Formulation and Characterization of PLGA Minocycline Microneedles for Enhanced Skin Deposition and Antibacterial Activity in Acne Treatment
Abstract
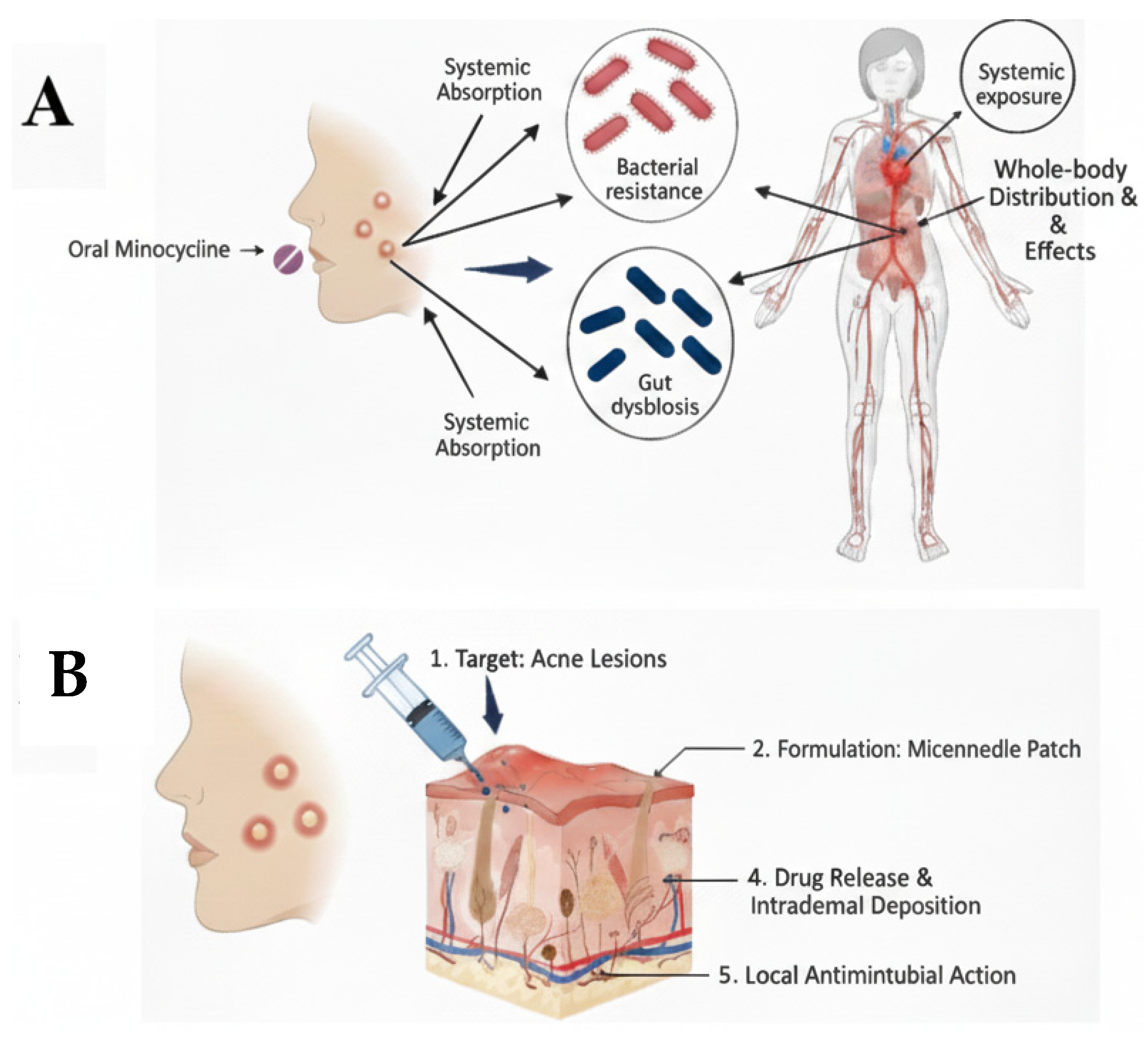
1. Introduction
2. Materials
3. Methods
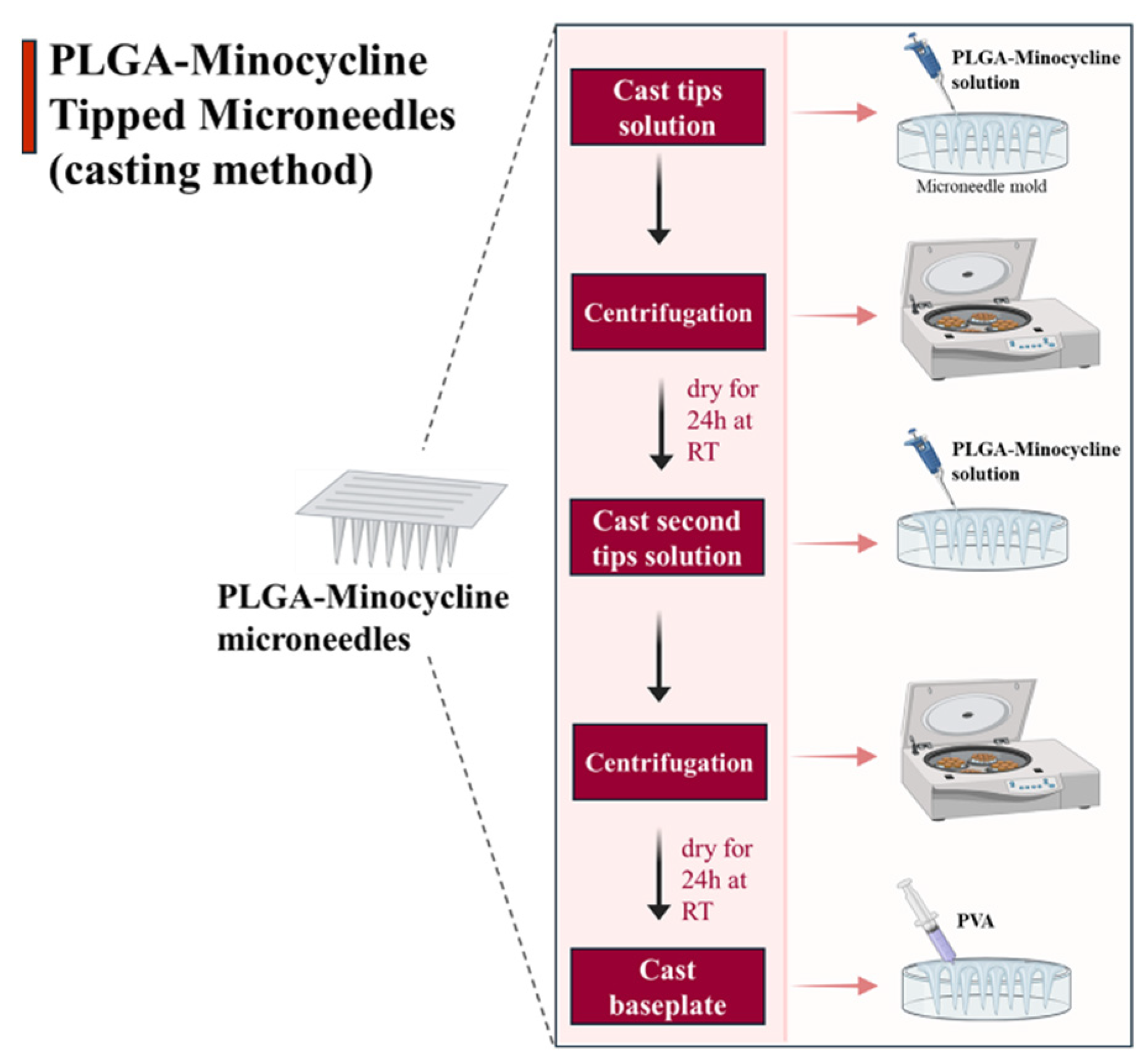
3.1. PLGA-Minocycline Tipped Microneedles Fabrication
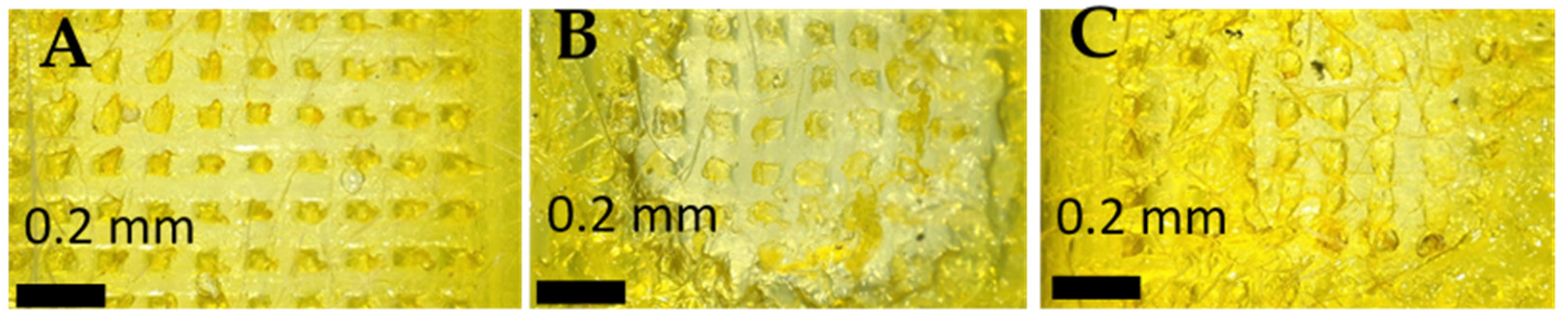
3.2. PLGA-Minocycline Tipped Microneedles Characterization
3.3. PLGA-Minocycline Tipped Microneedles Drug Content
3.4. PLGA-MNC Tipped Microneedles In Vitro and Ex Vivo Dissolution Time
3.5. PLGA-Minocycline Tipped Microneedles Ex Vivo Skin Deposition Study
3.6. PLGA-Minocycline Tipped Microneedles Antibacterial Potency
3.6.1. Agar Diffusion Method
3.6.2. Minimum Inhibitory Concentration (MIC)
3.6.3. Minimum Bactericidal Concentration (MBC)
3.7. Statistical Analysis
4. Results
4.1. PLGA-Minocycline Tipped Microneedles Fabrication
4.2. PLGA-Minocycline Tipped Microneedles Characterisation
4.3. PLGA-Minocycline Tipped Microneedles Drug Content
4.4. PLGA-Minocycline Tipped Microneedles In Vitro and Ex Vivo Dissolution Time
4.5. PLGA-Minocycline Tipped Microneedles Ex Vivo Skin Deposition Study
4.6. PLGA-Minocycline Tipped Microneedles Antibacterial Potency
4.6.1. Agar Diffusion Method
4.6.2. Minimum Inhibitory Concentration (MIC)
4.6.3. Minimum Bactericidal Concentration (MBC)
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MNs | Microneedles |
| MNC | Minocycline |
| PLGA | poly lactic-co-glycolic acid |
| UV | Ultra violet |
| DMSO | Dimethyl sulfoxide |
References
- Zaenglein, A.L. Acne vulgaris. N. Engl. J. Med. 2018, 379, 1343–1352. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H.; Xu, A.; He, L. A review of advancement on influencing factors of acne: An emphasis on environment characteristics. Front. Public Health 2020, 8, 450. [Google Scholar] [CrossRef]
- Ak, M. A comprehensive review of acne vulgaris. J. Clin. Pharm. 2019, 1, 17–45. [Google Scholar]
