Selective Gold Ion Sorption from Iron-Containing Solution Using an Interpolymer System of Industrial Ion Exchangers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Experimental Procedures
3. Results
3.1. Sorption Kinetics
Sorption of Au(I) and Fe(II) Ions from a Gold-Iron Mixed Solution
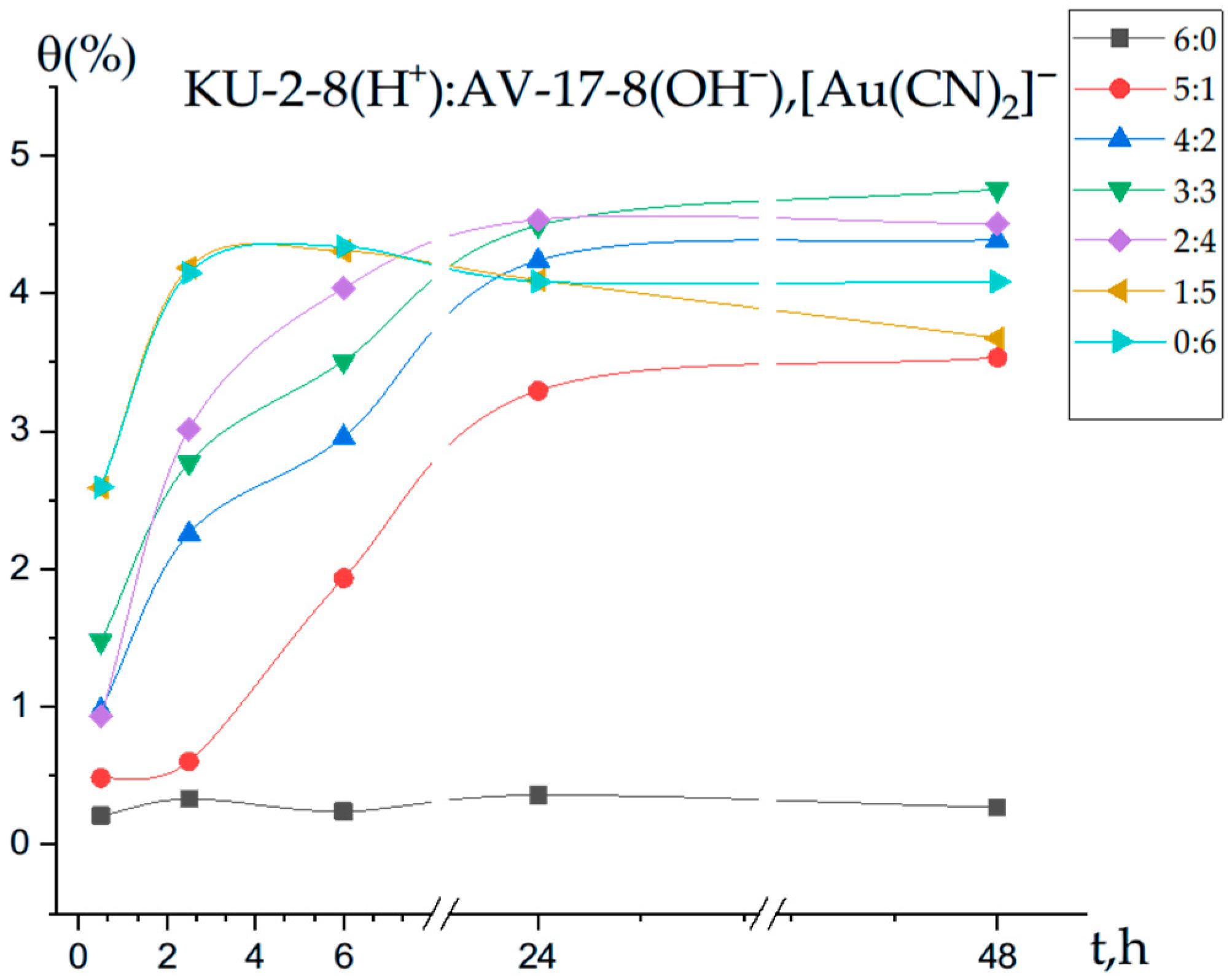
3.2. Polymer Chain Binding Degree (θ) of Gold Ions by the Interpolymer System
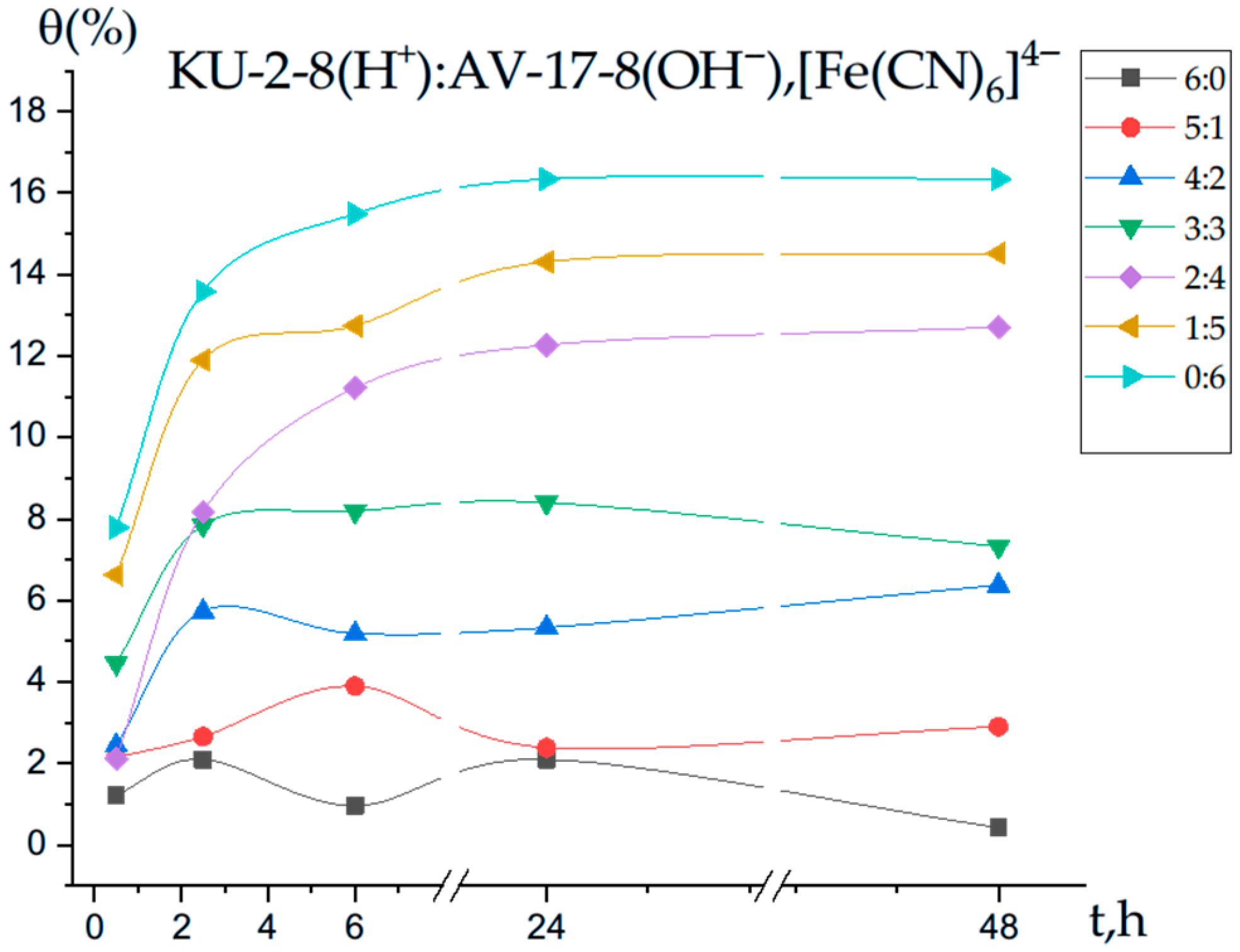
3.3. Polymer Chain Binding Degree (θ) of Iron Ions by the Interpolymer System
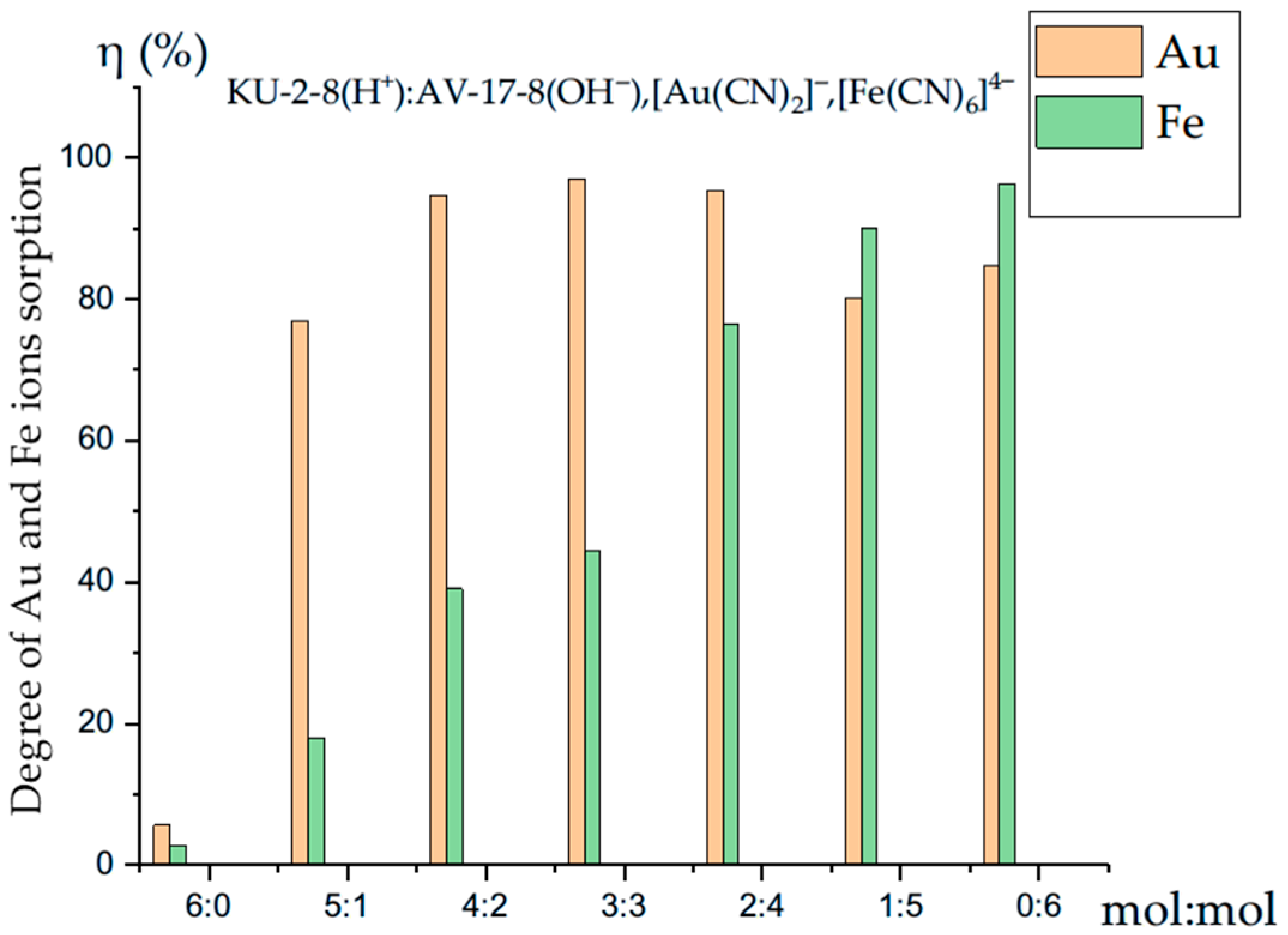
3.4. Competitive Sorption of the Gold and Iron Ions by the Interpolymer System KU-2-8(H+):AV-17-8(OH−)
3.5. Investigation of the Desorption of Au(I) and Fe(II) Ions
3.5.1. Mathematical Evaluation of Desorption Kinetics
3.5.2. Ion Exchange Mechanisms
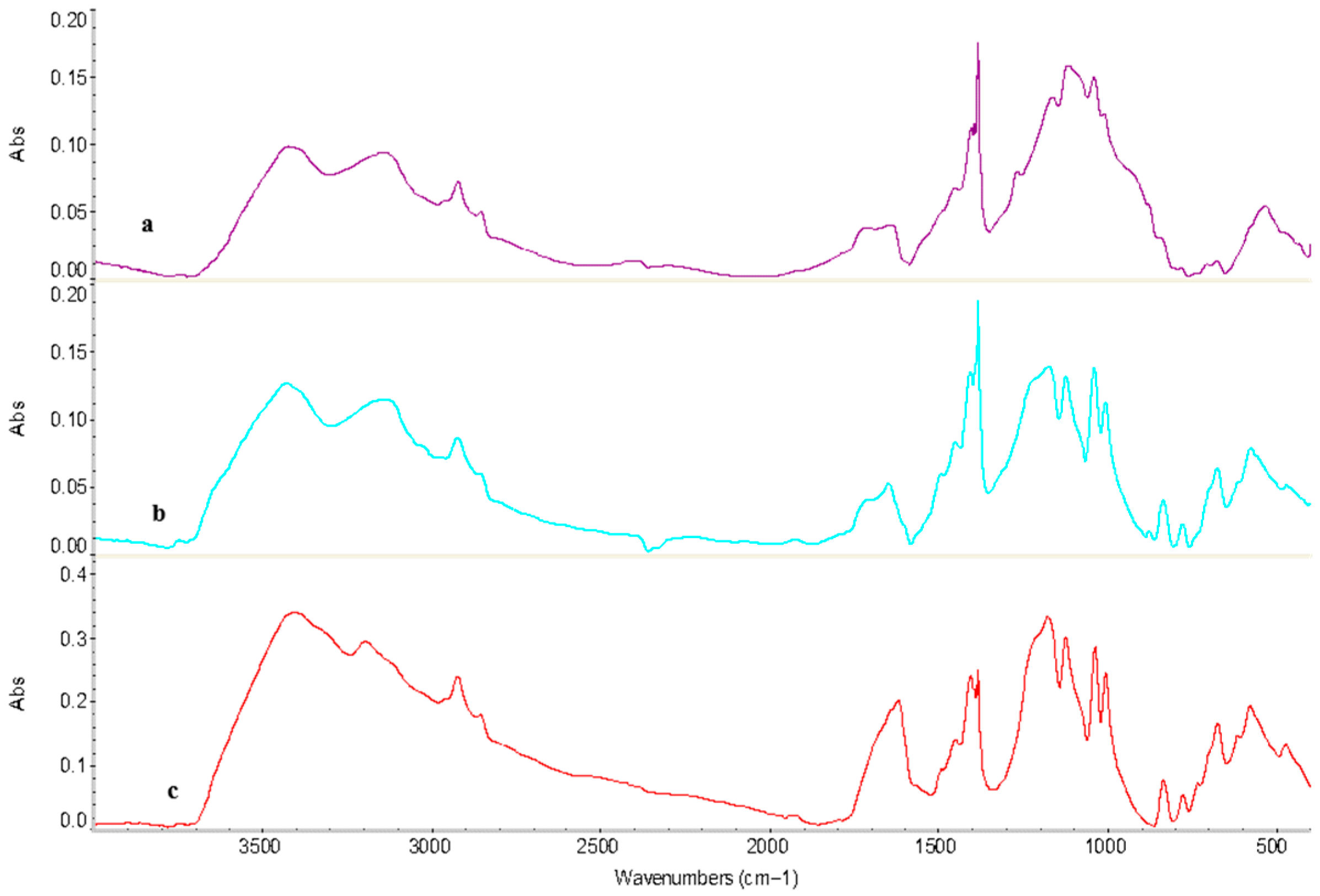
3.6. Fourier Transform Infrared (FTIR) Spectroscopy Studies
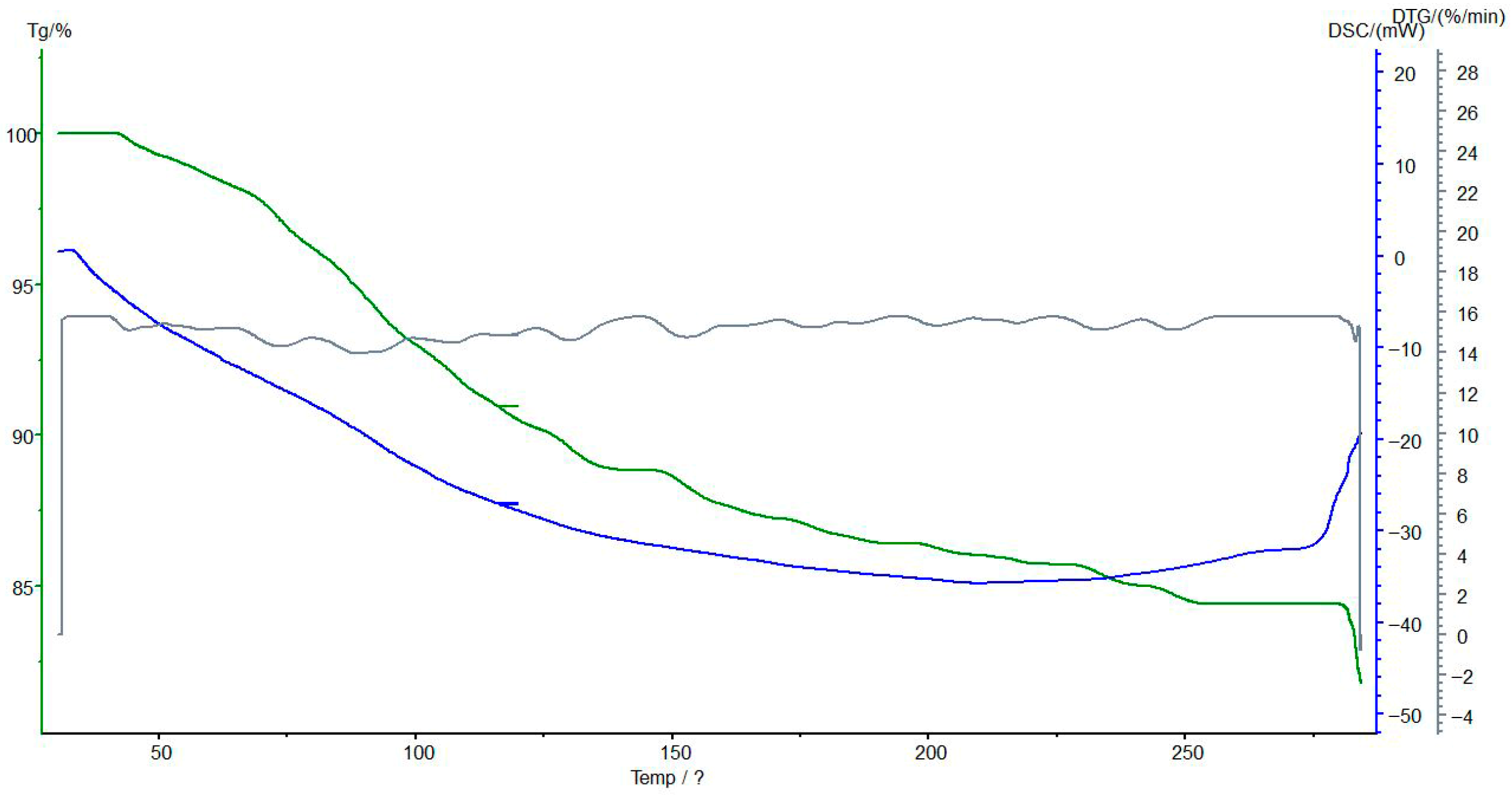
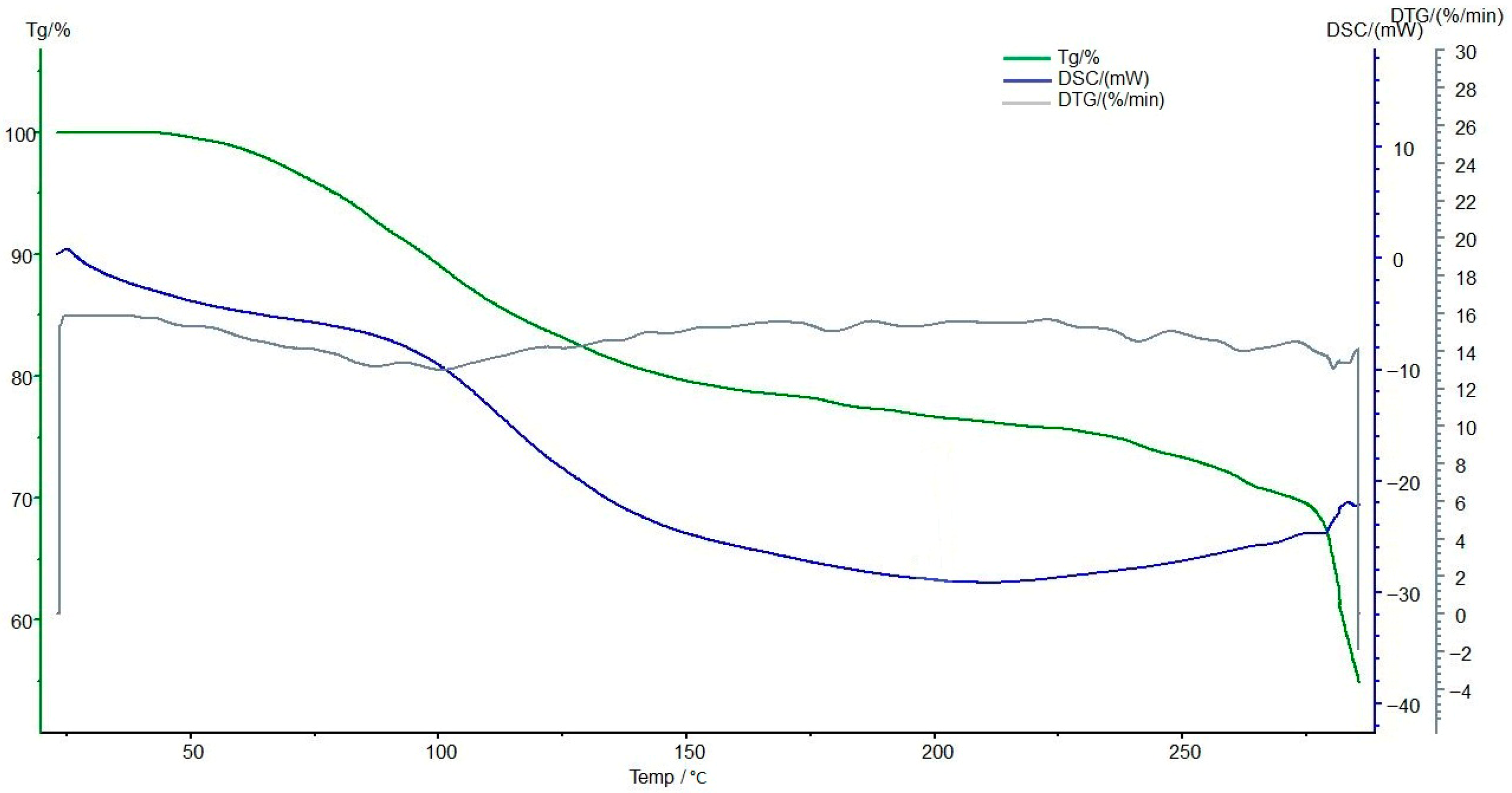
3.7. Thermogravimetric and Differential Scanning Calorimetric Analyses of the Interpolymer System KU-2-8(H+):AV-17-8(OH−) (3:3) and Its Complexes
3.8. Determination of Ion Distribution and Separation Coefficients
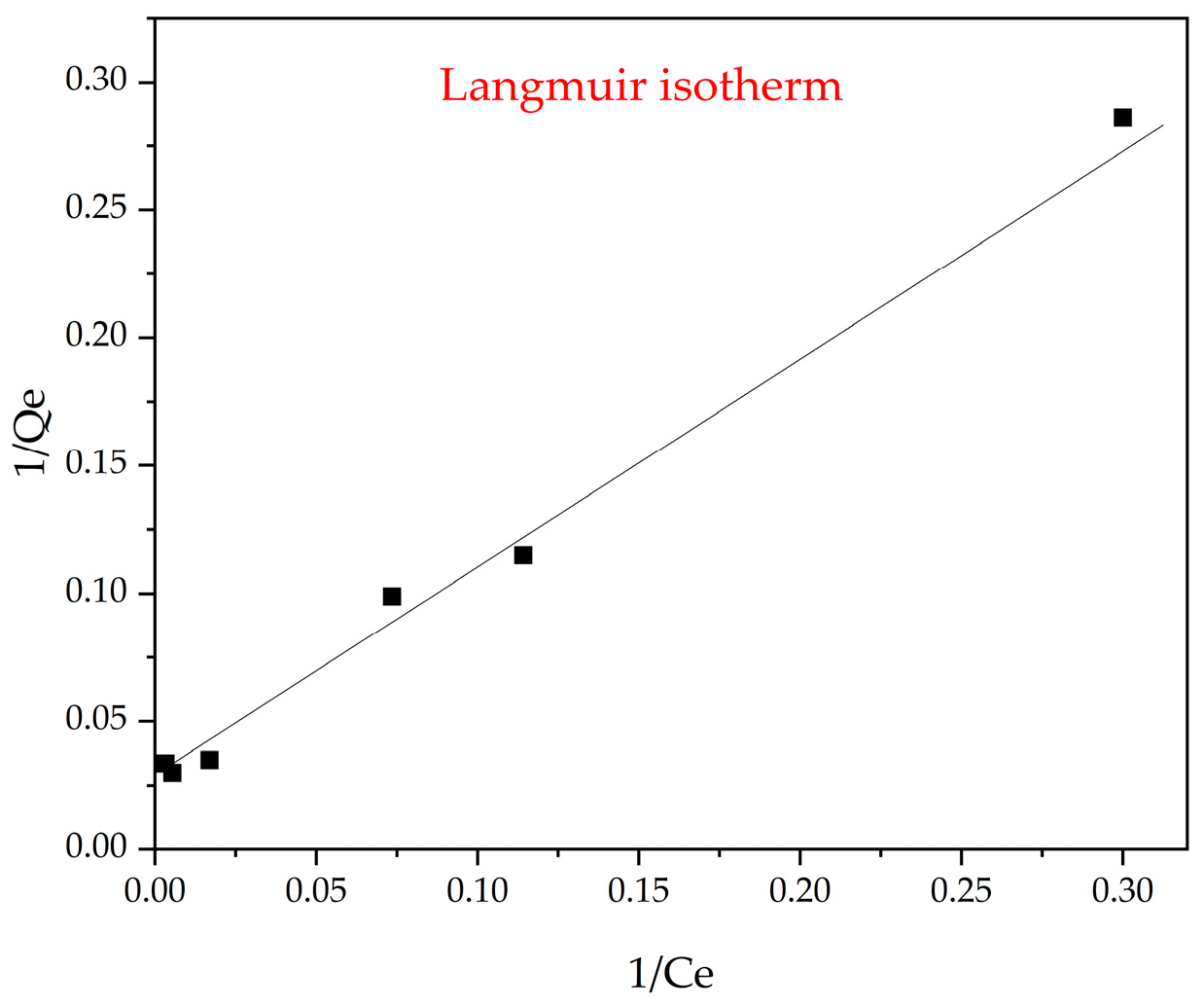
3.9. Langmuir Isothermal Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez, J.P.; Folens, K.; Leus, K.; Vanhaecke, F.; Van Der Voort, P.; Du Laing, G. Progress in hydrometallurgical technologies to recover critical raw materials and precious metals from low-concentrated streams. Resour. Conserv. Recycl. 2019, 142, 177–188. [Google Scholar] [CrossRef]
- Dodson, J.R.; Parker, H.L.; Muñoz García, A.; Hicken, A.; Asemave, K.; Farmer, T.J.; He, H.; Clark, J.H.; Hunt, A.J. Bio-derived materials as a green route for Precious & Critical Metal Recovery and re-use. Green Chem. 2015, 17, 1951–1965. [Google Scholar] [CrossRef]
