3.1. Characterization of Halloysite
As-received halloysite may contain absorbed water that may affect the extent of its dispersion into the PU matrix. Therefore, before characterization, the halloysite was heated at 120 °C overnight.
The ATR-IR spectrum of halloysite (
Figure 6) shows two low and intense broad stretching bands of OH groups at 3685 and 3610 cm
−1, and Si–O and Si–O–Si stretching bands at 1103 and 1032 cm
−1, respectively. Si–O bending bands appear at 808 and 740 cm
−1, and two additional bands at 911 cm
−1 (Al–OH) and 554 cm
−1 (Al–O–Si bending) can be distinguished. The assignment of these bands agrees well with the existing literature [
34].
The chemical composition on the halloysite surface was assessed by XPS. The halloysite surface contained 71 at.% oxygen, 16 at.% silicon, and 13 at.% aluminium. Thus, the experimental Si/Al ratio was 1.2, which is higher than the stoichiometric Si/Al ratio in halloysite (Al
2Si
2O
5(OH)
4·nH
2O), because Si atoms were predominantly located on the outer surfaces of the nanotubes [
47]. On the other hand, both the stoichiometric O/Si and O/Al ratios in halloysite are 0.40, while the experimental ratios were 0.26 and 0.18, respectively; therefore, the number of oxygen atoms on the halloysite surface was significantly lower than the stoichiometric one.
The X-ray diffractogram of halloysite (
Figure S2 and Table S1 in the Supplementary Materials) shows intense crystalline diffraction peaks at 2θ values of 12°, 20°, 25°, 35°, and 63°, which correspond to the (001), (100), (002), (100), and (300) planes, respectively [
40].
The thermal properties of halloysite were characterized by DSC and TGA.
Figure 7 shows the DSC curves of halloysite corresponding to the first and second heating runs. An endothermic event corresponding to a melting peak at 168 °C with a melting enthalpy of 4.6 J/g was observed. This melting peak corresponds to the onset of the dehydroxylation of inner hydroxyl groups in halloysite [
41].
The TGA curve of halloysite shows 15 wt.% loss and exhibits two thermal decompositions at 44 °C (1 wt.% loss due to adsorbed water) and 455 °C (14 wt.% attributed to structural dehydroxylation) (
Figure S3 in the Supplementary Materials).
3.2. Polyurethanes Made with 0.5 wt.% As-Received and Thermally Treated Halloysite (E0.5-20 and E0.5)
In
Section 3.1, it was concluded that the thermal treatment of halloysite at 120 °C caused 1 wt.% water loss and the onset of the dehydroxylation of inner hydroxyl groups. These structural changes may affect the structure and properties of the filled polyurethanes. So, a comparison of the self-healing, structural/morphological, and mechanical properties of two PUs synthesized similarly and containing 0.5 wt.% as-received (E0.5-20) and thermally treated (120 °C/overnight) (E0.5) halloysite was carried out.
Both E0.5-20 and E0.5 exhibit similar and fast intrinsic room-temperature self-healing (
Figure 8,
Video S1 in the Supplementary Materials), so the addition of as-received and thermally treated halloysite does not affect the self-healing ability of the unfilled polyurethane.
The stress–strain curves reveal differences in the mechanical performance of E0.5-20 and E0.5 (
Figure 9). Both halloysite-filled PUs exhibit reasonable mechanical properties. E0.5 has a higher Young’s modulus (39.8 MPa), yield point (2.9 MPa), and elongation-at-break value (559%) than E0.5-20 (Young’s modulus, 14.0 MPa; yield point, 2.2 MPa; elongation-at-break value, 396%). However, in the plastic region, E0.5-20 has higher stress and tensile strength (2.3 MPa) than E0.5 (1.6 MPa). The differences in the mechanical properties align with distinct halloysite–PU interactions in E0.5-20 and E0.5.
