Synergistic Therapeutic Effects of Chitosan and Royal Jelly
Abstract
1. Introduction
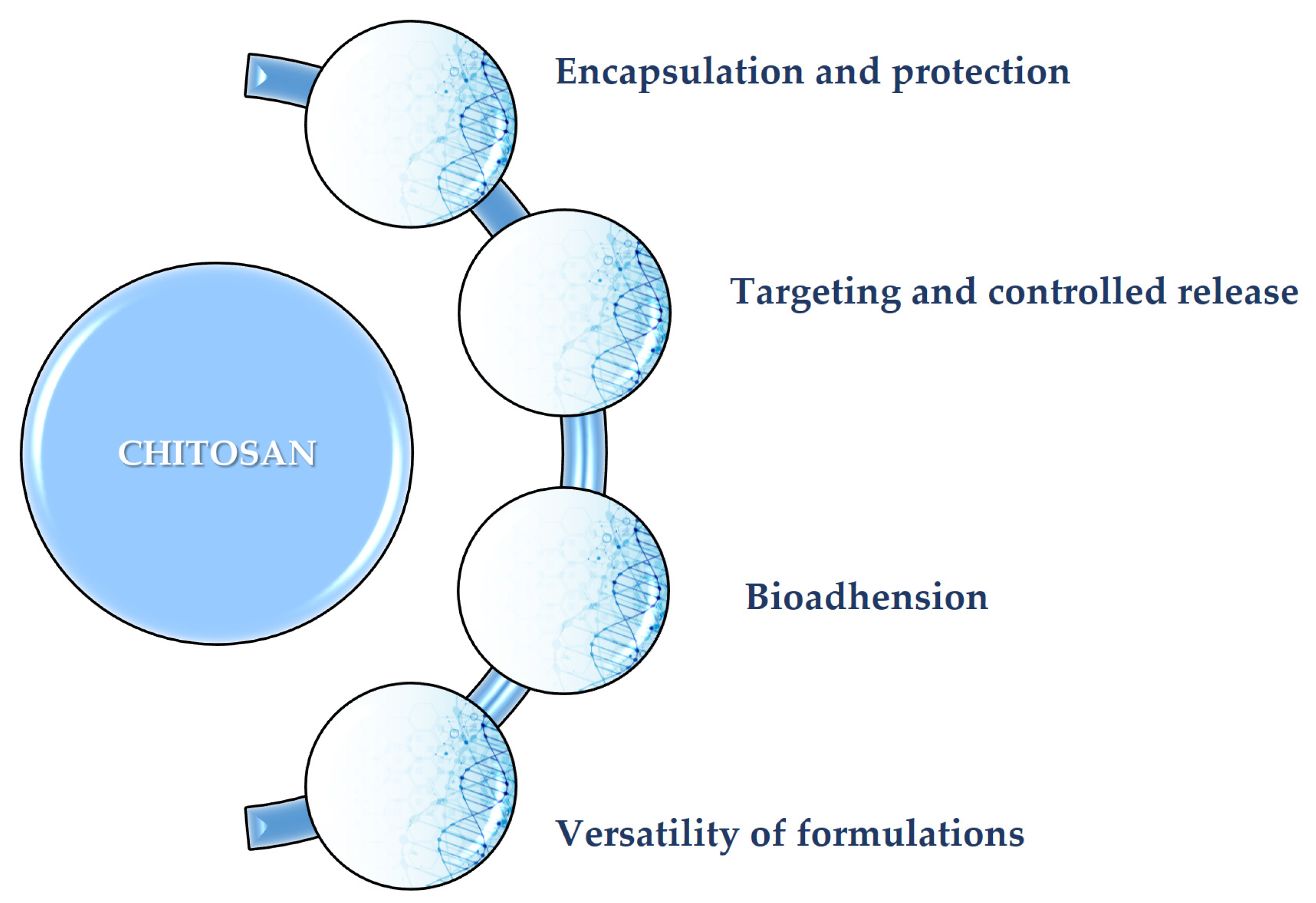
2. Chitosan in Therapeutics
2.1. Structure and Key Properties
2.2. Role of CS in Wound Healing Treatments
2.3. Essentials in Drug Delivery
2.4. Role of Chitosan in Cosmetics and Applied Dermatology
3. Royal Jelly: Composition and Therapeutic Potential
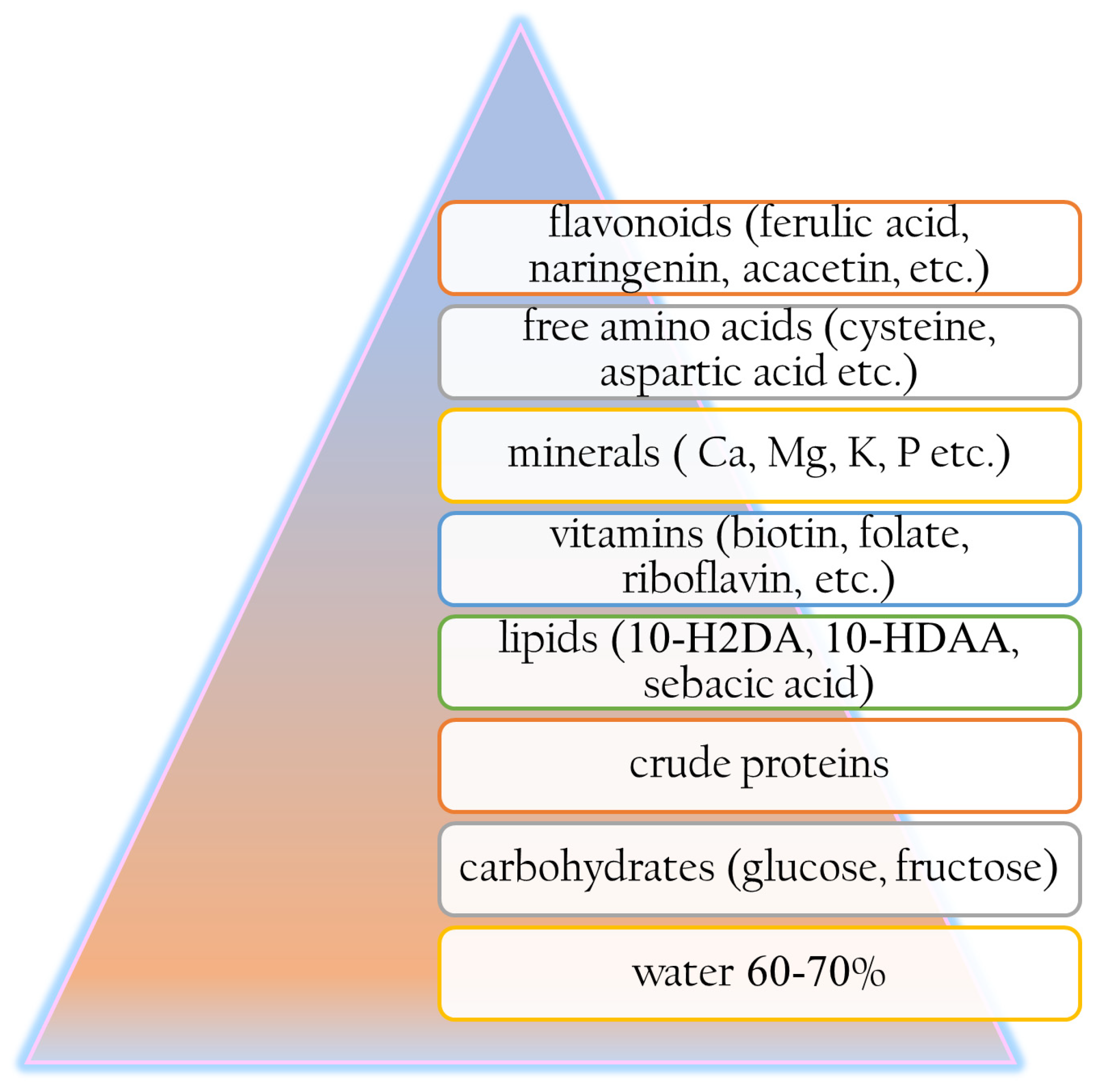
3.1. Origin and Composition of Royal Jelly
| Solvent | Solubility and Additional Notes |
|---|---|
| aqueous solution | slightly soluble [91] |
| saline solution, NaCl 0.9% | slightly soluble [92] |
| ethanol | large amounts of lipids and proteins are soluble [93] |
| acetonitrile | the most suitable solvent for the extraction of fatty acids (10-HDA and 10-H2DA acids) [90] |
| chloroform/methanol | fatty acid extraction [90] |
| Protein | Contributions | References |
|---|---|---|
| MRJP1 | immune modulator | [88,94] |
| MRJP2 | proliferative effect | [95] |
| MRJP3 | anti-inflammatory properties and protein effector | [96] |
| MRJP4 | nutritional function | [97] |
| MRJP5 | high protein content | [98] |
| MRJP6 | anti-inflammatory, immunomodulatory, antimicrobial, anticancer, and antihypertensive properties | [99] |
| MRJP7 | antimicrobial, anti-inflammatory, and antioxidant properties | [99] |
| MRJP8 | broad-spectrum antimicrobial activity | [100] |
| MRJP9 | antibacterial and antifungal activities | [100] |
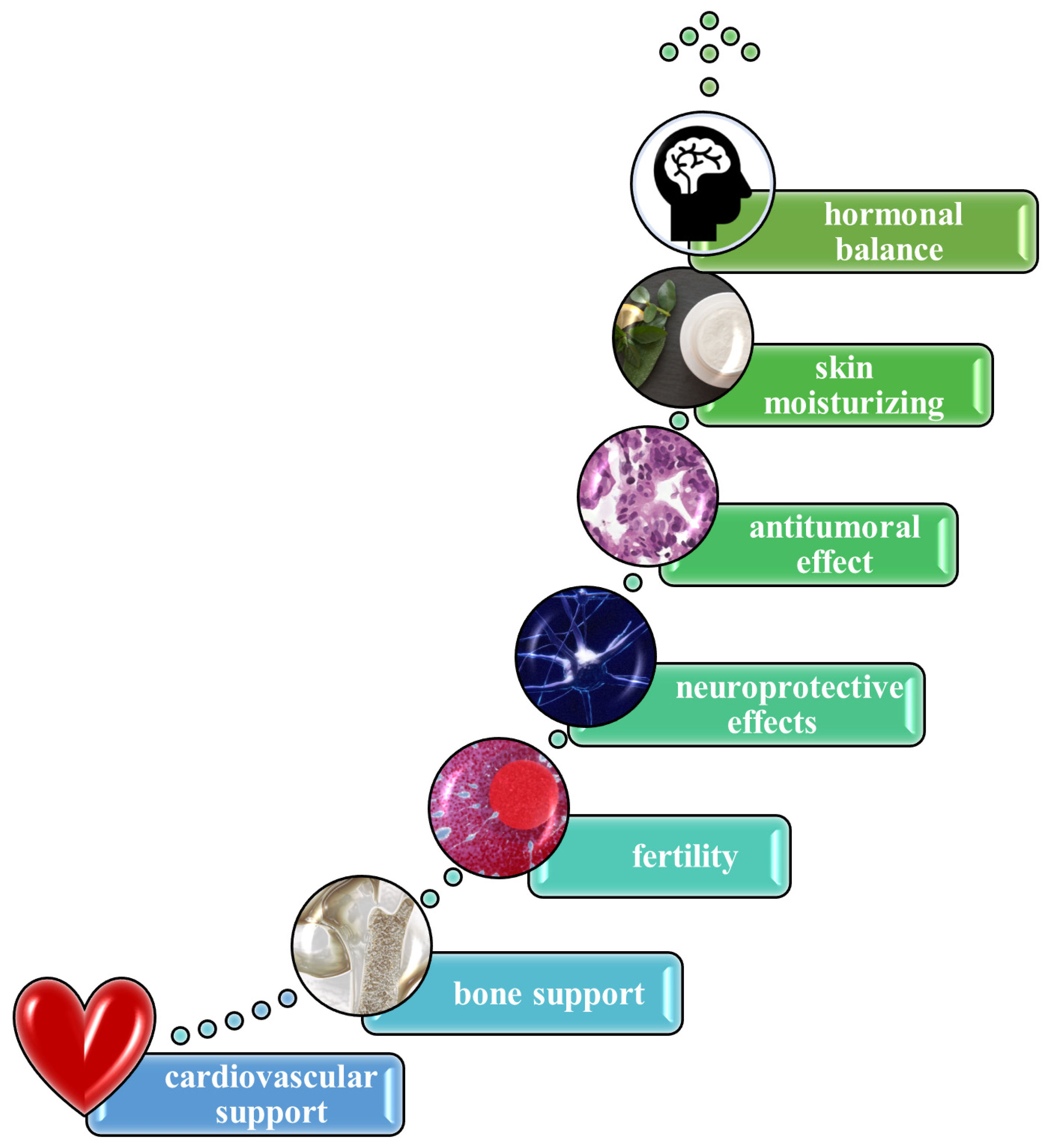
3.2. Therapeutic Effects of Royal Jelly
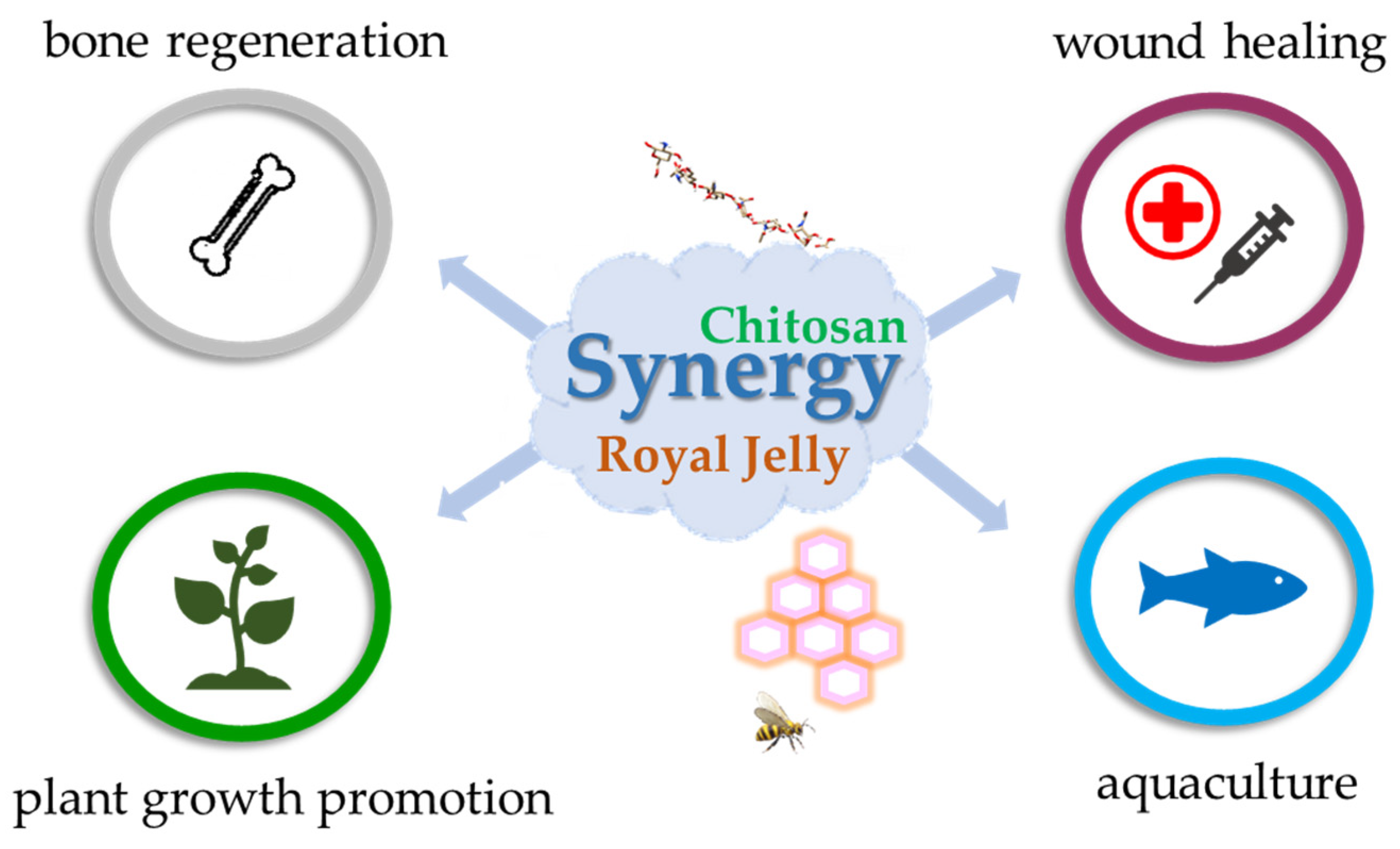
4. Interaction and Synergistic Potential of CS and Royal Jelly
4.1. Structural and Functional Synergy in Nanocomposite Materials
4.2. Antimicrobial and Cytocompatibility Enhancements
4.3. Bone Regeneration
4.4. Broader Applications in Agriculture and Aquaculture
5. Key Conclusions and Outlook for Future Research
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Serra, J.; Reizabal, A.; Correia, D.M.; Fernandes, L.C.; Brito-Pereira, R.; Lizundia, E.; Costa, C.M.; Lanceros-Méndez, S. Biobased polymers for advanced applications: Towards a sustainable future. Prog. Polym. Sci. 2025, 162, 101934. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Ahmed, J.; Vahora, A.; Pathania, S. Effect of pectin incorporation on characteristics of chitosan based edible films. J. Food Meas. Charact. 2023, 17, 5569–5581. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Thai, T.L.A. Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films. Food Hydrocoll. 2017, 71, 141–148. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Maniruzzaman, M. Rheological and Dielectric Behavior of 3D-Printable Chitosan/Graphene Oxide Hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 88–99. [Google Scholar] [CrossRef]
- Troy, E.; Tilbury, M.A.; Power, A.M.; Wall, J.G. Nature-Based Biomaterials and Their Application in Biomedicine. Polymers 2021, 13, 3321. [Google Scholar] [CrossRef]
- Yahya, E.B.; Ali, S.R.; Lalung, J.; Zain, M.S.C.; Danish, M.; John, A. Exploring the potential of biopolymers in cosmetic applications: Sustainable, biocompatible, and high-performance materials for future innovations. Polym. Eng. Sci. 2025, 65, 2789–2802. [Google Scholar] [CrossRef]
- Rech, A.; Daugaard, A.E. Thermoprocessing Biopolymers and Bio-Waste-Based Materials. ACS Sustain. Resour. Manag. 2025, 2, 4–28. [Google Scholar] [CrossRef]
- Ruxandra Leontieș, A.; Răducan, A.; Cristina Culiță, D.; Alexandrescu, E.; Moroșan, A.; Eduard Mihaiescu, D.; Aricov, L. Laccase immobilized on chitosan-polyacrylic acid microspheres as highly efficient biocatalyst for naphthol green B and indigo carmine degradation. Chem. Eng. J. 2022, 439, 135654. [Google Scholar] [CrossRef]
