Nanomedicines for Delivery of Cytarabine: Effect of Carrier Structure and Spacer on the Anti-Lymphoma Efficacy
Abstract
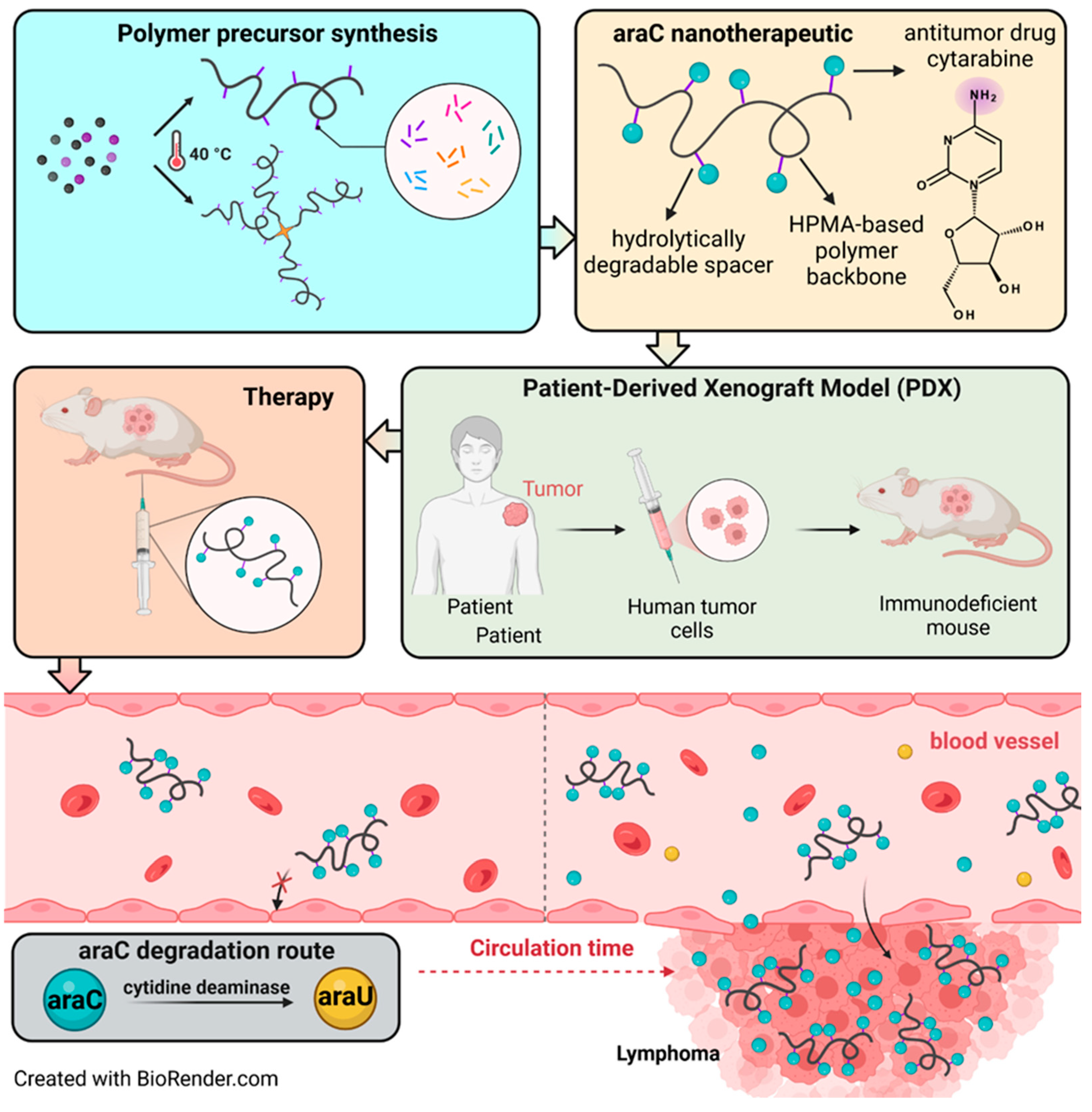
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
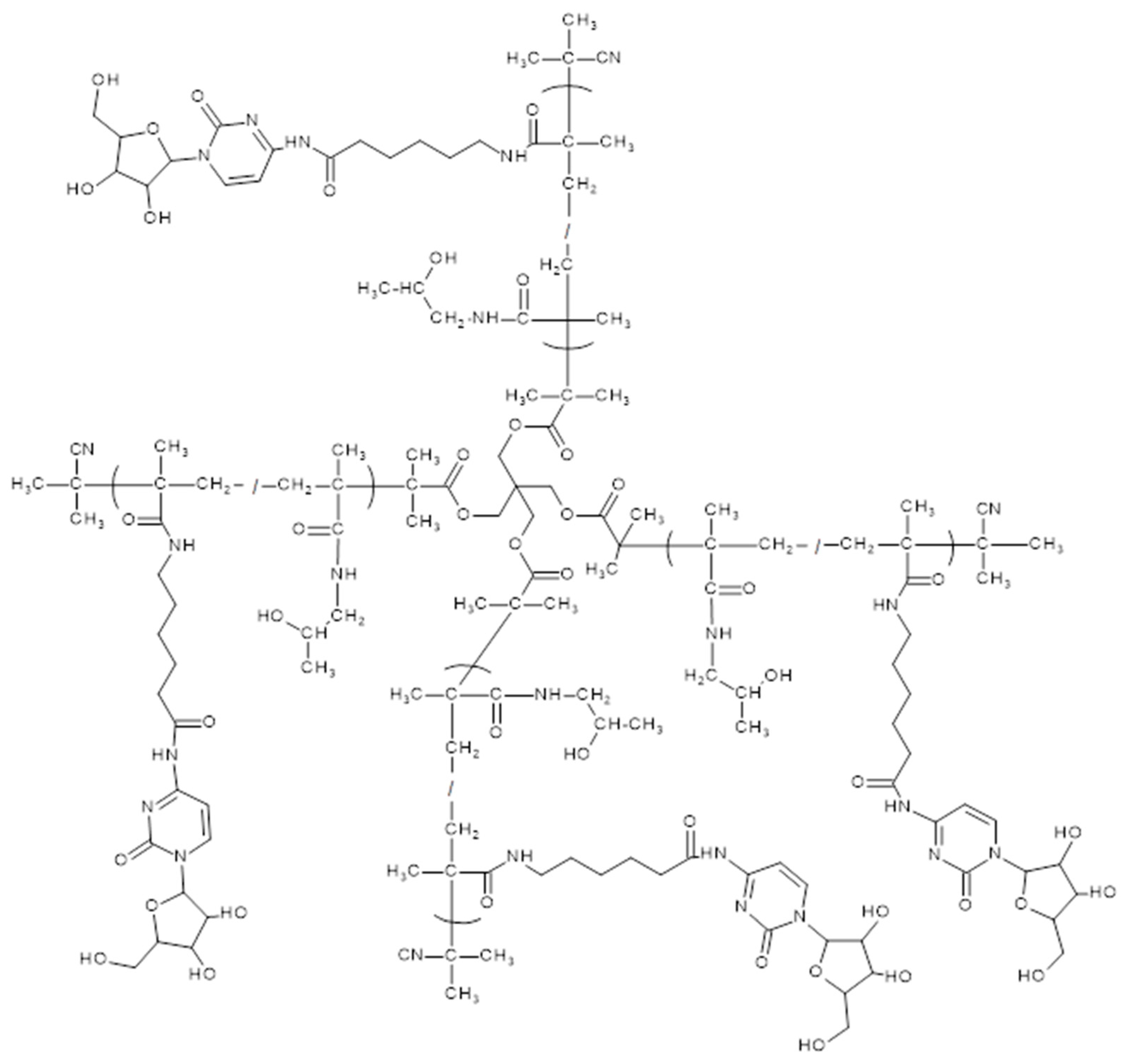
2.3. Synthesis of Monomers, Polymer Precursors and araC-Conjugates
2.4. In Vitro Release of araC from the Polymer Conjugates in PBS Buffer
2.5. In Vitro Release of araC from the Polymer Conjugates in Serum
2.6. Mouse Model
3. Results and Discussion
3.1. Synthesis and Physico-Chemical Characterization of the Polymer Precursors and Conjugates
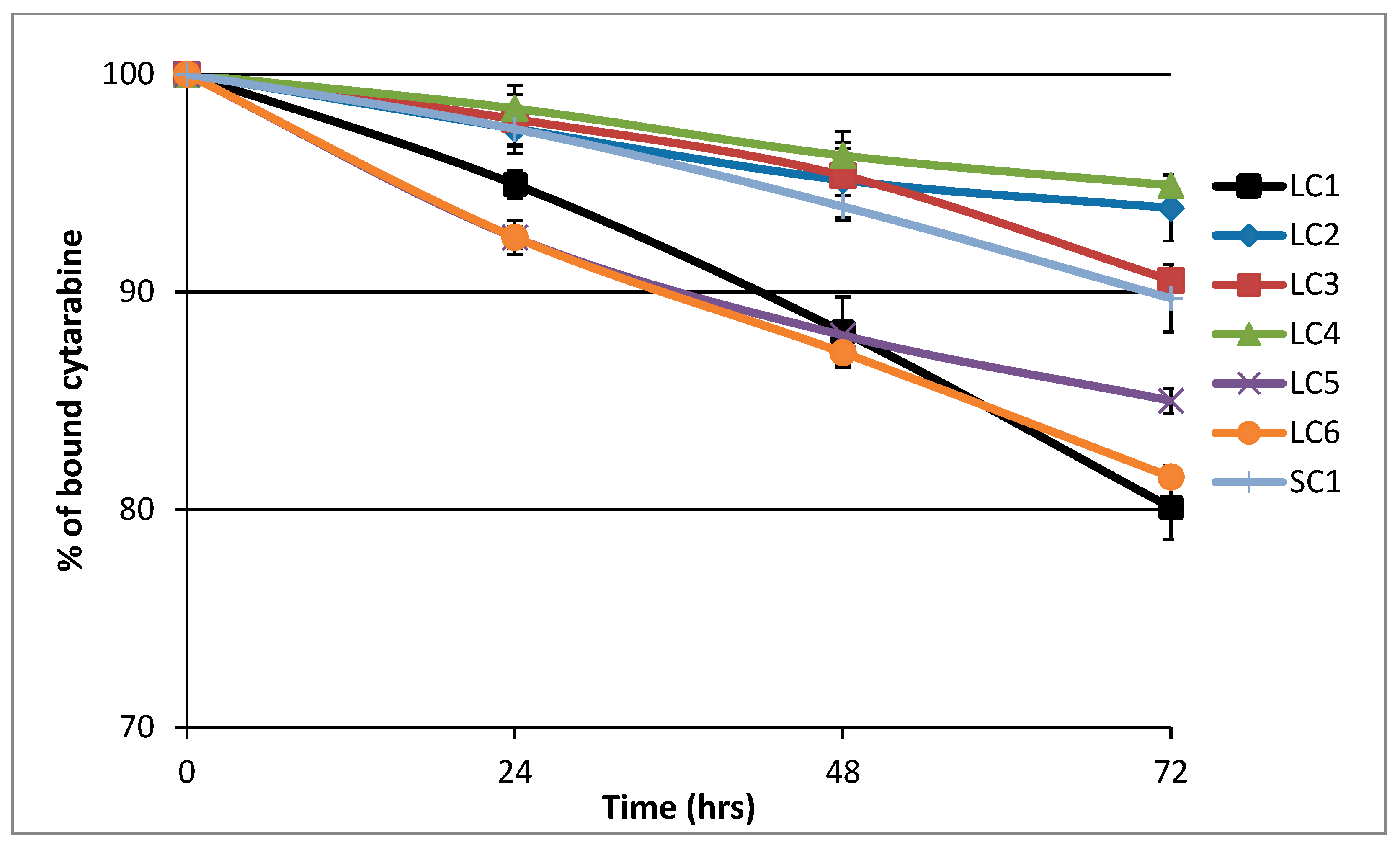
3.2. Hydrolytic Release of araC from the Polymer Conjugates in PBS Buffer
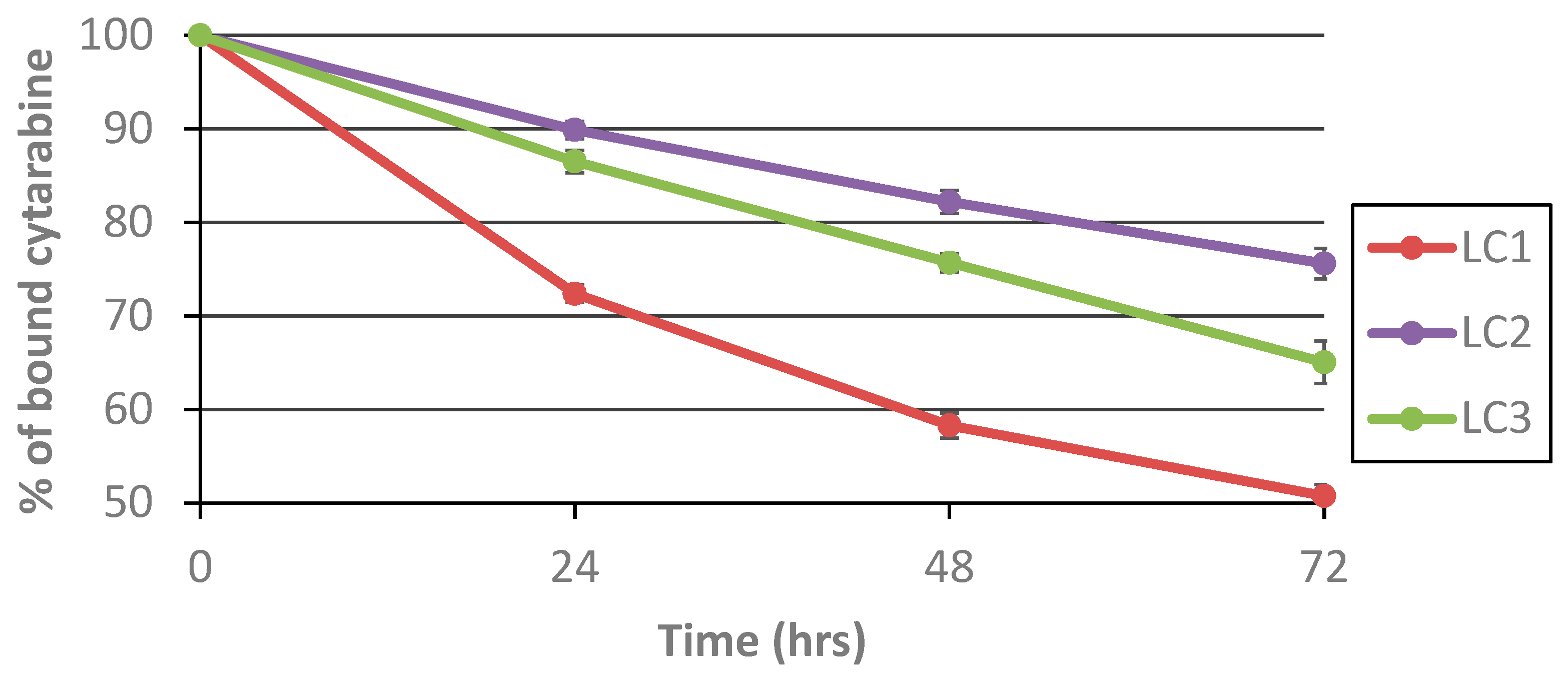
3.3. Hydrolytic Release of araC from the Polymer Conjugates in Human Serum
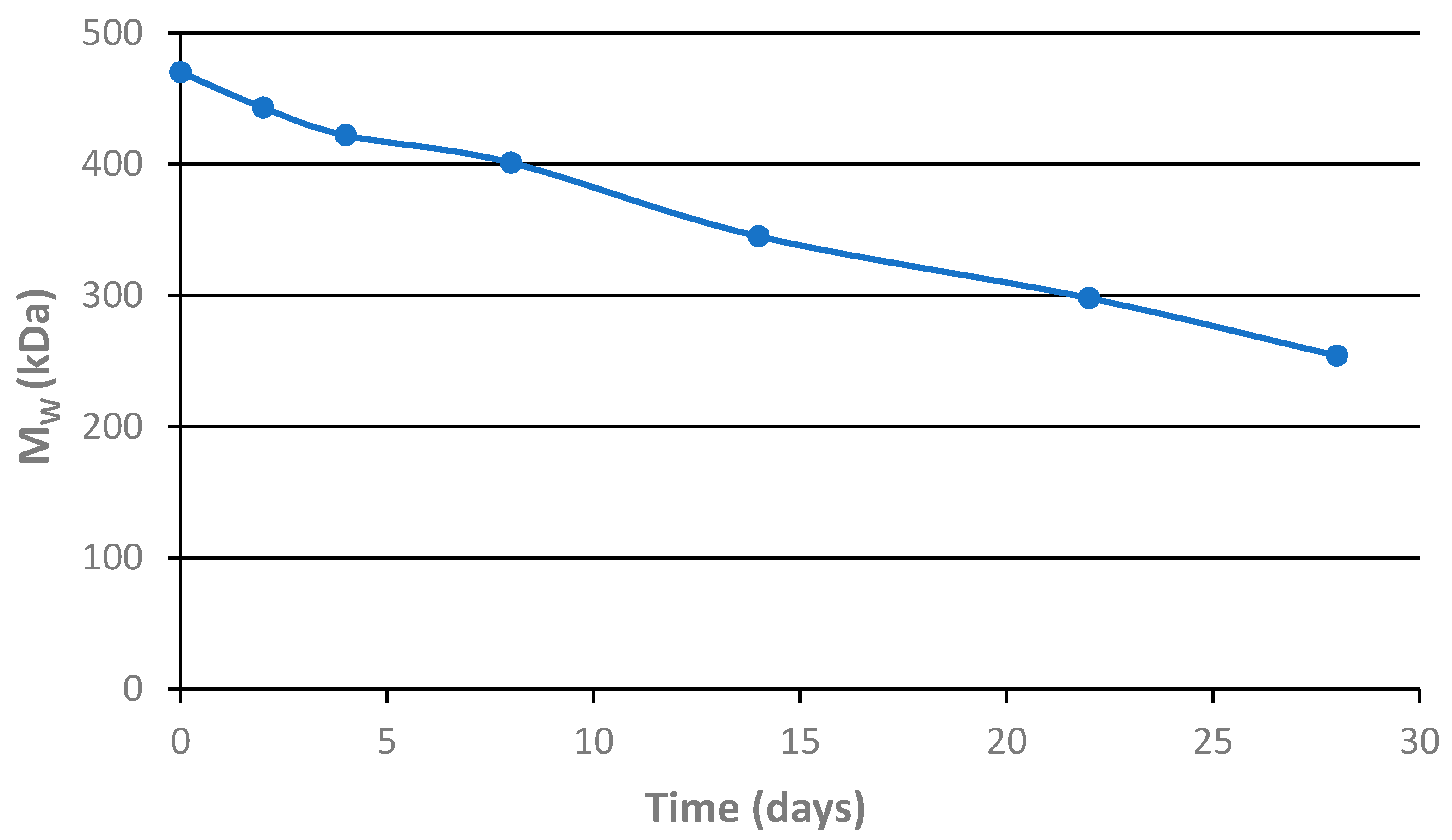
3.4. Hydrolytic Degradation of the Star Polymer Conjugate
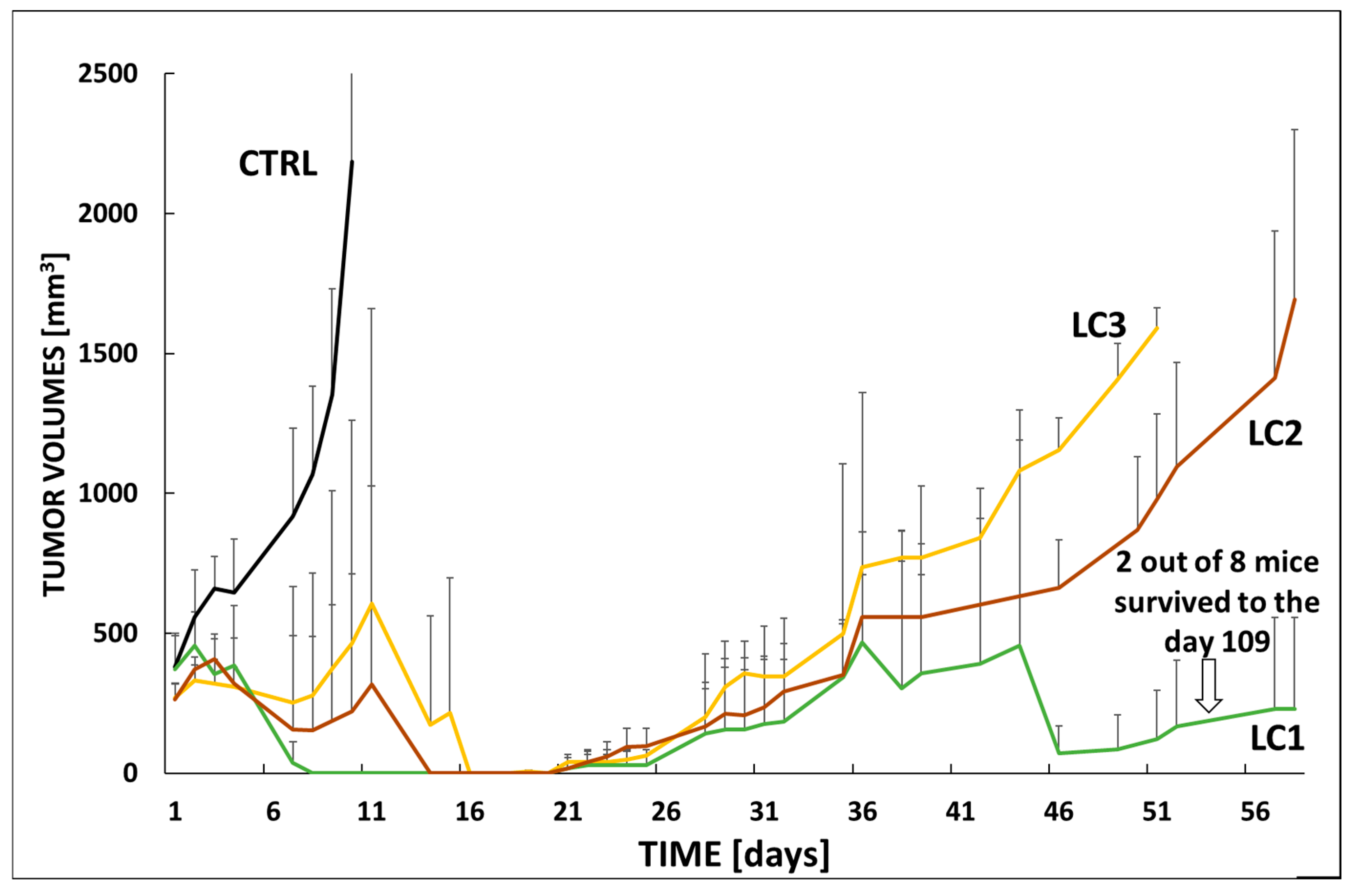
3.5. In Vivo Efficacy and Toxicity in VFN-D1 DLBCL Model
3.6. In Vivo Efficacy in VFN-B2 Lymphoma Model
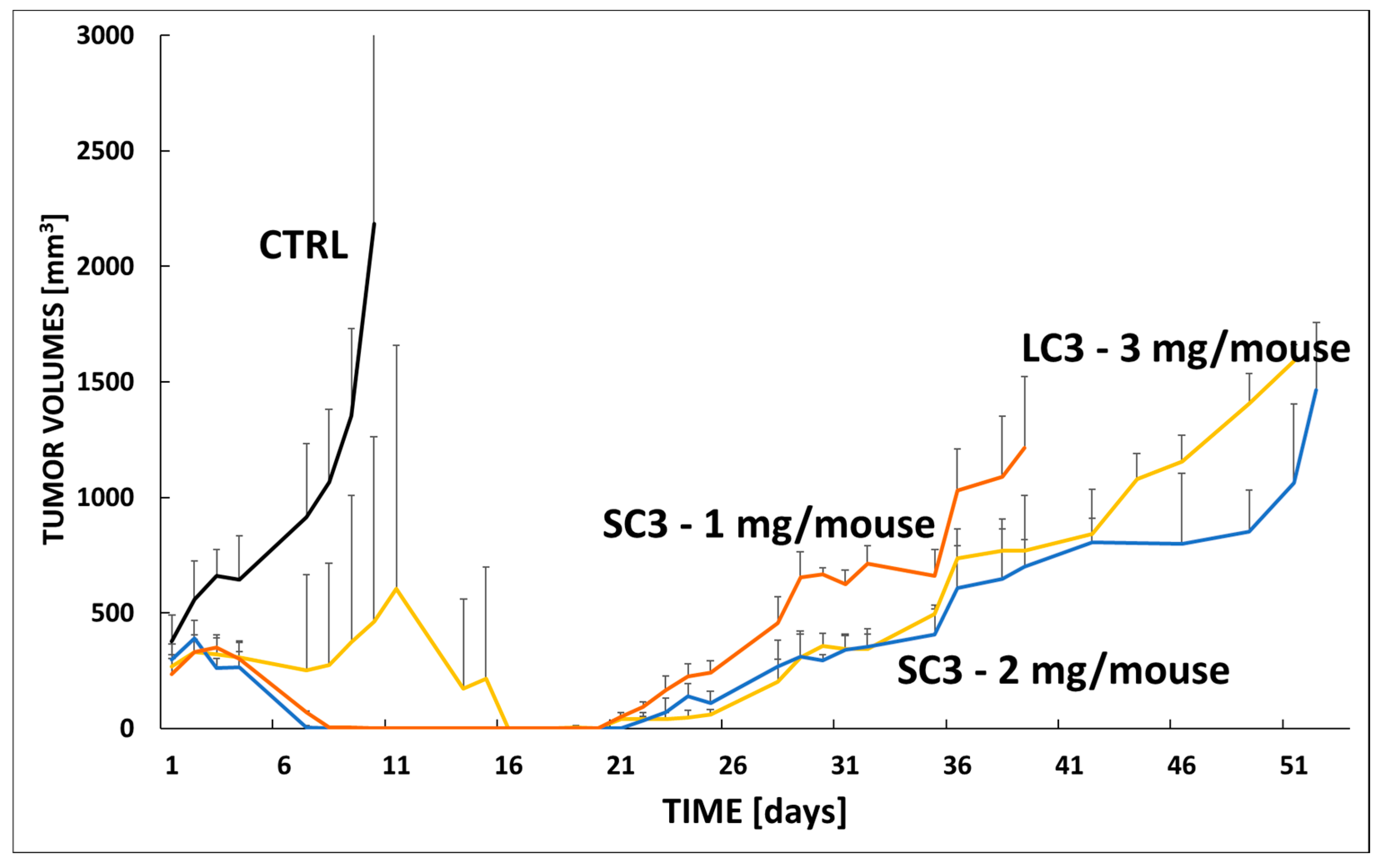