To assess the different mechanical properties between E0.5-20 and E.05, their chemical compositions were analyzed by ATR-IR and XPS. The ATR-IR spectra (
Figure S4 in the Supplementary Material file) of E0.5-20 and E0.5 are very similar, but they mainly differ in the carbonyl stretching region (1800–1600 cm
−1) and the intensity of the OH stretching band of halloysite (more intense in E0.5-20). The curve fitting of the carbonyl stretching region of the ATR-IR spectra of E0.5-20 and E0.5 (
Figure 10 and
Figure S5 in the Supplementary Materials) was carried out by using a Gaussian function. The C=O stretching regions of the ATR-IR spectra of both PUs exhibit the same contributions due to free carbonate (1745 cm
−1), free urethane and carbonyl–carbonyl interaction (1730 cm
−1), hydrogen-bonded urethane (1710 cm
−1), free urea (1695 cm
−1), and hydrogen-bonded urea (1660 cm
−1) groups. This assignment was made according to a previous study [
12]. Both PUs show similar percentages of bonded urethane (14–15%), free urea (10–11%), and bonded urea (2%), indicating that the addition of as-received or thermally treated halloysite does not affect the formation of free and hydrogen-bonded urea (
Table 1). However, E0.5 has a higher percentage of free carbonate groups and a lower percentage of free urethane/carbonate–carbonate interactions than E0.5-20 (
Table 1). Because the hard segment contents in E0.5-20 and E0.5 are similar (22 wt.%), the differences in the percentages of free and associated carbonate groups are due to lower interactions between the carbonate groups in E0.5, leading to the higher mobility of the polyurethane chains.
The structural differences between E0.5-20 and E0.5 were confirmed by DSC. The DSC curves of both PUs (
Figure S6 in the Supplementary Materials) show the same thermal events (the glass transition of the soft segments at −20–−23 °C, the melting of the soft segments at 42 °C, and one small melting event at 91–100 °C) (
Table 2). E0.5 shows a significantly lower (0.20 J/g °C) heat capacity at constant pressure (Δc
p) in the glass transition region and significantly higher melting enthalpy (7.5 J/g) due to the higher amount of free carbonate groups and, thus, the higher mobility of the soft segments. E0.5-20 shows a Δc
p value of 0.67 J/g °C and a melting enthalpy of 2.0 J/g, which correspond to a lower number of free carbonate groups and lower mobility of the soft segments (
Table 2). Therefore, E0.5 shows a more ordered internal structure and stronger interactions between soft segments—likely reinforced by more effective halloysite–PU interfacial interactions—than E0.5-20. On the other hand, the small melting event at 91–100 °C in the DSC curves of both PUs is likely a partial relaxation or reorganization of the polyurethane chains around the halloysite surface because the melting in the DSC curve of halloysite (
Figure 7) is produced at a higher temperature. It should be noted that the glass transition temperatures of E0.5 and E0.5-20 cannot be obtained due to the overlap with the end of the melting peak due to the onset of the dehydroxylation of inner hydroxyl groups in halloysite (
Figure 7).
Because the DSC curves show a different ordering of the soft segments in E0.5-20 and E0.5, they should exhibit different crystalline structures. The X-ray diffractogram of E0.5-20 shows a higher number of peaks than E0.5 and exhibits more intense peaks at 2θ = 20° (3018 a.u. vs. 1918 a.u.) and 2θ = 23° (1918 a.u. vs. 352 a.u.) (
Figure 11), indicating higher crystalline ordering of the soft segments. On the other hand, the intense halloysite peak at 2θ = 12° appears only in E0.5-20, whereas the X-ray diffractogram of E0.5 shows the peaks of halloysite at 2θ values of 17°, 19°, and 25°. Therefore, the halloysite–PU interactions are different in E0.5-20 and E0.5.
The different structures of E0.5-20 and E0.5 also determine their viscoelastic properties.