- Mullah, M.F.; Joseph, L.; Arfat, Y.A.; Ahmed, J. Thermal Properties of Gelatin and Chitosan. In Glass Transition and Phase Transitions in Food and Biological Materials; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 281–304. ISBN 9781118935682. [Google Scholar]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R. Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics 2022, 9, 99. [Google Scholar] [CrossRef]
- Wang, J.; Duan, X.; Zhong, D.; Zhang, M.; Li, J.; Hu, Z.; Han, F. Pharmaceutical applications of chitosan in skin regeneration: A review. Int. J. Biol. Macromol. 2024, 261, 129064. [Google Scholar] [CrossRef] [PubMed]
- Gonciarz, W.; Balcerczak, E.; Brzeziński, M.; Jeleń, A.; Pietrzyk-Brzezińska, A.J.; Narayanan, V.H.B.; Chmiela, M. Chitosan-based formulations for therapeutic applications. A recent overview. J. Biomed. Sci. 2025, 32, 62. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Marín, R.; Fernandes, S.C.M.; McReynolds, C.; Labidi, J.; Sánchez, M.Á.A. Chapter 22—Chitosan-based materials as templates for essential oils. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 689–720. ISBN 978-0-12-817966-6. [Google Scholar]
- Niu, Y.; Hu, W. Preparation, characterization and application in environmental protection of low-molecular-weight chitosan: A review. Sustain. Environ. Res. 2024, 34, 29. [Google Scholar] [CrossRef]
- Kong, S.; Lv, L.; Guo, J.; Yang, X.; Liao, M.; Zhao, T.; Sun, H.; Zhang, S.; Li, W. Preparation of Cod Skin Collagen Peptides/Chitosan-Based Temperature-Sensitive Gel and Its Anti-Photoaging Effect in Skin. Drug Des. Devel. Ther. 2023, 17, 419–437. [Google Scholar] [CrossRef]
- Naveedunissa, S.; Meenalotchani, R.; Manisha, M.; Ankul Singh, S.; Nirenjen, S.; Anitha, K.; Harikrishnan, N.; Prajapati, B.G. Advances in chitosan based nanocarriers for targetted wound healing therapies: A review. Carbohydr. Polym. Technol. Appl. 2025, 11, 100891. [Google Scholar] [CrossRef]
- Mehmood, A.; Javaid, S.; Rehman, S.U.; Ahmed, N.; Kanwal, S.; Baig, M.M. Exploring drug administration routes using chitosan-based polymeric nanoparticles: A comprehensive review. J. Drug Deliv. Sci. Technol. 2025, 113, 107347. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Bai, B.; Song, G.; Zhang, J.; Yu, H.; Huang, S.; Wang, Z.; Lu, G. Topical Drug Delivery Strategies for Enhancing Drug Effectiveness by Skin Barriers, Drug Delivery Systems and Individualized Dosing. Front. Pharmacol. 2023, 14, 1333986. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M. Royal Jelly: Biological Action and Health Benefits. Int. J. Mol. Sci. 2024, 25, 6023. [Google Scholar] [CrossRef] [PubMed]
- Maxim, M.-E.; Toma, R.-M.; Aricov, L.; Leonties, A.-R.; Precupas, A.; Tatia, R.; Oprita, E.I. Unlocking the Rich Potential of a Soft Gel-Cream Enriched with Royal Jelly for Topical Use. Gels 2025, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Ohba, K.; Matsuo, T.; Mitsunari, K.; Sakai, H. A randomized, double-blinded clinical trial of royal jelly intake for anticancer effects and suppressing adverse events in renal cell carcinoma patients treated with tyrosine kinase inhibitors. J. Clin. Oncol. 2020, 38, 697. [Google Scholar] [CrossRef]
- Tao, J.; Bi, Y.; Luo, S.; Quan, S.; He, J.; Dong, P.; Tian, W.; Fang, X. Chitosan nanoparticles loaded with royal jelly: Characterization, antioxidant, antibacterial activities and in vitro digestion. Int. J. Biol. Macromol. 2024, 280, 136155. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, D.; Lu, W.; Zhang, C.; Wang, H.; Peng, Z.; Jiang, H.; Xiao, C. Characterization of polyvinyl alcohol/chitosan nanofibers loaded with royal jelly by blending electrospinning for potential wound dressings. Int. J. Biol. Macromol. 2025, 307, 141977. [Google Scholar] [CrossRef]
