K.; Rao, J.R.; Nair, B.U. Studies on the physico-chemical characteristics of collagen–pectin composites. RSC Adv. 2014, 4, 63840–63849. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Alhadhrami, A.S.; Shah, Y.A.; Kotta, S.; Iqbal, J.; Anwer, M.K.; Nair, A.K.; Koca, E.; Aydemir, L.Y. Physical, Chemical, Barrier, and Antioxidant Properties of Pectin/Collagen Hydrogel-Based Films Enriched with Melissa officinalis. Gels 2023, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Filho, J.G.D.; Duarte, L.G.R.; Belo, L.; Cordeiro de Souza, T.F.; Bitencourt, A.H.; Yamim, S.T.; Egea, M.B. Recent advances in kefiran polymer to produce nanofibers and films for food packaging applications. Food Humanit. 2025, 4, 100603. [Google Scholar] [CrossRef]
- Khan, M.R.; Volpe, S.; Valentino, M.; Miele, N.A.; Cavella, S.; Torrieri, E. Active Casein Coatings and Films for Perishable Foods: Structural Properties and Shelf-Life Extension. Coatings 2021, 11, 899. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Dehghannya, J. Development and characterization of kefiran-gelatin bio-nanocomposites containing Zhumeria majdae essential oil nanoemulsion to use as active food packaging in sponge cakes. Int. J. Biol. Macromol. 2024, 279, 135120. [Google Scholar] [CrossRef]
- Bhatia, S.; Shah, Y.A.; Al-Harrasi, A.; Jawad, M.; Dıblan, S.; Khan, T.S.; Koca, E.; Aydemir, L.Y. Gelatin/calcium-caseinate films loaded with petitgrain essential oil for sustainable food packaging. Int. J. Food Sci. Technol. 2024, 59, 2430–2445. [Google Scholar] [CrossRef]
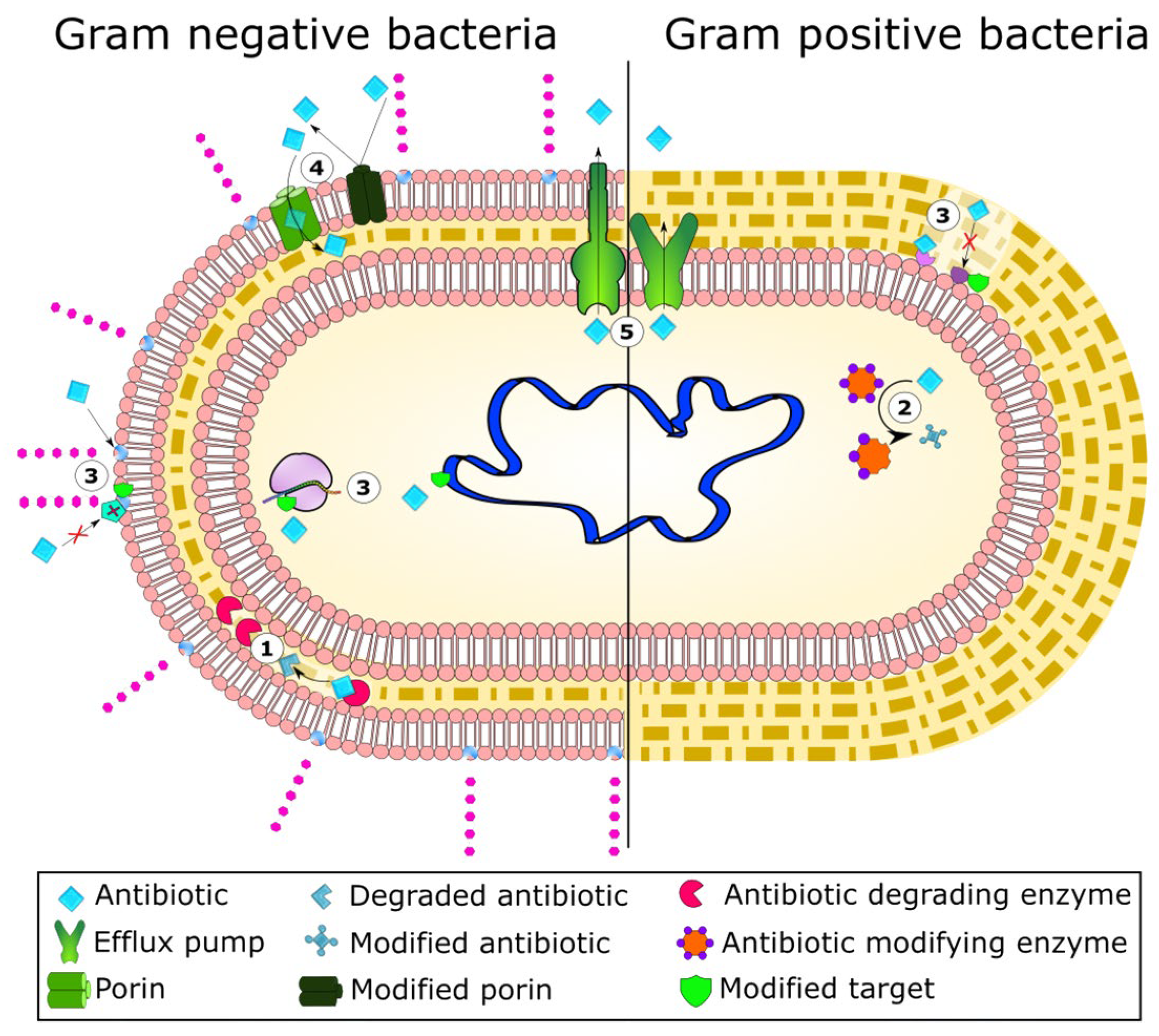