- Shyam Sunder, G.S.; Adhikari, S.; Rohanifar, A.; Poudel, A.; Kirchhoff, J.R. Evolution of environmentally friendly strategies for Metal Extraction. Separations 2020, 7, 4. [Google Scholar] [CrossRef]
- Kerr, R.A. Is the world tottering on the precipice of peak gold? Science 2012, 335, 1038–1039. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Takatori, K.; Matsuno, Y. Environmentally sound recovery of gold from Waste Electrical and electronic equipment using organic aqua regia. Int. J. Autom. Technol. 2020, 14, 999–1004. [Google Scholar] [CrossRef]
- Kurganbekov, Z.; Utebayev, A.; Domatskiy, V.; Mukataeva, Z.; Zhetpisbay, D. Accumulation of Heavy Metals in Soil and Cultivated Crops. J. Environ. Account. Manag. 2021, 9, 391–402. [Google Scholar] [CrossRef]
- Milone, C.; Ingoglia, R.; Galvagno, S. Gold supported on Iron Oxy-hydroxides: A versatile tool for the synthesis of Fine Chemicals. Gold Bull. 2006, 39, 54–65. [Google Scholar] [CrossRef]
- Corti, C.W.; Holliday, R.J. Commercial aspects of gold applications: From materials science to chemical science. Gold Bull. 2004, 37, 20–26. [Google Scholar] [CrossRef]
- Schwank, J. Gold in bimetallic catalysts. Gold Bull. 1985, 18, 2–10. [Google Scholar] [CrossRef]
- Sun, Z.; Xiao, Y.; Sietsma, J.; Agterhuis, H.; Yang, Y. A cleaner process for selective recovery of valuable metals from electronic waste of complex mixtures of end-of-life electronic products. Environ. Sci. Technol. 2015, 49, 7981–7988. [Google Scholar] [CrossRef]
- Goc, K.; Kluczka, J.; Benke, G.; Malarz, J.; Pianowska, K.; Leszczyńska-Sejda, K. Application of ion exchange for recovery of noble metals. Minerals 2021, 11, 1188. [Google Scholar] [CrossRef]
- Chakraborty, S.C.; Zaman, M.W.; Hoque, M.; Qamruzzaman, M.; Zaman, J.U.; Hossain, D.; Pramanik, B.K.; Nguyen, L.N.; Nghiem, L.D.; Mofijur, M.; et al. Metals extraction processes from electronic waste: Constraints and opportunities. Environ. Sci. Pollut. Res. 2022, 29, 32651–32669. [Google Scholar] [CrossRef] [PubMed]