- Meimandi-Parizi, A.; Oryan, A.; Bigham-Sadegh, A.; Sayahi, E. Effects of Chitosan Scaffold along with Royal Jelly or Bee Venom in Regeneration of Critical Sized Radial Bone Defect in Rat. Iran. J. Vet. Res. 2018, 19, 246–254. [Google Scholar]
- Alghuthaymi, M.A. Antifungal Nanocomposites from Honeybee Chitosan and Royal Jelly-Mediated Nanosilver for Suppressing Biofilm and Hyphal Formation of Candida albicans. Polymers 2025, 17, 1916. [Google Scholar] [CrossRef]
- Kudłacik-Kramarczyk, S.; Krzan, M.; Jamroży, M.; Przybyłowicz, A.; Drabczyk, A. Exploring the Potential of Royal-Jelly-Incorporated Hydrogel Dressings as Innovative Wound Care Materials. Int. J. Mol. Sci. 2023, 24, 8738. [Google Scholar] [CrossRef]
- Sultan, M.; Hafez, O.M.; Saleh, M.A.; Youssef, A.M. Smart edible coating films based on chitosan and beeswax–pollen grains for the postharvest preservation of Le Conte pear. RSC Adv. 2021, 11, 9572–9585. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- de Sousa Victor, R.; Marcelo da Cunha Santos, A.; Viana de Sousa, B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rodrigues Menezes, R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef] [PubMed]
- Arpa, M.D.; Akbuğa, F.J. Chitosan-Based Nanogels in Modern Drug Delivery: Focus on Protein and Gene Applications. Gels 2025, 11, 735. [Google Scholar] [CrossRef]
- Visan, R.M.; Leonties, A.R.; Anastasescu, M.; Angelescu, D.G. Towards understanding the interaction of quercetin with chitosan-phytate complex: An experimental and computational investigation. J. Mol. Liq. 2023, 380, 121673. [Google Scholar] [CrossRef]
- Visan, R.M.; Leonties, A.R.; Aricov, L.; Chihaia, V.; Angelescu, D.G. Polymorphism of chitosan-based networks stabilized by phytate investigated by molecular dynamics simulations. Phys. Chem. Chem. Phys. 2021, 23, 22601–22612. [Google Scholar] [CrossRef]
- Visan, R.M.; Angelescu, D.G. Coarse-Grained Model of Phytic Acid for Predicting the Supramolecular Architecture of Ionically Cross-Linked Chitosan Hydrogels. J. Phys. Chem. B 2023, 127, 5718–5729. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Chapter 4—Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 67–89. ISBN 978-0-12-396983-5. [Google Scholar]
- Muhammad, R.; Rosiyah, Y.; Aziz, H.; Muhammad, Y.; Ahmad, D.A.; Vidhya, S.; Faridah, S.; Cheyma, N.A. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar]
- Araújo, F.; Magalhães, S.; Medronho, B.; Eivazi, A.; Dahlström, C.; Norgren, M.; Alves, L. Effect of Chitosan Properties and Dissolution State on Solution Rheology and Film Performance in Triboelectric Nanogenerators. Gels 2025, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- Attasgah, R.B.; Velasco-Rodríguez, B.; Pardo, A.; Fernández-Vega, J.; Arellano-Galindo, L.; Rosales-Rivera, L.C.; Prieto, G.; Barbosa, S.; Soltero, J.F.A.; Mahmoudi, M.; et al. Development of functional hybrid scaffolds for wound healing applications. iScience 2022, 25, 104019. [Google Scholar] [CrossRef] [PubMed]
- Manohar, M.; Shetty, A.; Lobo, C.L.; Jain, P.; Hebbar, S.; Dhas, N.; Sutar, K.P.; Sukeewandhi, J.; Perumalsamy, H.; Balusamy, S.R.; et al. Emerging trends in chitosan based colloidal drug delivery systems: A translational journey from research to practice. Carbohydr. Polym. 2025, 360, 123604. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Alizad, S.; Kaseem, M.; Dikici, B. A review of smart polymeric materials: Recent developments and prospects for medicine applications. Hybrid Adv. 2024, 5, 100178. [Google Scholar] [CrossRef]