3.7. In Vivo Efficacy Star Conjugate SC1 in VFN-B2 Lymphoma Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minko, T.; Dharap, S.S.; Pakunlu, R.I.; Wang, Y. Molecular targeting of drug delivery systems to cancer. Curr. Drug Targets 2004, 5, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Nan, A.; Ghandehari, H.; Hebert, C.; Siavash, H.; Nikitakis, N.; Reynolds, M.; Sauk, J.J. Water-soluble polymers for targeted drug delivery to human squamous carcinoma of head and neck. J. Drug Target. 2005, 13, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Etrych, T.; Kovář, L.; Strohalm, J.; Chytil, P.; Ríhová, B. Ulbrich. Biodegradable star HPMA polymer-drug conjugates: Biodegradability, distribution and anti-tumor efficacy. J. Control. Release 2011, 154, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Pechar, M.; Pola, R.; Studenovský, M.; Bláhová, M.; Grosmanová, E.; Dydowiczová, A.; Filipová, M.; Islam, R.; Gao, S.; Fang, J.; et al. Polymer nanomedicines with enzymatically triggered activation: A comparative study of in vitro and in vivo anti-cancer efficacy related to the spacer structure. Nanomed. Nanotechnol. Biol. Med. 2022, 46, 102597. [Google Scholar] [CrossRef]
- Zhang, R.; Luo, K.; Yang, J.; Sima, M.; Sun, Y.; Janát-Amsbury, M.M.; Kopeček, J. Synthesis and evaluation of a backbone biodegradable multiblock HPMA copolymer nanocarrier for the systemic delivery of paclitaxel. J. Control. Release 2013, 166, 66–74. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Z.; Chau, Y. Synthesis, characterization, and in vivo evaluation of poly(ethylene oxide-co-glycidol)-platinate conjugate. Eur. J. Pharm. Sci. 2010, 41, 464–472. [Google Scholar] [CrossRef]
- Nichifor, M.; Schacht, E.H.; Seymour, L.W.; Anderson, D.; Shoaibi, M. Cytotoxicity and anticancer activity of macromolecular prodrugs of 5-fluorouracil. J. Bioact. Compat. Polym. 1997, 12, 265–281. [Google Scholar] [CrossRef]
- Takemoto, H.; Inaba, T.; Nomoto, T.; Matsui, M.; Liu, X.; Toyoda, M.; Honda, Y.; Taniwaki, K.; Yamada, N.; Kim, J.; et al. Polymeric modification of gemcitabine via cyclic acetal linkage for enhanced anticancer potency with negligible side effects. Biomaterials 2020, 235, 119804. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Polymer nanomedicines. Adv. Drug Deliv. Rev. 2020, 156, 40–64. [Google Scholar] [CrossRef]
- Kantarjian, H.; Barlogie, B.; Plunkett, W.; Velasquez, W.; McLaughlin, P.; Riggs, S.; Cabanillas, F. High-dose cytosine arabinoside in non-Hodgkin’s lymphoma. J. Clin. Oncol. 1983, 1, 689–694. [Google Scholar] [CrossRef]
- Abraham, A.; Varatharajan, S.; Abbas, S.; Zhang, W.; Shaji, R.V.; Ahmed, R.; Abraham, A.; George, B.; Srivastava, A.; Chandy, M.; et al. Cytidine deaminase genetic variants influence RNA expression and