Figure 12 shows the variation in the storage modulus (G′) as a function of the temperature for E0.5 and E0.5-20. Both PUs show a continuous decrease in the G′ value by increasing the temperature, and the G′ values are higher in E0.5-20 in all temperature ranges. Therefore, E0.5-20 shows more elastic rheological behavior than E0.5 due to stronger halloysite–polyurethane chain interactions that may constrain the mobility of polymer chains.
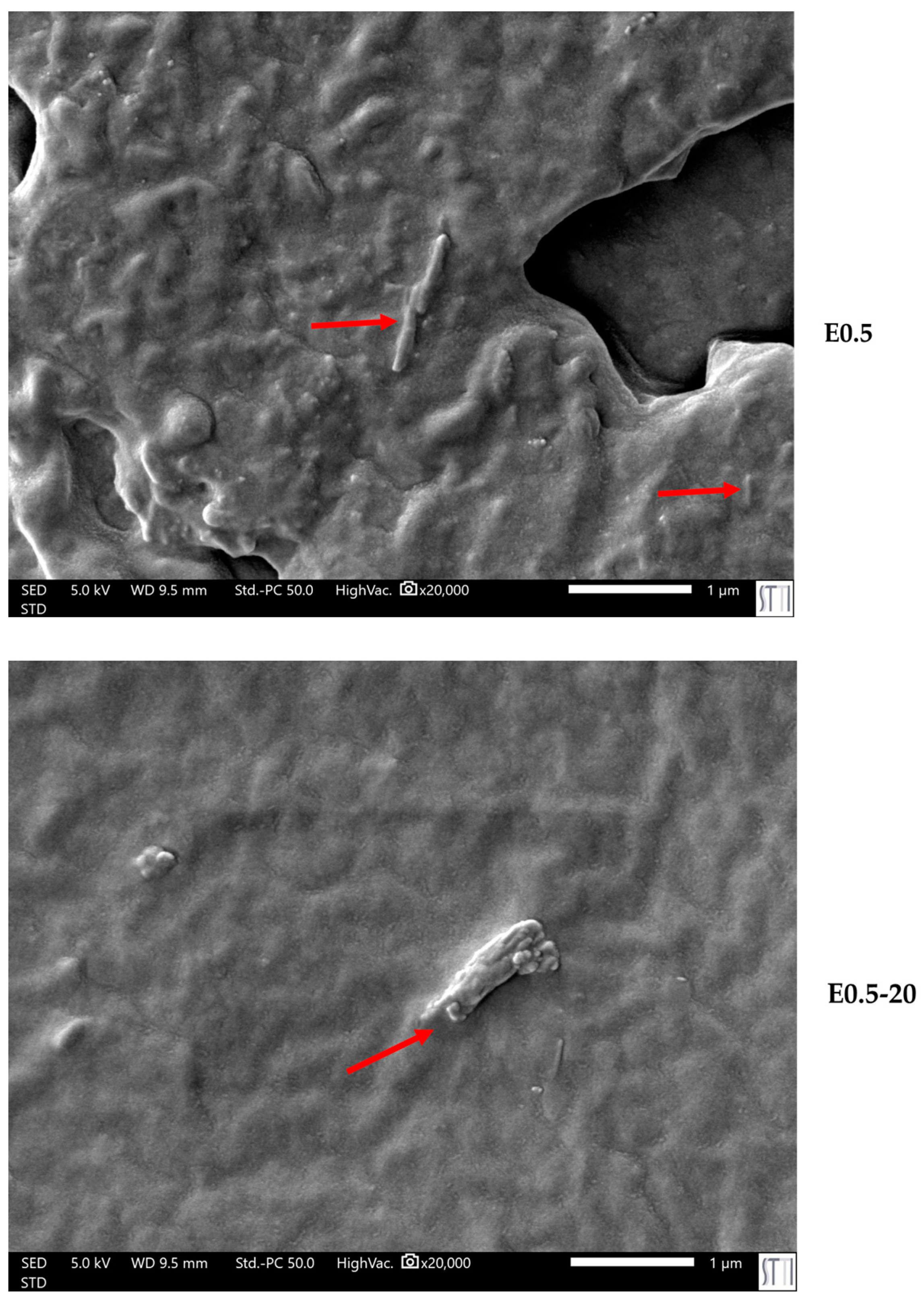
The differences in the viscoelastic behavior are closely related to the microstructural morphology of the polyurethanes. SEM micrographs (
Figure 13) reveal differences in the dispersion and morphology of halloysite particles within the matrix. While E0.5 exhibits a homogeneous dispersion of tubular halloysite particles embedded within the polymer network, E0.5-20 presents less well-dispersed halloysite aggregates, which tend to form small clusters. This structural disparity supports the different chain mobilities and viscoelastic responses in E0.5-20 and E0.5.