- Yadav, H.; Malviya, R.; Kaushik, N. Chitosan in biomedicine: A comprehensive review of recent developments. Carbohydr. Polym. Technol. Appl. 2024, 8, 100551. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing natural polymers for skin wound healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef]
- Keast, D.H.; Janmohammad, A. The Hemostatic and Wound Healing Effect of Chitosan Following Debridement of Chronic Ulcers. Wounds-A Compend. Clin. Res. Pract. 2021, 33, 263–270. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases; Statpearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Springer: Singapore, 2020; pp. 457–489. [Google Scholar]
- Adair, T.; Montani, J.-P. Angiogenesis. Colloq. Ser. Integr. Syst. Physiol. Mol. Funct. 2010, 2, 1–84. [Google Scholar] [CrossRef]
- St Denis, T.G.; Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Wound-Healing Properties of Chitosan and Its Use in Wound Dressing Biopharmaceuticals. In Chitosan-Based Systems for Biopharmaceuticals; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2012; pp. 429–450. ISBN 9781119962977. [Google Scholar]
- Le, L.T.T.; Giang, N.N.; Chien, P.N.; Trinh, X.-T.; Long, N.-V.; VANAnh, L.E.T.; Nga, P.T.; Zhang, X.-R.; Nam, S.-Y.; Heo, C.-Y. Enhancement of Wound Healing Efficacy by Chitosan-Based Hydrocolloid on Sprague Dawley Rats. Vivo 2023, 37, 1052–1064. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. J. Compos. Sci. 2025, 9, 133. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.; Dai, W.-Q.; Cui, N.; Liu, J.-Q.; Wang, M.-G.; Zhou, Y.-F.; Fang, L.-X.; Sun, J.; Jiang, G.-B.; et al. Lutein-loaded multifunctional hydrogel dressing based on carboxymethyl chitosan for chronic wound healing. Int. J. Biol. Macromol. 2025, 300, 140219. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.M.; Zaghloul, R.A.; El-Dahhan, M.S. Formulation, optimization and characterization of allantoin-loaded chitosan nanoparticles to alleviate ethanol-induced gastric ulcer: In-vitro and in-vivo studies. Sci. Rep. 2021, 11, 2216. [Google Scholar] [CrossRef]
- Silva, J.M.; Carvalho, J.P.F.; Teixeira, M.C.; Facchinatto, W.M.; Braz, M.; Almeida, A.; Oliveira, H.; Vilela, C.; Branco, P.C.; Martins, J.; et al. Xylan-chitosan based films with deep eutectic solvents for wound healing applications. Int. J. Biol. Macromol. 2025, 320, 145482. [Google Scholar] [CrossRef]
- Chen, R.; Du, F.; Yuan, Q. Multifunctional Sodium Hyaluronate/Chitosan Foam Used as an Absorbable Hemostatic Material. Bioengineering 2023, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Zhou, F. Electrospun chitosan nanofibers for wound healing application. Eng. Regen. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D.; et al. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021, 12, 4733. [Google Scholar] [CrossRef]
- Pandian, M.; Kumar, V.A.; Jayakumar, R. Antiseptic chitosan bandage for preventing topical skin infections. Int. J. Biol. Macromol. 2021, 193, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Enache, A.-C.; Samoila, P.; Cojocaru, C.; Bele, A.; Bostanaru, A.-C.; Mares, M.; Harabagiu, V. Amphiphilic Chitosan Porous Membranes as Potential Therapeutic Systems with Analgesic Effect for Burn Care. Membranes 2022, 12, 973. [Google Scholar] [CrossRef]
- Cui, H.; Cai, J.; He, H.; Ding, S.; Long, Y.; Lin, S. Tailored chitosan/glycerol micropatterned composite dressings by 3D printing for improved wound healing. Int. J. Biol. Macromol. 2024, 255, 127952. [Google Scholar] [CrossRef]
