Porphyrin-Based Bio-Sourced Materials for Water Depollution Under Light Exposure
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis of Porphyrin Derivatives
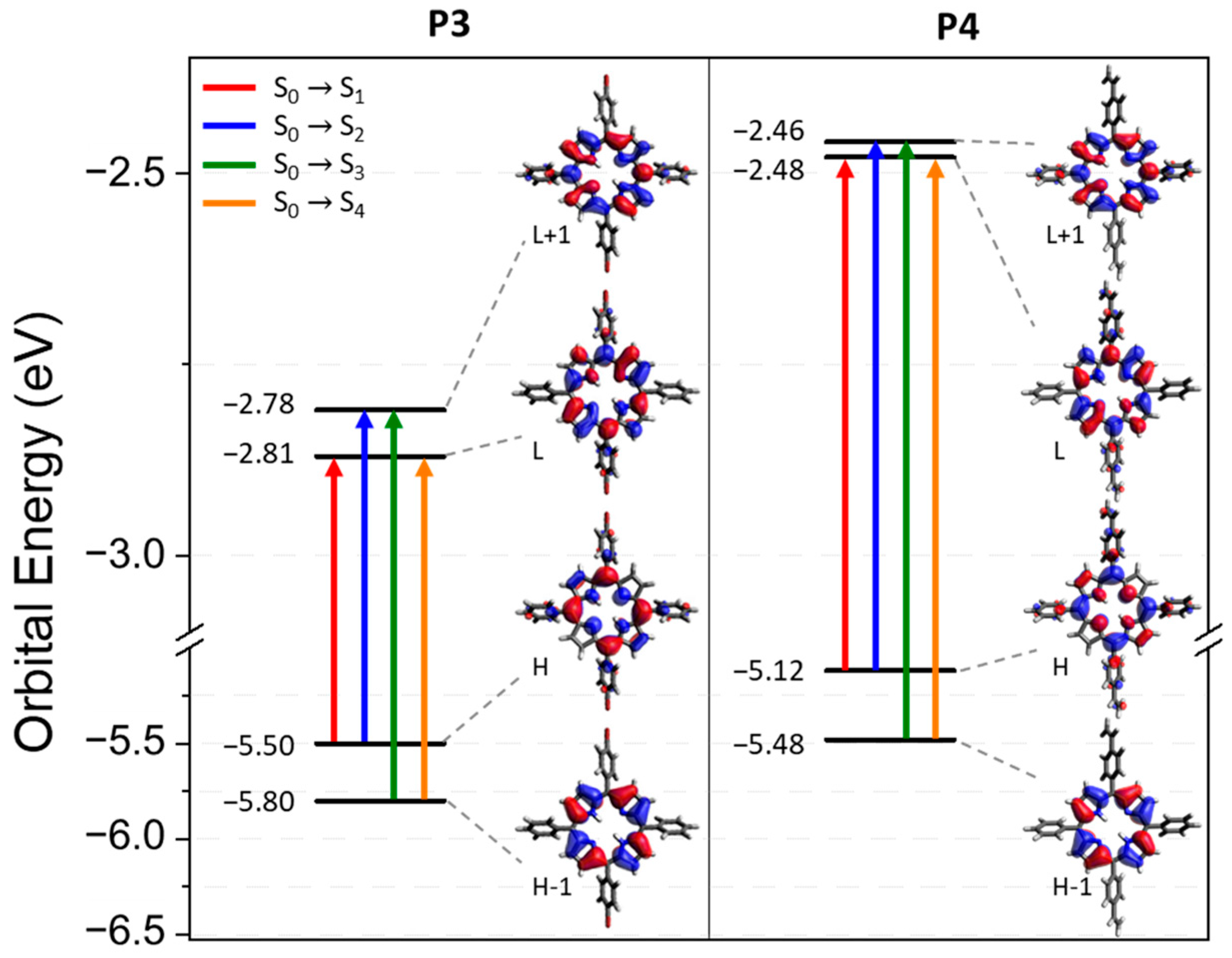
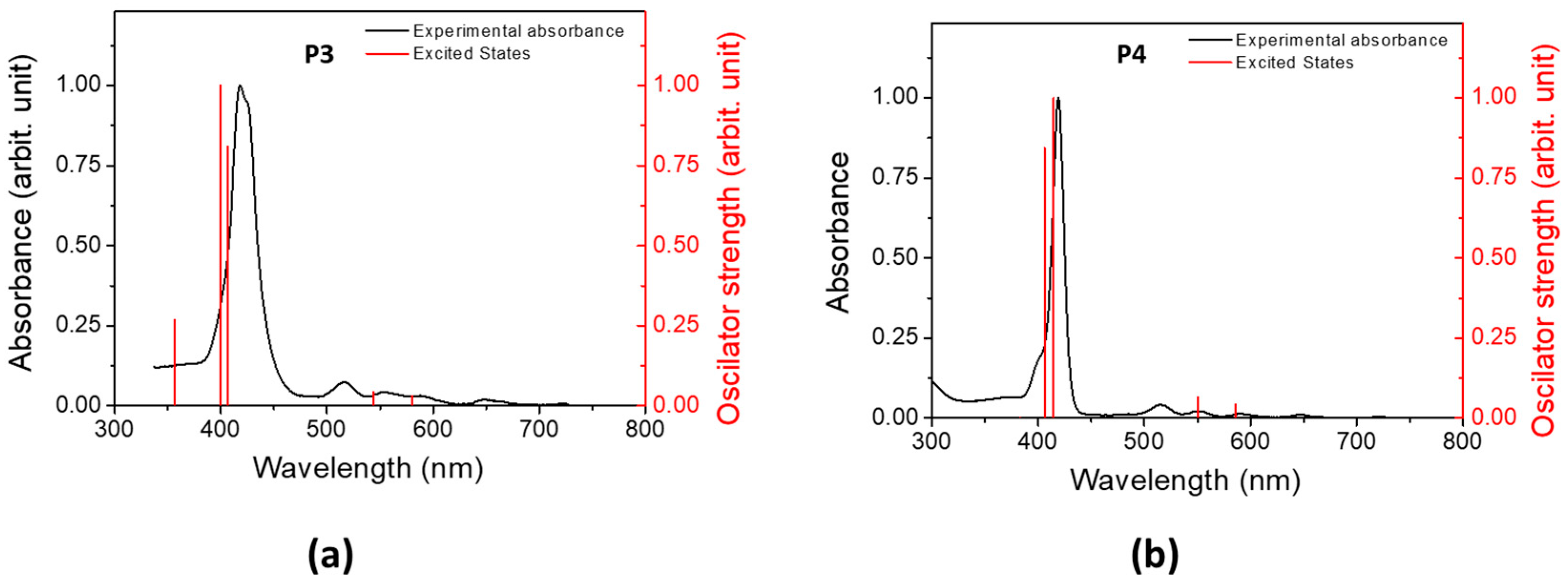
3.2. Computational Study and Absorbance Properties of Both Porphyrins
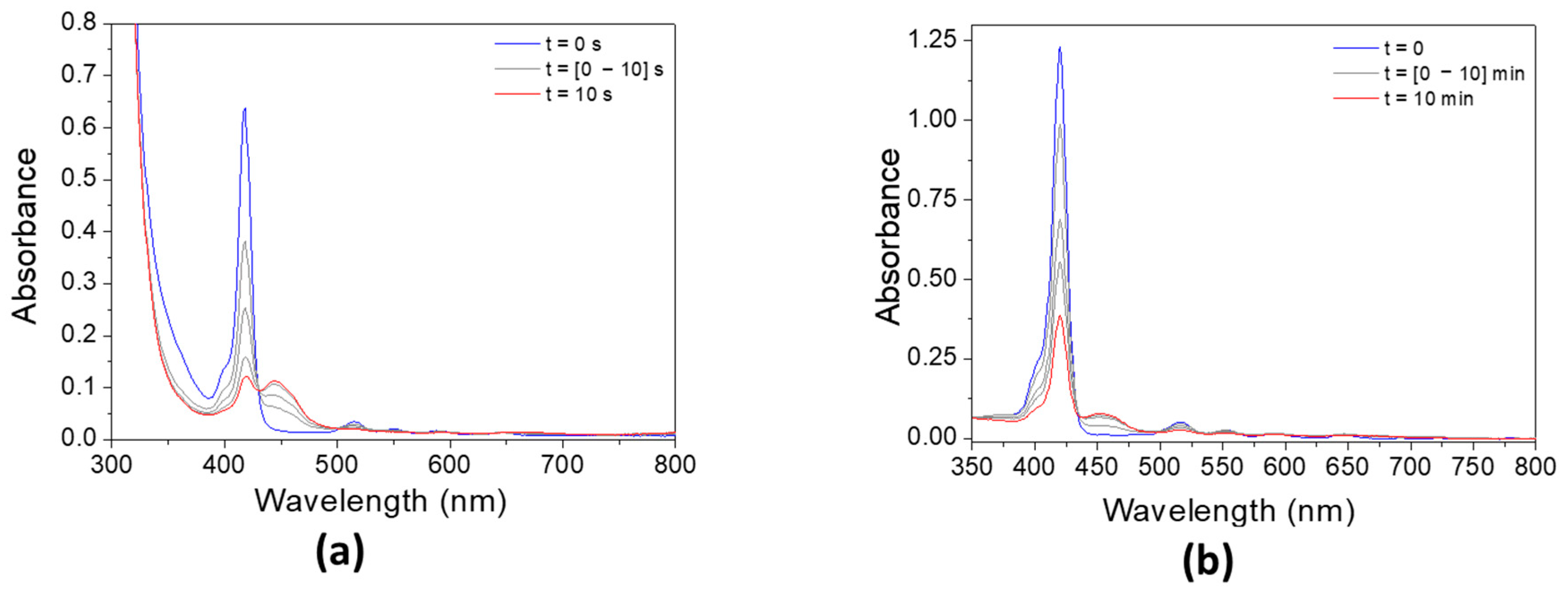
3.3. Experimental Absorbance and Fluorescence Spectra
3.4. Reactivity of P3 and P4-Based Photoinitiating Systems Under Light Irradiation
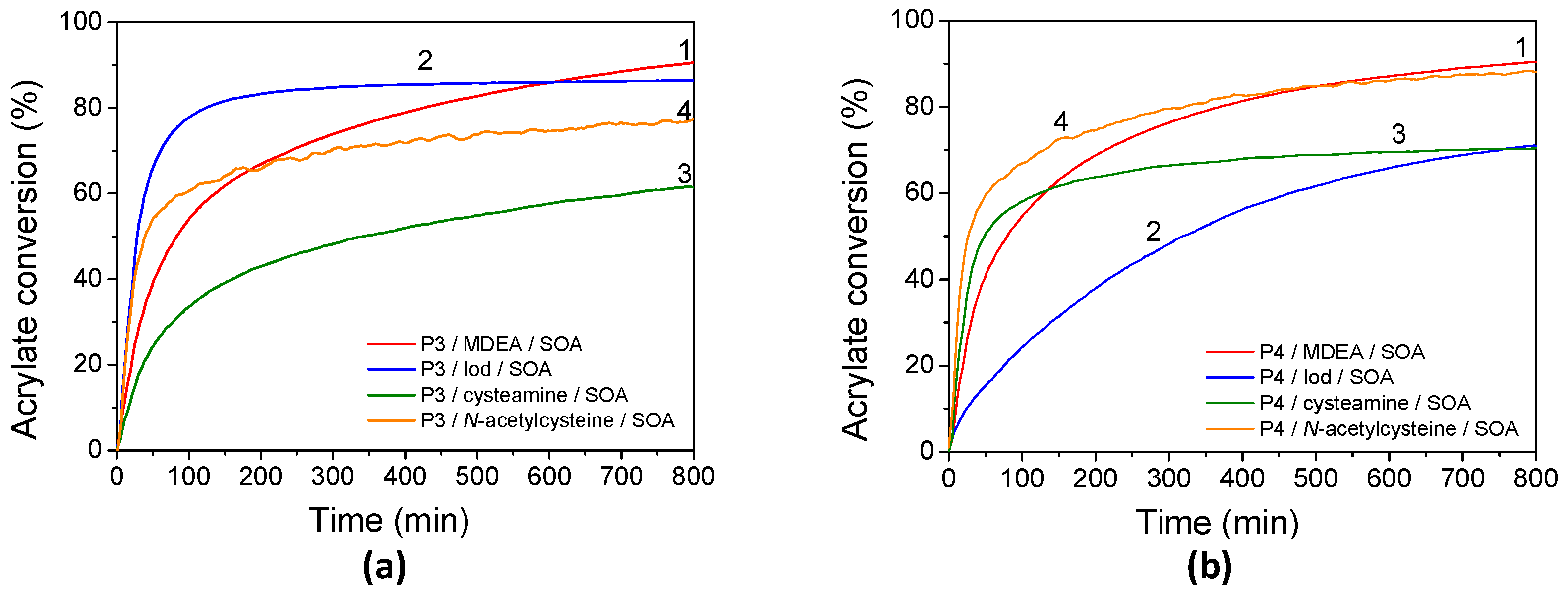
3.4.1. Effect of the Addition of MDEA
3.4.2. Effect of the Addition of Iod
3.4.3. Effect of the Addition of Cysteamine
3.4.4. Effect of the Addition of N-Acetylcysteine (NAC)
3.5. Free-Radical Polymerization of SOA
3.6. Formation of ROS Under UV Light
3.7. Photo-Oxidation of AR14
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOP | Advanced oxidation process |
| AR14 | Acid red 14 |
| DCM | Dichloromethane |
| DDQ | 2,3-dichloro-5,6-dicyano-1,4-benzoquinone |
| DFT | Density Functional Theory |
| DMPO | 5,5-dimethylpyrroline-N-oxide |
| EPR ST | Electron paramagnetic resonance spin trapping |
| FRP | Free-radical photopolymerization |
| Iod | 4-(2-methylpropyl)phenyliodonium hexafluorophosphate |
| LED | Light-emitting diode |
| MDEA | N-methyldiethanolamine |
| NAC | N-acetylcysteine |
| NBS | N-bromosuccinimide |
| NMR | Nuclear Magnetic Resonance |
| PET-RAFT | Photoinduced electron/energy transfer reversible addition–fragmentation chain transfer |
| POP | Porous organic polymer |
| ROS | Reactive oxygen species |
| RT-FTIR | Real-Time Fourier-Transformed Infrared spectroscopy |
| SOA | Soybean oil acrylate |
| TD-DFT | Time-Dependent Density Functional Theory |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| TLC | Thin-layer chromatography |
| TPCPD | Tetraphenylcyclopentadienone |
| UV | Ultraviolet |
References
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Chan, B.P. Biomedical Applications of Photochemistry. Tissue Eng. Part B Rev. 2010, 16, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Claus, H. Ozone Generation by Ultraviolet Lamps†. Photochem. Photobiol. 2021, 97, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The Impact of Ultraviolet Radiation on Skin Photoaging—Review of in Vitro Studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, H. Changes of Polymer Morphology Caused by u.v. Irradiation: 2. Surface Destruction of Polymer Blends. Polymer 1996, 37, 547–553. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Contreras, A.; Andaloussi, S.A.; Coradin, T.; Hélary, C.; Razza, N.; Sangermano, M.; Mazeran, P.-E.; Malval, J.-P.; Versace, D.-L. Eosin-Mediated Synthesis of Polymer Coatings Combining Photodynamic Inactivation and Antimicrobial Properties. J. Mater. Chem. B 2017, 5, 7572–7582. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Brezová, V.; Malval, J.-P.; Chiappone, A.; Breloy, L.; Abbad-Andaloussi, S.; Versace, D.-L. Purpurin Derivatives as Visible-Light Photosensitizers for 3D Printing and Valuable Biological Applications. Polym. Chem. 2021, 12, 2627–2642. [Google Scholar] [CrossRef]