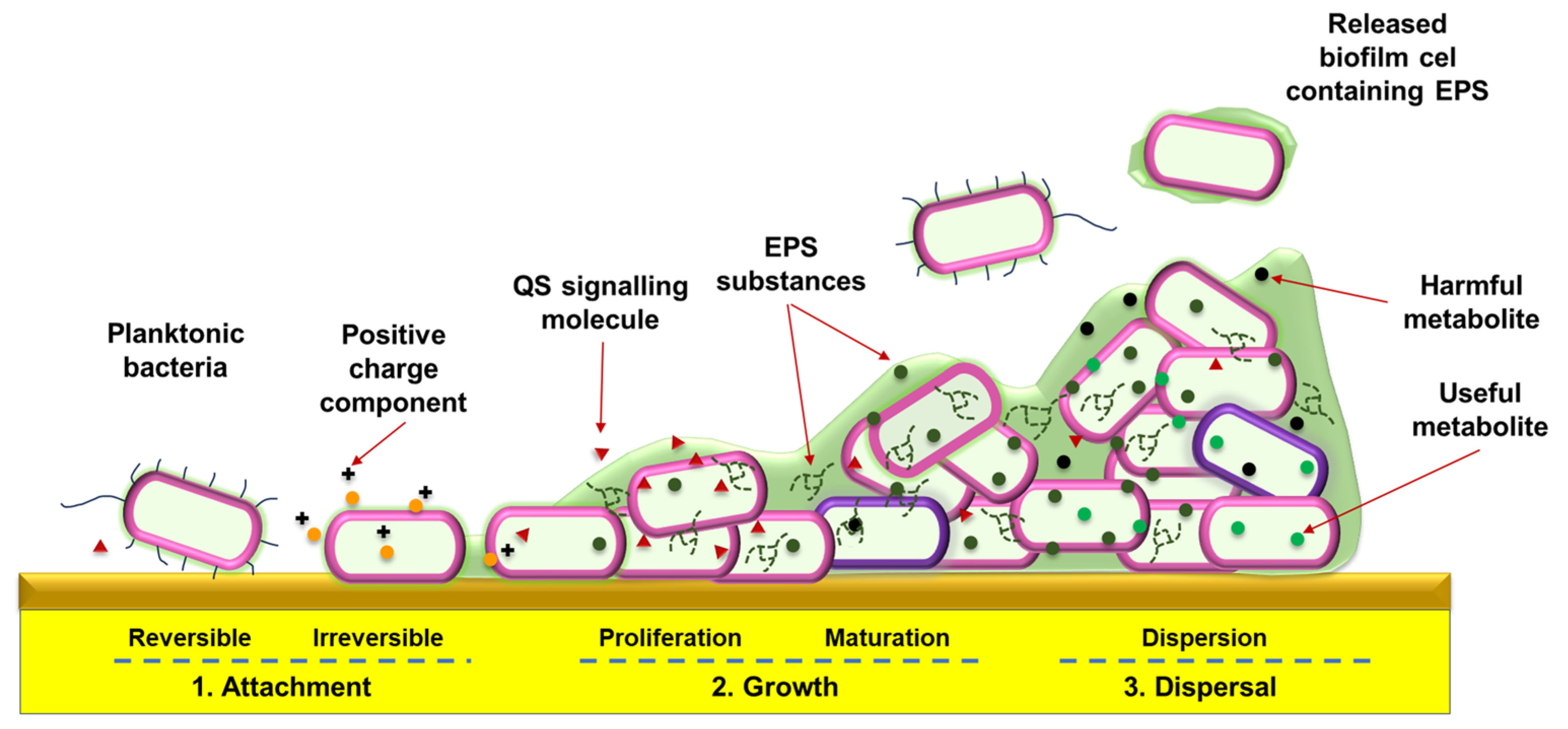

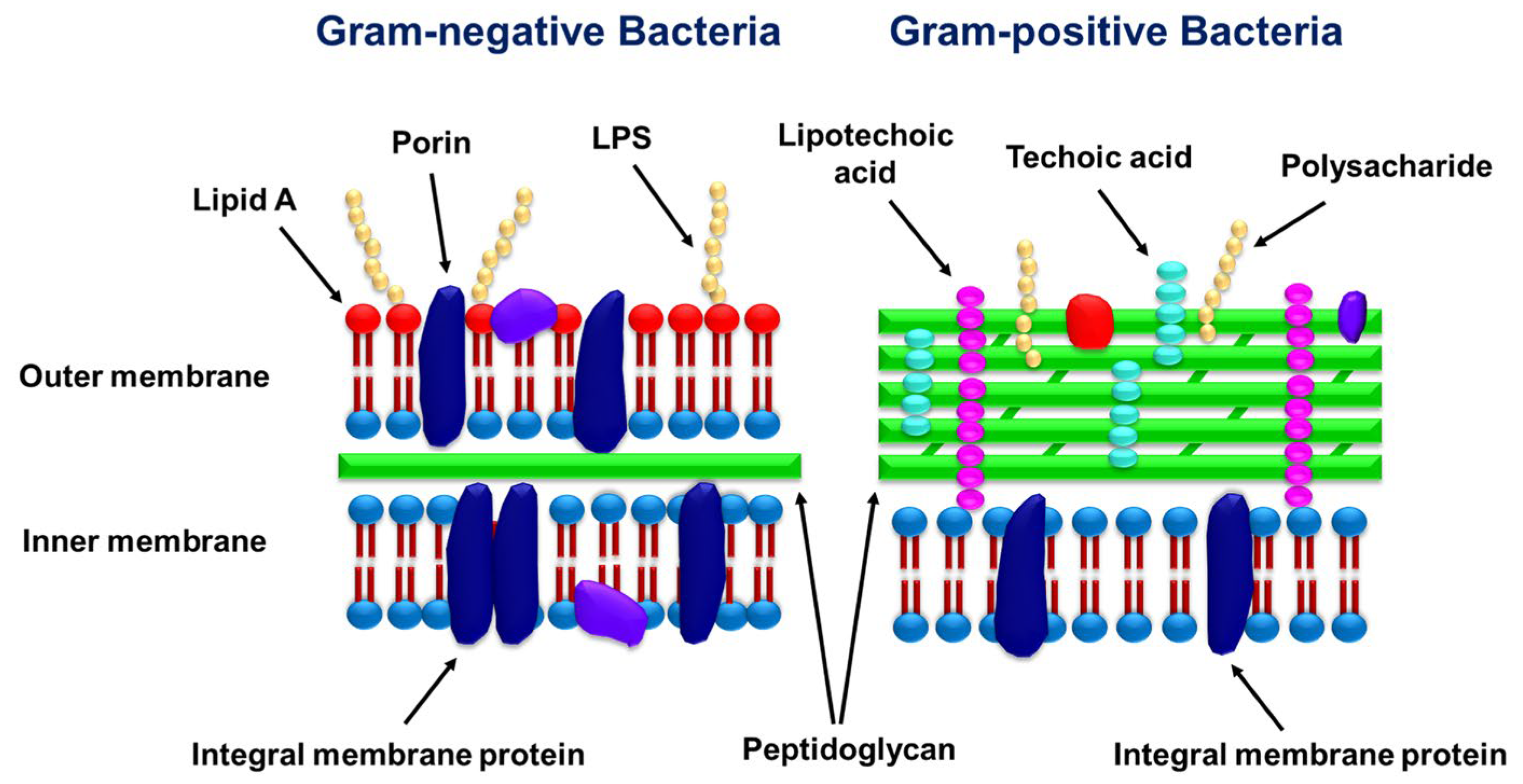
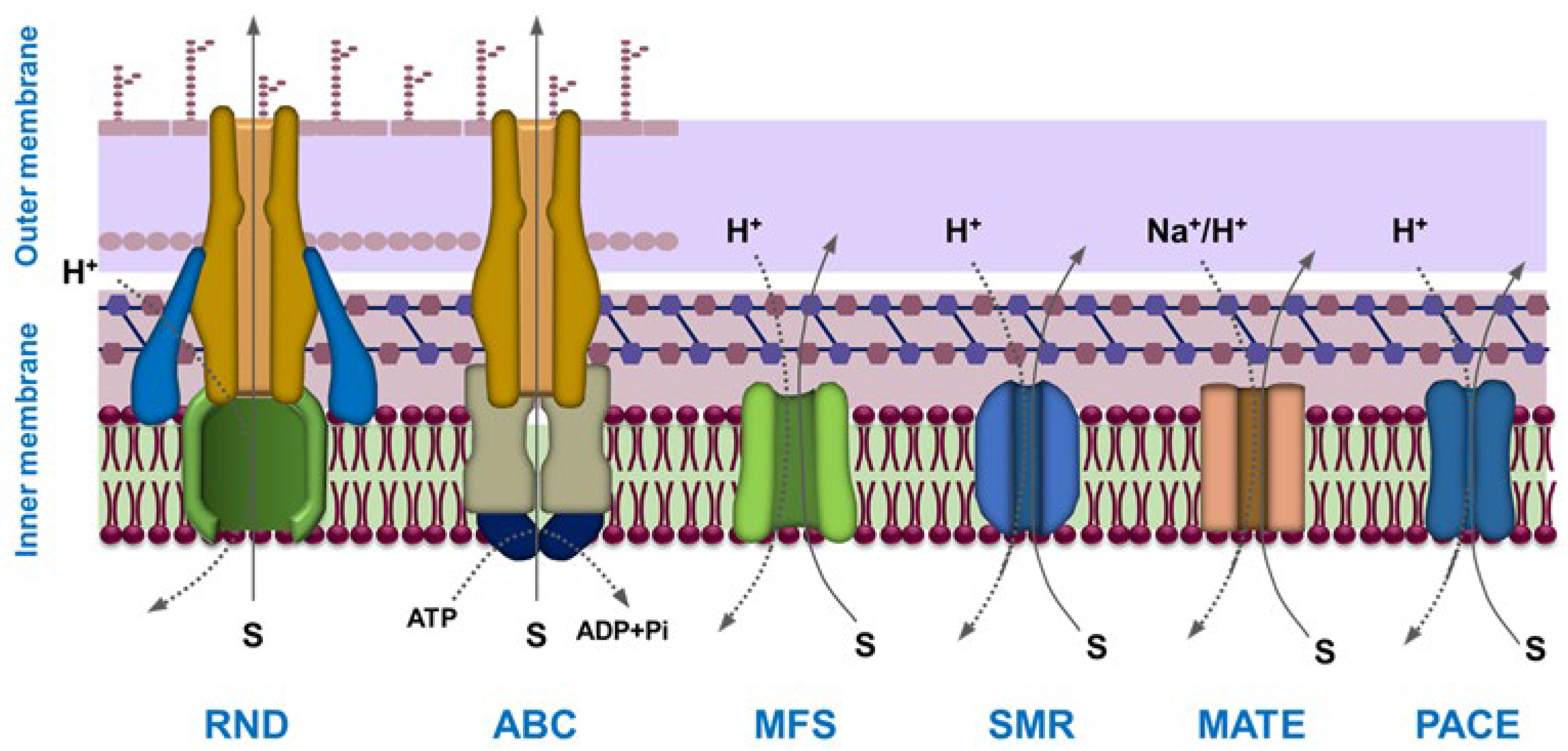

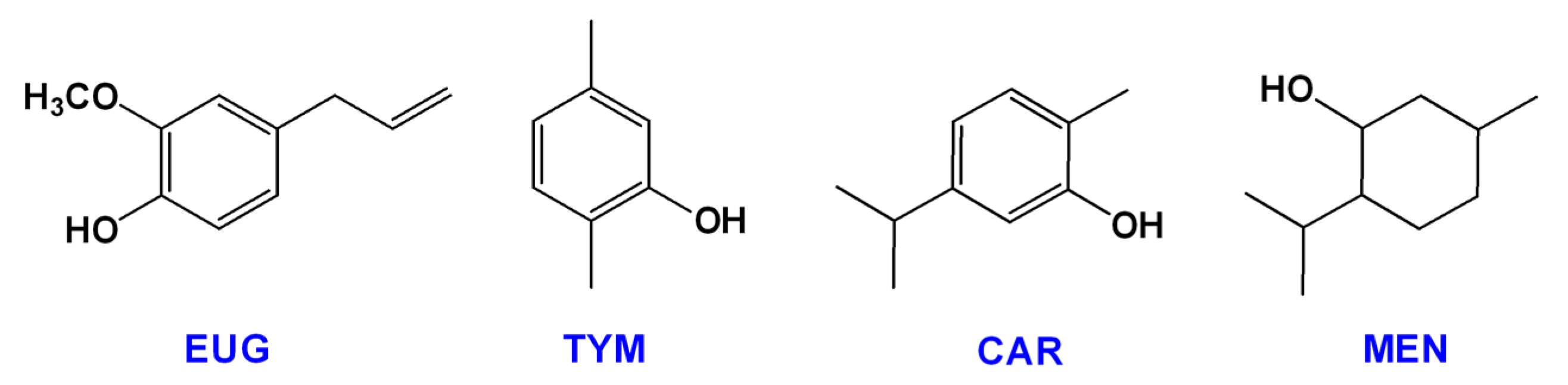
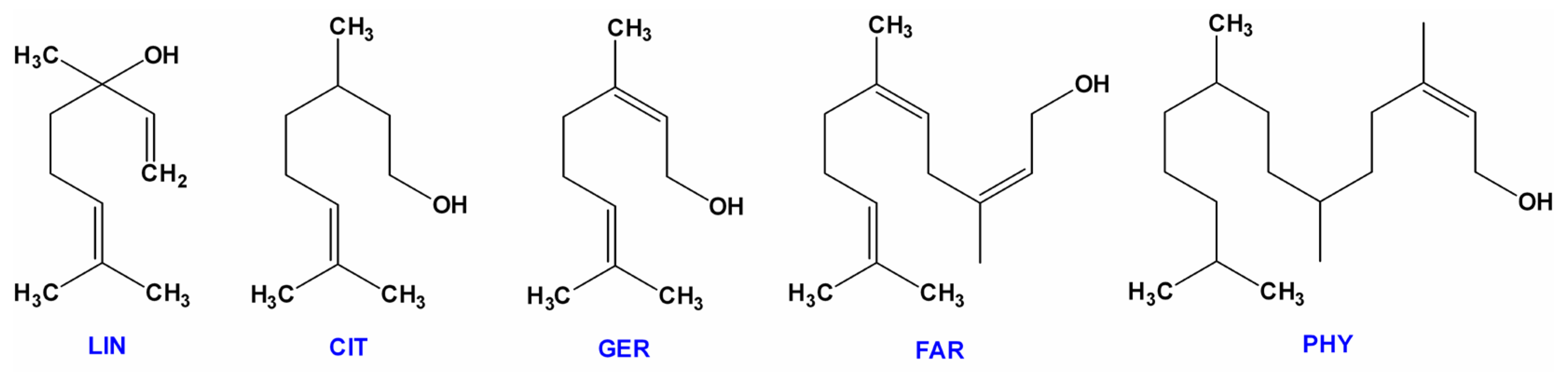
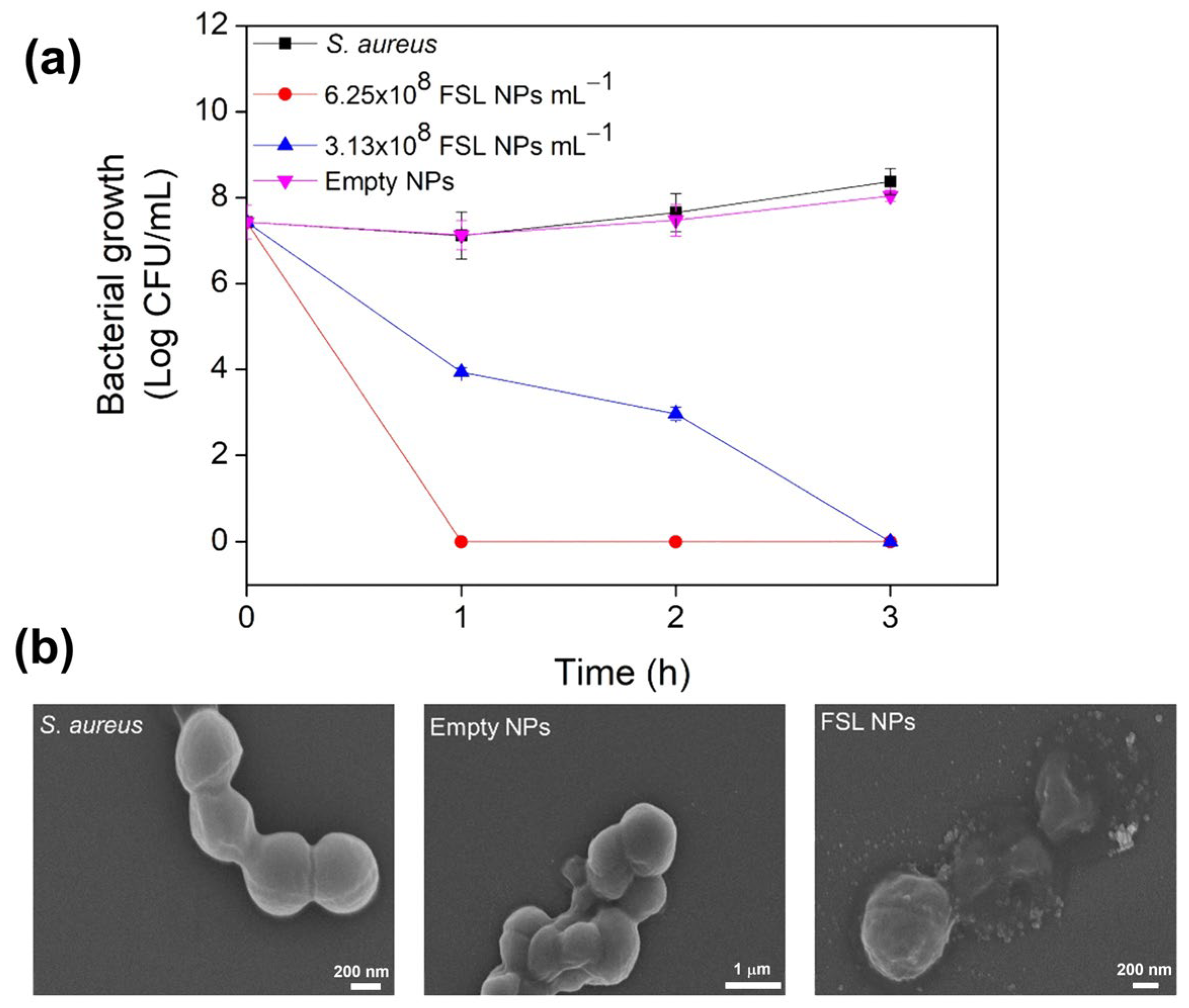
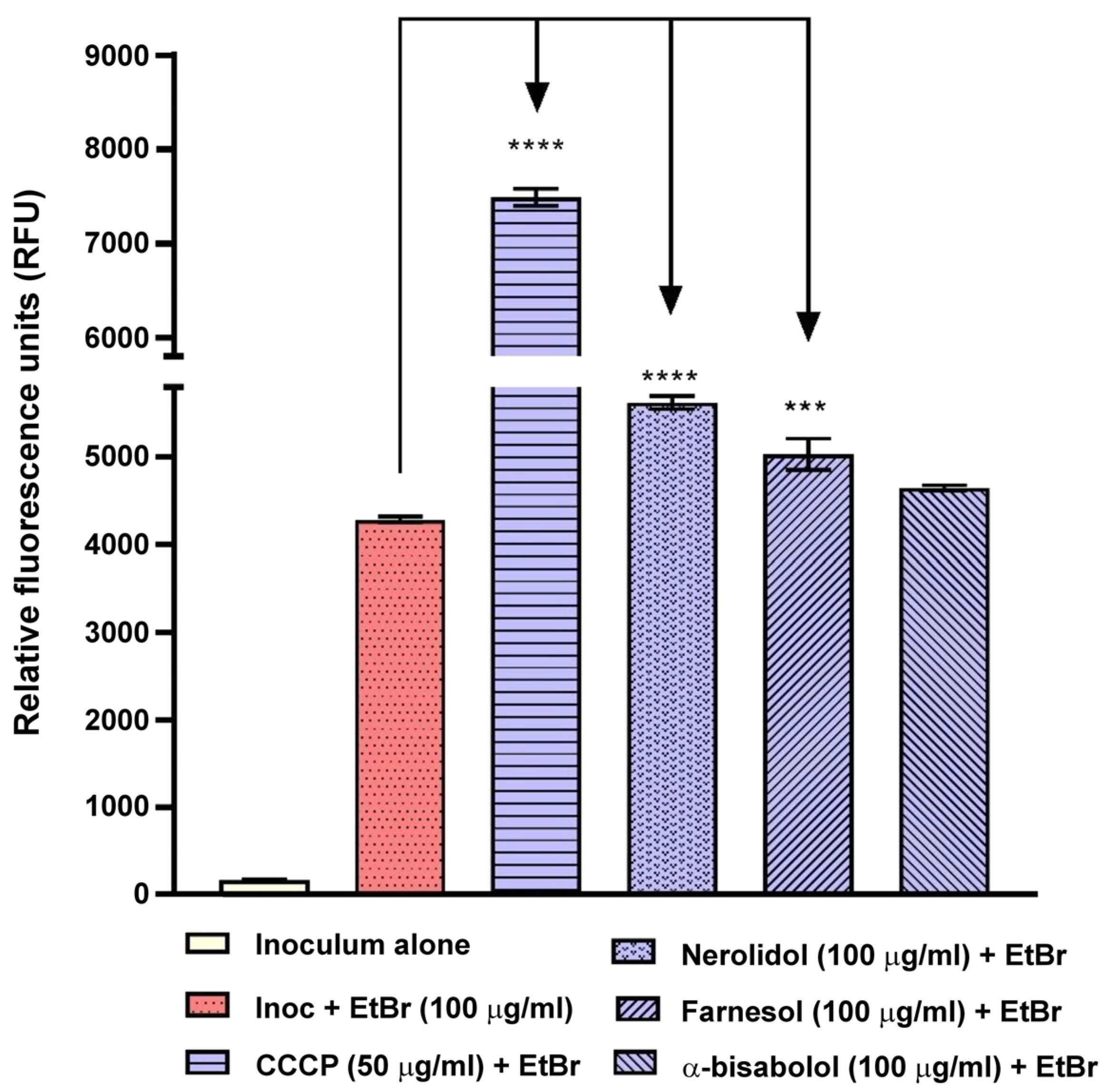
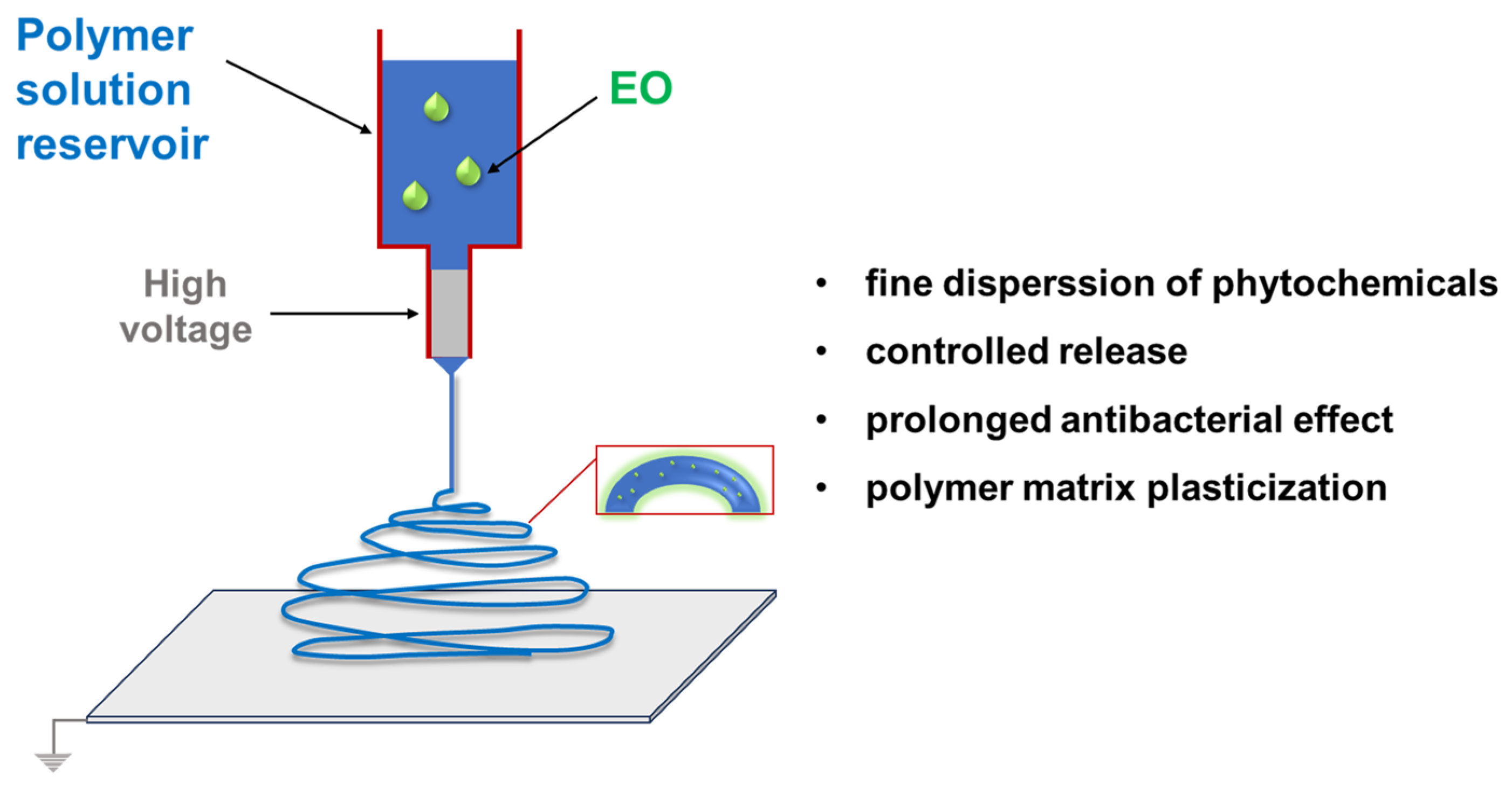
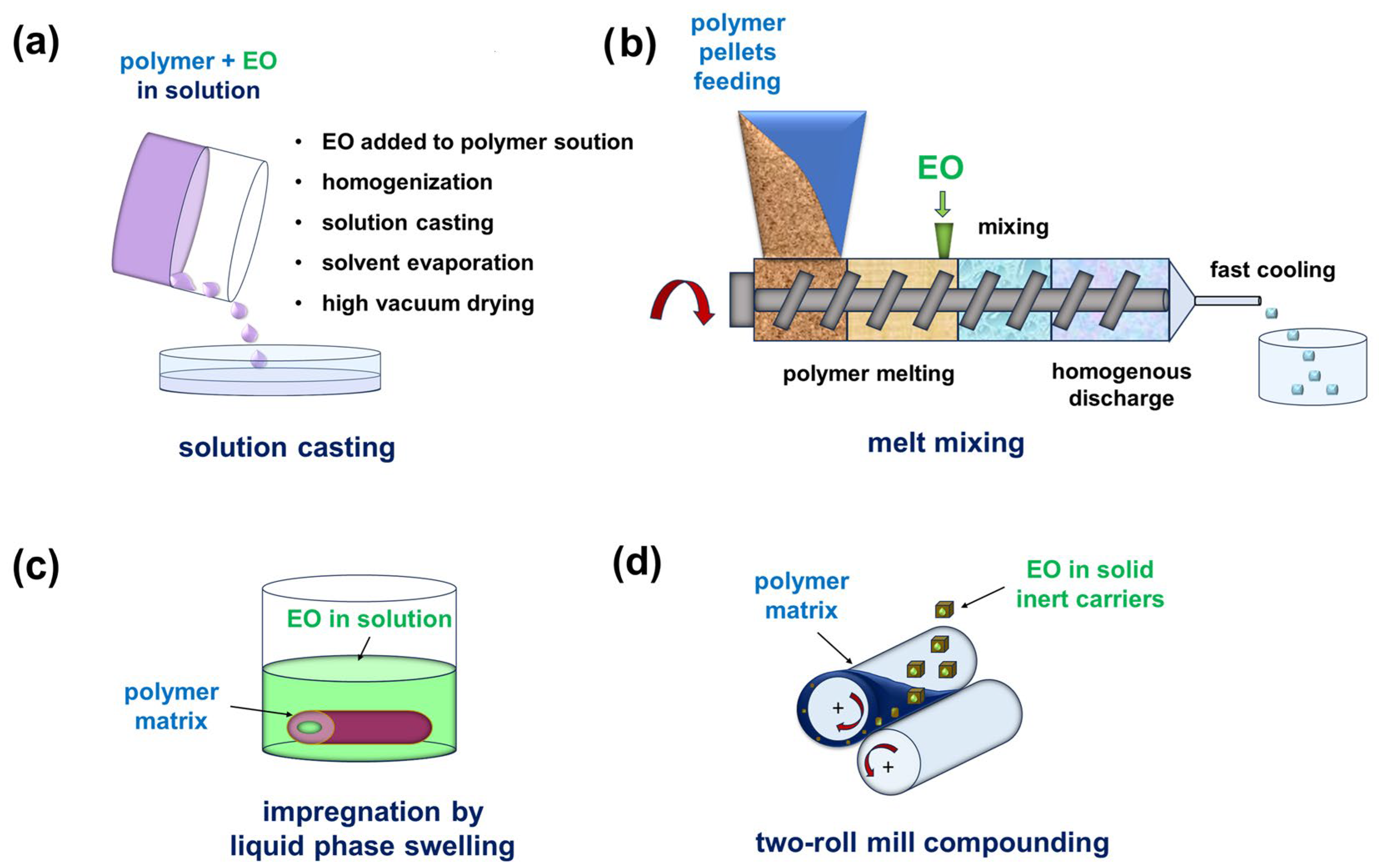
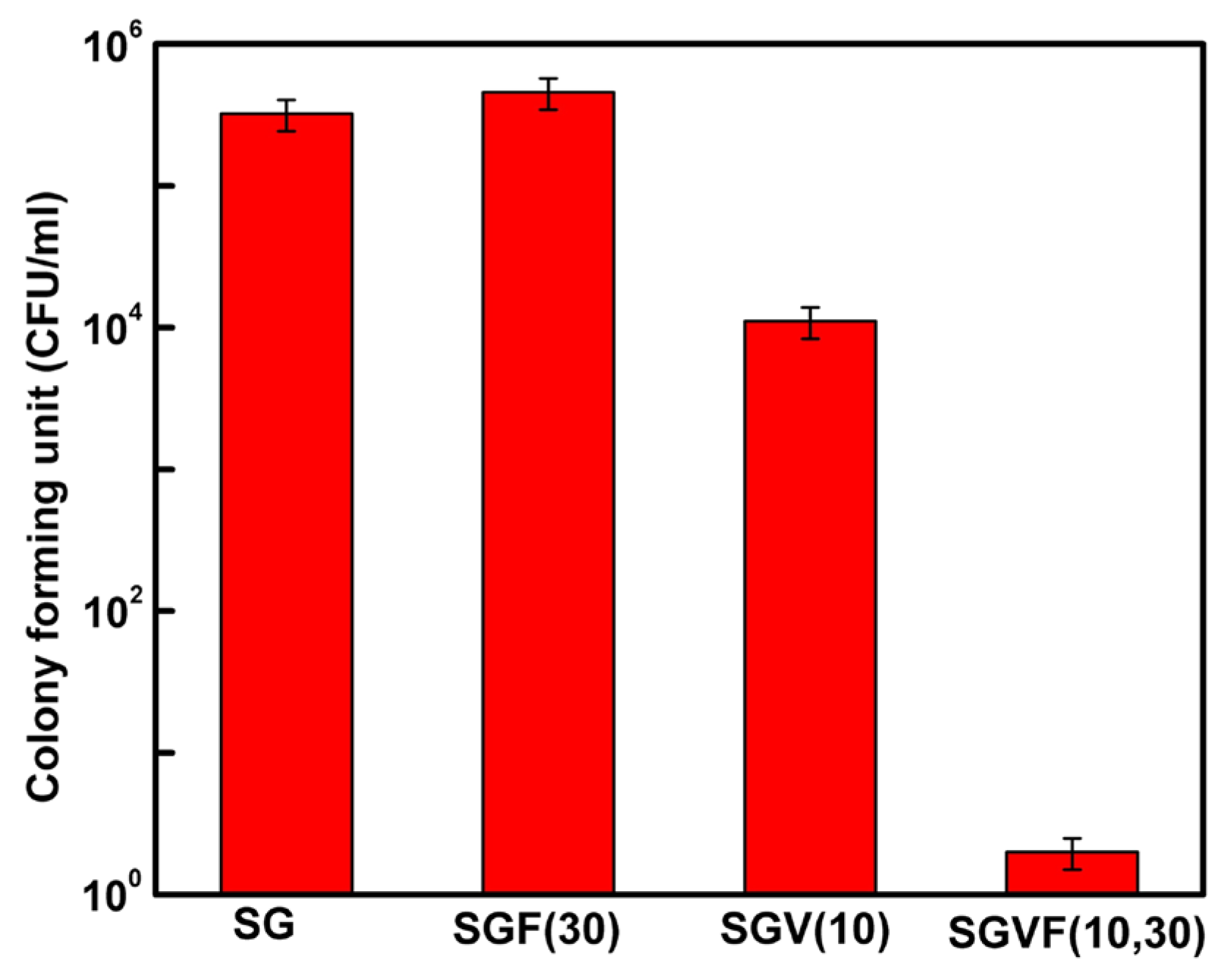


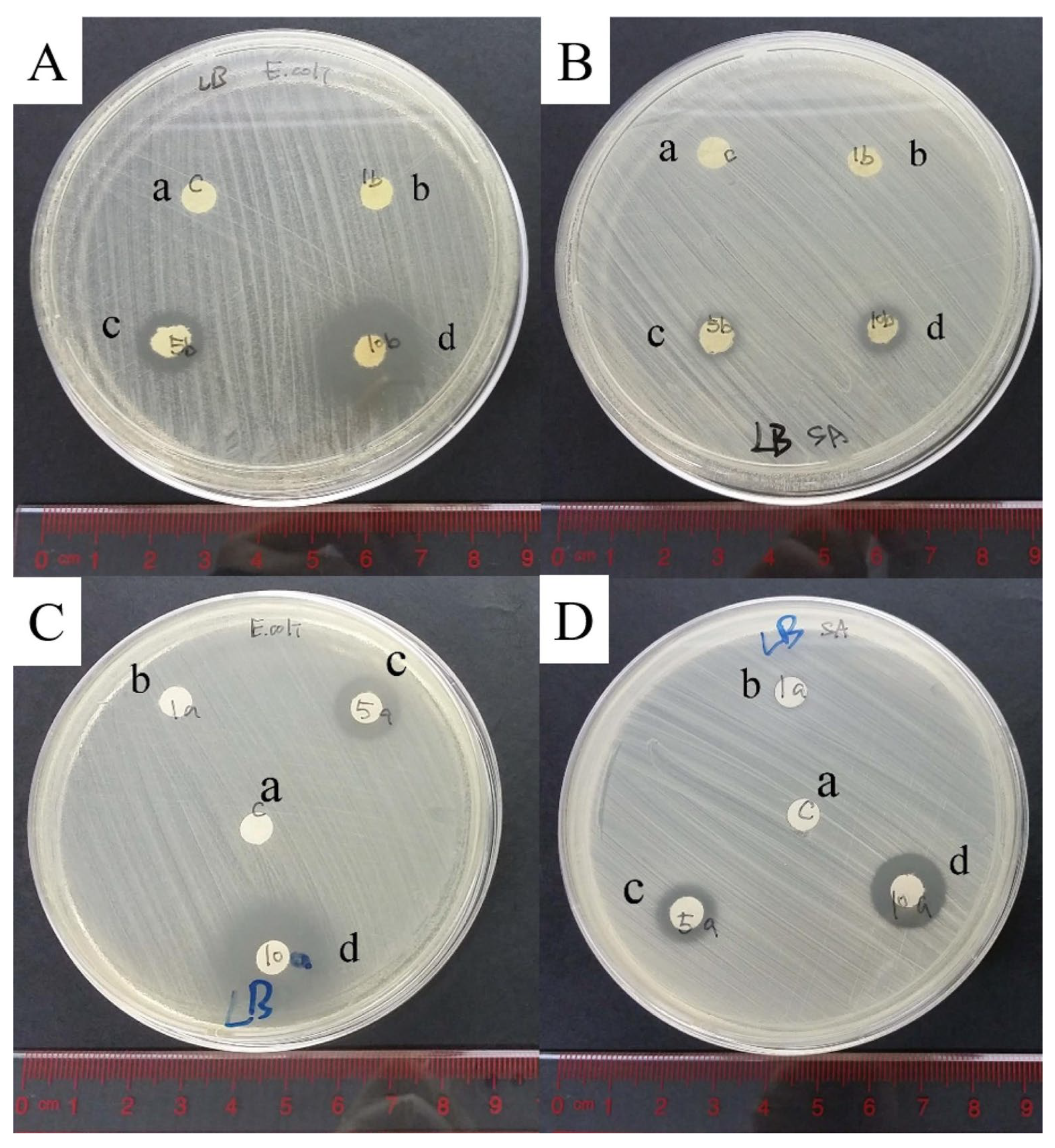
| Name | Type * | Advantages | Ref. | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Eugenol | A | Active against both G+ and G- bacteria. | [38,44,45,46] | Masking phenolic group changes antimicrobial activity of EUG. | [38,44] |
