Modulating the Behavior of Schwann Cells with NGF Exposure Combined with Different Energy Densities of Photobiomodulation Cultured on Polyhydroxybutyrate (PHB) Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Schwann Cell Culture
- Control group: Schwann cells without exposure to LLLT and NGF.
- L4 group: Schwann cells irradiated with LLLT with 4 J/cm2.
- L80 group: Schwann cells irradiated with LLLT with 80 J/cm2.
- L4 + NGF group: Schwann cells exposed to LLLT with 4 J/cm2 and NGF (25 ng/mL).
- L80 + NGF group: Schwann cells exposed to LLLT with 80 J/cm2 and NGF (25 ng/mL).
2.2. Schwann Cell Viability—MTT Assay
2.3. Schwann Cell Proliferation—Crystal Violet Assay
2.4. Cell Morphology, Morphometry, and Quantification—Scanning Electron Microscope Analysis
2.5. Data Analysis
3. Results
3.1. Schwann Cell Viability—MTT Assay
3.2. Schwann Cell Proliferation—Crystal Violet Cell Mass Assay
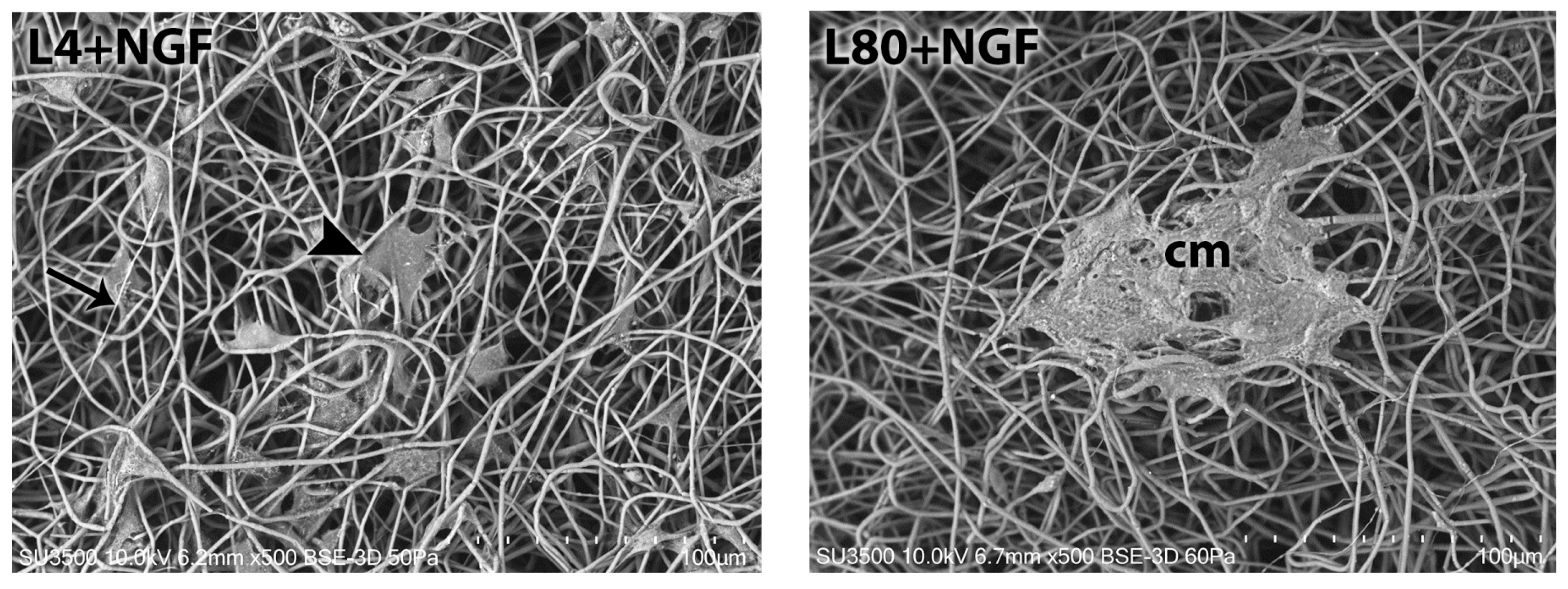
3.3. Schwann Cell Morphology—Scanning Electron Microscopy
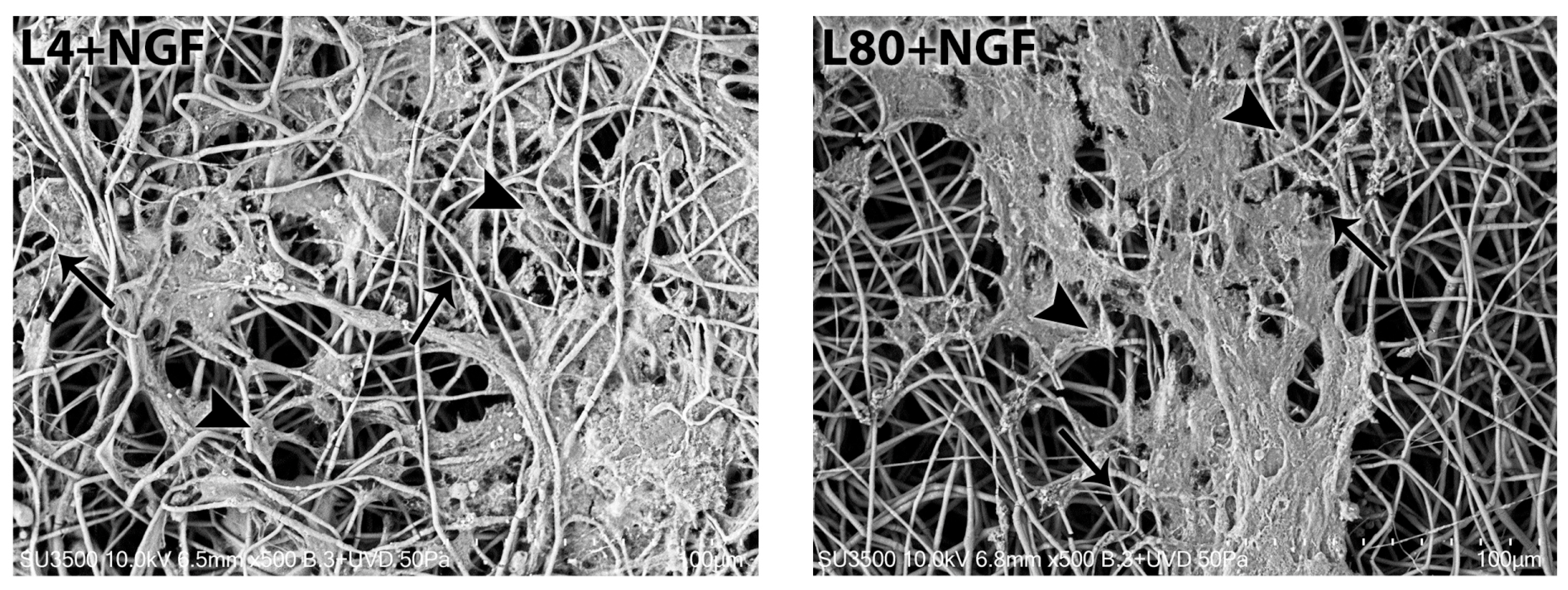
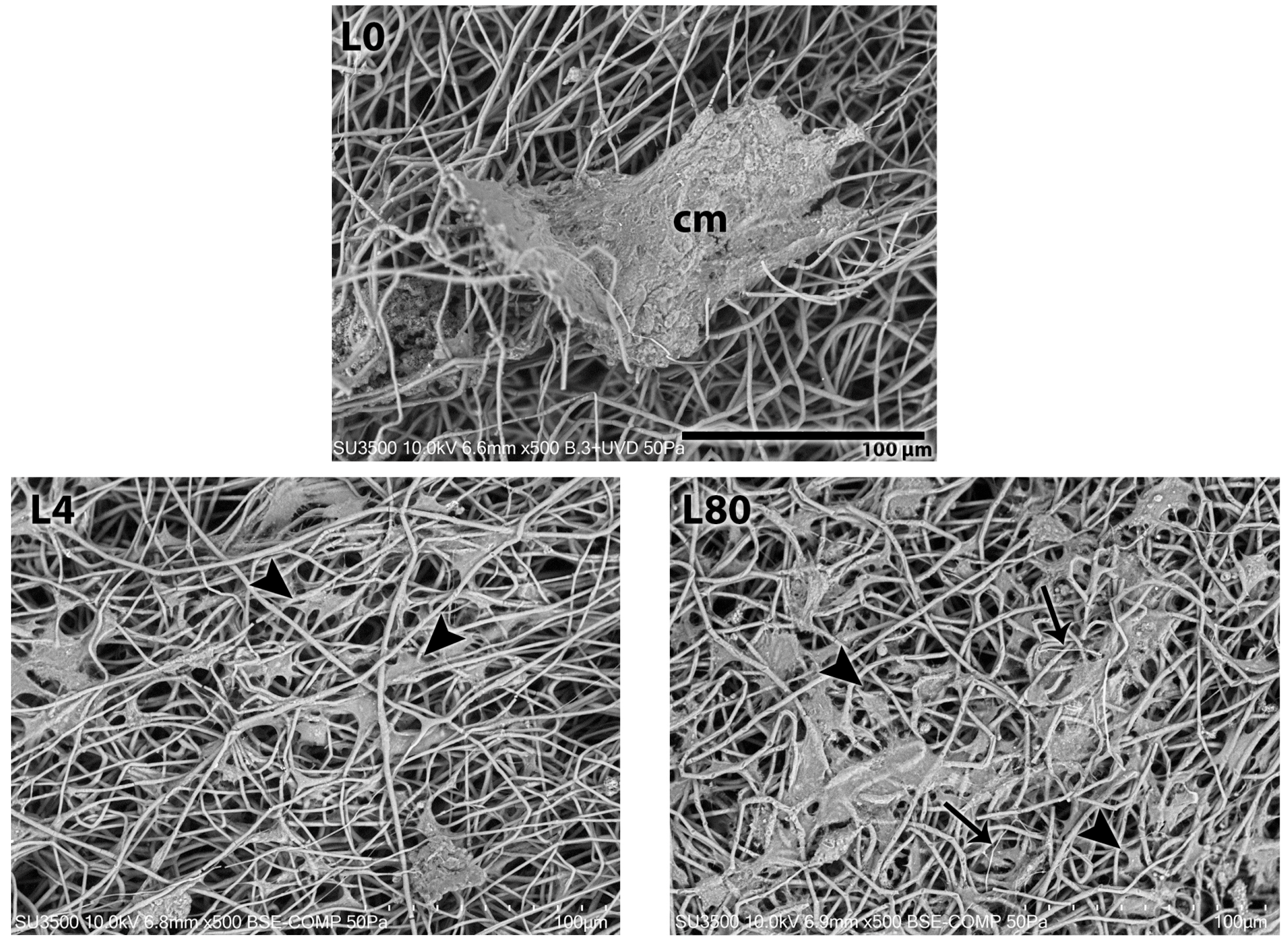
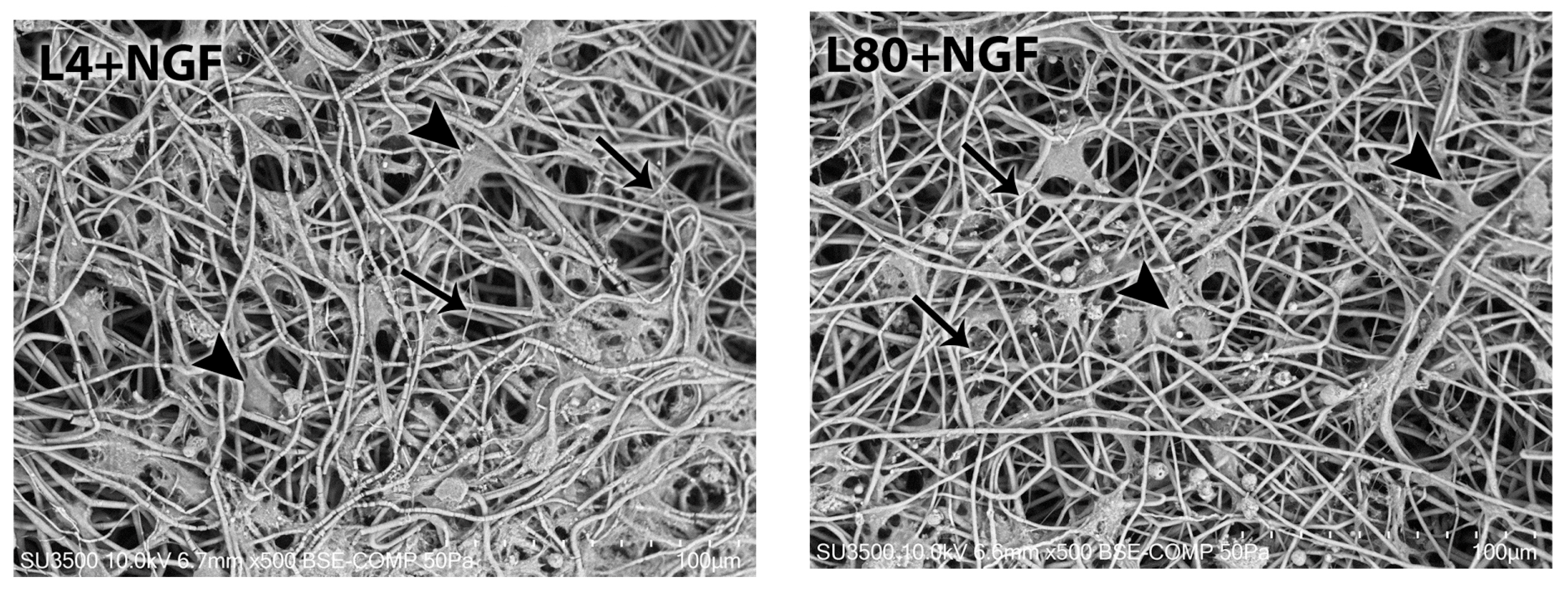
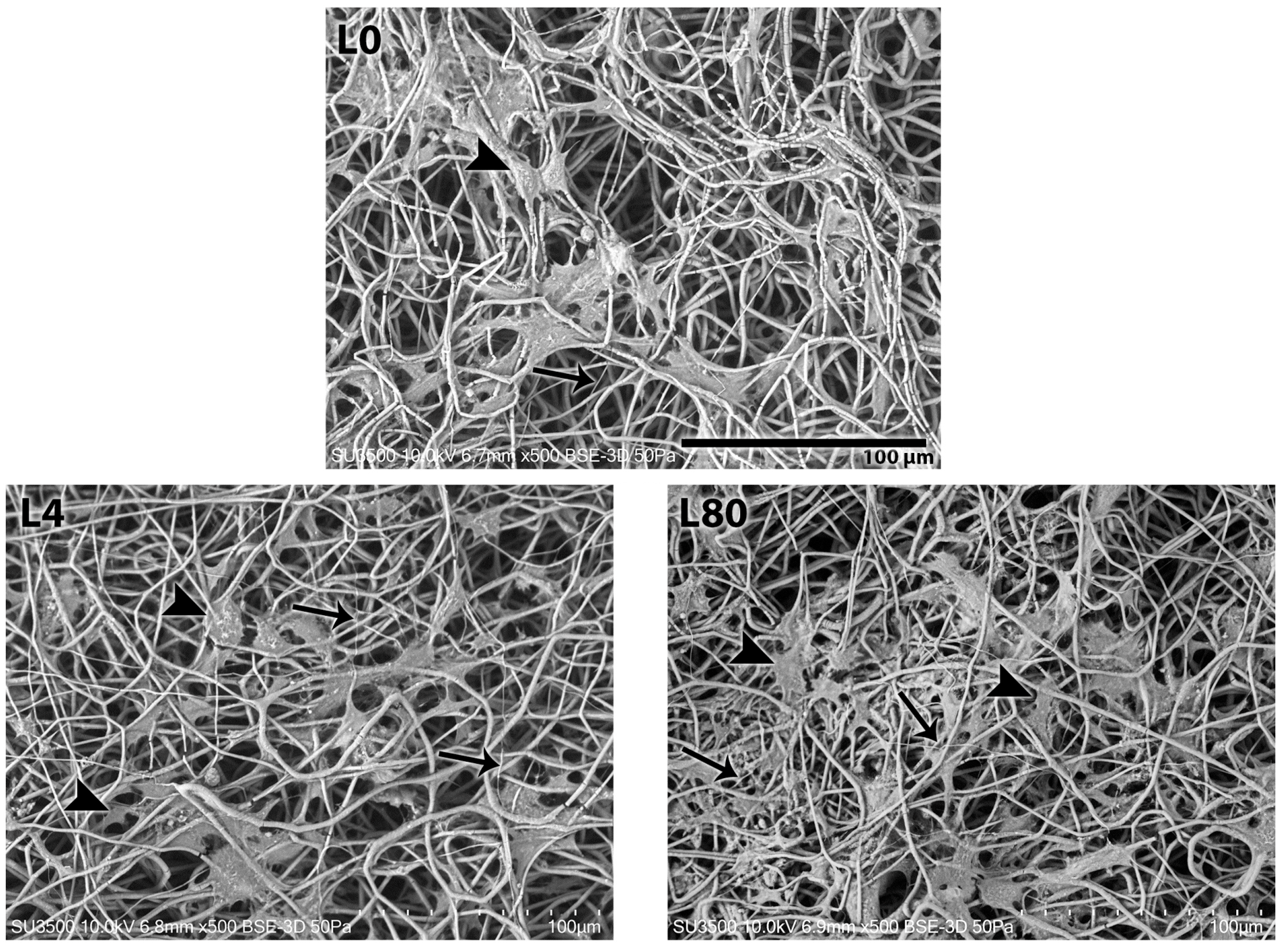
3.3.1. Morphological Analysis—1 Day After LLLT Irradiation and NGF Exposure
3.3.2. Morphological Analysis—3 Days After LLLT Irradiation and NGF Exposure
3.3.3. Morphological Analysis—7 Days After LLLT Irradiation and NGF Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NGF | Nerve growth factor |
| SC | Schwann cell |
| LLLT | Low-level laser therapy |
| PBMT | Photobiomodulation therapy |
| PHB | Polyhydroxybutyrate |
| L0 | Control group—LLLT 0 J/cm2 |
| L4 | LLLT 4 J/cm2 study group |
| L80 | LLLT 80 J/cm2 study group |
| L4 + NGF | LLLT 4 J/cm2 & NGF (25 ng/mL) study group |
| L80 + NGF | LLLT 80 J/cm2 & NGF (25 ng/mL) study group |
References
- García, O.; Massieu, L. Interacción entre las células gliales y neuronales y su papel en la muerte y sobrevida neuronal. Arch. Neurocien. 2004, 9, 39–46. [Google Scholar]
- Chen, C.Z.; Neumann, B.; Förster, S.; Franklin, R.J.M. Schwann cell remyelination of the central nervous system: Why does it happen and what are the benefits? Open Biol. 2021, 11, 200352. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Sheng, Q.S.; Qi, F.Y.; Hu, X.Y.; Zhao, W.; Wang, Y.Q.; Lan, L.F.; Huang, J.H.; Luo, Z.J. Schwann cell-seeded scaffold with longitudinally oriented micro-channels for reconstruction of sciatic nerve in rats. J. Mater. Sci. Mater. Med. 2013, 24, 1767–1780. [Google Scholar] [CrossRef]