The different mechanical properties and structures of E0.5-20 and E0.5 could be ascribed to the different reactivities of water and hydroxyl groups of halloysite with isocyanate. Because the as-received halloysite contains two molecules of water and surface hydroxyl groups, urea bonds may form by reaction with isocyanate. Thermally treated halloysite does not contain significant amounts of water and has a lower number of hydroxyl groups than as-received halloysite, so the formation of urea bonds should be inhibited. However, the curve fitting of the ATR-IR spectra of E0.5-20 and E0.5 shows similar numbers of urea groups, indicating similar reactivities of halloysite with isocyanate in both PUs. Considering that, during PU synthesis, halloysite was initially dispersed between the polyol chains, it is quite unlikely that a reaction of halloysite with isocyanate can be produced (the number of OH groups is significantly higher in the polyol than in halloysite), so the differences between E0.5-20 and E0.5 derive from the different interactions of the as-received and thermally treated halloysite with the soft segments. This is supported by the higher percentage of free carbonate groups, the higher heat capacity at constant pressure (Δcp) in the glass transition region, the lower crystallinity, and the lower rheological elastic modulus in E0.5.
3.3. Characterization of Polyurethanes Without and with Different Amounts of Halloysite
The experimental evidence shown in
Section 3.2 indicated that the thermally treated halloysite is better dispersed in PU than the as-received one, so different amounts (0.5 to 10 wt.%) of thermally treated halloysite were added during PU synthesis to determine the optimal halloysite content that offers the best balance between mechanical reinforcement and room-temperature intrinsic self-healing.
All PUs exhibited fast intrinsic self-healing at room temperature (90 s under mild pressure) (
Figure 14). E0 (without halloysite) exhibited self-healing due to the segmental mobility of the polycarbonate soft segments [
12]. Similarly, all halloysite-filled PUs (E0.5, E1, E3, and E10) exhibited intrinsic self-healing at room temperature, indicating that the addition of halloysite does not inhibit the dynamic non-covalent interactions between carbonate groups in E0 (
Figure 2), even by adding 10 wt.% halloysite.
The stress–strain curves (
Figure 15) show that all PUs containing halloysite have significantly improved mechanical properties with respect to E0 (
Table 3). The Young’s modulus, yield strength, and elongation-at-break values increased noticeably by adding 0.5 wt.% halloysite only, and E0.5 also exhibited room-temperature self-healing. In general, Young’s moduli and yield point values did not change noticeably by increasing the halloysite loading in the PUs, but the tensile strength and elongation-at-break values were higher in E1 and E3. However, the addition of 10 wt.% halloysite caused a stiffening of the polyurethane, i.e., higher tensile strength and lower elongation-at-break values than in E3 were obtained. This suggests that the addition of an excessive amount of halloysite filler leads to particle aggregation and reduced matrix flexibility, offsetting the reinforcement benefits, in agreement with previous studies [
40,
41,
42,
43,
44]. This can be ascribed to the higher elastic modulus of halloysite than the one of E0 and strong halloysite–PU interfacial interactions, which limited the motion of polyurethane chains. The mechanical reinforcement of PUs by adding halloysite did not compromise their intrinsic room-temperature self-healing ability. However, even though they improved with respect to E0, the values of the mechanical parameters of the fast room-temperature self-healing halloysite-filled PUs were lower than the ones of the thermally self-healing halloysite-filled PUs [
42,
43,
44].