cytarabine cytotoxicity in acute myeloid leukemia. Pharmacogenomics 2012, 13, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Neurotoxicity of intra-CSF liposomal cytarabine (DepoCyt) administered for the treatment of leptomeningeal metastases: A retrospective case series. J. Neuro-Oncology 2012, 108, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, K.; Pels, H.; Kowoll, A.; Kuhnhenn, J.; Schlegel, U. Neurologic complications after intrathecal liposomal cytarabine in combination with systemic polychemotherapy in primary CNS lymphoma. J. Neuro-Oncology 2011, 103, 635–640. [Google Scholar] [CrossRef]
- Salehi, B.; Selamoglu, Z.; Mileski, K.S.; Pezzani, R.; Redaelli, M.; Cho, W.C.; Kobarfard, F.; Rajabi, S.; Martorell, M.; Kumar, P.; et al. Liposomal cytarabine as cancer therapy: From chemistry to clinical applications. Biomolecules 2019, 9, 773. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Parang, K. Development of cytarabine prodrugs and delivery systems for leukemia treatment. Expert Opin. Drug Deliv. 2010, 7, 1225–1238. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, J.; Zhang, D.; Wang, K.; Luan, Y. Self-assembling nanoparticles based on cytarabine prodrugs: Synthesis, characterization and antitumor evaluation. J. Mol. Liq. 2018, 251, 178–184. [Google Scholar] [CrossRef]
