Thermal, Rheological, and Moisture Absorption Behaviours of Polyvinyl Alcohol (PVA)/Lignin Composites
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Manufacturing of PVA/Lignin Films
2.3. Characterisation
3. Results and Discussions
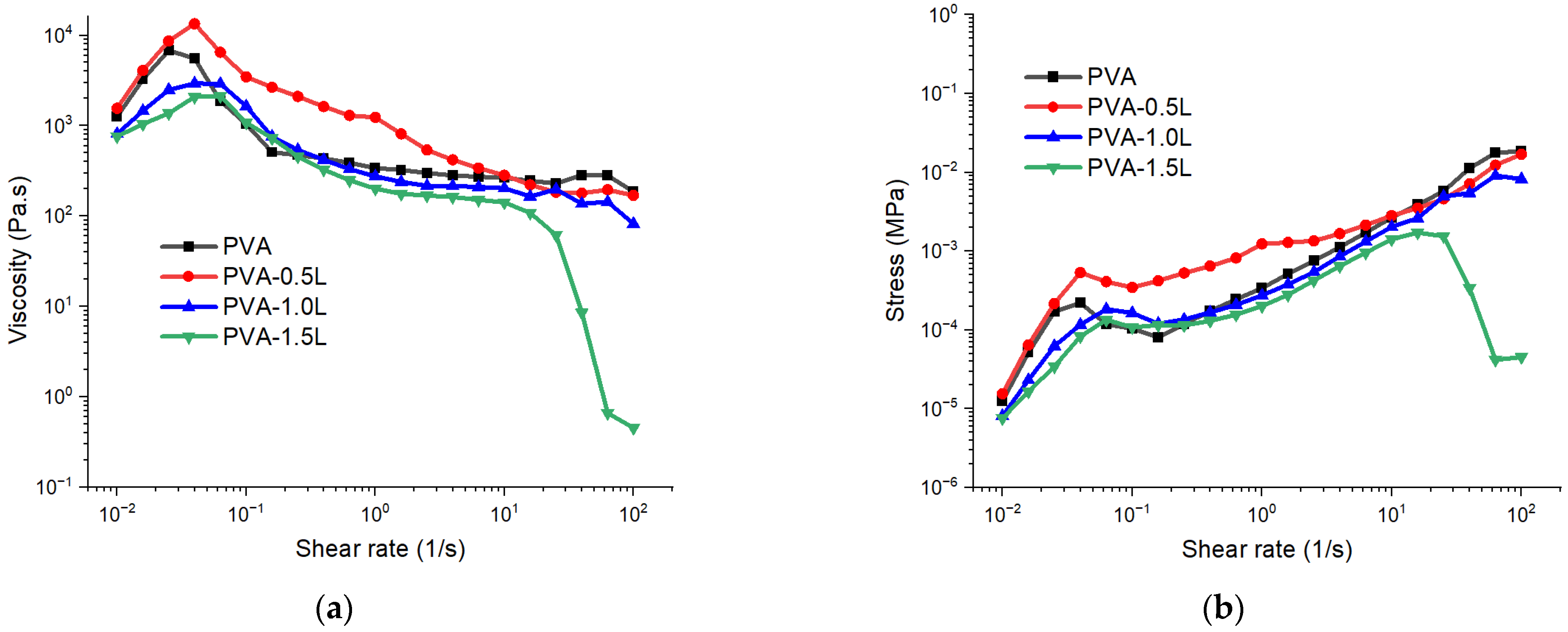
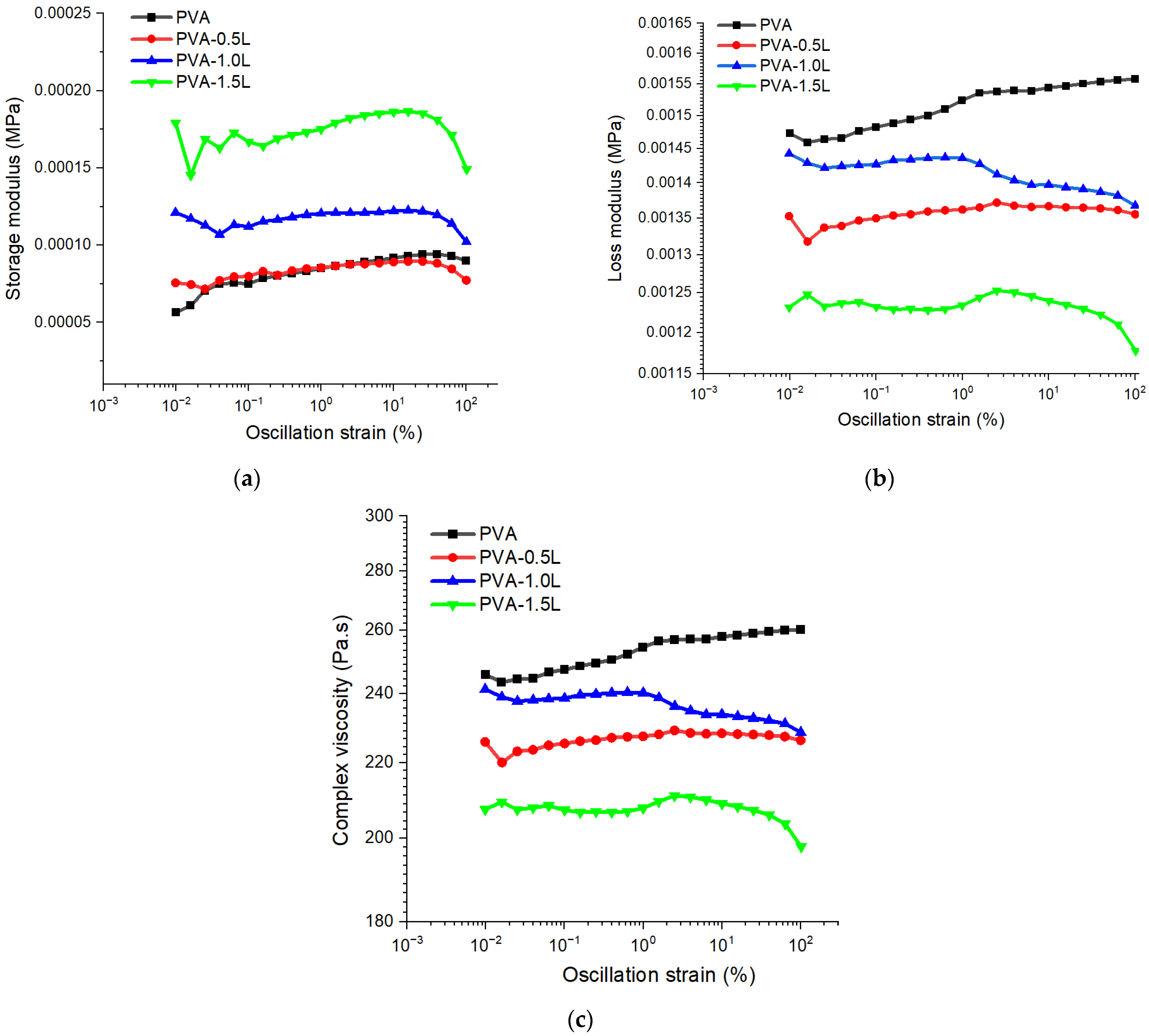
3.1. Rheological Test Results
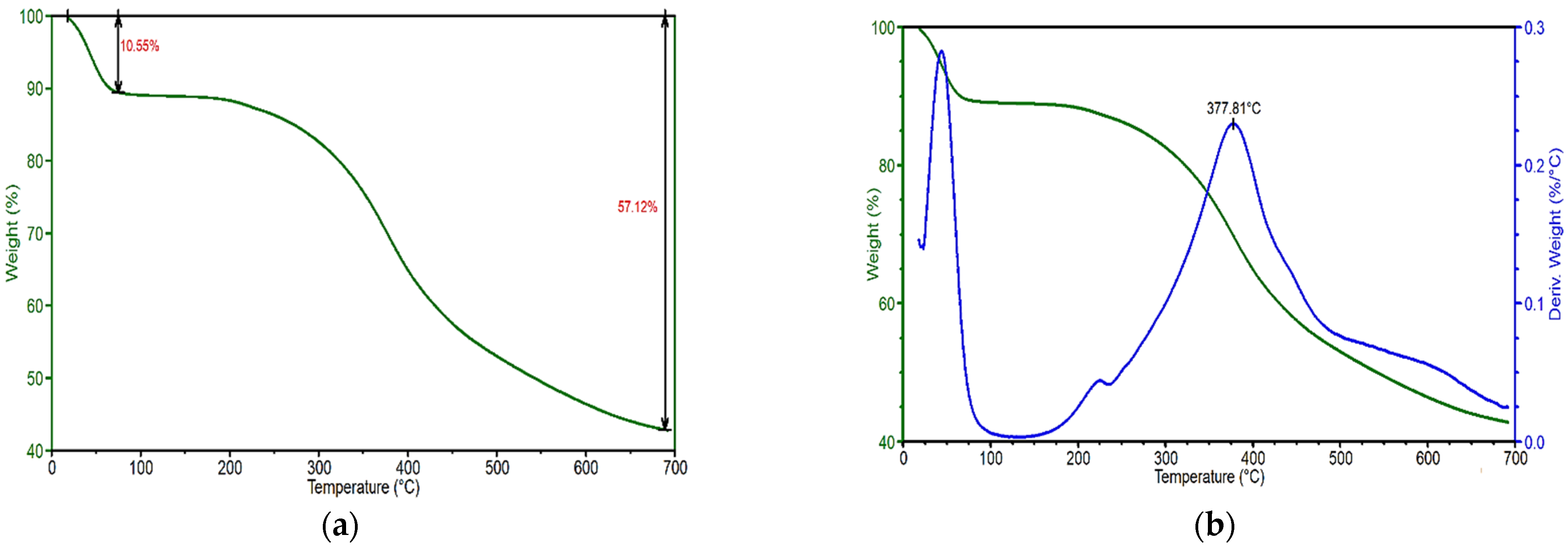
3.2. Thermal Test Results
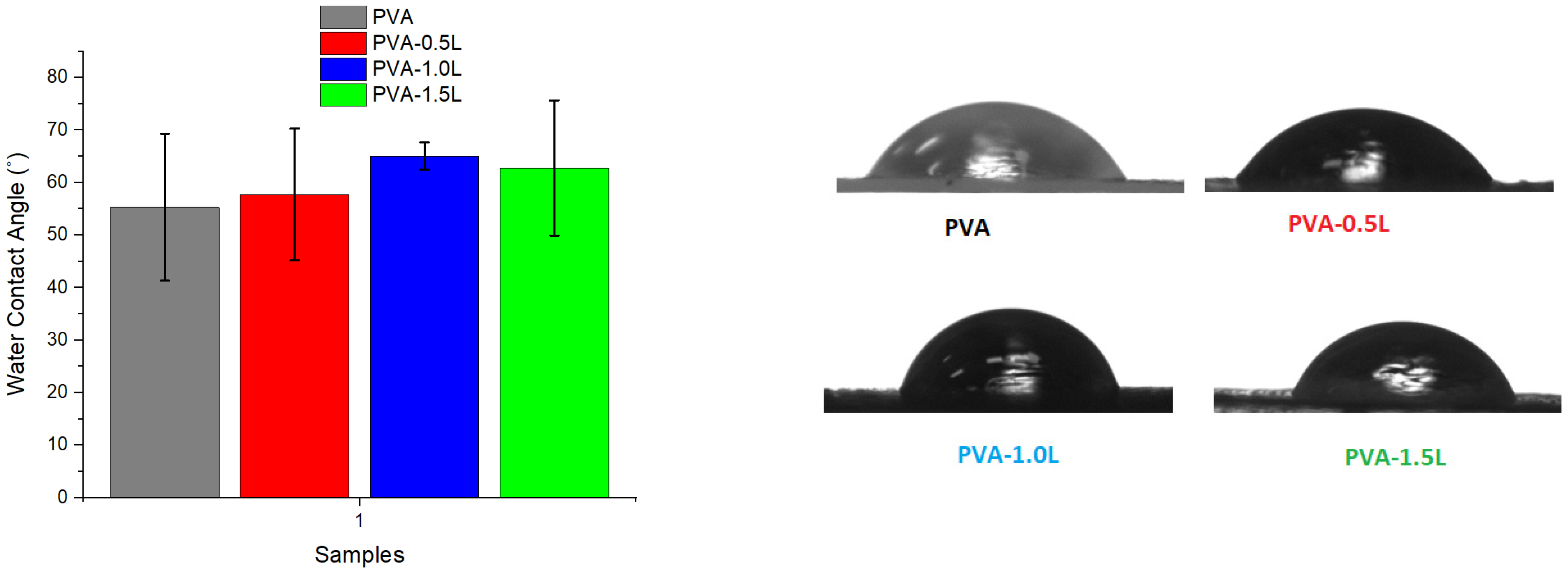
3.3. Humidity and Contact Angle Test Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelaziz, M.; Ghannam, M.M. Influence of titanium chloride addition on the optical and dielectric properties of PVA films. Phys. B Condens. Matter 2010, 405, 958–964. [Google Scholar] [CrossRef]
- Virtanen, S.; Vartianen, J.; Setälä, H.; Tammelin, T.; Vuoti, S. Modified nanofibrillated cellulose–polyvinyl alcohol films with improved mechanical performance. RSC Adv. 2014, 4, 11343–11350. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, X.; Li, C.; Liang, M.; Lu, C.; Deng, Y. Mechanochemical activation of cellulose and its thermoplastic polyvinyl alcohol ecocomposites with enhanced physicochemical properties. Carbohydr. Polym. 2011, 83, 257–263. [Google Scholar] [CrossRef]
- Mok, C.F.; Ching, Y.C.; Muhamad, F.; Abu Osman, N.A.; Hai, N.D.; Che Hassan, C.R. Adsorption of Dyes Using Poly(vinyl alcohol) (PVA) and PVA-Based Polymer Composite Adsorbents: A Review. J. Polym. Environ. 2020, 28, 775–793. [Google Scholar] [CrossRef]
- Jain, N.; Singh, V.K.; Chauhan, S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mater. 2017, 26, 213–222. [Google Scholar] [CrossRef]
- Helmi, M. Chapter 7—Polymeric adsorbents for heavy metal removal. In Polymeric Adsorbents; Ghaemi, A., Norouzbeigi, R., Masoumi, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 259–296. [Google Scholar] [CrossRef]
- Park, N.; Friest, M.A.; Liu, L. Enhancing the Properties of Polyvinyl Alcohol Films by Blending with Corn Stover-Derived Cellulose Nanocrystals and Beeswax. Polymers 2023, 15, 4321. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA Blends, and Their Nanocomposites for Biodegradable Packaging Application. Polym. Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef]