- Versace, D.-L.; Moran, G.; Belqat, M.; Spangenberg, A.; Méallet-Renault, R.; Abbad-Andaloussi, S.; Brezová, V.; Malval, J.-P. Highly Virulent Bactericidal Effects of Curcumin-Based μ-Cages Fabricated by Two-Photon Polymerization. ACS Appl. Mater. Interfaces 2020, 12, 5050–5057. [Google Scholar] [CrossRef]
- Elian, C.; Quienne, B.; Lajnef, S.; Peyrot, F.; Moilleron, R.; Abbad Andaloussi, S.; Caillol, S.; Versace, D.-L. Photoactivable Alizarin and Eugenol-Based Materials for Antibacterial Applications. Eur. Polym. J. 2023, 197, 112369. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Malval, J.-P.; Weiss-Maurin, M.; Paul, J.; Blacha-Grzechnik, A.; Tomane, S.; Mazeran, P.-E.; Lalevée, J.; Langlois, V.; Versace, D.-L. Paprika, Gallic Acid, and Visible Light: The Green Combination for the Synthesis of Biocide Coatings. ACS Sustain. Chem. Eng. 2018, 6, 104–109. [Google Scholar] [CrossRef]
- Balta, D.K.; Temel, G.; Goksu, G.; Ocal, N.; Arsu, N. Thioxanthone–Diphenyl Anthracene: Visible Light Photoinitiator. Macromolecules 2012, 45, 119–125. [Google Scholar] [CrossRef]
- Breloy, L.; Brezová, V.; Blacha-Grzechnik, A.; Presset, M.; Yildirim, M.S.; Yilmaz, I.; Yagci, Y.; Versace, D.-L. Visible Light Anthraquinone Functional Phthalocyanine Photoinitiator for Free-Radical and Cationic Polymerizations. Macromolecules 2020, 53, 112–124. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Poriel, C.; Dumur, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.P.; Lalevée, J. Zinc Tetraphenylporphyrin as High Performance Visible Light Photoinitiator of Cationic Photosensitive Resins for LED Projector 3D Printing Applications. Macromolecules 2017, 50, 746–753. [Google Scholar] [CrossRef]
- Kim, D.; Stansbury, J.W. A Photo-Oxidizable Kinetic Pathway of Three-Component Photoinitiator Systems Containing Porphrin Dye (Zn-Tpp), an Electron Donor and Diphenyl Iodonium Salt: A Photo-Oxidizable Kinetic Pathway. J. Polym. Sci. A Polym. Chem. 2009, 47, 3131–3141. [Google Scholar] [CrossRef]
- Marcille, H.; Malval, J.-P.; Presset, M.; Bogliotti, N.; Blacha-Grzechnik, A.; Brezová, V.; Yagci, Y.; Versace, D.-L. Diphenyl Functional Porphyrins and Their Metal Complexes as Visible-Light Photoinitiators for Free-Radical, Cationic and Thiol–Ene Polymerizations. Polym. Chem. 2020, 11, 4237–4249. [Google Scholar] [CrossRef]
- Noirbent, G.; Xu, Y.; Bonardi, A.-H.; Gigmes, D.; Lalevée, J.; Dumur, F. Metalated Porphyrins as Versatile Visible Light and NIR Photoinitiators of Polymerization. Eur. Polym. J. 2020, 139, 110019. [Google Scholar] [CrossRef]
- Wayland, B.B.; Poszmik, G.; Mukerjee, S.L.; Fryd, M. Living Radical Polymerization of Acrylates by Organocobalt Porphyrin Complexes. J. Am. Chem. Soc. 1994, 116, 7943–7944. [Google Scholar] [CrossRef]