- Pola, R.; Pokorná, E.; Vočková, P.; Böhmová, E.; Pechar, M.; Karolová, J.; Pankrác, J.; Šefc, L.; Helman, K.; Trněný, M.; et al. Cytarabine nanotherapeutics with increased stability and enhanced lymphoma uptake for tailored highly effective therapy of mantle cell lymphoma. Acta Biomater. 2021, 119, 349–359. [Google Scholar] [CrossRef]
- Ulbrich, K.; Šubr, V.; Strohalm, J.; Plocová, D.; Jelınková, M.; Řıhová, B. Polymeric drugs based on conjugates of synthetic and natural macromolecules: I. Synthesis and physico-chemical characterisation. J. Control. Release 2000, 64, 63–79. [Google Scholar] [CrossRef]
- Pola, R.; Laga, R.; Ulbrich, K.; Sieglová, I.; Král, V.; Fábry, M.; Kabešová, M.; Kovář, M.; Pechar, M. Polymer Therapeutics with a Coiled Coil Motif Targeted against Murine BCL1 Leukemia. Biomacromolecules 2013, 14, 881–889. [Google Scholar] [CrossRef]
- Pola, R.; Janoušková, O.; Etrych, T. Etrych. The pH-Dependent and Enzymatic Release of Cytarabine From Hydrophilic Polymer Conjugates. Physiol. Res. 2016, 65, S225–S232. [Google Scholar] [CrossRef]
- Perrier, S.; Takolpuckdee, P.; Mars, C.A. Reversible Addition−Fragmentation Chain Transfer Polymerization: End Group Modification for Functionalized Polymers and Chain Transfer Agent Recovery. Macromolecules 2005, 38, 2033–2036. [Google Scholar] [CrossRef]