- Guanarathne, V.; Rajapaksha, A.U.; Vithanage, M.; Alessi, D.S.; Selvasembian, R.; Naushad, M.; You, S.; Oleszczuk, P.; Ok, Y.S. Hydrometallurgical processes for heavy metals recovery from industrial sludges. Crit. Rev. Environ. Sci. Technol. 2020, 52, 1022–1062. [Google Scholar] [CrossRef]
- Xu, W.; Mo, X.; Zhou, S.; Zhang, P.; Xiong, B.; Liu, Y.; Huang, Y.; Li, H.; Tang, K. Highly efficient and selective recovery of Au (III) by a new metal-organic polymer. J. Hazard Mater. 2019, 380, 120844. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, L.; Wang, S.; Zhang, B.; Peng, J. Selective recovery of Au (III) from aqueous solutions by nanosilica grafted with cationic polymer: Kinetics and isotherm. J. Taiwan Inst. Chem. Eng. 2017, 80, 342–348. [Google Scholar] [CrossRef]
- Kuhar, L.L.; Breuer, P.L.; Haque, N.; Robinson, D.J. Considerations and potential economic advantages for the in-situ recovery of gold from deep, hard-rock deposits. Miner. Eng. 2018, 121, 14–22. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Y. Sustainable electrochemical extraction of metal resources from waste streams: From removal to recovery. ACS Sustain. Chem. Eng. 2020, 8, 4693–4707. [Google Scholar] [CrossRef]
- Goodall, W.R.; Scales, P.J. An overview of the advantages and disadvantages of the determination of gold mineralogy by Automated Mineralogy. Miner. Eng. 2007, 20, 506–517. [Google Scholar] [CrossRef]
- Jumadilov, T.; Malimbayeva, Z.; Khimersen, K.; Saparbekova, I.; Imangazy, A.; Suberlyak, O. Specific features of praseodymium extraction by intergel system based on polyacrylic acid and poly-4-vinylpyridine hydrogels. Bull. Karaganda Univ. Chem. Ser. 2021, 103, 53–59. [Google Scholar] [CrossRef]