- Eichenfield, D.Z.; Sprague, J.; Eichenfield, L.F. Management of acne vulgaris: A review. J. Am. Med. Assoc. 2021, 326, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Chukwudi, C.U. rRNA binding sites and the molecular mechanism of action of the tetracyclines. Antimicrob. Agents Chemother. 2016, 60, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Qian, W.; Zhang, J.; Chen, D.; Yeung, K.W.; Liu, X. Minocycline hydrochloride loaded graphene oxide enables enhanced osteogenic activity in the presence of Gram-positive bacteria, Staphylococcus aureus. J. Mater. Chem. B 2019, 7, 3590–3598. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M.; Johnson, J.L.; Graber, E.M. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics 2021, 10, 757. [Google Scholar] [CrossRef]
- Colovic, M.; Caccia, S. Liquid chromatographic determination of minocycline in brain-to-plasma distribution studies in the rat. J. Chromatogr. B 2003, 791, 337–343. [Google Scholar] [CrossRef]
- Lebrun-Vignes, B.; Kreft-Jais, C.; Castot, A.; Chosidow, O.; French Network of Regional Centers of Pharmacovigilance. Comparative analysis of adverse drug reactions to tetracyclines: Results of a French national survey and review of the literature. Br. J. Dermatol. 2012, 166, 1333–1341. [Google Scholar] [CrossRef]
- Garner, S.E.; Eady, A.; Bennett, C.; Newton, J.N.; Thomas, K.; Popescu, C.M. Minocycline for acne vulgaris: Efficacy and safety. Cochrane Database Syst. Rev. 2012, 8, CD002086. [Google Scholar] [CrossRef]
- Ullah, I.; Ali, E. Bioavailability of antibiotics and their toxicity. In Antibiotics and Antimicrobial Resistance Genes: Environmental Occurrence and Treatment Technologies; Springer: Berlin/Heidelberg, Germany, 2020; pp. 211–238. [Google Scholar]
- Toor, D.; Wasson, M.K.; Kumar, P.; Karthikeyan, G.; Kaushik, N.K.; Goel, C.; Singh, S.; Kumar, A.; Prakash, H. Dysbiosis disrupts gut immune homeostasis and promotes gastric diseases. Int. J. Mol. Sci. 2019, 20, 2432. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.G.; Rainer, B.M.; Antonescu, C.; Florea, L.; Mongodin, E.F.; Kang, S.; Chien, A.L. Minocycline and its impact on microbial dysbiosis in the skin and gastrointestinal tract of acne patients. Ann. Dermatol. 2020, 32, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Dominic, M.R. Adverse reactions induced by minocycline: A review of literature. Curr. Drug Saf. 2021, 16, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Ershaid, J.M.A.; Abudoleh, S.M.; Lafi, D.N. Freeze-dried erythromycin nanocrystals: Preparation, characterisation, antimicrobial activity, and aerodynamic properties. Pharmacia 2024, 71, 1–10. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; Abudoleh, S.M.; Abualassal, Q.I.A.; Abudayeh, Z.; Aldalahmah, Y.; Hussein, M.Z. Preparation and characterisation of ciprofloxacin-loaded silver nanoparticles for drug delivery. IET Nanobiotechnol. 2022, 16, 92–101. [Google Scholar] [CrossRef]