- Hernández, A.; González, B.; Orellana, A.; Martín, J.; Berty, J. Láser de baja potencia en el tratamiento de las calcificaciones de hombro. Rev. Soc. Esp. Dolor. 2009, 16, 230–238. [Google Scholar] [CrossRef]
- España-Tost, A.; Arnabat-Domínguez, J.; Berini-Aytés, L.; Gay-Escoda, C. Aplicaciones del láser en Odontología. RCOE 2004, 9, 497–511. [Google Scholar] [CrossRef]
- Yazdani, S.O.; Golestaneh, A.F.; Shafiee, A.; Hafizi, M.; Omrani, H.A.; Soleimani, M. Effects of low level laser therapy on proliferation and neurotrophic factor gene expression of human schwann cells in vitro. J. Photochem. Photobiol. B 2012, 107, 9–13. [Google Scholar] [CrossRef]
- Benítez, E.; Ferreiro, M. Calibración y mantenimiento de aparatos de láser de baja potencia: Estudio descriptivo. Enfermería 2022, 11, 2855. [Google Scholar] [CrossRef]
- Ziago, E.K.; Fazan, V.P.; Iyomasa, M.M.; Sousa, L.G.; Yamauchi, P.Y.; Da Silva, E.A.; Borie, E.; Fuentes, R.; Dias, F.J. Analysis of the variation in low-level laser energy density on the crushed sciatic nerves of rats: A morphological, quantitative, and morphometric study. Lasers Med. Sci. 2017, 32, 369–378. [Google Scholar] [CrossRef]
- Andreo, L.; Mesquita-Ferrari, R.; Grenho, L.; Gomes, P.; Bussadori, S.K.; Fernandes, K.; Fernandes, M. Effects of 660-nm and 780-nm Laser Therapy on ST88-14 Schwann Cells. Photochem. Photobiol. 2021, 97, 198–204. [Google Scholar] [CrossRef]
- Wang, C.Z.; Chen, Y.J.; Wang, Y.H.; Yeh, M.L.; Huang, M.H.; Ho, M.L.; Liang, J.I.; Chen, C.H. Low-level laser irradiation improves functional recovery and nerve regeneration in sciatic nerve crush rat injury model. PLoS ONE 2014, 9, e103348. [Google Scholar] [CrossRef]
- Gigo-Benato, D.; Russo, T.L.; Tanaka, E.H.; Assis, L.; Salvini, T.F.; Parizotto, N.A. Effects of 660 and 780 nm low-level laser therapy on neuromuscular recovery after crush injury in rat sciatic nerve. Lasers Surg. Med. 2010, 42, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S.; Geuna, S.; Shainberg, A. Chapter 25: Phototherapy in peripheral nerve injury: Effects on muscle preservation and nerve regeneration. Int. Rev. Neurobiol. 2009, 87, 445–464. [Google Scholar]