- Rahmadiawan, D.; Abral, H.; Railis, R.M.; Iby, I.C.; Mahardika, M.; Handayani, D.; Natrana, K.D.; Juliadmi, D.; Akbar, F. The Enhanced Moisture Absorption and Tensile Strength of PVA/Uncaria gambir Extract by Boric Acid as a Highly Moisture-Resistant, Anti-UV, and Strong Film for Food Packaging Applications. J. Compos. Sci. 2022, 6, 337. [Google Scholar] [CrossRef]
- Priya; Rakesh, S.; Singh, V.K. Water absorption behavior of functionalized graphene reinforced PVA based composite crosslinked using citric acid. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Khan, P.; Ali, S.; Jan, R.; Kim, K.M. Lignin Nanoparticles: Transforming Environmental Remediation. Nanomaterials 2024, 14, 1541. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly(lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef]
- Ou, W.-X.; Weng, Y.; Zeng, J.-B.; Li, Y.-D. Fully biobased poly(lactic acid)/lignin composites compatibilized by epoxidized natural rubber. Int. J. Biol. Macromol. 2023, 236, 123960. [Google Scholar] [CrossRef]
- Yang, W.; Qi, G.; Ding, H.; Xu, P.; Dong, W.; Zhu, X.; Zheng, T.; Ma, P. Biodegradable poly (lactic acid)-poly (ε-caprolactone)-nanolignin composite films with excellent flexibility and UV barrier performance. Compos. Commun. 2020, 22, 100497. [Google Scholar] [CrossRef]
- Lopez Camas, K.; Ullah, A. Depolymerization of lignin into high-value products. Biocatal. Agric. Biotechnol. 2022, 40, 102306. [Google Scholar] [CrossRef]
- Johansson, M.; Skrifvars, M.; Kadi, N.; Dhakal, H.N. Lignin-polylactic acid biopolymer blends for advanced applications—Effect of impact modifier. Composites Part C Open Access 2024, 14, 100502. [Google Scholar] [CrossRef]
- Johansson, M.; Skrifvars, M.; Kadi, N.; Dhakal, H.N. Effect of lignin acetylation on the mechanical properties of lignin-poly-lactic acid biocomposites for advanced applications. Ind. Crops Prod. 2023, 202, 117049. [Google Scholar] [CrossRef]
- Mariana, M.; Alfatah, T.; HPS, A.K.; Yahya, E.B.; Olaiya, N.G.; Nuryawan, A.; Mistar, E.M.; Abdullah, C.K.; Abdulmadjid, S.N.; Ismail, H. A current advancement on the role of lignin as sustainable reinforcement material in biopolymeric blends. J. Mater. Res. Technol. 2021, 15, 2287–2316. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin valorization: Lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Corradini, E.; Pineda, E.A.G.; Hechenleitner, A.A.W. Lignin-poly (vinyl alcohol) blends studied by thermal analysis. Polym. Degrad. Stab. 1999, 66, 199–208. [Google Scholar] [CrossRef]