| Eradication of planktonic cells of S. epidermidis and P. aeruginosa. | [39] | Less active against biofilms of S. epidermidis and P. aeruginosa. | [39] | ||
| Hinders quorum sensing in E. coli and S. aureus. | [48,54] | ||||
| Affects virulence and NorA efflux pump in S. aureus. | [49,52] | ||||
| Affects MexA and AcrA efflux pumps in P. aeruginosa and E. coli. | [53] | ||||
| Carvacrol | A | Active against both G+ and G- bacteria. | [38,44,45,46] | Less active against biofilms of S. epidermidis and P. aeruginosa. | [39] |
| Eradication of planktonic cells of S. epidermidis and P. aeruginosa. | [39] | Weaker effect on P. aeruginosa biofilms at sublethal concentrations. | [61] | ||
| Eradication of KPC-producing K. pneumoniae. | [59] | ||||
| Antibiofilm and antivirulence action against UPEC E. coli. | [60] | ||||
| S. aureus biofilm inhibition. | [61] | ||||
| Inhibiting/modulating biofilm formation in P. aeruginosa | [62] | ||||
| amplification of the action of antibiotics. | [64] | ||||
| Linalool | B | Cell membrane destruction and alteration of bacteria metabolism. | [68] | ||
| Inhibition of P. aeruginosa, A. hydrophila, and S. aureus bacterial growth. | [69,70,71,75,76] | ||||
| Eradication of E. coli. | [73] | ||||
| Hinders quorum sensing in A. baumannii and P. aeruginosa. | [72,74] | ||||
| Inhibition of B. amyloliquefaciens motility. | [77] | ||||
| Geraniol | B | Antibiotic activity against Streptococcus spp., Staphylococcus spp., and S. aureus (MRSA). | [80,81,82,83,84] | Less active against Staphylococci than citral and LIN. | [66] |
| Effective (almost as citral) against Enterococci. | [66] | ||||