- Schnetz, F.; Knysh, I.; Jacquemin, D.; Andaloussi, S.A.; Presset, M.; Lajnef, S.; Peyrot, F.; Versace, D.-L. Porphyrin-Based Photosensitizers for Visible-Light Polymerization and Antibacterial Applications. Polym. Chem. 2024, 15, 1377–1392. [Google Scholar] [CrossRef]
- Schnetz, F.; Versace, D.-L.; Richeter, S. Porphyrin Derivatives: Promising Perspectives in Visible/IR Light Photopolymerization. Polym. Chem. 2025, 16, 1732–1791. [Google Scholar] [CrossRef]
- Corrigan, N.; Rosli, D.; Jones, J.W.J.; Xu, J.; Boyer, C. Oxygen Tolerance in Living Radical Polymerization: Investigation of Mechanism and Implementation in Continuous Flow Polymerization. Macromolecules 2016, 49, 6779–6789. [Google Scholar] [CrossRef]
- Ng, G.; Jung, K.; Li, J.; Wu, C.; Zhang, L.; Boyer, C. Screening RAFT Agents and Photocatalysts to Mediate PET-RAFT Polymerization Using a High Throughput Approach. Polym. Chem. 2021, 12, 6548–6560. [Google Scholar] [CrossRef]
- Shanmugam, S.; Xu, J.; Boyer, C. A Logic Gate for External Regulation of Photopolymerization. Polym. Chem. 2016, 7, 6437–6449. [Google Scholar] [CrossRef]
- Shanmugam, S.; Xu, J.; Boyer, C. Exploiting Metalloporphyrins for Selective Living Radical Polymerization Tunable over Visible Wavelengths. J. Am. Chem. Soc. 2015, 137, 9174–9185. [Google Scholar] [CrossRef]
- Athar, M.; Mukhtar, H.; Bickers, D.R. Differential Role of Reactive Oxygen Intermediates in Photofrin-I- and Photofrin-II-Mediated Photoenhancement of Lipid Peroxidation in Epidermal Microsomal Membranes. J. Investig. Dermatol. 1988, 90, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, Q.; Liu, F.; Zhou, P.; Gu, Y.; Zeng, J.; An, J.; Dai, W.; Li, X. Hematoporphyrin Monomethyl Ether Photodynamic Damage on HeLa Cells by Means of Reactive Oxygen Species Production and Cytosolic Free Calcium Concentration Elevation. Cancer Lett. 2004, 216, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.M.; Silva, P.R.; Vono, L.L.R.; Fernandes, A.U.; Tada, D.B.; Baptista, M.S. Protoporphyrin IX Nanoparticle Carrier: Preparation, Optical Properties, and Singlet Oxygen Generation. Langmuir 2008, 24, 12534–12538. [Google Scholar] [CrossRef]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. Photodynamic Therapeutics: Basic Principles and Clinical Applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef]
- Zhou, H.; Smith, D.W. Advanced Technologies in Water and Wastewater Treatment. Can. J. Civ. Eng. 2001, 28, 49–66. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, Z.; Shi, H.; Wang, D. UV Photodegradation of Azo Dye Diacryl Red X-GRL. J. Photochem. Photobiol. A Chem. 2005, 171, 97–106. [Google Scholar] [CrossRef]
- Langhals, H. Color Chemistry. Synthesis, Properties and Applications of Organic Dyes and Pigments. 3rd Revised Edition. By Heinrich Zollinger. Ang. Chem. Int. Ed. 2004, 43, 5291–5292. [Google Scholar] [CrossRef]