- Jumadilov, T.; Totkhuskyzy, B.; Imangazy, A.; Gražulevičius, J. Anomalous sorption of yttrium ions by the mutual activated hydrogels in the interpolymer system of poly(methacrylic acid) and poly(4-vinylpyridine). Chem. Chem. Technol. 2023, 17, 52–59. [Google Scholar] [CrossRef]
- Jumadilov, T.K.; Imangazy, A.M.; Khimersen, K.; Haponiuk, J.T. Remote interaction effect of industrial ion exchangers on the electrochemical and sorption equilibrium in scandium sulfate solution. Polym. Bull. 2024, 81, 2023–2041. [Google Scholar] [CrossRef]
- Imangazy, A.; Jumadilov, T.; Khimersen, K.; Bayshibekov, A. Enhanced sorption of europium and scandium ions from nitrate solutions by remotely activated Ion Exchangers. Polymers 2023, 15, 1194. [Google Scholar] [CrossRef]
- Jumadilov, T.; Khimersen, K.; Haponiuk, J.; Totkhuskyzy, B. Enhanced Lutetium Ion Sorption from Aqueous Solutions Using Activated Ion Exchangers. Polymers 2024, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Jumadilov, T.; Utesheva, A.; Grazulevicius, J.; Imangazy, A. Selective Sorption of Cerium Ions from Uranium-Containing Solutions by Remotely Activated Ion Exchangers. Polymers 2023, 15, 816. [Google Scholar] [CrossRef]
- Bektenov, N.; Baidullayeva, A.; Chalov, T.; Jumadilov, T.; Kanat, S. Modified Adsorbents Based on Glycidyl Methacrylate Copolymers for the Removal of Copper and Lead Ions from Wastewater. Eng. Sci. 2024, 31, 1237. [Google Scholar] [CrossRef]
- Kon’kova, T.V.; Quynh, T.N. Sorption recovery of lanthanum, iron, aluminum, and calcium ions from phosphoric acid. Russ. J. Appl. Chem. 2020, 93, 1868–1872. [Google Scholar] [CrossRef]
- Sert, S.; Altas, Y.; Tel, H.; Inan, S.; Cetinkaya, B.; Sengul, S.; Ozkan, B. Investigation of sorption behaviors of La, Pr, Nd, Sm, Eu and Gd on D2EHPA-impregnated XAD7 resin in nitric acid medium. Sep. Sci. Tech. 2021, 56, 26–35. [Google Scholar] [CrossRef]
- Yelemessova, Z.; Imangazy, A.; Tulepov, M.; Mansurov, Z. Energetic Metal–Organic Frameworks: Thermal Behaviors and Combustion of Nickel Oxide (II) Based on Activated Carbon Compositions. J. Eng. Phys. Thermophys. 2021, 94, 804–811. [Google Scholar] [CrossRef]