- Lidický, O.; Renešová, N.; Kudláčová, J.; Filipová, M.; Běláková, T.; Kovaříková, P.; Manakov, D.; Pankrác, J.; Dolníková, A.; Pokorná, E.; et al. Antibody polymer drug conjugates with increased drug to antibody ratio: CD38-targeting nanomedicines for innovative therapy of relapsed lymphomas. J. Control. Release 2025, 384, 113876. [Google Scholar] [CrossRef]
- Jakša, R.; Karolová, J.; Svatoň, M.; Kazantsev, D.; Grajciarová, M.; Pokorná, E.; Tonar, Z.; Klánová, M.; Winkowska, L.; Maláriková, D.; et al. Complex genetic and histopathological study of 15 patient-derived xenografts of aggressive lymphomas. Lab. Investig. 2022, 102, 957–965. [Google Scholar] [CrossRef]
- Willig, F.; Greiner, I.; Stork, H.; Schmidt, F.H. Leucinaminopeptidase-(Arylamidase-) Aktivität im Serum: Bestimmung mit Leucin-p-nitroanilid als Substrat. Klin. Wochenschr. 1967, 45, 474–481. [Google Scholar] [CrossRef]
- Laizure, S.C.; Herring, V.; Hu, Z.; Witbrodt, K.; Parker, R.B. The role of human carboxylesterases in drug metabolism: Have we overlooked their importance? Pharmacotherapy 2013, 33, 210–222. [Google Scholar] [CrossRef]
- Jasiecki, J.; Szczoczarz, A.; Cysewski, D.; Lewandowski, K.; Skowron, P.; Waleron, K.; Wasąg, B. Butyrylcholinesterase–Protein Interactions in Human Serum. Int. J. Mol. Sci. 2021, 22, 10662. [Google Scholar] [CrossRef]