- Sabbagh, H.A.K.; Hussein-Al-Ali, S.H.; Hussein, M.Z.; Abudayeh, Z.; Ayoub, R.; Abudoleh, S.M. A statistical study on the development of metronidazole-chitosan-alginate nanocomposite formulation using the full factorial design. Polymers 2020, 12, 772. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Geilich, B.M.; Webster, T.J. Synthesis, characterization, and antimicrobial activity of an ampicillin-conjugated magnetic nanoantibiotic for medical applications. Int. J. Nanomed. 2014, 9, 3801–3814. [Google Scholar] [CrossRef]
- Bonati, L.M.; Dover, J.S. Treating acne with topical antibiotics: Current obstacles and the introduction of topical minocycline as a new treatment option. J. Drugs Dermatol. 2019, 18, 240–244. [Google Scholar]
- Abu Ershaid, J.M.; Vora, L.K.; Volpe-Zanutto, F.; Sabri, A.H.; Peng, K.; Anjani, Q.K.; McKenna, P.E.; Ripolin, A.; Larrañeta, E.; McCarthy, H.O.; et al. Microneedle array patches for sustained delivery of fluphenazine: A micron scale approach for the management of schizophrenia. Biomater. Adv. 2023, 153, 213526. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, C.; Abu-Ershaid, J.M.; Li, M.; Li, Y.; Naser, Y.; Dai, X.; Abbate, M.T.A.; Donnelly, R.F. Smart responsive microarray patches for transdermal drug delivery and biological monitoring. Adv. Healthc. Mater. 2021, 10, 2100996. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Malek-Khatabi, A.; Razavi, M.S.; Abdollahi, A.; Rahimzadeghan, M.; Moammeri, F.; Sheikhi, M.; Tavakoli, M.; Rad-Malekshahi, M.; Rad, Z.F. Recent progress in PLGA-based microneedle-mediated transdermal drug and vaccine delivery. Biomater. Sci. 2023, 11, 5390–5409. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Abu Ammar, A.; Himawan, A.; Dai, X.; Duncan, R.; Gilmore, B.F.; Donnelly, R.F.; Vora, L.K. Amphotericin B PLGA nanoparticles loaded dissolving microneedle patches in treating cutaneous fungal infections. J. Drug Deliv. Sci. Technol. 2025, 106, 106697. [Google Scholar] [CrossRef]
