Color-Tunable Intrinsically Black Polyimides: A Facile Strategy via In Situ Oxidation Color Control
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of 2,4-Dinitrodiphenylamine
2.3. Synthesis of 2,4-Diaminodiphenylamine
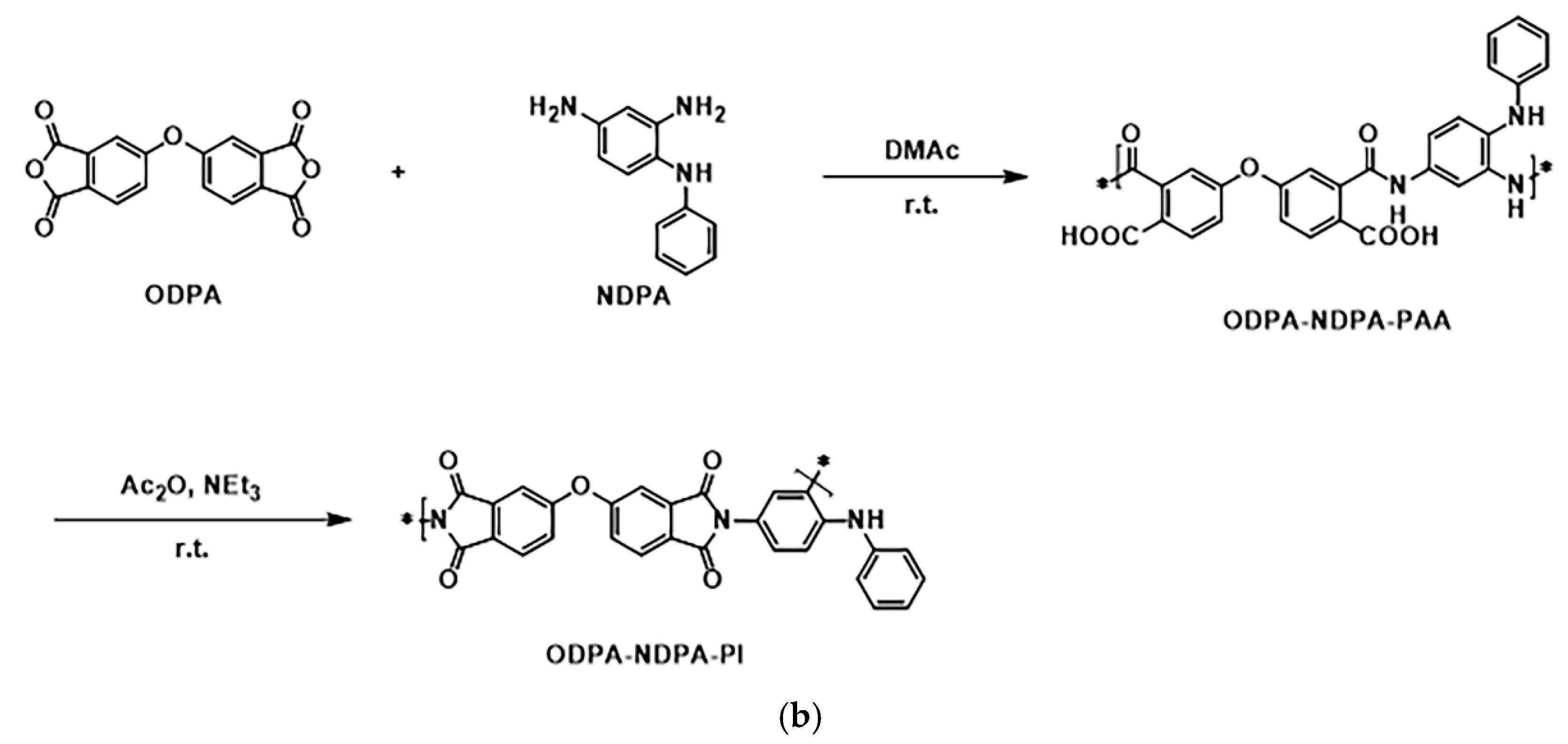
2.4. Synthesis of PI
2.5. Preparation of PI Films
2.6. Characterization
3. Result and Discussion
3.1. Synthesis and Characterization of NPDA
3.2. Synthesis and Characterization of PI
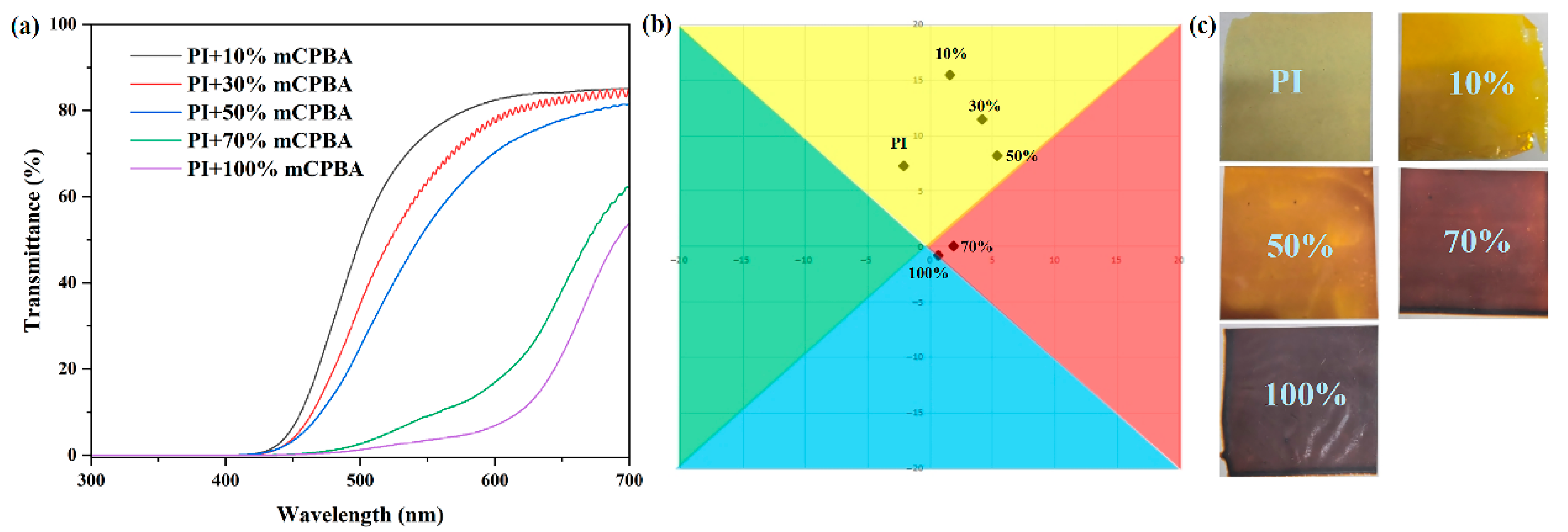
3.3. Effect of Reaction Conditions on Optical Properties
3.4. Oxidation-Dependent Optical Properties of PI
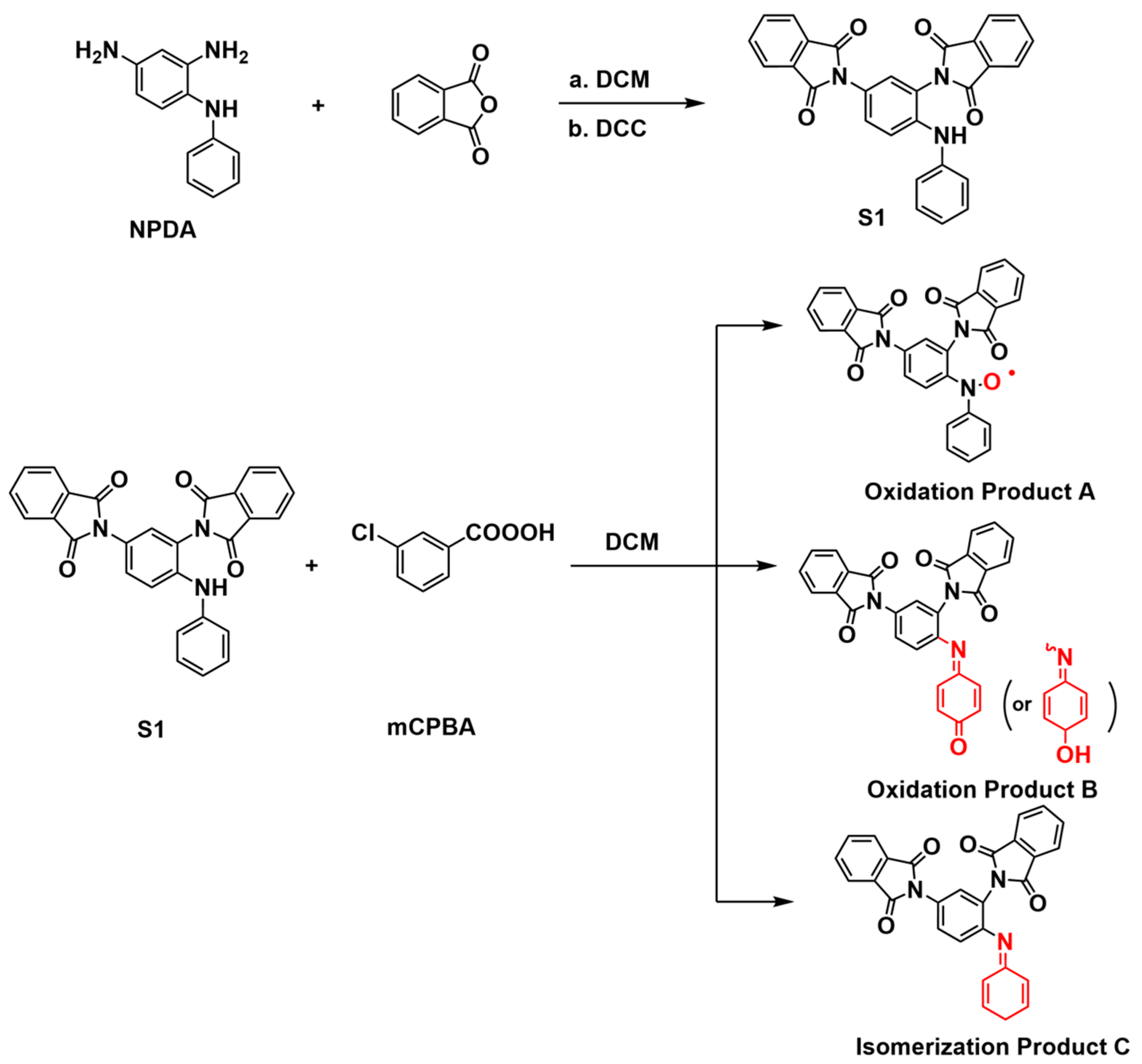
3.5. Mechanistic Investigation of mCPBA Oxidation
3.6. Thermal Properties and Mechanical Properties of PI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, X.; Zhang, F.; Leng, J. Metal mesh embedded in colorless shape memory polyimide for flexible transparent electric-heater and actuators. Appl. Mater. Today 2020, 21, 100797. [Google Scholar] [CrossRef]
- Tsurusaki, Y.; Sawada, R.; Liu, H.; Ando, S. Optical, Dielectric, and Thermal Properties of Bio-Based Polyimides Derived from An Isosorbide-Containing Diamine. Macromol. Rapid Commun. 2025, 46, 2401113. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, J.; Yang, H.; Yang, S. Progress in Aromatic Polyimide Films for Electronic Applications: Preparation, Structure and Properties. Polymers 2022, 14, 1269. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.; Wan, J.; Yuan, J.; Lu, Q. Precise colored 3D-structure of polyimide printed by digital light processing. Chem. Eng. J. 2025, 515, 163433. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.-Y.; Liu, J.-G.; Zhi, X.-X.; Huangfu, M.-G.; Jiang, G.-L.; Wu, X.; Zhang, X. Molecular design, synthesis and characterization of intrinsically black polyimide films with high thermal stability and good electrical properties. J. Polym. Res. 2019, 26, 171. [Google Scholar] [CrossRef]
- Ni, H.-J.; Liu, J.-G.; Wang, Z.-H.; Yang, S.-Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Liu, T.-Q.; Zheng, F.; Ding, T.-M.; Zhang, S.-Y.; Lu, Q. Design and synthesis of a novel quinoxaline diamine and its polyimides with high-Tg and red color. Polymer 2019, 179, 121612. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, T.; Qin, J.; Yan, J.; Wang, Z. Intrinsically Colored Polyimide Films Prepared by Embedding Chromophores on the Polymer Backbones. Macromol. Chem. Phys. 2024, 225, 2300405. [Google Scholar] [CrossRef]