- Korbag, I.; Saleh, S.M. Studies on the formation of intermolecular interactions and structural characterization of polyvinyl alcohol/lignin film. Int. J. Environ. Stud. 2016, 73, 226–235. [Google Scholar] [CrossRef]
- Fazeli, M.; Mukherjee, S.; Baniasadi, H.; Abidnejad, R.; Mujtaba, M.; Lipponen, J.; Seppälä, J.; Rojas, O.J. Lignin beyond the status quo: Recent and emerging composite applications. Green Chem. 2024, 26, 593–630. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Liu, W.; Qiu, X. High performance PVA/lignin nanocomposite films with excellent water vapor barrier and UV-shielding properties. Int. J. Biol. Macromol. 2020, 142, 551–558. [Google Scholar] [CrossRef]
- Posoknistakul, P.; Tangkrakul, C.; Chaosuanphae, P.; Deepentham, S.; Techasawong, W.; Phonphirunrot, N.; Bairak, S.; Sakdaronnarong, C.; Laosiripojana, N. Fabrication and Characterization of Lignin Particles and Their Ultraviolet Protection Ability in PVA Composite Film. ACS Omega 2020, 5, 20976–20982. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ren, X. Transparent and ultra-tough PVA/alkaline lignin films with UV shielding and antibacterial functions. Int. J. Biol. Macromol. 2022, 216, 86–94. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, L.; Wang, L.; Qi, Y.; Zhao, Y.; Lu, H.; Lu, L.; Zhang, D.; Wang, Z.; Zhang, H. Preparation and application of degradable lignin/poly (vinyl alcohol) polymers as urea slow-release coating materials. Molecules 2024, 29, 1699. [Google Scholar] [CrossRef]
- Bethel, K.; Buck, A.; Tindall, G.; Thies, M.C.; Davis, E.M. Fabrication of physically crosslinked lignin–PVA hydrogels containing high concentrations of fractionated and cleaned lignins. MRS Commun. 2022, 12, 624–631. [Google Scholar] [CrossRef]
- Dou, Y.; Hassan, E.A.M.; Wang, S.; Gibril, M.E.; Kong, F. Enhancing PVA mulching films: Leveraging modified lignin as a bio-based crosslinking agent for improved mechanical strength, UV barrier, and biodegradability. Ind. Crops Prod. 2024, 222, 119766. [Google Scholar] [CrossRef]