- Paredes, A.J.; McKenna, P.E.; Ramöller, I.K.; Naser, Y.A.; Volpe-Zanutto, F.; Li, M.; Abbate, M.T.A.; Zhao, L.; Zhang, C.; Abu-Ershaid, J.M.; et al. Microarray patches: Poking a hole in the challenges faced when delivering poorly soluble drugs. Adv. Funct. Mater. 2021, 31, 2005792. [Google Scholar] [CrossRef]
- Li, H.; Wen, X.; Gong, X.; Wu, Y.; Zhao, P.; Zhang, Y.; Sha, Z.; Chang, H.; Chen, X. Facile minocycline deployment in gingiva using a dissolvable microneedle patch for the adjunctive treatment of periodontal disease. Bioeng. Transl. Med. 2025, 10, e10730. [Google Scholar] [CrossRef]
- Song, Y.W.; Nam, J.; Kim, J.; Lee, Y.; Choi, J.; Min, H.S.; Yang, H.; Cho, Y.; Hwang, S.; Son, J.; et al. Hyaluronic acid-based minocycline-loaded dissolving microneedle: Innovation in local minocycline delivery for periodontitis. Carbohydr. Polym. 2025, 349 Pt B, 122976. [Google Scholar] [CrossRef]
- Abu Ershaid, J.M.; Zhang, H.; Tayyem, M.; Sabri, A.H.; Donnelly, R.F.; Vora, L.K. Sodium alginate microneedles loaded with vancomycin for skin infections. J. Funct. Biomater. 2024, 15, 316. [Google Scholar] [CrossRef]
- Tarawneh, O.; Almasri, S.; Alhusban, A.A.; Hailat, M.; Hamadneh, L.; Ershaid, J.M.A.; Hailat, Z.; Makableh, Y.F. Innovative dissolving microneedles for enhanced delivery of alpha arbutin and ascorbic acid: A novel LC-MS quantification approach. Mater. Adv. 2025, 6, 1006–1019. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.I.; Vora, L.K.; Ershaid, J.A.; Peng, K.; Tekko, I.A.; Donnelly, R.F. Nanoemulsion-based dissolving microneedle arrays for enhanced intradermal and transdermal delivery. Drug Deliv. Transl. Res. 2022, 12, 881–896. [Google Scholar] [CrossRef]
- Cordeiro, A.S.; Tekko, I.A.; Jomaa, M.H.; Vora, L.; McAlister, E.; Volpe-Zanutto, F.; Nethery, M.; Baine, P.T.; Mitchell, N.; McNeill, D.W.; et al. Two-photon polymerisation 3D printing of microneedle array templates with versatile designs: Application in the development of polymeric drug delivery systems. Pharm. Res. 2020, 37, 174. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.L.; Saiman, L.; Whittier, S.; Larone, D.; Krzewinski, J.; Liu, Z.; Marshall, S.A.; Jones, R.N. Comparison of agar diffusion methodologies for antimicrobial susceptibility testing of Pseudomonas aeruginosa isolates from cystic fibrosis patients. J. Clin. Microbiol. 