- Chen, X.; Sawa, M.; Mobley, W. Dysregulation of neurotrophin signaling in the pathogenesis of Alzheimer disease and of Alzheimer disease in Down syndrome. Free Radic. Biol. Med. 2018, 114, 52–61. [Google Scholar] [CrossRef]
- Lin, L.; Doherty, D.; Lile, J.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Meeker, R.; Williams, K. Dynamic nature of the p75 neurotrophin receptor in response to injury and disease. J. Neuroimmune Pharmacol. 2014, 9, 615–628. [Google Scholar] [CrossRef]
- Pfister, L.; Papaloïzos, M.; Merkle, H.; Gander, B. Nerve conduits and growth factor delivery in peripheral nerve repair. J. Peripher. Nerv. Syst. 2007, 12, 65–82. [Google Scholar] [CrossRef]
- Richner, M.; Ulrichsen, M.; Elmegaard, S.; Dieu, R.; Pallesen, L.; Vaegter, C. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol. Neurobiol. 2014, 50, 945–970. [Google Scholar] [CrossRef] [PubMed]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef]
- Negro, S.; Pirazzini, M.; Rigoni, M. Models and methods to study Schwann cells. J. Anat. 2022, 241, 1235–1258. [Google Scholar] [CrossRef]
- Hörner, S.J.; Couturier, N.; Gueiber, D.C.; Hafner, M.; Rudolf, R. Development and In Vitro Differentiation of Schwann Cells. Cells 2022, 11, 3753. [Google Scholar] [CrossRef]
- Lezcano, M.F.; Martínez-Rodríguez, P.; Godoy, K.; Hermosilla, J.; Acevedo, F.; Gareis, I.E.; Dias, F.J. Exploring Schwann Cell Behavior on Electrospun Polyhydroxybutyrate Scaffolds with Varied Pore Sizes and Fiber Thicknesses: Implications for Neural Tissue Engineering. Polymers 2023, 15, 4625. [Google Scholar] [CrossRef]
- Gutiérrez, B.; González-Quijón, M.E.; Martínez-Rodríguez, P.; Alarcón-Apablaza, J.; Godoy, K.; Cury, D.P.; Lezcano, M.F.; Vargas-Chávez, D.; Dias, F.J. Comprehensive Development of a Cellulose Acetate and Soy Protein-Based Scaffold for Nerve Regeneration. Polymers 2024, 16, 216. [Google Scholar] [CrossRef]
- Hyung, G.Y.; Kim, K.H.; Baek, S.H.; Jang, G.Y.; Cho, C.G. Effect of Nerve Growth Factor on Cultured Rat Schwann Cells in Hyperglycemic Condition. Diabetes Metab. J. 2000, 24, 10–18. [Google Scholar]
