3. Results and Discussion
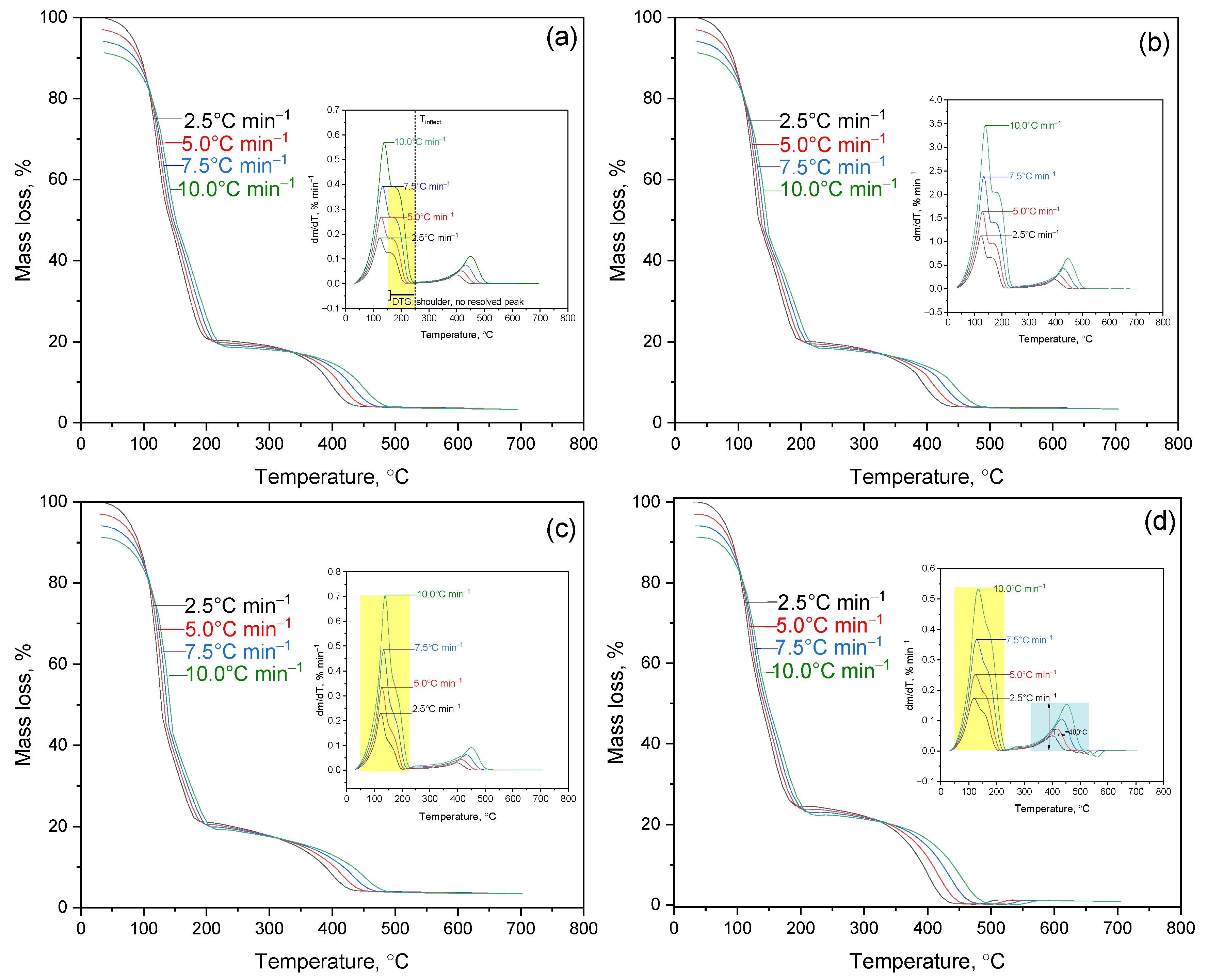
Figure 3 presents the TGA/DTG curves of PVA/PEGDA–PEGMA hydrogels (PEGMA 11 wt.% in all samples, H
2O 75–81 wt.%) with the following compositions: (a) 1/7, (b) 2/7, (c) 3/7, and (d) 3/11 (PVA/PEGDA wt.%).