The influence of adding different amounts of halloysite on the chemical structure of the PUs was determined by ATR-IR spectroscopy and XPS.
The ATR-IR spectra of PUs without and with different amounts of halloysite (
Figure S7 in the Supplementary Materials) show a broad band at 3351 cm
−1 (N–H stretching of urethane + O–H stretching of halloysite) that becomes more intense and broader by increasing the halloysite content. Furthermore, the intensity of the Al–O–Si band at 554 cm
−1 increases by increasing the halloysite content in the PUs. The bands at 2928 and 2847 cm
−1 are due to the symmetric and asymmetric stretching of –CH
2 groups and the bands at 1400 and 1457 cm
−1 correspond to –CH
2 groups of the soft segments and the chain extender. These bands are slightly less intense in the PUs containing higher amounts of halloysite. On the other hand, the C-O stretching bands of the soft segments at 789, 975, 1020, and 1049 cm
−1 show higher intensities in E3 and E10, indicating the interaction of halloysite with the soft segments.
All ATR-IR spectra show an intense C=O stretching band at 1741 cm
−1 due to carbonate and urethane groups and a band of N–H bending + C–N stretching at 1534 cm
−1 of urethane groups. The curve fitting of the carbonyl region (1800–1600 cm
−1) of the PUs shows similar C=O species (
Figure S8 in the Supplementary Materials), but, depending on their halloysite content, they differ in their percentages.
Table 4 shows that the percentages of hydrogen-bonded urethane at 1710 cm
−1 (14–15%), free urea at 1695 cm
−1 (9–11%), and hydrogen-bonded urea at 1660 cm
−1 (2–4%) are similar in unfilled and filled PUs, and they remain unchanged with the addition of different amounts of halloysite. On the other hand, the addition of 0.5 wt.% halloysite does not affect the percentages of free carbonate at 1745 cm
−1 (48–49%) and carbonate–carbonate interactions at 1730 cm
−1 (24–25%) in E0. However, a decrease in the percentage of free carbonate from 49% in E0 to 39% and an increase in the percentage of carbonate–carbonate interactions from 25% in E0 to 33% are observed in E1 and E3, indicating the disruption of carbonate–carbonate interactions between the soft segments by halloysite addition, i.e., new halloysite–PU interactions are produced by constrained polymer regions near the filler interface. Comparable percentages of free carbonate and carbonate–carbonate interactions in E3 and E10 indicate that the addition of more than 3 wt.% halloysite does not further alter the chemical structure of E0.
The surface chemical composition of selected PUs was further examined by XPS. The presence of halloysite was confirmed by the detection of 0.5 and 1 at.% Si on E0.5 and E1 surfaces, respectively (
Table 5). The atomic percentages of carbon and oxygen are slightly different on E0.5 and E1 surfaces. The E0.5 surface exhibits a lower carbon and a higher oxygen content compared with the E1 surface. This trend correlates with the higher free carbonate group content on the E0.5 surface with respect to the E1 surface (in agreement with the ATR-IR spectra), suggesting a higher proportion of accessible oxygen-rich species on the E0.5 surface.
These differences are also reflected in the high-resolution C1s spectra of the halloysite-filled PUs (
Figure S9 in the Supplementary Materials). The chemical species on E0 and E1 surfaces are similar: 87 at.% C-C/C-H (binding energy, BE = 284.7 eV), 9 at.% C–N due to urethane (BE = 286.4 eV), 3 at.% C=O due to urethane and carbonate (BE = 288.6 eV), and 1 at.% O–(C=O)–O due to carbonate (BE = 290.4 eV) (
Table 6). Therefore, the addition of 1 wt.% halloysite does not significantly change the chemical species on the E1 surface, i.e., the carbonate–carbonate interactions are not affected by adding filler. However, the addition of 0.5 wt.% halloysite increases the percentages of C–N and O–(C=O)–O species and decreases the percentage of C=O species with respect to the E0 surface (
Table 6). The increase in O–(C=O)–O content, combined with the reduction in the C=O percentage, implies a greater presence of free carbonate groups and a partial disruption of carbonate–carbonate interactions on the E0.5 surface, possibly caused by the interaction of halloysite with the soft segments.