- Kim, H.S.; Kim, J.Y.; Song, C.L.; Jeong, J.E.; Cho, Y.S. Directly induced human Schwann cell precursors as a valuable source of Schwann cells. Stem Cell Res. Ther. 2020, 11, 257. [Google Scholar] [CrossRef]
- Vistica, V.T.; Skehan, P.; Scudiero, D.; Monks, A.; Pittman, A.; Boyd, M.R. Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res. 1991, 51, 2515–2520. [Google Scholar] [PubMed]
- Maehara, Y.; Anai, H.; Tamada, R.; Sugimachi, K. The ATP assay is more sensitive than the succinate dehydrogenase inhibition test for predicting cell viability. Eur. J. Cancer Clin. Oncol. 1987, 23, 273–276. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, 087379. [Google Scholar] [CrossRef]
- Ramezani, F.; Neshasteh-Riz, A.; Ghadaksaz, A.; Fazeli, S.M.; Janzadeh, A.; Hamblin, M.R. Mechanistic aspects of photobiomodulation therapy in the nervous system. Lasers Med. Sci. 2022, 37, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.P.O.; Buchaim, D.V.; Kawano, N.; Furlanette, G.; Pomini, K.T.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef]
- Cheng, M.; Lv, X.; Zhang, C.; Du, W.; Liu, Y.; Zhu, L.; Hao, J. DNMT1, a Novel Regulator Mediating mTORC1/mTORC2 Pathway-Induced NGF Expression in Schwann Cells. Neurochem. Res. 2018, 43, 2141–2154. [Google Scholar] [CrossRef]
- Viader, A.; Golden, J.P.; Baloh, R.H.; Schmidt, R.E.; Hunter, D.A.; Milbrandt, J. Schwann cell mitochondrial metabolism supports long-term axonal survival and peripheral nerve function. J. Neurosci. 2011, 31, 10128–10140. [Google Scholar] [CrossRef]
- Ino, D.; Iino, M. Schwann cell mitochondria as key regulators in the development and maintenance of peripheral nerve axons. Cell Mol. Life Sci. 2017, 74, 827–835. [Google Scholar] [CrossRef]
- Wan, T.; Zhang, F.S.; Qin, M.Y.; Jiang, H.R.; Zhang, M.; Qu, Y.; Wang, Y.L.; Zhang, P.X. Growth factors: Bioactive macromolecular drugs for peripheral nerve injury treatment—Molecular mechanisms and delivery platforms. Biomed. Pharmacother. 2024, 170, 116024. [Google Scholar] [CrossRef]
- Jiang, L.; Jones, S.; Jia, X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int. J. Mol. Sci. 2017, 18, 94. [Google Scholar] [CrossRef]