- Han, S.; Ren, X.; Li, D.; Song, Z.; Yang, C.; Wang, Z.; Liu, J. Preparation and Characterizations of Intrinsically Black Polyesterimide Films with Good Thermal Endurance at Elevated Temperatures for Potential Two-Layer Flexible Copper Clad Laminate Applications. Polymers 2025, 17, 304. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Z.; Liu, P.; Su, Y.; Huang, T.; Liu, R.; Xi, X.; Wu, D. A facile in-situ polymerization strategy towards polyimide/carbon black composites as high performance lithium ion battery cathodes. Electrochim. Acta 2018, 260, 598–605. [Google Scholar] [CrossRef]
- Tan, J.; Xie, F.; Huang, J.; Li, H.; Liu, X.; Zhao, C.; Yuan, J.; Liu, Y. High-performance black polyimide with improved solubility for flexible printed circuit boards. J. Mater. Sci. 2023, 58, 16855–16867. [Google Scholar] [CrossRef]
- Tan, Y.-Y.; Zhang, Y.; Jiang, G.-L.; Zhi, X.-X.; Xiao, X.; Wu, L.; Jia, Y.-J.; Liu, J.-G.; Zhang, X.-M. Preparation and Properties of Inherently Black Polyimide Films with Extremely Low Coefficients of Thermal Expansion and Potential Applications for Black Flexible Copper Clad Laminates. Polymers 2020, 12, 576. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Ji, X.; Zhang, X. Suspending Light-Absorbing Nanoparticles in Silica Aerogel Enables Numerous Superblacks. Adv. Mater. 2025, 37, 2412385. [Google Scholar] [CrossRef]
- Kim, J.; Kim, G.; Kim, S.-Y.; Lee, S.; Kim, Y.; Lee, J.; Kim, J.; Jung, Y.C.; Kwon, J.; Han, H. Fabrication of highly flexible electromagnetic interference shielding polyimide carbon black composite using hot-pressing method. Compos. Part B Eng. 2021, 221, 109010. [Google Scholar] [CrossRef]
- Weng, L.; Liu, J.; Zhang, X.; Xu, H.; Guan, L.; Yu, Y. A facile in-situ fabrication of black polyimide films with ultrahigh electrical properties. J. Appl. Polym. Sci. 2024, 141, e55267. [Google Scholar] [CrossRef]
- Lin, J.-S.; Chiu, H.-T. Preparation and Properties of Conductive Polyimide Films. J. Polym. Res. 2002, 9, 189–194. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, J.-M.; Kim, J.-S.; Shim, E.-G.; Lee, S.-Y. Polyimide/carbon black composite nanocoating layers as a facile surface modification strategy for high-voltage lithium ion cathode materials. J. Mater. Chem. A 2013, 1, 12441. [Google Scholar] [CrossRef]
- Jiaping, S. High temperature resistant coating-type black matt polyimide film. Patent CN102529262A 4 July.