Similar findings are evidenced in the high-resolution O1s XPS spectra of the halloysite-filled PUs (
Figure S10 in the Supplementary Materials). All PUs show C=O (531.6–531.8 eV) and C–O (533.5–533.7 eV) species. The addition of 0.5 wt.% halloysite led to a decrease (from 75% to 67%) in C=O and an increase (from 25% to 33%) in C–O species on the E0.5 surface compared with the E0 surface (
Table 7), suggesting a higher content of free carbonate groups and partial disruption of carbonate–carbonate interactions due to halloysite interactions with the soft segments. By contrast, on the E1 surface, the percentages of C=O and C–O species remained nearly unchanged relative to the E0 surface, indicating that the addition of 1 wt.% halloysite does not significantly affect the carbonate interactions among the soft segments.
Halloysite interactions affect the thermal properties of PUs. The DSC curve of the unfilled PU—E0—only shows the glass transitions of the soft segments at −21 °C and the hard segments at 223 °C (
Figure S11 in the Supplemental Materials). The addition of any amount of halloysite does not change the glass transition temperature (T
g) of the soft segments, but the heat capacity at constant pressure (∆c
p) values are higher in E1 and E3 (0.32–0.37 J/g. °C) (
Table 8), indicating the confinement of the soft segments between halloysite particles. Furthermore, two new melting transitions at 39–42 °C and 61–100 °C appear in filled PUs, indicating the existence of halloysite–PU interactions (
Table 8). The thermal event at 39–42 °C is due to the melting of halloysite–soft segment interactions and the melting enthalpy is significantly higher in E0.5, indicating more effective intercalation of halloysite particles among the soft segments of E0. The melting at 61–100 °C is associated with halloysite (the DSC curve of halloysite shows a melting event at 168 °C with an enthalpy of 4.6 J/g,
Figure 7) and, in general, the melting temperature and enthalpy are lower in the filled PUs. This evidence supports the intercalation of halloysite particles among the soft segments.
The addition of halloysite increases the thermal stability of the PUs (
Figure S12 in the Supplementary Materials) in a similar manner irrespective of the amount added. Furthermore, the TGA curves of halloysite-filled PUs shift toward higher temperatures compared with the unfilled PU (E0), indicating the existence of halloysite–PU interactions. The better thermal stability of halloysite-filled PUs has been previously stated in thermally self-healing PUs [
43]. However, the increase in thermal stability by adding 0.5–10 wt.% halloysite does not agree with a previous study dealing with thermal self-healing PUs in which the weight loss was shifted to lower temperatures for 0.5–1 wt.% halloysite [
45]; conversely, increased thermal stability was found by adding more than 1 wt.% halloysite, in agreement with the results of this study. This discrepancy is caused by the different synthesis procedures, the addition of halloysite to the polyol, and the composition of PUs.
As shown in
Figure 16, all filled PUs exhibit an additional thermal degradation step at 324–330 °C with increasing mass losses by increasing the halloysite loading, attributed to new interactions between halloysite and PU. Furthermore, the hard segments decompose at lower temperatures (281–283 °C) and with lower mass loss (7%) in all PUs containing halloysite with respect to E0, indicating the intercalation of halloysite among the polyurethane chains that facilitate earlier decomposition (
Table 9). Also, the degradation of the soft segments at 313 °C is gradually displaced to lower temperatures and with lower mass loss by increasing the halloysite content in the PUs. Additionally, the thermal degradation of the carbonate–carbonate interactions at 387 °C in E0 is displaced to lower temperatures (346–348 °C) with mass losses of 27–33% in all filled PUs because of the intercalation of halloysite nanoparticles among the polyurethane chains.