- Chidchai, P.; Singpanna, K.; Opanasopit, P.; Patrojanasophon, P.; Pornpitchanarong, C. Development of photo-crosslinked chitosan-methacrylate hydrogel incorporated with ciprofloxacin as dressing for infected wounds. Carbohydr. Polym. Technol. Appl. 2024, 7, 100478. [Google Scholar] [CrossRef]
- Anushree, U.; Punj, P.; Vasumathi; Bharati, S. Phosphorylated chitosan accelerates dermal wound healing in diabetic wistar rats. Glycoconj. J. 2023, 40, 19–31. [Google Scholar] [CrossRef]
- Jayabal, P.; Kannan Sampathkumar, V.; Vinothkumar, A.; Mathapati, S.; Pannerselvam, B.; Achiraman, S.; Venkatasubbu, G.D. Fabrication of a Chitosan-Based Wound Dressing Patch for Enhanced Antimicrobial, Hemostatic, and Wound Healing Application. ACS Appl. Bio Mater. 2023, 6, 615–627. [Google Scholar] [CrossRef]
- Herdiana, Y.; Febrina, E.; Nurhasanah, S.; Gozali, D.; Elamin, K.M.; Wathoni, N. Drug Loading in Chitosan-Based Nanoparticles. Pharmaceutics 2024, 16, 1043. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef] [PubMed]
- Medina-Moreno, A.; El-Hammadi, M.M.; Arias, J.L. pH-dependent, extended release and enhanced in vitro efficiency against colon cancer of Tegafur formulated using chitosan-coated poly(ε-caprolactone) nanoparticles. J. Drug Deliv. Sci. Technol. 2023, 86, 104594. [Google Scholar] [CrossRef]
- Ritu; Pannu, P.; Pooja; Das, A.; Chandra, P. Chapter 14—Safety and biocompatibility of nanogels: Addressing current concerns. In Nanogels; Singh, A.K., Chaturvedi, V.K., Singh, S.K., Singh, J., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2025; pp. 401–437. ISBN 978-0-443-30016-5. [Google Scholar]
- Bayer, I.S. Recent Advances in Mucoadhesive Interface Materials, Mucoadhesion Characterization, and Technologies. Adv. Mater. Interfaces 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M.; Mennini, N. Multiple Roles of Chitosan in Mucosal Drug Delivery: An Updated Review. Mar. Drugs 2022, 20, 335. [Google Scholar] [CrossRef]
- Yadav, D.; Malviya, R.; Rizg, W.Y.; Warsi, M.H. Potential of chitosan for targeted mitochondrial delivery of therapeutic agents. Carbohydr. Polym. Technol. Appl. 2025, 9, 100634. [Google Scholar] [CrossRef]
- MWays, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Kulka, K.; Sionkowska, A. Chitosan Based Materials in Cosmetic Applications: A Review. Molecules 2023, 28, 1817. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shang, J.; Chen, Y.; Feng, X. Potential Applications of Chitosan in Seborrheic Dermatitis and Other Skin Diseases: A Comprehensive Review. Clin. Cosmet. Investig. Dermatol. 2025, 18, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.R.; Hirano, R.S.; Gaspar, A.L.; Chagas, E.; Carvalho, R.A.; Silva, F.V.; Leonardi, G.R.; Lopes, P.; Silva, C.; Yoshida, C. Biodegradable antioxidant chitosan films useful as an anti-aging skin mask. Int. J. Biol. Macromol. 2019, 132, 1262–1273. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yu, D.; Eom, S.-H.; Kim, S.-H.; Oh, J.; Jung, W.-K.; Kim, Y.-M. Synergistic Antibacterial Effects of Chitosan-Caffeic Acid Conjugate against Antibiotic-Resistant Acne-Related Bacteria. Mar. Drugs 2017, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.-H.; Loo, H.-L.; Goh, C.F.; Fu, J.-Y.; Ng, S.-F. Chitosan-Based Drug Delivery Systems for Skin Atopic Dermatitis: Recent Advancements and Patent Trends. Drug Deliv. Transl. Res. 2023, 13, 1436–1455. [Google Scholar] [CrossRef] [PubMed]
- Gaetano, V.; Gagliardi, A.; Giuliano, E.; Longo, E.; Cosco, D. Chitosan Nanoparticles Loaded with Polyphenols for Cosmeceutical Applications: A State-of-the-Art Review. Pharmaceutics 2025, 17, 1068. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Ferreira, V.F.; da Silva, F.d.C.; Freitas, C.S.; Pereira, P.R.; Paschoalin, V.M.F. Chitosans and Nanochitosans: Recent Advances in Skin Protection, Regeneration, and Repair. Pharmaceutics 2022, 14, 1307. [Google Scholar] [CrossRef]