2000, 38, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-C.; Liang, J.; Li, Z.-E.; Duan, M.-H.; Dai, Y.; Jin, Y.-G.; Zhang, Y.-N.; Liu, Y.; Zhang, M.; Wang, G.-Y.; et al. In vitro antibacterial activity of danofloxacin against Escherichia coli in Gushi chickens and its residue depletion following multiple oral administration. Poult. Sci. 2024, 103, 103493. [Google Scholar] [CrossRef]
- Abudoleh, S.M.; Abu Ershaid, J.M.; Lafi, D.; Dahshan, N.A.; Talhouni, A.; Abuirmeileh, A. Minocycline nanocrystals: A new approach for treating acne with reduced systemic side effects. Pharmaceutics 2025, 17, 727. [Google Scholar] [CrossRef]
- Asawahame, C.; Sutjarittangtham, K.; Eitssayeam, S.; Tragoolpua, Y.; Sirithunyalug, B.; Sirithunyalug, J. Antibacterial activity and inhibition of adherence of Streptococcus mutans by propolis electrospun fibers. AAPS PharmSciTech 2015, 16, 182–191. [Google Scholar] [CrossRef]
- Valan, A.S.; Kolli, S.; Eswaramoorthy, R.; Krithikadatta, J.; Sureshbabu, N.M. Comparison of antibacterial efficacy of triple antibiotic-loaded hydrogel versus modified triple antibiotic-loaded hydrogel as intracanal medicament against Enterococcus faecalis: An in vitro study. Eur. Endod. J. 2024, 9, 154. [Google Scholar] [CrossRef]
- Russell, L.M.; Wiedersberg, S.; Delgado-Charro, M.B. The determination of stratum corneum thickness: An alternative approach. Eur. J. Pharm. Biopharm. 2008, 69, 861–870. [Google Scholar] [CrossRef]
- Vora, L.K.; Donnelly, R.F.; Larrañeta, E.; González-Vázquez, P.; Thakur, R.R.S.; Vavia, P.R. Novel bilayer dissolving microneedle arrays with concentrated PLGA nano-microparticles for targeted intradermal delivery: Proof of concept. J. Control. Release 2017, 265, 93–101. [Google Scholar] [CrossRef]
- Sen, O.; Poddar, P.; Sarkar, P.; Das, S.; Manna, S. Current advancements in microneedle technology for therapeutic and biomedical applications. Sens. Int. 2025, 6, 100325. [Google Scholar] [CrossRef]
- Leone, M.; Romeijn, S.; Slütter, B.; O’mAhony, C.; Kersten, G.; Bouwstra, J.A. Hyaluronan molecular weight: Effects on dissolution time of dissolving microneedles in the skin and on immunogenicity of antigen. Eur. J. Pharm. Sci. 2020, 146, 105269. [Google Scholar] [CrossRef]