- Wang, A.; Qu, J.; Ru, J.; Liu, H.; Ge, J. Mineralization of an Azo Dye Acid Red 14 by Electro-Fenton’s Reagent Using an Activated Carbon Fiber Cathode. Dye. Pigment. 2005, 65, 227–233. [Google Scholar] [CrossRef]
- Hsueh, C.L.; Huang, Y.H.; Wang, C.C.; Chen, C.Y. Degradation of Azo Dyes Using Low Iron Concentration of Fenton and Fenton-like System. Chemosphere 2005, 58, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Lodha, B.; Chaudhari, S. Optimization of Fenton-Biological Treatment Scheme for the Treatment of Aqueous Dye Solutions. J. Hazard. Mater. 2007, 148, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Muruganandham, M.; Swaminathan, M. Decolourisation of Reactive Orange 4 by Fenton and Photo-Fenton Oxidation Technology. Dye. Pigment. 2004, 63, 315–321. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, L.-C.; Lin, H. A Novel Kind of Thin Film Composite Nanofiltration Membrane with Sulfated Chitosan as the Active Layer Material. Chem. Eng. Sci. 2013, 87, 152–159. [Google Scholar] [CrossRef]
- Huang, L.; He, M.; Chen, B.; Cheng, Q.; Hu, B. Facile Green Synthesis of Magnetic Porous Organic Polymers for Rapid Removal and Separation of Methylene Blue. ACS Sustain. Chem. Eng. 2017, 5, 4050–4055. [Google Scholar] [CrossRef]
- Asgharinezhad, A.A.; Ebrahimzadeh, H. A Simple and Fast Method Based on Mixed Hemimicelles Coated Magnetite Nanoparticles for Simultaneous Extraction of Acidic and Basic Pollutants. Anal. Bioanal. Chem. 2016, 408, 473–486. [Google Scholar] [CrossRef]
- Arola, K.; Ward, A.; Mänttäri, M.; Kallioinen, M.; Batstone, D. Transport of Pharmaceuticals during Electrodialysis Treatment of Wastewater. Water Res. 2019, 161, 496–504. [Google Scholar] [CrossRef]
- Slokar, Y.M.; Majcen Le Marechal, A. Methods of Decoloration of Textile Wastewaters. Dye. Pigment. 1998, 37, 335–356. [Google Scholar] [CrossRef]
- Galindo, C.; Jacques, P.; Kalt, A. Photooxidation of the Phenylazonaphthol AO20 on TIO2: Kinetic and Mechanistic Investigations. Chemosphere 2001, 45, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Tünay, O.; Kabdasli, I.; Eremektar, G.; Orhon, D. Color Removal from Textile Wastewaters. Water Sci. Technol. 1996, 34, 9–16. [Google Scholar] [CrossRef]
- Kuo, W.S.; Ho, P.H. Solar Photocatalytic Decolorization of Methylene Blue in Water. Chemosphere 2001, 45, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical Processes for Water Treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Carra, I.; Malato, S.; Jiménez, M.; Maldonado, M.I.; Sánchez Pérez, J.A. Microcontaminant Removal by Solar Photo-Fenton at Natural pH Run with Sequential and Continuous Iron Additions. Chem. Eng. J. 2014, 235, 132–140. [Google Scholar] [CrossRef]
- Zhao, W.; Liang, C.; Wang, B.; Xing, S. Enhanced Photocatalytic and Fenton-like Performance of CuOx-Decorated ZnFe2O4. ACS Appl. Mater. Interf. 2017, 9, 41927–41936. [Google Scholar] [CrossRef]
- Perkowski, J.; Ledakowicz, S. Decomposition of Anthraquinone Dye in the Aqueous Solution by Ozone, Hydrogen Peroxide or UV Radiation. Fibres Text. East. Eur. 2002, 10, 72–77. [Google Scholar]