- Mizrahi, A.; Lensky, Y. Bee Products: Properties, Applications, and Apitherapy; Springer: New York, NY, USA, 2013; ISBN 9781475793710. [Google Scholar]
- Jones, R. Honey and healing through the ages. J. ApiProduct ApiMedical Sci. 2009, 1, 2–5. [Google Scholar] [CrossRef]
- Baptista, B.G.; Lima, L.S.; Ribeiro, M.; Britto, I.K.; Alvarenga, L.; Kemp, J.A.; Cardozo, L.F.; Berretta, A.A.; Mafra, D. Royal Jelly: A Predictive, Preventive and Personalised Strategy for Novel Treatment Options in Non-Communicable Diseases. EPMA J. 2023, 14, 381–404. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An Ancient Remedy with Remarkable Antibacterial Properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef]
- Kumar, R.; Thakur, A.; Kumar, S.; Hajam, Y.A. Royal Jelly a Promising Therapeutic Intervention and Functional Food Supplement: A Systematic Review. Heliyon 2024, 10, e37138. [Google Scholar] [CrossRef]
- Alkindi, F.; El-Keblawy, A.; Ridouane, F.; Mirza, S. Factors influencing the quality of Royal jelly and its components: A review. Cogent Food Agric. 2024, 10, 2348253. [Google Scholar] [CrossRef]
- Shen, L.; Wang, Y.; Zhai, L.; Zhou, W.; Tan, L.; Li, M.; Liu, D.; Xiao, F. Determination of Royal Jelly Freshness by ELISA with a Highly Specific Anti-Apalbumin 1, Major Royal Jelly Protein 1 Antibody. J. Zhejiang Univ. Sci. B 2015, 16, 155–166. [Google Scholar] [CrossRef]
- Schmitzova, J.; Klaudiny, J.; Albert, S.; Schröder, W.; Schreckengost, W.; Hanes, J.; Judova, J.; Simúth, J. A family of mayor royal jelly protein of the honeybee Apis mellifera L. Cell. Mol. Life Sci. 1998, 54, 1020–1030. [Google Scholar] [CrossRef]
- Yu, X.; Tu, X.; Tao, L.; Daddam, J.; Li, S.; Hu, F. Royal Jelly Fatty Acids: Chemical Composition, Extraction, Biological Activity, and Prospect. J. Funct. Foods 2023, 111, 105868. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhang, X.; Ming, H.; Tian, L.; Luo, L. Exploration of quality deterioration mechanisms of royal jelly proteins during storage by LC-MS/MS-based peptidomics. J. Food Compos. Anal. 2025, 140, 107202. [Google Scholar] [CrossRef]
- Hata, T.; Furusawa-Horie, T.; Arai, Y.; Takahashi, T.; Seishima, M.; Ichihara, K. Studies of royal jelly and associated cross-reactive allergens in atopic dermatitis patients. PLoS ONE 2020, 15, e0233707. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Fan, R.; Jiang, J.; Feng, S.; Yin, H.; Luo, S.-Z.; Chen, L. Ethanol-Soluble Proteins from the Royal Jelly of Xinjiang Black Bees. Protein Sci. 2021, 30, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Lin, Y.; Shao, Q.; Zhang, M.; Lu, C.; Fleming, J.; Su, S. Royal jelly-derived proteins enhance proliferation and migration of human epidermal keratinocytes in an in vitro scratch wound model. BMC Complement. Altern. Med. 2019, 19, 175. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Feng, M.; Ma, C.; Rueppell, O.; Li, J. Major royal jelly proteins influence the neurobiological regulation of the division of labor among honey bee workers. Int. J. Biol. Macromol. 2023, 225, 848–860. [Google Scholar] [CrossRef]
- Zhuang, S.; Ming, H.; Yu, W.; Luo, L. Identification of freshness and metabolite changes of royal jelly during storage using Nano-ESI-MS and UPLC-Q/TOF-MS. J. Food Compos. Anal. 2025, 139, 107092. [Google Scholar] [CrossRef]
- Furusawa, T.; Rakwal, R.; Nam, H.W.; Shibato, J.; Agrawal, G.K.; Kim, Y.S.; Ogawa, Y.; Yoshida, Y.; Kouzuma, Y.; Masuo, Y.; et al. Comprehensive Royal Jelly (RJ) Proteomics Using One- and Two-Dimensional Proteomics Platforms Reveals Novel RJ Proteins and Potential Phospho/Glycoproteins. J. Proteome Res. 2008, 7, 3194–3229. [Google Scholar] [CrossRef]