- Vorobyev, P.; Mikhailovskaya, T.; Yugay, O.; Serebryanskaya, A.; Chukhno, N.; Imangazy, A. Catalytic Oxidation of 4-Methylpyridine on Modified Vanadium Oxide Catalysts. Iran. J. Chem. Chem. Eng. 2018, 37, 81–89. [Google Scholar] [CrossRef]
- Yelemessova, Z.; Kydyrbekova, S.; Yerken, A. Thermal Characteristics Enhancement of AN/Mg/NC Composite Using Activated Carbon/Cobalt Oxide as Highly Effective Catalytic Additive. J. Compos. Sci. 2023, 7, 471. [Google Scholar] [CrossRef]
- Kamunur, K.; Jandosov, J.M.; Abdulkarimova, R.G.; Hori, K.; Yelemessova, Z.K. Combustion study of different transitional metal oxide based on AN/MgAl composites gas generators. Eurasian Chem. Technol. J. 2017, 19, 341–346. [Google Scholar] [CrossRef]
- Yelemessova, Z.; Yerken, A.; Zhaxlykova, D.; Milikhat, B. The Impact of Activated Carbon–MexOy (Me = Bi, Mo, Zn) Additives on the Thermal Decomposition Kinetics of the Ammonium Nitrate–Magnesium–Nitrocellulose Composite. J. Compos. Sci. 2024, 8, 420. [Google Scholar] [CrossRef]
- Tileuberdi, Y.; Ongarbayev, Y.; Mukatayeva, Z.; Zhanbekov, K.; Mukhambetkaliyev, K.; Akkazin, Y.; Shadin, N.; Imanbayev, Y. Studying Characteristics of Hot Fine-Grained Asphalt Concrete with the Addition of Coked Sands from the Pyrolysis of Oil Sands. Processes 2024, 12, 2540. [Google Scholar] [CrossRef]
- Imangazy, A.; Smagulova, G.; Kaidar, B.; Mansurov, Z.; Kerimkulova, A.; Umbetkaliev, K.; Zakhidov, A.; Vorobyev, P.; Jumadilov, T. Compositional Fibers Based on Coal Tar Mesophase Pitch Obtained by Electrospinning Method. Chem. Chem. Technol. 2021, 15, 403–407. [Google Scholar] [CrossRef]
- Jumadilov, T.; Suleimenova, M.; Gražulevičius, J.V. Selective Sorption of Gold Ions from Iron-Rich Solutions Using a Dual-Phase Interpolymer System. Eng. Sci. 2025, 35, 1482. [Google Scholar] [CrossRef]