- Zou, S.-L.; Xiao, L.-P.; Yin, W.-Z.; Gui, T.; Sun, R.-C. Fabrication of biodegradable polyvinyl alcohol-based plastics toward technical lignin valorization. Int. J. Biol. Macromol. 2025, 284, 138123. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Ye, D.-Z.; Tang, J.-B.; Zhang, L.-J.; Zhang, X. From waste to functional additives: Thermal stabilization and toughening of PVA with lignin. RSC Adv. 2016, 6, 13797–13802. [Google Scholar] [CrossRef]
- Korbag, I.; Saleh, S.M. Studies on mechanical and biodegradability properties of PVA/lignin blend films. Int. J. Environ. Stud. 2016, 73, 18–24. [Google Scholar] [CrossRef]
- Xu, G.; Ren, S.; Wang, D.; Su, L.; Fang, G. Fabrication and Properties of Alkaline Lignin/Poly (Vinyl Alcohol) Blend Membranes. Bioresources 2013, 8, 2510–2520. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Ji, X.; Yang, G.; Chen, J.; Yoo, C.G.; Janaswamy, S.; Lyu, G. Innovative production of lignin nanoparticles using deep eutectic solvents for multifunctional nanocomposites. Int. J. Biol. Macromol. 2021, 183, 781–789. [Google Scholar] [CrossRef]
- He, X.; Luzi, F.; Hao, X.; Yang, W.; Torre, L.; Xiao, Z.; Xie, Y.; Puglia, D. Thermal, antioxidant and swelling behaviour of transparent polyvinyl (alcohol) films in presence of hydrophobic citric acid-modified lignin nanoparticles. Int. J. Biol. Macromol. 2019, 127, 665–676. [Google Scholar] [CrossRef]
- Bush, J.D. Rheology of Lignin and Lignin/PET Blends; University of Tennessee: Knoxville, TN, USA, 2006. [Google Scholar]
- Yang, X.; Zhong, S. Properties of maleic anhydride-modified lignin nanoparticles/polybutylene adipate-co-terephthalate composites. J. Appl. Polym. Sci. 2020, 137, 49025. [Google Scholar] [CrossRef]
- Porkodi, P.; Abhilash, J.K.; Shukla, H.K.; Rawat, J. Rheological properties of concentrated polyacrylonitrile co-polymer and lignin blend solution. Polym. Bull. 2020, 77, 3937–3951. [Google Scholar] [CrossRef]
- Liu, H.C.; Tuan, C.C.; Davijani, A.A.B.; Wang, P.H.; Chang, H.; Wong, C.P.; Kumar, S. Rheological behavior of polyacrylonitrile and polyacrylonitrile/lignin blends. Polymer 2017, 111, 177–182. [Google Scholar] [CrossRef]