- Kang, S.-F.; Liao, C.-H.; Chen, M.-C. Pre-Oxidation and Coagulation of Textile Wastewater by the Fenton Process. Chemosphere 2002, 46, 923–928. [Google Scholar] [CrossRef]
- Neamţu, M.; Catrinescu, C.; Kettrup, A. Effect of Dealumination of Iron(III)—Exchanged Y Zeolites on Oxidation of Reactive Yellow 84 Azo Dye in the Presence of Hydrogen Peroxide. Appl. Catal. B 2004, 51, 149–157. [Google Scholar] [CrossRef]
- Guzmán-Vargas, A.; Delahay, G.; Coq, B.; Lima, E.; Bosch, P.; Jumas, J.-C. Influence of the Preparation Method on the Properties of Fe-ZSM-5 for the Selective Catalytic Reduction of NO by n-Decane. Catal. Today 2005, 107–108, 94–99. [Google Scholar] [CrossRef]
- Makhotkina, O.A.; Kuznetsova, E.V.; Preis, S.V. Catalytic Detoxification of 1,1-Dimethylhydrazine Aqueous Solutions in Heterogeneous Fenton System. Appl. Catal. B 2006, 68, 85–91. [Google Scholar] [CrossRef]
- Idel-aouad, R.; Valiente, M.; Yaacoubi, A.; Tanouti, B.; López-Mesas, M. Rapid Decolourization and Mineralization of the Azo Dye C.I. Acid Red 14 by Heterogeneous Fenton Reaction. J. Hazard. Mater. 2011, 186, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, T.; Liu, Y.; Cheng, F.; Zhang, M.; Guo, M. Novel Efficient Heterogeneous Visible Light Assisted Fenton-like Catalyst (Ni,Mg,Cu)Fe2O4 from Nickel Sulfide Concentrate. Mater. Lett. 2019, 253, 1–4. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, W.; Zhao, Y.; Zhang, G.; Zhang, W. Enhanced Catalytic Degradation of Methylene Blue by α-Fe2O3/Graphene Oxide via Heterogeneous Photo-Fenton Reactions. Appl. Catal. B 2017, 206, 642–652. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, L.; Lei, J.; Liu, Y.; Zhang, J. Photo-Fenton Degradation of Phenol by CdS/rGO/Fe2+ at Natural pH with in Situ-Generated H2O2. Appl. Catal. B 2019, 241, 367–374. [Google Scholar] [CrossRef]
- Liu, X.; Yan, Z.; Zhang, Y.; Liu, Z.; Sun, Y.; Ren, J.; Qu, X. Two-Dimensional Metal–Organic Framework/Enzyme Hybrid Nanocatalyst as a Benign and Self-Activated Cascade Reagent for in Vivo Wound Healing. ACS Nano 2019, 13, 5222–5230. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic Degradation of Azo Dye Acid Red 14 in Water: Investigation of the Effect of Operational Parameters. J. Photochem. Photobiol. A Chem. 2003, 157, 111–116. [Google Scholar] [CrossRef]
- Nouacer, S.; Djellabi, R. Easy-Handling Semi-Floating TiO2-Based Aerogel for Solar Photocatalytic Water Depollution. Environ. Sci. Pollut. Res. 2022, 30, 22388–22395. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Le Pivert, M.; Zerelli, B.; Martin, N.; Capochichi-Gnambodoe, M.; Leprince-Wang, Y. Smart ZnO Decorated Optimized Engineering Materials for Water Purification under Natural Sunlight. Constr. Build. Mater. 2020, 257, 119592. [Google Scholar] [CrossRef]