- Jumadilov, T.K.; Khimersen, K.; Totkhuskyzy, B.; Haponiuk, J. Adsorption methods for the extraction and separation of rare earth elements. Review. Kompl. Ispolz. Min. Syra 2021, 318, 12–23. [Google Scholar] [CrossRef]
- Jumadilov, T.; Yskak, L.; Imangazy, A.; Suberlyak, O. Ion Exchange Dynamics in Cerium Nitrate Solution Regulated by Remotely Activated Industrial Ion Exchangers. Materials. 2021, 14, 3491. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Kontoleon, K.J.; Ashraf, M.A.; Petru, M. Polymer-Based Composites for Sorption, Energy Storage and Sensing Applications. Sep. Purif. Rev. 2024, 53, 1–14. [Google Scholar] [CrossRef]
- Jiang, Y.; Tan, P.; Liu, X.-Q.; Sun, L.-B. Process-Oriented Smart Adsorbents: Tailoring the Properties Dynamically as Demanded by Adsorption/Desorption. Acc. Chem. Res. 2021, 55, 75–86. [Google Scholar] [CrossRef]
- Ouimet, J.A.; Xu, J.; Flores-Hansen, C.; Phillip, W.A.; Boudouris, B.W. Design Considerations for Next-Generation Polymer Sorbents: From Polymer Chemistry to Device Configurations. Macromol. Chem. Phys. 2022, 223, 2200032. [Google Scholar] [CrossRef]
- Nesterov, Y.V. Ionites and Ion Exchange. Sorption Technology in the Extraction of Uranium and Other Metals by Underground Leaching; Unicor Publishing House: Moscow, Russia, 2007; 480p. [Google Scholar]
- Donnan, F.G. Theorie der Membrangleichgewichte und Membranpotentiale bei Vorhandensein von nicht dialysierenden Elektrolyten. Ein Beitrag zur physikalisch-chemischen Physiologie. Z. Elektrochem. Angew. Phys. Chem. 1911, 17, 572–581. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; Wiley: Chichester, UK, 2004; 368p. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 1–464. [Google Scholar]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press: San Diego, CA, USA, 1990; pp. 1–547. [Google Scholar]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules, 3rd ed.; Halsted Press, a Division of John Wiley & Sons, Inc.: New York, NY, USA, 1975; pp. 1–433. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–400. [Google Scholar]