- Guo, J.; Guo, S.; Wang, Y.; Yu, Y. Promoting potential of adipose derived stem cells on peripheral nerve regeneration. Mol. Med. Rep. 2017, 16, 7297–7304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, M.; Yan, C.; Lu, Q.; Wei, S.; Gao, R.; Yu, M.; Zou, Y.; Sriram, G.; Tong, H.J.; et al. Directed Differentiation of Human Embryonic Stem Cells to Neural Crest Stem Cells, Functional Peripheral Neurons, and Corneal Keratocytes. Biotechnol. J. 2017, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Furusho, M.; Bansal, R. Mek/ERK1/2-MAPK and PI3K/Akt/mTOR signaling plays both independent and cooperative roles in Schwann cell differentiation, myelination and dysmyelination. Glia 2021, 69, 2429–2446. [Google Scholar] [CrossRef] [PubMed]
- Ulrichsen, M.; Gonçalves, N.P.; Mohseni, S.; Hjæresen, S.; Lisle, T.L.; Molgaard, S.; Madsen, N.K.; Andersen, O.M.; Svenningsen, Å.F.; Glerup, S.; et al. Sortilin Modulates Schwann Cell Signaling and Remak Bundle Regeneration Following Nerve Injury. Front. Cell Neurosci. 2022, 16, 856734. [Google Scholar] [CrossRef]
- Li, R.; Li, D.; Wu, C.; Ye, L.; Wu, Y.; Yuan, Y.; Yang, S.; Xie, L.; Mao, Y.; Jiang, T.; et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics 2020, 10, 1649–1677. [Google Scholar] [CrossRef]
- Ma, T.; Yang, Y.; Quan, X.; Lu, L.; Xia, B.; Gao, J.; Qi, F.; Li, S.; Zhao, L.; Mei, L.; et al. Oxygen carrier in core-shell fibers synthesized by coaxial electrospinning enhances Schwann cell survival and nerve regeneration. Theranostics 2020, 10, 8957–8973. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Wang, F.; Huang, J.J.; Huang, J.J.; Li, Z.A.; Kong, Y.; Zhang, Z.J. Microfluidic hollow fiber with improved stiffness repairs peripheral nerve injury through noninvasive electromagnetic induction and controlled release of NGF. Chem. Eng. J. 2021, 426, 131826. [Google Scholar] [CrossRef]
- Soilu-Hänninen, M.; Ekert, P.; Bucci, T.; Syroid, D.; Bartlett, P.F.; Kilpatrick, T.J. Nerve growth factor signaling through p75 induces apoptosis in Schwann cells via a Bcl-2-independent pathway. J. Neurosci. 1999, 19, 4828–4838. [Google Scholar] [CrossRef]
- Lackington, W.A.; Kočí, Z.; Alekseeva, T.; Hibbitts, A.J.; Kneafsey, S.L.; Chen, G.; O'Brien, F.J. Controlling the dose-dependent, synergistic and temporal effects of NGF and GDNF by encapsulation in PLGA microparticles for use in nerve guidance conduits for the repair of large peripheral nerve defects. J. Control Release 2019, 304, 51–64. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Pilch, K.S.; van der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef]