- Martin, N.; Lacour, V.; Perrault, C.M.-T.; Roy, E.; Leprince-Wang, Y. High Flow Rate Microreactors Integrating in Situ Grown ZnO Nanowires for Photocatalytic Degradation. React. Chem. Eng. 2022, 7, 750–757. [Google Scholar] [CrossRef]
- Habba, Y.G.; Capochichi-Gnambodoe, M.; Serairi, L.; Leprince-Wang, Y. Enhanced Photocatalytic Activity of ZnO Nanostructure for Water Purification. Phys. Status Solidi B Basic Res. 2016, 253, 1480–1484. [Google Scholar] [CrossRef]
- Leprince-Wang, Y.; Martin, N.; Ghozlane Habba, Y.; Pivert, M.L.; Capochichi-Gnambodoe, M. ZnO Nanostructure Based Photocatalysis for Water Purification. NanoWorld J. 2020, 6, 1–6. [Google Scholar] [CrossRef]
- Le Pivert, M.; Poupart, R.; Capochichi-Gnambodoe, M.; Martin, N.; Leprince-Wang, Y. Direct Growth of ZnO Nanowires on Civil Engineering Materials: Smart Materials for Supported Photodegradation. Microsyst. Nanoeng. 2019, 5, 57. [Google Scholar] [CrossRef]
- Wang, M.-R.; Deng, L.; Liu, G.-C.; Wen, L.; Wang, J.-G.; Huang, K.-B.; Tang, H.-T.; Pan, Y.-M. Porous Organic Polymer-Derived Nanopalladium Catalysts for Chemoselective Synthesis of Antitumor Benzofuro [2,3-b]Pyrazine from 2-Bromophenol and Isonitriles. Org. Lett. 2019, 21, 4929–4932. [Google Scholar] [CrossRef]
- Shit, S.C.; Koley, P.; Joseph, B.; Marini, C.; Nakka, L.; Tardio, J.; Mondal, J. Porous Organic Polymer-Driven Evolution of High-Performance Cobalt Phosphide Hybrid Nanosheets as Vanillin Hydrodeoxygenation Catalyst. ACS Appl. Mater. Interf. 2019, 11, 24140–24153. [Google Scholar] [CrossRef]
- Leng, F.; Liu, H.; Ding, M.; Lin, Q.-P.; Jiang, H.-L. Boosting Photocatalytic Hydrogen Production of Porphyrinic MOFs: The Metal Location in Metalloporphyrin Matters. ACS Catal. 2018, 8, 4583–4590. [Google Scholar] [CrossRef]
- Liu, T.-T.; Liang, J.; Huang, Y.-B.; Cao, R. A Bifunctional Cationic Porous Organic Polymer Based on a Salen-(Al) Metalloligand for the Cycloaddition of Carbon Dioxide to Produce Cyclic Carbonates. Chem. Commun. 2016, 52, 13288–13291. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Breloy, L.; Brezová, V.; Malval, J.-P.; Rios de Anda, A.; Bourgon, J.; Kurogi, T.; Mindiola, D.J.; Versace, D.-L. Well-Defined Titanium Complex for Free-Radical and Cationic Photopolymerizations under Visible Light and Photoinduction of Ti-Based Nanoparticles. Macromolecules 2019, 52, 3716–3729. [Google Scholar] [CrossRef]
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and Functionalization of Porphyrins through Organometallic Methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef]
- Vaz, B.; Alvarez, R.; Nieto, M.; Paniello, A.I.; de Lera, A.R. Suzuki Cross-Coupling of Meso-Dibromoporphyrins for the Synthesis of Functionalized A2B2 Porphyrins. Tetrahedron Lett. 2001, 42, 7409–7412. [Google Scholar] [CrossRef]
- Boyle, R.W.; Bruckner, C.; Posakony, J.; James, B.R.; Dolphin, D. 5-Phenyldipyrromethane and 5,15-Diphenylporphyrin. In Organic Syntheses; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; p. 287. ISBN 978-0-471-26422-4. [Google Scholar]