- Zavidovskiy, I.A.; Streletskiy, O.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Savchenko, N.F.; Tatarintsev, A.A.; Pavlikov, A.V. Highly Selective Polyene-Polyyne Resistive Gas Sensors: Response Tuning by Low-Energy Ion Irradiation. J. Compos. Sci. 2023, 7, 156. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–286. [Google Scholar]
- Roeges, N.P.G. A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures; Wiley: Chichester, UK, 1994; pp. 1–360. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 1991; 503p. [Google Scholar]
- Wang, S.H.; Griffiths, P.R. Resolution Enhancement of Diffuse Reflectance, I.R. Spectra of Coals by Fourier Self-Deconvolution. 1. C–H Stretching and Bending Modes. Fuel 1985, 64, 1220–1227. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Co., Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2004; pp. 1–200. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; 436p. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Watters, K.L.; Risen, W.M. Spectroscopic Studies of Metal–Metal Bonded Compounds. Inorg. Chim. Acta Rev. 1969, 3, 129–154. [Google Scholar] [CrossRef]
- Elschenbroich, C. Organometallics, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 1–818. [Google Scholar]
- Atkins, P.W. Molecular Quantum Mechanics; Oxford University Press: Oxford, UK, 1983; pp. 1–500. [Google Scholar]
- Günzler, H.; Gremlich, H.-U. IR Spectroscopy: An Introduction; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002; pp. 1–365. [Google Scholar]
- Koenig, J.L. Spectroscopy of Polymers, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 1999; pp. 1–500. [Google Scholar]