- Zhang, P.; Li, H.; Liang, H.; Wang, H.; Yang, C.; Shan, X.; Zhang, Q.; Chen, Y. Polyimide aerogels with a dual electrically conductive network for electromagnetic interference shielding, piezoresistive sensing, and thermal management. Mater. Today Commun. 2024, 38, 108506. [Google Scholar] [CrossRef]
- Kong, D.; Li, J.; Guo, A.; Xiao, X. High temperature electromagnetic shielding shape memory polymer composite. Chem. Eng. J. 2021, 408, 127365. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide-Based Materials for Space Applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef]
- Cui, Z.; Zhou, J.; Liu, T.; Wang, Y.; Hu, Y.; Wang, Y.; Zou, Z. Porphyrin-containing Polyimide with Enhanced Light Absorption and Photocatalysis Activity. Chem.-Asian J. 2019, 14, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, Y.; Liu, Y.; Yang, C.; Dai, S.; Wang, X.; Liu, J. Preparation and Properties of Intrinsically Black Polyimide Films with CIE Lab Color Parameters Close to Zero and High Thermal Stability for Potential Applications in Flexible Printed Circuit Boards. Polymers 2022, 14, 3881. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, S.; Yuan, J.; Lu, Q. New developments in intrinsic black photosensitive polyimide for advanced display applications. Mater. Today Chem. 2024, 42, 102346. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, S.; Zheng, F.; Lu, Q. Intrinsically Black Polyimide with Retained Insulation and Thermal Properties: A Black Anthraquinone Derivative Capable of Linear Copolymerization. Macromolecules 2021, 54, 9307–9318. [Google Scholar] [CrossRef]
- Tan, J.; Shen, J.; Huang, J.; Xie, F.; Liu, X.; Zhao, C.; Li, H.; Liu, Y. Rational design of soluble intrinsic black polyimide containing tetraphenylcyclopentadienone-based chromophore. Polymer 2023, 285, 126354. [Google Scholar] [CrossRef]
- Tan, J.; Shen, J.; Huang, J.; Yuan, J.; Liu, X.; Li, H.; Xie, F.; Zhao, C.; Liu, Y. Design, synthesis, and properties of soluble intrinsic black polyimide bearing tetraphenylcyclopentadienone units. J. Polym. Sci. 2023, 61, 2965–2978. [Google Scholar] [CrossRef]
- Tan, J.; Shen, J.; Huang, J.; Zhao, C.; Li, H.; Liu, X.; Xie, F.; Liu, Y. Synthesis and characterization of soluble intrinsic black polyimide with excellent comprehensive properties. Sci. China Technol. Sci. 2023, 66, 3604–3614. [Google Scholar] [CrossRef]
- Tan, J.; Xie, F.; Huang, J.; Liu, X.; Li, H.; Yuan, J.; Zhao, C.; Liu, Y. Rational design and coloration mechanism of soluble intrinsic black polyimide with high comprehensive performance for black flexible copper clad laminates. Dye. Pigment. 2023, 216, 111351. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Tan, J.; Huang, J.; Yuan, J.; Li, H.; Guo, J.; Yu, P.; Chen, Y. Synthesis and Characterization of Polyimide with High Blackness and Low Thermal Expansion by Introducing 3,6-bis(thiophen-2-yl)diketopyrrolopyrrole-Based Chromophores. Polymers 2024, 16, 3365. [Google Scholar] [CrossRef]
- Wu, W.; Niu, H.; Lai, S.; Liu, C.; Zhou, L.; Huang, X. Synthesis, characterization, and gas separation properties of novel fluorinated co-polyimides with bulky side groups. Polymer 2022, 257, 125273. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Lan, Q.; Liu, S.; Qin, Z.; Chen, L.; Zhao, C.; Chi, Z.; Xu, J.; Economy, J. High-Performance Functional Polyimides Containing Rigid Nonplanar Conjugated Triphenylethylene Moieties. Chem. Mater. 2012, 24, 1212–1222. [Google Scholar] [CrossRef]