- Wood-Yang, A.J.; Sankaranarayanan, A.; Freidlin, M.J.; Prausnitz, M.R. Highly water-soluble microneedle patch for short wear time and rapid drug delivery. Mol. Pharm. 2025, 22, 573–582. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]



| Microneedles |
S. aureus
(ATCC 9144) |
S. epidermidis
(ATCC 51625) |
C. acnes
(ATCC 11827) |
|---|---|---|---|
| PLGA-Minocycline MNs | 2.8 ± 0.11 | 2.3 ± 0.07 | 3.1 ± 0.11 |
| Control MNs (PLGA-DMSO) | No inhibition | No inhibition | Slight inhibition |
| Samples |
S. aureus
(ATCC 9144) |
S. epidermidis
(ATCC 51625) |
C. acnes
(ATCC 11827) |
|---|---|---|---|
| Minocycline-PLGA-DMSO solution | <0.146 µg/mL | <0.146 µg/mL | <0.146 µg/mL |
| Minocycline solution | <0.146 µg/mL | <0.146 µg/mL | <0.146 µg/mL |
| PLGA-DMSO solution | 150 µg/mL | 75 µg/mL | 75 µg/mL |
| Sample |
S. aureus
(ATCC 9144) |
S. epidermidis
(ATCC 51625) |
C. acnes
(ATCC 11827) |
|---|---|---|---|
| Minocycline-PLGA-DMSO solution | 9.375 µg/mL | 18.75 µg/mL | 18.75 µg/mL |
| Minocycline solution | 9.375 µg/mL | 18.75 µg/mL | 18.75 µg/mL |
| PLGA-DMSO solution | 300 µg/mL | 300 µg/mL | 300 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Ershaid, J.M.; Abudoleh, S.M.; Lafi, D.N.; Dahshan, N.A. Formulation and Characterization of PLGA Minocycline Microneedles for Enhanced Skin Deposition and Antibacterial Activity in Acne Treatment. Polymers 2025, 17, 2912. https://doi.org/10.3390/polym17212912
Abu Ershaid JM, Abudoleh SM, Lafi DN, Dahshan NA. Formulation and Characterization of PLGA Minocycline Microneedles for Enhanced Skin Deposition and Antibacterial Activity in Acne Treatment. Polymers. 2025; 17(21):2912. https://doi.org/10.3390/polym17212912
Chicago/Turabian StyleAbu Ershaid, Juhaina M., Suha M. Abudoleh, Dima N. Lafi, and Nisreen A. Dahshan. 2025. "Formulation and Characterization of PLGA Minocycline Microneedles for Enhanced Skin Deposition and Antibacterial Activity in Acne Treatment" Polymers 17, no. 21: 2912. https://doi.org/10.3390/polym17212912
APA StyleAbu Ershaid, J. M., Abudoleh, S. M., Lafi, D. N., & Dahshan, N. A. (2025). Formulation and Characterization of PLGA Minocycline Microneedles for Enhanced Skin Deposition and Antibacterial Activity in Acne Treatment. Polymers, 17(21), 2912. https://doi.org/10.3390/polym17212912