- Possari, L.T.; Bretas, R.E.S.; Rigolin, T.R.; Bettini, S.H.P. Dualistic effect of Kraft lignin on the viscoelastic behavior of biodegradable biobased PBSA. Mater. Today Commun. 2021, 29, 102847. [Google Scholar] [CrossRef]
- Barzegari, M.R.; Alemdar, A.; Zhang, Y.; Rodrigue, D. Mechanical and rheological behavior of highly filled polystyrene with lignin. Polym. Compos. 2012, 33, 353–361. [Google Scholar] [CrossRef]
- Bian, H.; Wei, L.; Lin, C.; Ma, Q.; Dai, H.; Zhu, J.Y. Lignin-containing cellulose nanofibril-reinforced polyvinyl alcohol hydrogels. ACS Sustain. Chem. Eng. 2018, 6, 4821–4828. [Google Scholar] [CrossRef]
- Schreiber, M.; Vivekanandhan, S.; Mohanty, A.K.; Misra, M. A study on the electrospinning behaviour and nanofibre morphology of anionically charged lignin. Adv. Mater. Lett. 2012, 3, 476–480. [Google Scholar] [CrossRef]
- Seydibeyoglu, M.O.; Uysalman, T.; Yakkan, E.; Atagür, M.; Sever, K. The influence of coupling agents on mechanical properties of lignin-filled polypropylene composites. Turk. J. For. 2018, 19, 308–316. [Google Scholar] [CrossRef]
- Alassod, A.; Gibril, M.; Islam, S.R.; Huang, W.; Xu, G. Polypropylene/lignin blend monoliths used as sorbent in oil spill cleanup. Heliyon 2020, 6, e04591. [Google Scholar] [CrossRef]
- Acosta, J.L.E.; Torres Chavez, P.I.; Ramírez-Wong, B.; Bello-Pérez, L.A.; Vega Ríos, A.; Carvajal Millan, E.; Plascencia Jatomea, M.; Ledesma Osuna, A.I. Mechanical, thermal, and antioxidant properties of composite films prepared from durum wheat starch and lignin. Starch-Stärke 2015, 67, 502–511. [Google Scholar] [CrossRef]
- Lepifre, S.; Froment, M.; Cazaux, F.; Houot, S.; Lourdin, D.; Coqueret, X.; Lapierre, C.; Baumberger, S. Lignin incorporation combined with electron-beam irradiation improves the surface water resistance of starch films. Biomacromolecules 2004, 5, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bai, T.; Lu, J.; Wang, D.; Huan, S.; Yu, H.; Cheng, W.; Yue, Y.; Han, G. Pea-like inspired design: Lignin nanospheres-mediated PVA film with high strength, low water evaporation permeability, strong UV resistance and anti-oxidation properties for extending food freshness. Chem. Eng. J. 2024, 494, 152993. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Kenny, J.M.; Puglia, D. Effect of processing conditions and lignin content on thermal, mechanical and degradative behavior of lignin nanoparticles/polylactic (acid) bionanocomposites prepared by melt extrusion and solvent casting. Eur. Polym. J. 2015, 71, 126–139. [Google Scholar] [CrossRef]