- Nudy, L.R.; Hutchinson, H.G.; Schieber, C.; Longo, F.R. A Study of Bromoporphins. Tetrahedron 1984, 40, 2359–2363. [Google Scholar] [CrossRef]
- Locos, O.B.; Arnold, D.P. The Heck Reaction for Porphyrin Functionalisation: Synthesis of Meso-Alkenyl Monoporphyrins and Palladium-Catalysed Formation of Unprecedented Meso–β Ethene-Linked Diporphyrins. Org. Biomol. Chem. 2006, 004, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Gouterman, M.; Wagnière, G.H.; Snyder, L.C. Spectra of Porphyrins: Part II. Four Orbital Model. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar] [CrossRef]
- Mandal, A.K.; Taniguchi, M.; Diers, J.R.; Niedzwiedzki, D.M.; Kirmaier, C.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Photophysical Properties and Electronic Structure of Porphyrins Bearing Zero to Four Meso-Phenyl Substituents: New Insights into Seemingly Well Understood Tetrapyrroles. J. Phys. Chem. A 2016, 120, 9719–9731. [Google Scholar] [CrossRef]
- Boulmier, A.; Haouas, M.; Tomane, S.; Michely, L.; Dolbecq, A.; Vallée, A.; Brezová, V.; Versace, D.-L.; Mialane, P.; Oms, O. Photoactive Polyoxometalate/DASA Covalent Hybrids for Photopolymerization in the Visible Range. Chem. Eur. J. 2019, 25, 14349–14357. [Google Scholar] [CrossRef]
- Peyrot, F.; Lajnef, S.; Versace, D.-L. Electron Paramagnetic Resonance Spin Trapping (EPR–ST) Technique in Photopolymerization Processes. Catalysts 2022, 12, 772. [Google Scholar] [CrossRef]
- Conte, M.; Wilson, K.; Chechik, V. Radical Intermediates in Chloroform Reactions over Triphenylphosphine-Protected Au Nanoparticles. Org. Biomol. Chem. 2009, 7, 1361–1367. [Google Scholar] [CrossRef]
- O’Brien, A.K.; Cramer, N.B.; Bowman, C.N. Oxygen Inhibition in Thiol–Acrylate Photopolymerizations. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2007–2014. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–Enes: Chemistry of the Past with Promise for the Future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Elian, C.; Méallet, R.; Versace, D.-L. Photoactive Dye-Loaded Polymer Materials: A New Cutting Edge for Antibacterial Photodynamic Therapy. Adv. Funct. Mater. 2024, 34, 2407228. [Google Scholar] [CrossRef]
- Bonnett, R.; Martínez, G. Photobleaching of Sensitisers Used in Photodynamic Therapy. Tetrahedron 2001, 57, 9513–9547. [Google Scholar] [CrossRef]
- El-Hallag, I.S.; Al-Youbi, A.O.; Obaid, A.Y.; El-Mossalamy, E.H.; El-Daly, S.A.; Asiri, A.M. Electrochemical investigation of cysteamine at carbon fiber microdisk electrode. J. Chil. Chem. Soc. 2011, 56, 837–841. [Google Scholar] [CrossRef]







| Name | Chemical Structure |
|---|---|
| P3 5,15-diphenyl-10,20-bis(4-bromophenyl) porphyrin |  |
| P4 5,15-diphenyl-10,20-bis(4-vinylphenyl) porphyrin |  |
| MDEA N-methyldiethanol amine | 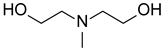 |
| Iod Bis(4-methylphenyl) iodonium hexafluorophosphate | 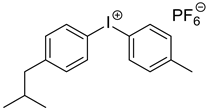 |
| Cysteamine | 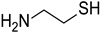 |