- Corfas, G.; Velardez, M.O.; Ko, C.P.; Ratner, N.; Peles, E. Mechanisms and roles of axon-Schwann cell interactions. J. Neurosci. 2004, 24, 9250–9260. [Google Scholar] [CrossRef]
- Wang, Z.H.; Walter, G.F.; Gerhard, L. The expression of nerve growth factor receptor on Schwann cells and the effect of these cells on the regeneration of axons in traumatically injured human spinal cord. Acta Neuropathol. 1996, 91, 180–184. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Cana, A.; Baumgärtner, W.; Spitzbarth, I. p75 Neurotrophin Receptor: A Double-Edged Sword in Pathology and Regeneration of the Central Nervous System. Vet. Pathol. 2018, 55, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Chan, J.R.; Shooter, E.M. Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proc. Natl. Acad. Sci. USA 2004, 101, 8774–8779. [Google Scholar] [CrossRef] [PubMed]
- Câmara, C.; Brito, M.; Silveira, E.; Silva, D.; Simões, V.; Pontes, R. Histological analysis of low-intensity laser therapy effects in peripheral nerve regeneration in Wistar rats. Acta Cir. Bras. 2011, 26, 12–18. [Google Scholar] [CrossRef][Green Version]
- Moges, H.; Wu, X.; McCoy, J.; Vasconcelos, O.; Bryant, H.; Grunberg, N.; Anders, J. Effect of 810 nm light on nerve regeneration after autograft repair of severely injured rat median nerve. Lasers Surg. Med. 2011, 43, 901–906. [Google Scholar] [CrossRef]
- Shen, C.C.; Yang, Y.C.; Huang, T.B.; Chan, S.C.; Liu, B.S. Low-Level Laser-Accelerated Peripheral Nerve Regeneration within a Reinforced Nerve Conduit across a Large Gap of the Transected Sciatic Nerve in Rats. Evid. Based Complement. Alternat Med. 2013, 2013, 175629. [Google Scholar] [CrossRef]
- Takhtfooladi, M.; Sharif, D. A comparative study of red and blue light-emitting diodes and low-level laser in regeneration of the transected sciatic nerve after an end to end neurorrhaphy in rabbits. Lasers Med. Sci. 2015, 30, 2319–2324. [Google Scholar] [CrossRef]
- Martins, D.; Dos Santos, F.; Ciena, A.; Watanabe, I.; De Britto, L.; Lemos, J.; Chacur, M. Neuropeptide expression and morphometric differences in crushed alveolar inferior nerve of rats: Effects of photobiomodulation. Lasers Med. Sci. 2017, 32, 833–840. [Google Scholar] [CrossRef]