- Belouadah, Z.; Nasri, K.; Toubal, L. The effects of lignin on the thermal and morphological properties and damage mechanisms after UV irradiation of polypropylene biocomposites reinforced with flax and pine Fibres: Acoustic emission analysis. Materials 2024, 17, 2474. [Google Scholar] [CrossRef]
- Cao, X.; Li, X.; Wu, R.; Liu, B.; Lin, W. Enhancing the performance of biodegradable lignin nanoparticle/PVA composite films via phenolation pretreatment of lignin using a novel ternary deep eutectic solvent. Coatings 2024, 14, 1544. [Google Scholar] [CrossRef]
- Van Erven, G.; Wang, J.; Sun, P.; De Waard, P.; Van Der Putten, J.; Frissen, G.E.; Gosselink, R.J.; Zinovyev, G.; Potthast, A.; Van Berkel, W.J.; et al. Structural motifs of wheat straw lignin differ in susceptibility to degradation by the white-rot fungus Ceriporiopsis subvermispora. ACS Sustain. Chem. Eng. 2019, 7, 20032–20042. [Google Scholar] [CrossRef]
- Zeng, J.; Helms, G.L.; Gao, X.; Chen, S. Quantification of wheat straw lignin structure by comprehensive NMR analysis. J. Agric. Food Chem. 2013, 61, 10848–10857. [Google Scholar] [CrossRef]
- van Erven, G.; Veersma, R.J.; Kabel, M.A. Comprehensive Structural Characterization of Wheat Bran Lignin. J. Agric. Food Chem. 2025, 73, 9136–9143. [Google Scholar] [CrossRef] [PubMed]
- ISO 11357-1:2023 (en); Plastics—Differential Scanning Calorimetry (DSC)—Part 1: General Principles. ISO: Geneva, Switzerland, 2023.
- Yang, W.; He, X.; Luzi, F.; Dong, W.; Zheng, T.; Kenny, J.M.; Puglia, D.; Ma, P. Thermomechanical, antioxidant and moisture behaviour of PVA films in presence of citric acid esterified cellulose nanocrystals. Int. J. Biol. Macromol. 2020, 161, 617–626. [Google Scholar] [CrossRef]
- ISO 11358-1:2022; Plastics—Thermogravimetry (TG) of Polymers, Part 1: General Principle. ISO: Geneva, Switzerland, 2022.
- IEC 60068-2; Environmental Testing. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2025.
- ISO 3219-1:2021; Vocabulary and Symbols for Rotational and Oscillatory Rheometry. ISO: Geneva, Switzerland, 2021.