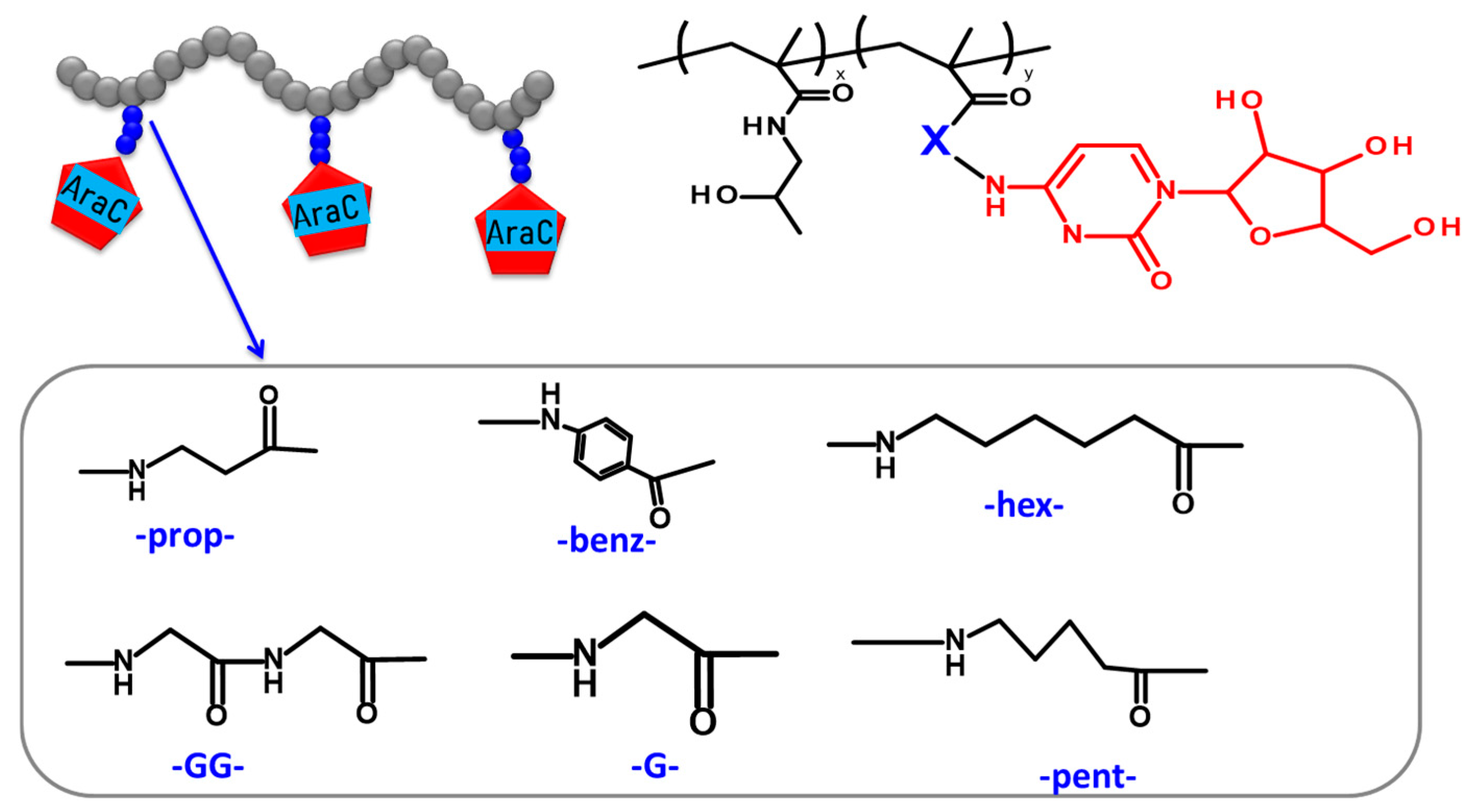


| Sample | Description | MW a [g‧mol−1] | Ð a [-] | Dh a [nm] | TT Content b [mol%] | Degree of Polymerization c |
|---|---|---|---|---|---|---|
| LP1 | p(HPMA-co-MA-prop-TT) | 37,100 | 1.07 | 8.8 | 14.0 | 233 |
| LP2 | p(HPMA-co-MA-pent-TT) | 34,500 | 1.08 | 9.0 | 10.0 | 219 |
| LP3 | p(HPMA-co-MA-hex-TT) | 44,500 | 1.12 | 8.6 | 11.3 | 277 |
| LP4 | p(HPMA-co-MA-benz-TT) | 43,000 | 1.16 | 9.2 | 9.8 | 270 |
| LP5 | p(HPMA-co-MA-G-TT) | 40,000 | 1.12 | 8.8 | 10.9 | 260 |
| LP6 | p(HPMA-co-MA-GG-TT) | 42,000 | 1.15 | 9.0 | 11.8 | 259 |
| SP1 | STAR-p(HPMA-co-MA-hex-TT) | 125,000 | 1.09 | 14.4 | 10.4 | 198/chain |
| Sample | Description | MW a [g‧mol−1] | Ð a [-] | Dh b [nm] | araC Content c [wt%] |
|---|---|---|---|---|---|
| LC1 | p(HPMA-co-MA-prop-araC) | 65,000 | 1.50 | 13.4 | 12.5 |
| LC2 | p(HPMA-co-MA-pent-araC) | 53,100 | 1.30 | 15.0 | 13.1 |
| LC3 | p(HPMA-co-MA-hex-araC) | 61,800 | 1.32 | 16.6 | 14.2 |
| LC4 | p(HPMA-co-MA-benz-araC) | 62,800 | 1.35 | 10.4 | 13.2 |
| LC5 | p(HPMA-co-MA-G-araC) | 62,600 | 1.39 | 10.6 | 13.5 |
| LC6 | p(HPMA-co-MA-GG-araC) | 63,300 | 1.35 | 11.0 | 13.9 |
| SC1 | STAR-p(HPMA-co-MA-hex-araC) | 470,000 | 3.9 | 27.8 | 14.7 |