- Lai, S.; Shi, Y.; Wu, W.; Wei, B.; Liu, C.; Zhou, L.; Huang, X. Highly soluble fluorinated polyimides with promising gas transport performance and optical transparency. Polym. Chem. 2023, 14, 359–373. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Jerković, I.; Sarais, G.; Congiu, F.; Marijanović, Z.; Kuś, P.M. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE L∗Cab∗hab∘ chromaticity coordinates. Food Chem. 2014, 145, 284–291. [Google Scholar] [CrossRef]
- Kanehashi, S.; Sato, S.; Nagai, K. Membrane color and gas permeability of 6FDA-TeMPD polyimide membranes prepared with various membrane preparation protocols. Polym. Eng. Sci. 2011, 51, 2360–2369. [Google Scholar] [CrossRef]



| PI a | λcut b (nm) | T600 c (%) | Tavg c (%) | L* d | a* d | b* d | c* d | Reference |
|---|---|---|---|---|---|---|---|---|
| PI-0 | 419 | 86.5 | 82.91 | 41.03 | −2.07 | 7.22 | 7.51 | this work |
| PI-10% | 446 | 82.4 | 72.61 | 40.01 | 1.63 | 15.42 | 15.5 | this work |
| PI-30% | 454 | 77.7 | 63.09 | 38.98 | 4.21 | 11.41 | 12.2 | this work |
| PI-50% | 456 | 70.2 | 46.43 | 36.71 | 5.42 | 8.14 | 9.78 | this work |
| PI-70% | 520 | 16.9 | 12.67 | 30.31 | 1.92 | −0.01 | 1.92 | this work |
| PI-100% | 591 | 5.9 | 5.87 | 29.94 | 0.70 | −0.84 | 1.09 | this work |
| PI-a | - | 0.0 | - | 20.8 | 0.8 | −2.6 | 2.72 | [26] |
| m/z (Measured Value) | Fragment Assignment | Molecular Formula | m/z (Theoretical Value) | Fragmentation Pathway |
|---|---|---|---|---|
| 476.1250 | [M]+• | C28H18N3O5 | 476.1246 | Molecular Ion Peak |
| 458.1145 | [M-H2O]+• | C28H16N3O4 | 458.1140 | Dehydration |
| 432.1354 | [M-CO2]+ | C27H18N3O3 | 432.1348 | Decarboxylation |
| 414.1249 | [M-CO2-H2O]+• | C27H16N3O2 | 414.1242 | Dehydration |
| 474.1093 | [M-H2]+• | C28H16N3O5 | 474.1090 | Dehydrogenation |
| PI | Tg a (°C) | T5% (°C) | CTE (ppm/K) | σ b (Mpa) | E c (Gpa) | εb d (%) |
|---|---|---|---|---|---|---|
| PI | 321.6 | 395.4 | 33 | 151 ± 4 | 2.4 ± 0.2 | 21 ± 1 |
| PI-30% | 324.1 | 375.5 | 35 | 128 ± 1 | 2.1 ± 0.2 | 17 ± 1 |
| PI-50% | 323.8 | 374.8 | 34 | 142 ± 4 | 2.6 ± 0.1 | 12 ± 1 |
| PI-70% | 330.2 | 375.2 | 33 | 122 ± 2 | 2.5 ± 0.1 | 10 ± 1 |
| PI-100% | 332.5 | 372.9 | 36 | 124 ± 4 | 3.1 ± 0.1 | 8.7 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Ma, J.; Li, Z.; Shi, S.; Yuan, T.; Qian, J.; Guo, H. Color-Tunable Intrinsically Black Polyimides: A Facile Strategy via In Situ Oxidation Color Control. Polymers 2025, 17, 2876. https://doi.org/10.3390/polym17212876
Kong D, Ma J, Li Z, Shi S, Yuan T, Qian J, Guo H. Color-Tunable Intrinsically Black Polyimides: A Facile Strategy via In Situ Oxidation Color Control. Polymers. 2025; 17(21):2876. https://doi.org/10.3390/polym17212876
Chicago/Turabian StyleKong, Desheng, Jiaojiao Ma, Zeyu Li, Shun Shi, Tong Yuan, Jianfeng Qian, and Haiquan Guo. 2025. "Color-Tunable Intrinsically Black Polyimides: A Facile Strategy via In Situ Oxidation Color Control" Polymers 17, no. 21: 2876. https://doi.org/10.3390/polym17212876
APA StyleKong, D., Ma, J., Li, Z., Shi, S., Yuan, T., Qian, J., & Guo, H. (2025). Color-Tunable Intrinsically Black Polyimides: A Facile Strategy via In Situ Oxidation Color Control. Polymers, 17(21), 2876. https://doi.org/10.3390/polym17212876