- Long, H.; Hu, L.; Yang, F.; Cai, Q.; Zhong, Z.; Zhang, S.; Guan, L.; Xiao, D.; Zheng, W.; Zhou, W.; et al. Enhancing the performance of polylactic acid composites through self-assembly lignin nanospheres for fused deposition modeling. Compos. B Eng. 2022, 239, 109968. [Google Scholar] [CrossRef]
- Poletto, M. Assessment of the thermal behavior of lignins from softwood and hardwood species. Maderas Cienc. Tecnol. 2017, 19, 63–74. [Google Scholar] [CrossRef]
- Durmaz, B.U.; Aytac, A. Effects of Polyol-Based Plasticizer Types and Concentration on the Properties of Polyvinyl Alcohol and Casein Blend Films. J. Polym. Environ. 2021, 29, 313–322. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Narron, R.; Wang, Y.; Yong, Q. Characterization of Kraft Lignin Fractions Obtained by Sequential Ultrafiltration and Their Potential Application as a Biobased Component in Blends with Polyethylene. ACS Sustain. Chem. Eng. 2017, 5, 11770–11779. [Google Scholar] [CrossRef]
- Dai, Z.; Shi, X.; Liu, H.; Li, H.; Han, Y.; Zhou, J. High-strength lignin-based carbon fibers via a low-energy method. RSC Adv. 2018, 8, 1218–1224. [Google Scholar] [CrossRef]
- Korbag, I.; Eghreibeel, H.; Mhmood, M.A.; Saleh, S.M. Thermal Properties of Poly (Vinyl Alcohol)/Lignin Blends. Al-Mukhtar J. Sci. 2018, 33, 273–280. [Google Scholar]
- Yang, W.; Ding, H.; Qi, G.; Li, C.; Xu, P.; Zheng, T.; Zhu, X.; Kenny, J.M.; Puglia, D.; Ma, P. Highly transparent PVA/nanolignin composite films with excellent UV shielding, antibacterial and antioxidant performance. React. Funct. Polym. 2021, 162, 104873. [Google Scholar] [CrossRef]
- Amit, T.A.; Roy, R.; Raynie, D.E. Thermal and structural characterization of two commercially available technical lignins for potential depolymerization via hydrothermal liquefaction. Curr. Res. Green Sustain. Chem. 2021, 4, 100106. [Google Scholar] [CrossRef]
- Siregar, A.H.; Gea, S.; Sitorus, L.M. The manufacture nanofiber from sugarcane bagasse lignin with polyvinyl alcohol by electrospinning method. AIP Conf. Proc. 2021, 2342, 060003. [Google Scholar] [CrossRef]
- De Freitas, A.S.M.; Rodrigues, J.S.; Fré, L.V.; Vieira, H.O.; Nascimento, M.E.; Amaro, S.F.; Emidio, L.S.; de Araujo, D.R.; Sepulveda, A.F.; Komatsu, D. A dual approach with lignin and nanolignin: Reinforcing UV stability and sustainability in PVA matrices. Int. J. Biol. Macromol. 2025, 314, 144386. [Google Scholar] [CrossRef] [PubMed]
- Chollet, B.; Lopez-Cuesta, J.-M.; Laoutid, F.; Ferry, L. Lignin Nanoparticles as A Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide. Materials 2019, 12, 2132. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Marais, S.; Kujawa, J.; Kujawski, W. Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior. Polymers 2019, 11, 1799. [Google Scholar] [CrossRef]