- Dobritzsch, D.; Aumer, D.; Fuszard, M.; Erler, S.; Buttstedt, A. The rise and fall of major royal jelly proteins during a honeybee (Apis mellifera) workers’ life. Ecol. Evol. 2019, 9, 8771–8782. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.S.; Ok, M.; Kim, B.Y.; Jin, B.R. Antimicrobial activity of major royal jelly protein 8 and 9 of honeybee (Apis mellifera) venom. J. Asia. Pac. Entomol. 2022, 25, 101964. [Google Scholar] [CrossRef]
- Zhi, D.; He, X.; Xue, Y.; Zhao, W.; Gong, X.; Guo, Y.; Luo, X.; Tian, Y.; Dong, K. Royal Jelly Acid: Preparation, Metabolism and Therapeutic Potential. Front. Pharmacol. 2025, 16, 1561351. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, E.; Varanasi, S.; Bukoski, S.; Mitschke, S.; Conger, S. Investigating the Effects of a Manuka Honey, Royal Jelly, and Bee Venom-Derived Face Serum on Skin Health and Signs of Aging. Cureus 2025, 17, e81244. [Google Scholar] [CrossRef] [PubMed]
- Omer, K.; Gelkopf, M.J.; Newton, G. Effectiveness of Royal Jelly Supplementation in Glycemic Regulation: A Systematic Review. World J. Diabetes 2019, 10, 96–113. [Google Scholar] [CrossRef]
- Bahari, H.; Taheri, S.; Rashidmayvan, M.; Hezaveh, Z.S.; Mousavi, S.E.; Malekahmadi, M. The effects of Royal Jelly consumption on lipid profile: A GRADE-assessed systematic review and dose-response meta-analysis. PharmaNutrition 2023, 25, 100351. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Royal Jelly as an Intelligent Anti-Aging Agent-A Focus on Cognitive Aging and Alzheimer’s Disease: A Review. Antioxidants 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, R.; Seres, A.B. Chapter 8—Royal jelly and fertility. In Bee Products and Their Applications in the Food and Pharmaceutical Industries; Boyacioglu, D., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 201–219. ISBN 978-0-323-85400-9. [Google Scholar]
- Zhu, F.; Yang, R.; He, B.; Xu, Y.; Wang, H.-L. Neuroregulatory effect of royal jelly. J. Nutr. Biochem. 2025, 145, 110028. [Google Scholar] [CrossRef]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef]
- Park, H.M.; Cho, M.H.; Cho, Y.; Kim, S.Y. Royal Jelly Increases Collagen Production in Rat Skin after Ovariectomy. J. Med. Food 2012, 15, 568–575. [Google Scholar] [CrossRef]
- Yan, C.-Y.; Zhu, Q.-Q.; Guan, C.-X.; Xiong, G.-L.; Chen, X.-X.; Gong, H.-B.; Li, J.-W.; Ouyang, S.-H.; Kurihara, H.; Li, Y.-F.; et al. Antioxidant and Anti-Inflammatory Properties of Hydrolyzed Royal Jelly Peptide in Human Dermal Fibroblasts: Implications for Skin Health and Care Applications. Bioengineering 2024, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, T.; Koga, H.; Nishimura, T.; Kobayashi, K. Royal Jelly Induces Thin Hair Shaft Formation by Suppressing Proliferation of Hair Follicle Stem Cells in Mice. ACS Omega 2025, 10, 17228–17236. [Google Scholar] [CrossRef]
- Liang, J.; Guo, C.; Li, Z.; Bi, J.; Wang, R.; Li, P.; Yang, X. Preparation, identification, and anti-melanogenesis activity of royal jelly protein peptides. J. Funct. Foods 2025, 129, 106897. [Google Scholar] [CrossRef]
- Royal Jelly Market Analysis by Type, Form, Application, and Region Forecast Through 2035. Available online: https://www.futuremarketinsights.com/reports/royal-jelly-market (accessed on 10 March 2025).
- Playmakers, R. Asia Pacific R. Jelly Heal. Prod. Mark. Share, Growth Forecast. 2026-2033. 2025. Available online: https://www.linkedin.com/pulse/asia-pacific-royal-jelly-health-products-market-share-nrixf/ (accessed on 10 March 2025).