The intercalation of halloysite particles among polymer chains should affect the crystallinity of PUs. The X-ray diffractogram of E0 (
Figure 17) shows several peaks at 2θ values of 14°, 17°, 19°, 20°, 23°, 25°, and 44° (
Table S2 in the Supplementary Materials), and the most intense peak is the one at 2θ = 20°. The X-ray diffractogram of E0.5 closely resembles that of the unfilled PU (E0), but with lower intensities of the peaks, except for the ones at 2θ values of 19° and 25°. Since these diffraction peaks are absent in the X-ray diffractogram of neat halloysite, their enhancement in E0.5 suggests deep intercalation of halloysite within the PU chains, disrupting the crystallite packing of the soft segments. In contrast, the X-ray diffractograms of E1, E3, and E10 do not exhibit the peaks at 2θ values of 17°, 19°, and 25°, indicating a reduced degree of intercalation and, potentially, filler aggregation. In fact, in E3 and E10, a peak at 2θ = 12°—characteristic of halloysite—is clearly visible, supporting the presence of halloysite agglomerates in the PUs at higher filler contents. Thus, low halloysite loadings (e.g., 0.5 wt.%) promote better dispersion and interfacial interactions with polymeric chains, while higher contents lead to reduced structural integration.
The viscoelastic properties of PUs are affected by the existence of halloysite–PU interactions. The rheological behavior of E0.5 differs notably from that of the other filled PUs. As shown in
Figure 18, all PUs exhibit a gradual decrease in the storage modulus (G′) by increasing temperature. E0.5 presents lower G′ values than E0 across most of the temperature range, indicating a more mobile network. Despite this, at 5 °C, E0.5 displays a higher G′ (1732 kPa) than E0 (1571 kPa), suggesting that the halloysite intercalation within the soft segments locally reinforces the structure while simultaneously disrupting carbonate–carbonate interactions. In contrast, the rheological curves of E1, E3, and E10 are similar and show consistently higher G′ values than E0, indicating that, at higher halloysite loadings, partial aggregation of halloysite particles is produced. These interactions may compromise uniform dispersion and polymer chain mobility.
In line with the rheological behavior, SEM micrographs of PUs (
Figure 19) confirm a distinct dispersion pattern in E0.5 in which well-dispersed nanotubular structures and small individual halloysite particles are embedded uniformly within the polymer matrix. As the halloysite content increases, particle agglomeration becomes more evident. In E1, spherical agglomerates appear, consistent with its broader particle size distribution (0.4–1.3 µm) as compared with E0.5 (
Figure S13 in the Supplementary Materials). This trend intensifies in E3 and E10, where larger clusters ranging from 3 to 7 µm are observed, and a significant fraction of halloysite particles appear to have accumulated at the PU surface, suggesting poor filler integration. In particular, E10 shows widespread surface migration and the highest degree of aggregation (
Figure 20), which correlates with the previously discussed reduction in polymer–filler interfacial quality and mechanical uniformity at high halloysite loadings. On the other hand, the surface of filled PUs becomes much rougher due to the strong bonding between the halloysite and the polymer matrix, which constrains the polyurethane chains surrounding the filler and increases the mechanical interlocking and physical entanglement density in the matrix; thus, the existence of halloysite favors energy dissipation during fracture.
The collective results from the thermal, mechanical, structural, and surface analyses suggest that halloysite interacts with the polyurethane matrix via two distinct mechanisms, depending on its concentration. As illustrated in
Figure 21, at low content (E0.5), halloysite nanotubes are sufficiently dispersed and capable of intercalating between the polycarbonate soft segments, locally disrupting carbonate–carbonate interactions and reinforcing the matrix. In contrast, at higher concentrations (E1 and PUs with 3–10 wt.% halloysite), halloysite predominantly interacts externally along the polymer chains, leading to more extensive but superficial contact, which limits its effectiveness as a reinforcing agent and disrupts the uniform phase morphology.