- Phansamarng, P.; Bacchus, A.; Pour, F.H.; Kongvarhodom, C.; Fatehi, P. Cationic Lignin and Cellulose Fiber Incorporated Polyvinyl Alcohol Film. J. Appl. Polym. Sci. 2025, 142, e56555. [Google Scholar] [CrossRef]
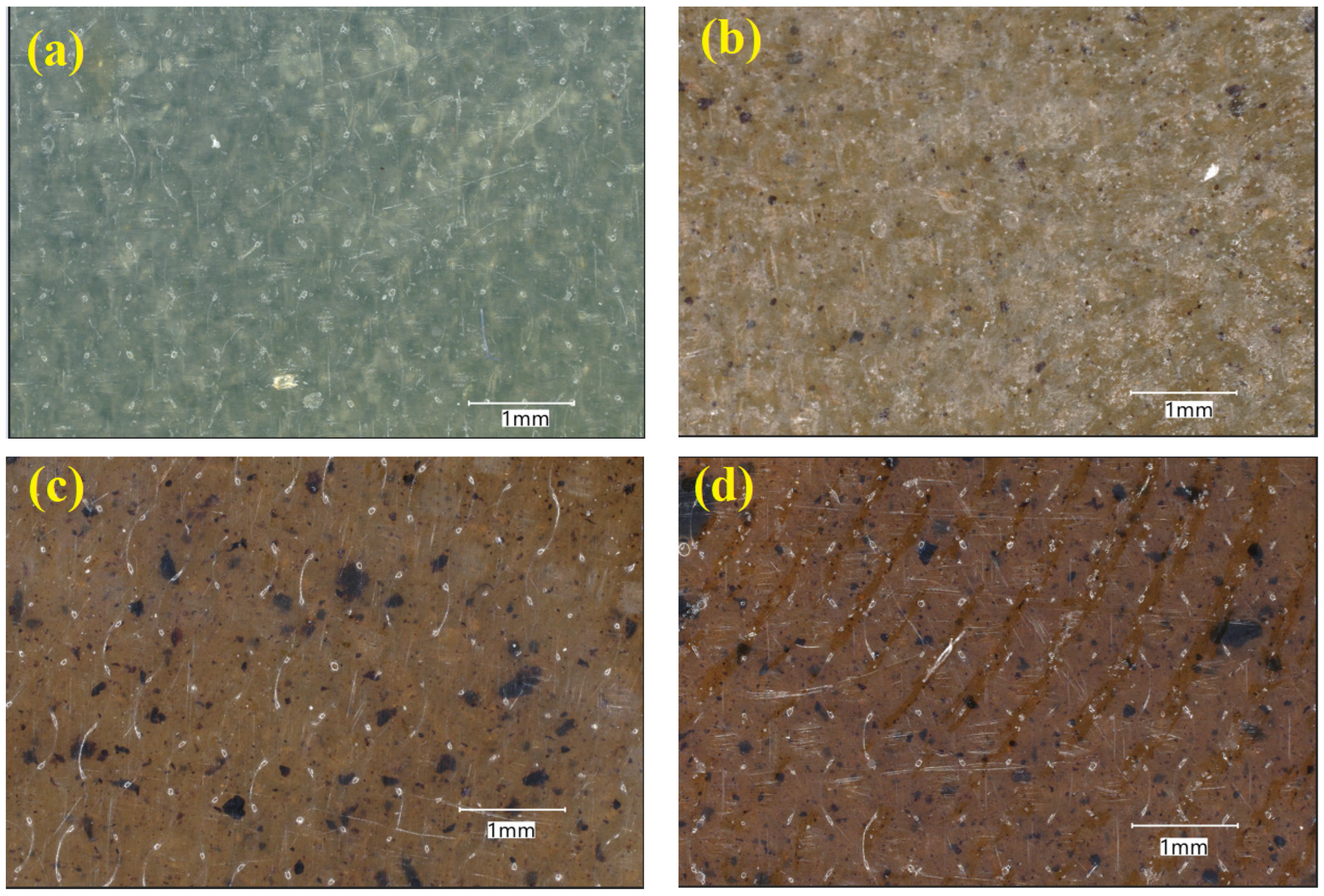




| Sample Code | PVA Content (%) | Lignin Content (%) |
|---|---|---|
| PVA | 100 | - |
| PVA-0.5L | 99.5 | 0.5 |
| PVA-1.0L | 99.0 | 1.0 |
| PVA-1.5L | 98.50 | 1.5 |
| PVA-3.0L | 97.00 | 3.0 |
| PVA-5.0L | 95.00 | 5.0 |
| PVA-10L | 90.00 | 10 |
| Samples | 1st Heating | Cooling | 2nd Heating | ||
|---|---|---|---|---|---|
| Tm (°C) | Xc (%) | Tc (°C) | Tm (°C) | Xc (%) | |
| PVA | 214.03 | 48.77 | 195.07 | 215.53 | 46.31 |
| PVA-0.5L | 213.91 | 49.66 | 194.31 | 214.45 | 46.00 |
| PVA-1.0L | 214.45 | 49.61 | 194.36 | 215.73 | 43.01 |
| PVA-1.5L | 214.07 | 48.03 | 192.96 | 214.90 | 45.97 |
| Samples | Degradation Onset Temperature (°C) | DTGA Peak (°C) | Residue at 700 °C (%) |
|---|---|---|---|
| Lignin | 257.9 (±8.3) | 378.3 (±0.7) | 42.75 (±0.40) |
| PVA | 351.9 (±5.7) | 393.0 (±1.6) | 0.48 (±0.03) |
| PVA-0.5L | 351.1 (±3.4) | 392.5 (±2.6) | 0.40 (±0.28) |
| PVA-1.0L | 358.9 (±2.2) | 396.4 (±6.5) | 1.42 (±0.59) |
| PVA-1.5L | 356.1 (±3.0) | 394.4 (±0.5) | 1.68 (±0.42) |
| PVA-3.0L | 355.1 (±0.1) | 387.7 (±5.6) | 1.70 (±0.91) |
| PVA-5.0L | 353.7 (±2.4) | 391.3 (±2.7) | 2.13 (±0.10) |
| PVA-10L | 352.1 (±0.5) | 389.9 (±0.6) | 4.15 (±0.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selver, E.; Selver, A.D.; Saifullah, A.; Zhang, Z.; Dhakal, H.N. Thermal, Rheological, and Moisture Absorption Behaviours of Polyvinyl Alcohol (PVA)/Lignin Composites. Polymers 2025, 17, 2918. https://doi.org/10.3390/polym17212918
Selver E, Selver AD, Saifullah A, Zhang Z, Dhakal HN. Thermal, Rheological, and Moisture Absorption Behaviours of Polyvinyl Alcohol (PVA)/Lignin Composites. Polymers. 2025; 17(21):2918. https://doi.org/10.3390/polym17212918
Chicago/Turabian StyleSelver, Erdem, Ayca Dogrul Selver, Abu Saifullah, Zhongyi Zhang, and Hom N. Dhakal. 2025. "Thermal, Rheological, and Moisture Absorption Behaviours of Polyvinyl Alcohol (PVA)/Lignin Composites" Polymers 17, no. 21: 2918. https://doi.org/10.3390/polym17212918
APA StyleSelver, E., Selver, A. D., Saifullah, A., Zhang, Z., & Dhakal, H. N. (2025). Thermal, Rheological, and Moisture Absorption Behaviours of Polyvinyl Alcohol (PVA)/Lignin Composites. Polymers, 17(21), 2918. https://doi.org/10.3390/polym17212918