- Dias, F.J.; Cury, D.P.; Dias, P.E.; Borie, E.; Alarcón-Apablaza, J.; Lezcano, M.F.; Martínez-Rodríguez, P.; Vargas, D.; Gutiérrez, B.; Fazan, V.P.S. Effects of Low-Level Laser Therapy and Purified Natural Latex (Hevea brasiliensis) Protein on Injured Sciatic Nerve in Rodents: Morpho-Functional Analysis. Int. J. Mol. Sci. 2023, 24, 14031. [Google Scholar] [CrossRef]
- Lee, J.H.; Carpena, N.T.; Kim, S.; Lee, M.Y.; Jung, J.Y.; Choi, J.E. Photobiomodulation at a wavelength of 633 nm leads to faster functional recovery than 804 nm after facial nerve injury. J. Biophotonics 2021, 14, e202100159. [Google Scholar] [CrossRef] [PubMed]
- Van Breugel, H.; Bär, P. He-Ne laser irradiation affects proliferation of cultured rat Schwann cells in a dose-dependent manner. J. Neurocytol. 1993, 22, 185–190. [Google Scholar] [CrossRef]
- Chan, J.R.; Watkins, T.A.; Cosgaya, J.M.; Zhang, C.; Chen, L.; Reichardt, L.F.; Shooter, E.M.; Barres, B.A. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron 2004, 43, 183–191. [Google Scholar] [CrossRef]
- Mata, M.; Alessi, D.; Fink, D.J. S100 is preferentially distributed in myelin-forming Schwann cells. J. Neurocytol. 1990, 19, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Windebank, A.J. Differentiation-specific regulation of Schwann cell expression of the major myelin glycoprotein. Proc. Natl. Acad. Sci USA 1985, 82, 5987–5991. [Google Scholar] [CrossRef]
- Suzuki, T.; Kadoya, K.; Endo, T.; Iwasaki, N. Molecular and Regenerative Characterization of Repair and Non-repair Schwann Cells. Cell Mol. Neurobiol. 2023, 43, 2165–2178. [Google Scholar] [CrossRef]
- Feng, C.; Deng, L.; Yong, Y.Y.; Wu, J.M.; Qin, D.L.; Yu, L.; Zhou, X.G.; Wu, A.G. The Application of Biomaterials in Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 816. [Google Scholar] [CrossRef] [PubMed]
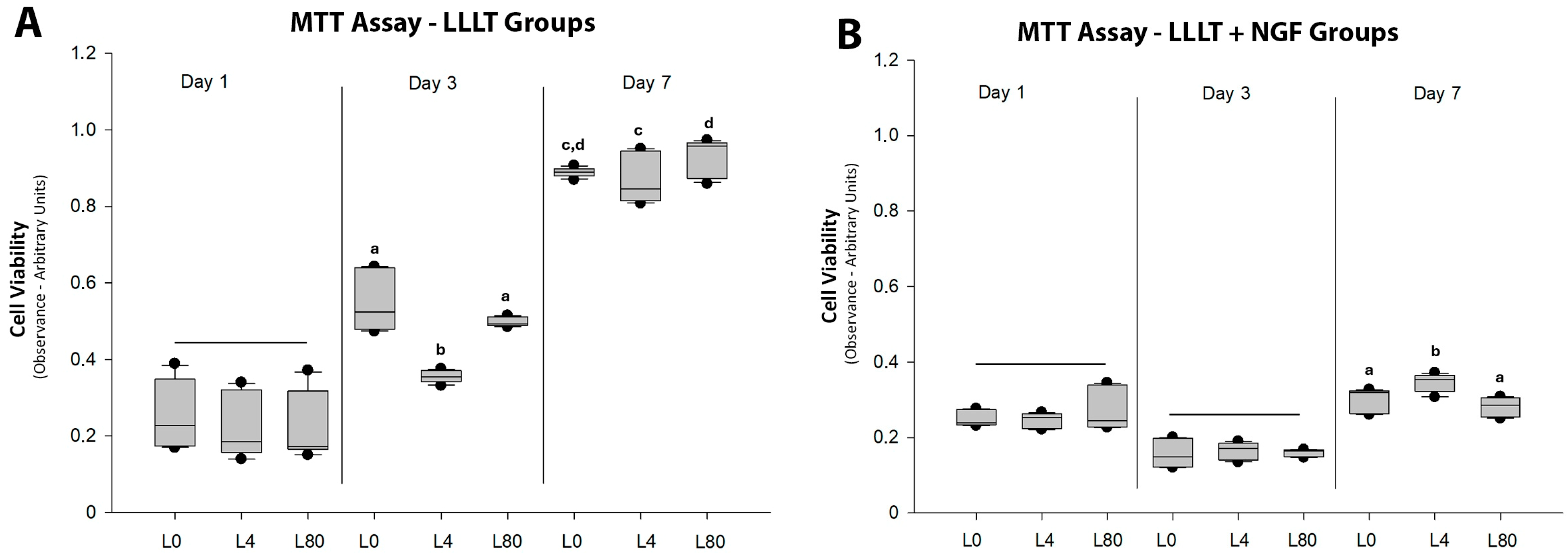
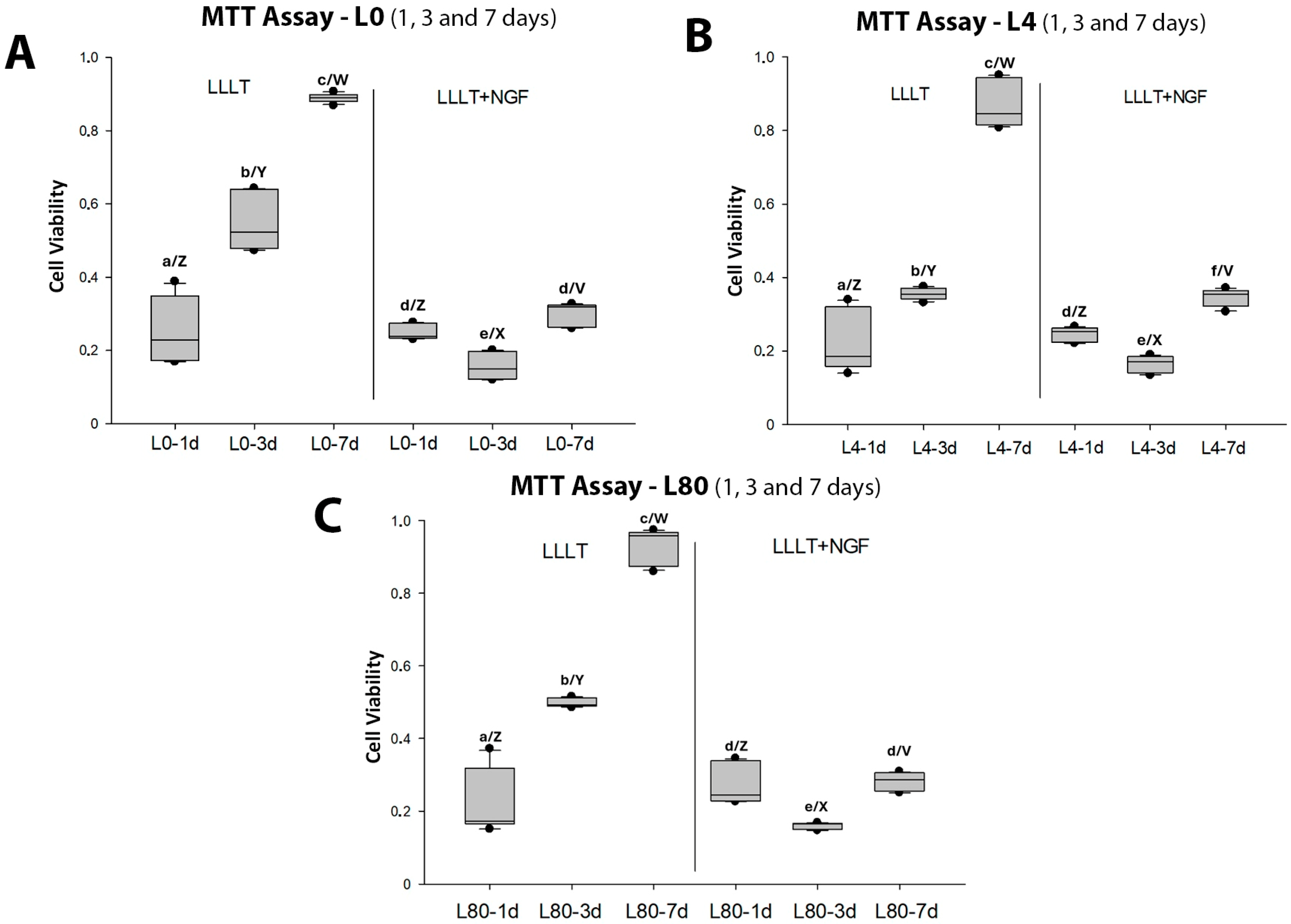
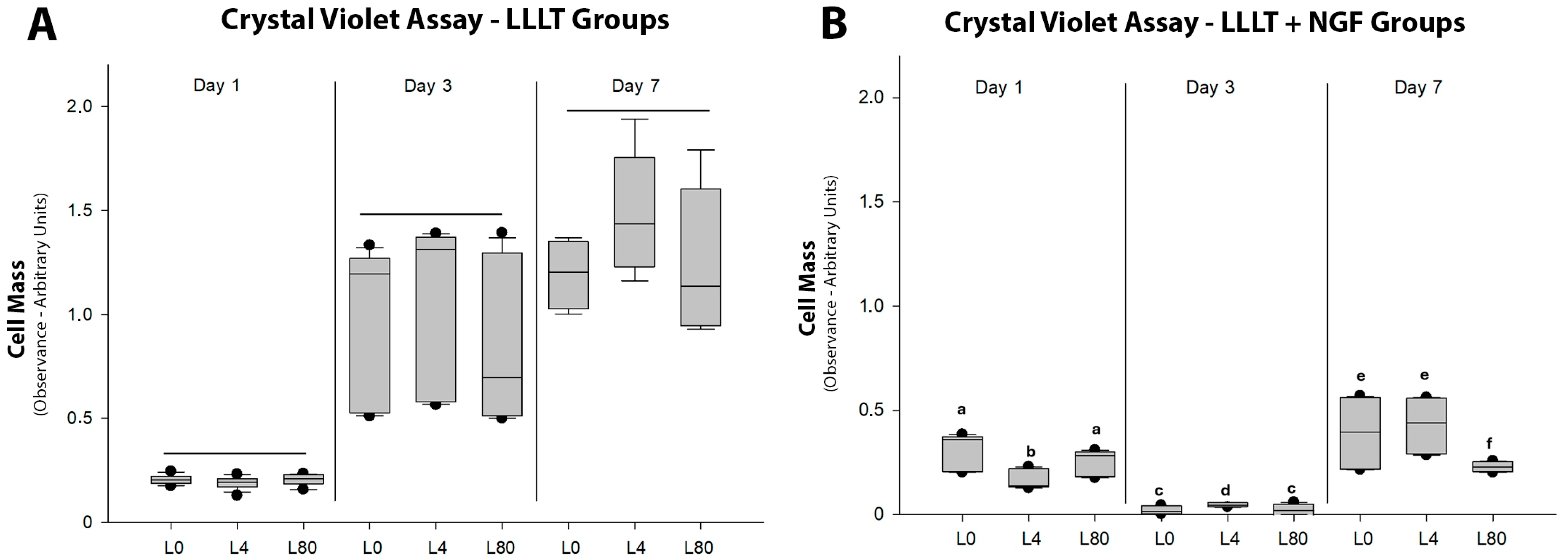

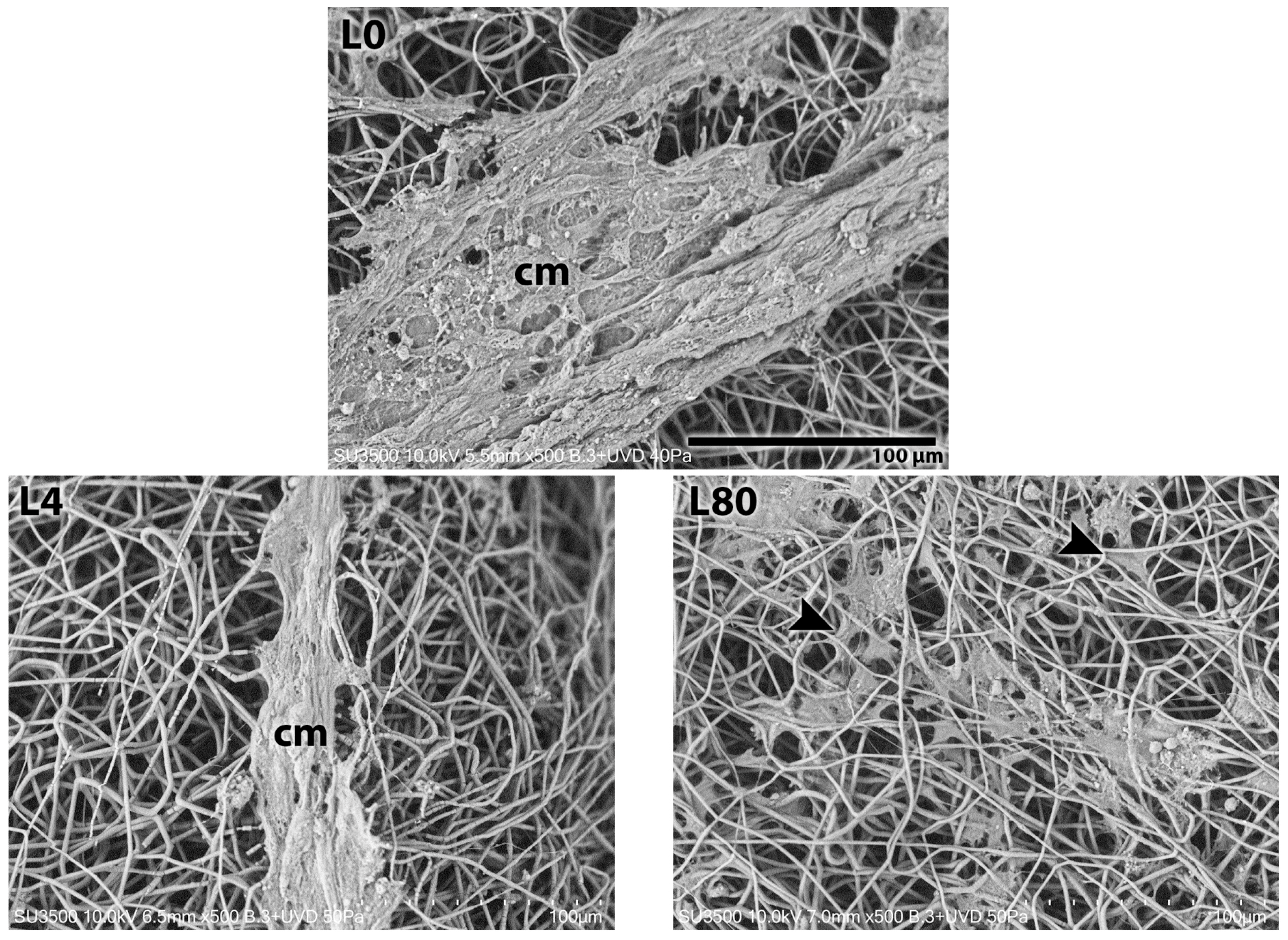
| Group | Counting (Number) | Mean ± SD (μm) | Sum (μm) | Min. (μm) | Max. (μm) |
|---|---|---|---|---|---|
| C-1d | 0 | 0 | 0 | 0 | 0 |
| L4-1d | 7 | 61.5 ± 62.0 | 492.0 | 18.8 | 188.3 |