| Bactericidal activity against E. coli and H. pylori. | [85,86] | ||||
| Inhibition of A. baumannii, S. epidermidis, E. carotovora. and P. fluorescens biofilm formation. | [55,81,87] | ||||
| Citronellol | B | Antimicrobial and antibiofilm action towards E. coli. | [89] | ||
| Antimicrobial action against E. coli, S. aureus, and Corynebacterium glutamicum bacterial strains. | [90] | ||||
| Inhibition of NorA efflux pump in S. aureus. | [91] | ||||
| Farnesol | C | Quorum-sensing molecule in Candida albicans biofilm (reactive oxygen species production, induction of cell apoptosis, and modulation of virulence factors). | [94] | Only E,E-isomer is active against C. albicans. | [95] |
| Sensitized S. aureus towards antibiotics (gentamycin and β-lactam antibiotic). | [96,97] | E,E-isomer acted only on G+ bacteria. | [96,97,98] | ||
| Ethanolic solutions of FAR-inhibited biofilms of S. aureus, P. aeruginosa, and S. epidermis. | [99,100] | Enhanced tolerance of S. aureus to some antimicrobials. | [101] | ||
| Phytol | D | Increased the level of ROS in P. aeruginosa. | [109] | Antagonistic interactions between PHY and norfloxacin in S. aureus. | [110] |
| Enhanced effects of norfloxacin against Salmonella spp. and E. coli. | [110,111] | ||||
| K. pneumoniae biofilm eradication. | [113] | ||||
| Inhibition of quorum sensing in P. fluorescens, P. aeruginosa, C. violaceum, Serratia marcescens, L. monocytogenes, S. enterica, and E. coli. | [114,116,117,118] | ||||
| Eradication of A. baumannii biofilm in combination with cefotaxime. | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska-Smolarek, K.; Kowalewska, A. Essential Oils as Green Antibacterial Modifiers of Polymeric Materials. Polymers 2025, 17, 2924. https://doi.org/10.3390/polym17212924
Majewska-Smolarek K, Kowalewska A. Essential Oils as Green Antibacterial Modifiers of Polymeric Materials. Polymers. 2025; 17(21):2924. https://doi.org/10.3390/polym17212924
Chicago/Turabian StyleMajewska-Smolarek, Kamila, and Anna Kowalewska. 2025. "Essential Oils as Green Antibacterial Modifiers of Polymeric Materials" Polymers 17, no. 21: 2924. https://doi.org/10.3390/polym17212924
APA StyleMajewska-Smolarek, K., & Kowalewska, A. (2025). Essential Oils as Green Antibacterial Modifiers of Polymeric Materials. Polymers, 17(21), 2924. https://doi.org/10.3390/polym17212924