- Evans, W.C.; Evans, D. Chapter 34—Miscellaneous products. In Trease and Evans’ Pharmacognosy, 6th ed.; Evans, W.C., Evans, D., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 477–482. ISBN 978-0-7020-2933-2. [Google Scholar]
- Kim, J.; Kim, Y.; Yun, H.; Park, H.; Kim, S.Y.; Lee, K.-G.; Han, S.-M.; Cho, Y. Royal Jelly Enhances Migration of Human Dermal Fibroblasts and Alters the Levels of Cholesterol and Sphinganine in an in Vitro Wound Healing Model. Nutr. Res. Pract. 2010, 4, 362–368. [Google Scholar] [CrossRef]
- Thewanjutiwong, S.; Phokasem, P.; Disayathanoowat, T.; Juntrapirom, S.; Kanjanakawinkul, W.; Chaiyana, W. Development of Film-Forming Gel Formulations Containing Royal Jelly and Honey Aromatic Water for Cosmetic Applications. Gels 2023, 9, 816. [Google Scholar] [CrossRef]
- Alhosin, M. Epigenetics Mechanisms of Honeybees: Secrets of Royal Jelly. Epigenetics Insights 2023, 16, 25168657231213716. [Google Scholar] [CrossRef]
- Wan, D.C.; Morgan, S.L.; Spencley, A.L.; Mariano, N.; Chang, E.Y.; Shankar, G.; Luo, Y.; Li, T.H.; Huh, D.; Huynh, S.K.; et al. Honey Bee Royalactin Unlocks Conserved Pluripotency Pathway in Mammals. Nat. Commun. 2018, 9, 5078. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Ghouizi, A.; Es-Safi, I.; Mechchate, H.; Lyoussi, B. New Insights into Potential Beneficial Effects of Bioactive Compounds of Bee Products in Boosting Immunity to Fight COVID-19 Pandemic: Focus on Zinc and Polyphenols. Nutrients 2022, 14, 942. [Google Scholar] [CrossRef]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a Novel Hypocholesterolemic Protein, Major Royal Jelly Protein 1, Derived from Royal Jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef]
- Zahmatkesh, E.; Najafi, G.; Nejati, V.; Heidari, R. Protective Effect of Royal Jelly on the Sperm Parameters and Testosterone Level and Lipid Peroxidation in Adult Mice Treated with Oxymetholone. Avicenna J. Phytomedicine 2014, 4, 43–52. [Google Scholar]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal Jelly Modulates Oxidative Stress and Apoptosis in Liver and Kidneys of Rats Treated with Cisplatin. Oxid. Med. Cell. Longev. 2011, 2011, 981793. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.; Shou, Q.; Abd El-Wahed, A.A.; Elias, N.; Xiao, J.; Swillam, A.; Umair, M.; Guo, Z.; Daglia, M.; Wang, K.; et al. Royal Jelly: Beneficial Properties and Synergistic Effects with Chemotherapeutic Drugs with Particular Emphasis in Anticancer Strategies. Nutrients 2022, 14, 4166. [Google Scholar] [CrossRef]
- Moskwa, J.; Naliwajko, S.K.; Dobiecka, D.; Socha, K. Bee Products and Colorectal Cancer—Active Components and Mechanism of Action. Nutrients 2023, 15, 1614. [Google Scholar] [CrossRef]
- You, M.; Pan, Y.; Liu, Y.; Chen, Y.; Wu, Y.; Si, J.; Wang, K.; Hu, F. Royal Jelly Alleviates Cognitive Deficits and β-Amyloid Accumulation in APP/PS1 Mouse Model Via Activation of the CAMP/PKA/CREB/BDNF Pathway and Inhibition of Neuronal Apoptosis. Front. Aging Neurosci. 2018, 10, 428. [Google Scholar] [CrossRef]
- Liang, H.; He, X.; Li, X.; Semiruomi, D.; Yan, F. Effect of Royal Gel addition to chitosan matrix for wound dress applications: Fabrication, characterization and artificial neural network analysis. Environ. Technol. Innov. 2023, 30, 103077. [Google Scholar] [CrossRef]
- Dumitru, C.D.; Ilie, C.-I.; Neacsu, I.A.; Motelica, L.; Oprea, O.C.; Ripszky, A.; Pițuru, S.M.; Voicu Bălașea, B.; Marinescu, F.; Andronescu, E. Antimicrobial Composite Films Based on Alginate–Chitosan with Honey, Propolis, Royal Jelly and Green-Synthesized Silver Nanoparticles. Int. J. Mol. Sci. 2025, 26, 6809. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, I.; Soltan, H.; Ezzat, A. Efficiency of foliar application by chitosan and royal jelly on growth, yield and quality of two garlic cultivars. SVU-Int. J. Agric. Sci. 2021, 3, 119–131. [Google Scholar] [CrossRef]
- Eissa, M.E.H.; Hendam, B.M.; ElBanna, N.I.; Aly, S.M. Bee venom loaded chitosan nanoparticles enhances growth, immunity and resistance to vibrio parahaemolyticus in pacific white shrimp. Sci. Rep. 2025, 15, 26179. [Google Scholar] [CrossRef]


| Formulations | Applications | References |
|---|---|---|
| Lutein-loaded carboxymethyl CS hydrogels | Acute/chronic wounds and burns | [53,54] |
| Allantoin-loaded CS nanoparticles | Gastric ulcers | [55] |
| Xylan–CS-based films | Antibacterial activity against MRSA strain | [56] |
| Sodium hyaluronate/CS foams | Hemostasis | [57] |
| CS nanofibers | Antibacterial activity and alleviating inflammatory responses | [58] |
| CS-based sponges | Preventing infection | [59] |
| CS bandages | Antiseptic, topical skin infections | [60] |
| CS porous membranes | Analgesic/burns | [61] |
| CS/glycerol micropatterned composite | Hemostasis and inflammations | [62] |
| CS-based-photo-crosslinked hydrogel | Treating infections | [63] |
| Phosphorylated CS | Dermal healing | [64] |
| CS patches | Antimicrobial activity, hemostasis | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, R.-M.; Băran, A. Synergistic Therapeutic Effects of Chitosan and Royal Jelly. Polymers 2025, 17, 2872. https://doi.org/10.3390/polym17212872
Toma R-M, Băran A. Synergistic Therapeutic Effects of Chitosan and Royal Jelly. Polymers. 2025; 17(21):2872. https://doi.org/10.3390/polym17212872
Chicago/Turabian StyleToma, Raluca-Marieta, and Adriana Băran. 2025. "Synergistic Therapeutic Effects of Chitosan and Royal Jelly" Polymers 17, no. 21: 2872. https://doi.org/10.3390/polym17212872
APA StyleToma, R.-M., & Băran, A. (2025). Synergistic Therapeutic Effects of Chitosan and Royal Jelly. Polymers, 17(21), 2872. https://doi.org/10.3390/polym17212872