| L80-1d | 32 | 44.2 ± 26.3 | 1415.6 | 8.8 | 141.4 |
| L4 + NGF-1d | 40 | 33.4 ± 21.0 | 1335.7 | 7.3 | 99.5 |
| L80 + NGF-1d | 42 | 40.2 ± 29.3 | 1686.2 | 8.9 | 142.1 |
| C-3d | 10 | 19.4 ± 11.4 | 193.7 | 4.7 | 44.2 |
| L4-3d | 42 | 53.9 ± 37.1 * | 2262.4 | 8.3 | 179.9 |
| L80-3d | 35 | 29.5 ± 17.9 a | 1033.3 | 5.7 | 64.9 |
| L4 + NGF-3d | 94 | 27.2 ± 16.7 | 2556.4 | 7.8 | 105.7 |
| L80 + NGF-3d | 69 | 30.5 ± 18.8 | 2104.7 | 10.6 | 91.8 |
| C-7d | 61 | 34.4 ± 21.6 | 2098.0 | 8.0 | 133.2 |
| L4-7d | 61 | 49.14 ± 32.5 | 2997.3 | 5.2 | 177.9 |
| L80-7d | 79 | 51.1 ± 40.4 | 4034.4 | 6.8 | 205.3 |
| L4 + NGF-7d | 72 | 43.5 ± 26.8 b | 3129.7 | 9.9 | 145.7 |
| L80 + NGF-7d | 48 | 43.4 ± 36.9 | 2082.4 | 7.7 | 164.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quidel-Necul, B.E.; Martínez-Rodríguez, P.; Godoy Sanchéz, K.; Nascimento, G.C.; de Paula, B.B.; Borie, E.; Dias, F.J. Modulating the Behavior of Schwann Cells with NGF Exposure Combined with Different Energy Densities of Photobiomodulation Cultured on Polyhydroxybutyrate (PHB) Scaffolds. Polymers 2025, 17, 2900. https://doi.org/10.3390/polym17212900
Quidel-Necul BE, Martínez-Rodríguez P, Godoy Sanchéz K, Nascimento GC, de Paula BB, Borie E, Dias FJ. Modulating the Behavior of Schwann Cells with NGF Exposure Combined with Different Energy Densities of Photobiomodulation Cultured on Polyhydroxybutyrate (PHB) Scaffolds. Polymers. 2025; 17(21):2900. https://doi.org/10.3390/polym17212900
Chicago/Turabian StyleQuidel-Necul, Bryan Enoc, Paulina Martínez-Rodríguez, Karina Godoy Sanchéz, Glauce Crivelaro Nascimento, Bruna Balbino de Paula, Eduardo Borie, and Fernando José Dias. 2025. "Modulating the Behavior of Schwann Cells with NGF Exposure Combined with Different Energy Densities of Photobiomodulation Cultured on Polyhydroxybutyrate (PHB) Scaffolds" Polymers 17, no. 21: 2900. https://doi.org/10.3390/polym17212900
APA StyleQuidel-Necul, B. E., Martínez-Rodríguez, P., Godoy Sanchéz, K., Nascimento, G. C., de Paula, B. B., Borie, E., & Dias, F. J. (2025). Modulating the Behavior of Schwann Cells with NGF Exposure Combined with Different Energy Densities of Photobiomodulation Cultured on Polyhydroxybutyrate (PHB) Scaffolds. Polymers, 17(21), 2900. https://doi.org/10.3390/polym17212900