| Sample | t50 (h) |
|---|---|
| LC1 | 72 |
| LC2 | 146 |
| LC3 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pola, R.; Grosmanová, E.; Pechar, M.; Kostka, L.; Pokorná, E.; Tušková, L.; Klener, P.; Etrych, T. Nanomedicines for Delivery of Cytarabine: Effect of Carrier Structure and Spacer on the Anti-Lymphoma Efficacy. Polymers 2025, 17, 2837. https://doi.org/10.3390/polym17212837
Pola R, Grosmanová E, Pechar M, Kostka L, Pokorná E, Tušková L, Klener P, Etrych T. Nanomedicines for Delivery of Cytarabine: Effect of Carrier Structure and Spacer on the Anti-Lymphoma Efficacy. Polymers. 2025; 17(21):2837. https://doi.org/10.3390/polym17212837
Chicago/Turabian StylePola, Robert, Eliška Grosmanová, Michal Pechar, Libor Kostka, Eva Pokorná, Liliana Tušková, Pavel Klener, and Tomáš Etrych. 2025. "Nanomedicines for Delivery of Cytarabine: Effect of Carrier Structure and Spacer on the Anti-Lymphoma Efficacy" Polymers 17, no. 21: 2837. https://doi.org/10.3390/polym17212837
APA StylePola, R., Grosmanová, E., Pechar, M., Kostka, L., Pokorná, E., Tušková, L., Klener, P., & Etrych, T. (2025). Nanomedicines for Delivery of Cytarabine: Effect of Carrier Structure and Spacer on the Anti-Lymphoma Efficacy. Polymers, 17(21), 2837. https://doi.org/10.3390/polym17212837