| N-acetylcysteine (NAC) | 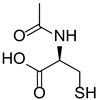 |
| SOA Soybean oil acrylate | 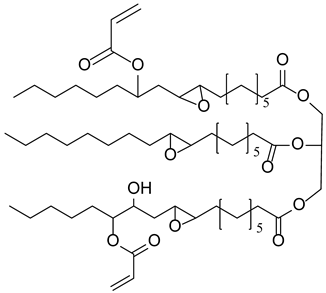 |
| Acid red 14 Disodium 4-hydroxy-2-[(E)-(4-sulfonato-1-naphthyl)diazenyl]naphthalene-1-sulfonate |  |
| Porphyrin | Soret Band: λabs/nm (ε/M−1·cm−1) | Q Bands: λabs/nm (ε/M−1·cm−1) | λem/nm (Intensity/Arb·Unit) | λphos/nm |
|---|---|---|---|---|
| P3 | 418 (62,825) | 516 (4660) | 653 (1), 717 (0.63) | 867 |
| 554 (2660) | ||||
| 588 (1993) | ||||
| 649 (1241) | ||||
| P4 | 419 (44,867) | 515 (1867) | 646 (1), 708 (0.86) | 867 |
| 551 (983) | ||||
| 589 (568) | ||||
| 646 (472) |
| MDEA | Iod | Cysteamine | N-Acetylcysteine (NAC) | |||||
|---|---|---|---|---|---|---|---|---|
| ΔGS | ΔGT | ΔGS | ΔGT | ΔGS | ΔGT | ΔGS | ΔGT | |
| P3 | +0.04 | +0.51 | −0.11 | +0.28 | +0.24 | +0.71 | +0.11 | +0.58 |
| P4 | −0.25 | +0.23 | −0.34 | +0.07 | −0.05 | +0.43 | −0.18 | +0.30 |
| Final Acrylate Conversion (%) of SOA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Photoinitiating systems | 385 nm | 405 nm | 455 nm | 530 nm | ||||
| P3/MDEA | 73a | 55 b | 91 a | 93 b | 64 a | 40 b | 43 a | 26 b |
| P4/MDEA | 76 a | 48 b | 91 a | 80 b | 66 a | 40 b | 47 a | 26 b |
| CQ/MDEA | 28 a | np b | 56 a | 38 b | 68 a | 44 b | 40 a | np b |
| P3/Iod | 77 a | 30 b | 86 a | 46 b | 66 a | 13 b | 70 a | 13 b |
| P4/Iod | 62 a | 28 b | 70 a | 46 b | 51 a | 9 b | 43 a | 2 b |
| CQ/Iod | 79 a | 13 b | 84 a | 51 b | 81 a | 64 b | 73 a | np b |
| P3/cysteamine | 47 a | 30 b | 61 a | 58 b | 29 a | 26 b | 21 a | 15 |
| P4/cysteamine | 45 a | 25 b | 70 a | 53 b | 31 a | 10 b | 17 a | np b |
| CQ/cysteamine | Polymerization at Rt | |||||||
| P3/NAC | 99 a | 37 b | 78 a | 96 b | 82 a | np b | 50 a | np b |
| P4/NAC | 92 a | np b | 78 a | 85 b | 92 a | np b | 61 a | np b |
| CQ/NAC | 78 a | 14 b | 98 a | 33 b | 95 a | 44 b | 52 a | np b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnetz, F.; Presset, M.; Malval, J.-P.; Leprince-Wang, Y.; Navizet, I.; Versace, D.-L. Porphyrin-Based Bio-Sourced Materials for Water Depollution Under Light Exposure. Polymers 2025, 17, 2882. https://doi.org/10.3390/polym17212882
Schnetz F, Presset M, Malval J-P, Leprince-Wang Y, Navizet I, Versace D-L. Porphyrin-Based Bio-Sourced Materials for Water Depollution Under Light Exposure. Polymers. 2025; 17(21):2882. https://doi.org/10.3390/polym17212882
Chicago/Turabian StyleSchnetz, Fanny, Marc Presset, Jean-Pierre Malval, Yamin Leprince-Wang, Isabelle Navizet, and Davy-Louis Versace. 2025. "Porphyrin-Based Bio-Sourced Materials for Water Depollution Under Light Exposure" Polymers 17, no. 21: 2882. https://doi.org/10.3390/polym17212882
APA StyleSchnetz, F., Presset, M., Malval, J.-P., Leprince-Wang, Y., Navizet, I., & Versace, D.-L. (2025). Porphyrin-Based Bio-Sourced Materials for Water Depollution Under Light Exposure. Polymers, 17(21), 2882. https://doi.org/10.3390/polym17212882