- Schrader, B. (Ed.) Infrared and Raman Spectroscopy: Methods and Applications; VCH: Weinheim, Germany, 1995; pp. 1–787. [Google Scholar]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley-Interscience: New York, NY, USA, 1997. [Google Scholar]
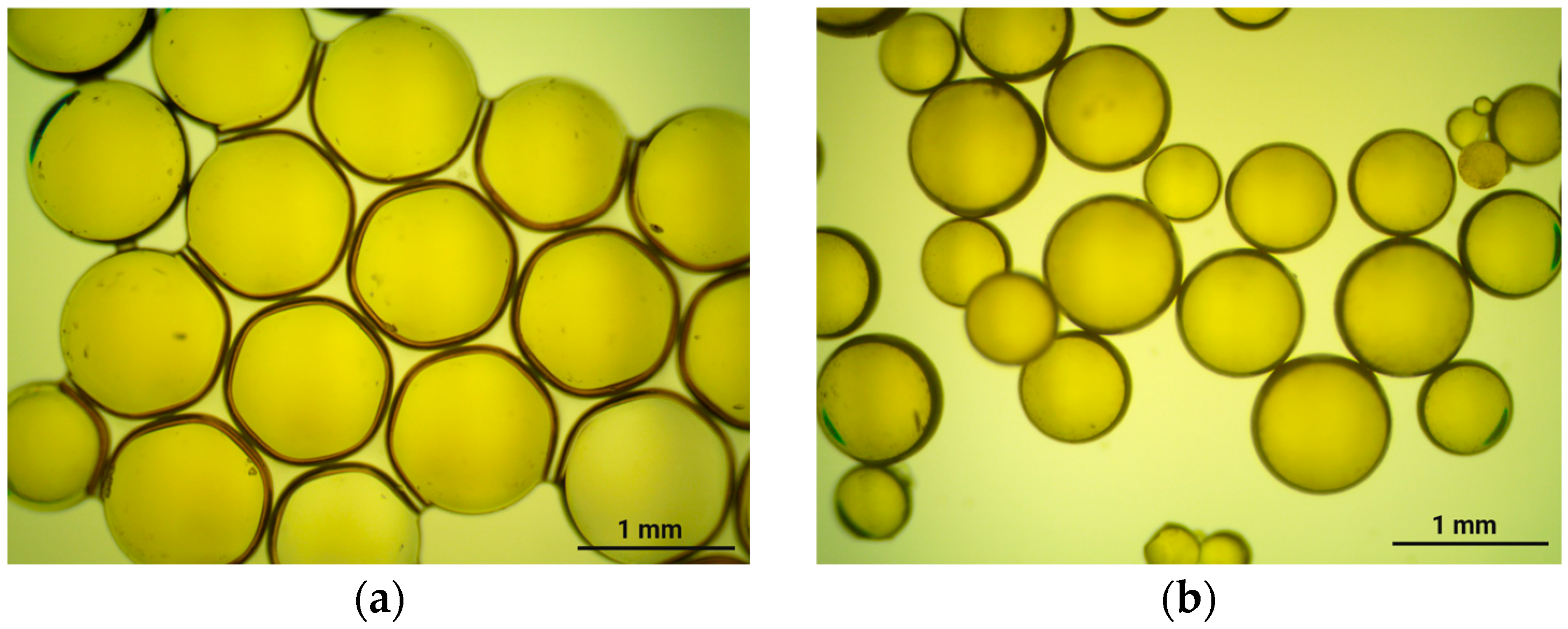






| Model | qe (mg/g) | Rate Constant | R2 |
|---|---|---|---|
| Pseudo-first-order | 27.08 | k1 = 0.937 h−1 | 0.936 |
| Pseudo-second-order | 28.49 | k2 = 0.050 g mg−1 h−1 | 0.999 |
| Molar Ratio (KU-2-8:AV-17-8) | Kd Au (mL/g) | Kd Fe (mL/g) | β (Kd Au/Kd Fe) |
|---|---|---|---|
| 6:0 | 6.0 | 2.7 | 2.22 |
| 5:1 | 334.8 | 22.0 | 15.22 |
| 4:2 | 1775.0 | 64.0 | 27.73 |
| 3:3 | 3233.3 | 79.6 | 40.62 |
| 2:4 | 2042.9 | 322.5 | 6.33 |
| 1:5 | 403.0 | 893.0 | 0.45 |
| 0:6 | 200.0 | 2627.3 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyussembayeva, G.; Jumadilov, T.; Gražulevicius, J.; Mukatayeva, Z.; Khimersen, K.; Totkhuskyzy, B. Selective Gold Ion Sorption from Iron-Containing Solution Using an Interpolymer System of Industrial Ion Exchangers. Polymers 2025, 17, 2808. https://doi.org/10.3390/polym17202808
Dyussembayeva G, Jumadilov T, Gražulevicius J, Mukatayeva Z, Khimersen K, Totkhuskyzy B. Selective Gold Ion Sorption from Iron-Containing Solution Using an Interpolymer System of Industrial Ion Exchangers. Polymers. 2025; 17(20):2808. https://doi.org/10.3390/polym17202808
Chicago/Turabian StyleDyussembayeva, Gulnur, Talkybek Jumadilov, Juozas Gražulevicius, Zhazira Mukatayeva, Khuangul Khimersen, and Bakytgul Totkhuskyzy. 2025. "Selective Gold Ion Sorption from Iron-Containing Solution Using an Interpolymer System of Industrial Ion Exchangers" Polymers 17, no. 20: 2808. https://doi.org/10.3390/polym17202808
APA StyleDyussembayeva, G., Jumadilov, T., Gražulevicius, J., Mukatayeva, Z., Khimersen, K., & Totkhuskyzy, B. (2025). Selective Gold Ion Sorption from Iron-Containing Solution Using an Interpolymer System of Industrial Ion Exchangers. Polymers, 17(20), 2808. https://doi.org/10.3390/polym17202808