Sample (a), containing 1 wt.% PVA and 7 wt.% PEGDA, exhibits the lowest crosslink density among the studied systems, resulting in the loosest polymer network. The first temperature interval (50–160 °C) corresponds to dehydration with a mass loss of 77.8%, while Tmax = 140 °C (DTG) reflects the removal of free and capillary water, i.e., moisture not bound to the polymer matrix. The second interval (160–240 °C) is weakly pronounced (Δm = 3.6%) and appears on the DTG curve only as a slight baseline inflection, corresponding to the onset of intramolecular dehydration of PVA and thermolysis of unstable side groups. These initial reactions indicate the beginning of chemical degradation, although still at a relatively low intensity. In samples (a) and (b), these processes are detected as a distinct but minor effect, whereas in (c) and (d) they overlap with the main decomposition stage. The principal stage of thermodestruction occurs between 376 and 492 °C with Tmax = 445 °C, and the DTG peak half-width (FWHM) of 68.1 °C indicates a relatively prolonged degradation process. Such a broad profile reflects gradual decomposition, which is favorable for systems designed for controlled release or slow degradation. The residual mass above 450 °C is only 0.4%, confirming the absence of thermally stable carbonaceous residue—an important characteristic for assessing long-term stability and “clean” thermal decomposition.
A comparison with sample (b), containing 2 wt.% PVA at the same PEGDA and PEGMA contents, shows that the additional introduction of hydrophilic fragments enhances moisture retention. The dehydration Tmax shifts to a higher temperature (187.7 °C), and the dehydration signal broadens (50–250 °C), with Δm = 75.0%. This behavior indicates stronger water binding, which is valuable for hydrogel matrices requiring moisture-retaining functionality. The main decomposition occurs between 376 and 488 °C with Tmax = 443 °C. The process becomes more localized, as evidenced by a narrower FWHM of 59.6 °C, suggesting a faster and more concentrated degradation. This may be advantageous for rapid-release systems but less favorable for long-term applications. The residual mass is 0.5%.
Sample (c), with 3 wt.% PVA and 7 wt.% PEGDA, demonstrates more intense dehydration (Δm = 83.9%) with Tmax = 135 °C, indicating stronger moisture binding and higher hydrophilicity. The main decomposition occurs in the range of 383–487 °C with Tmax = 453 °C, while the DTG peak exhibits a significant half-width (FWHM = 80.3 °C), suggesting a more extended and gradual degradation process. Such a broad DTG profile indicates enhanced resistance to thermal fluctuations, which is beneficial for sterilization and storage stability. The residual mass is 0.6%.
Sample (d), containing 3 wt.% PVA and 11 wt.% PEGDA (PEGMA = 11 wt.% and water = 75 wt.% kept constant), exhibits markedly different behavior. The main decomposition stage shifts to a lower temperature range (240–510 °C), with the maximum decomposition rate observed at T
max = 400 °C. The DTG peak is both broad and intense (FWHM = 87.3 °C), and the residual mass is minimal (0.3%). This behavior reflects local heat accumulation and accelerated degradation at high crosslink densities. While such dense networks enhance mechanical robustness prior to breakdown, they can also degrade more rapidly once decomposition begins. According to the literature [
9,
22], high crosslink density restricts heat transfer and limits the diffusion of volatile degradation products, leading to localized overheating and accelerated degradation—an effect that likely occurs in this system as well. This finding is of practical relevance, as it indicates that overly rigid hydrogel networks may undergo faster breakdown once decomposition is initiated.
The key parameters characterizing the thermal behavior of samples (a–d) are summarized in
Table 1.
The observed differences in the thermal behavior of PVA/PEGDA–PEGMA hydrogels (at a constant PEGMA content of 11 wt.% and water content of 75–81 wt.%) for the compositions (a) 1/7, (b) 2/7, (c) 3/7, and (d) 3/11 (wt.% PVA/PEGDA) are determined by the specific degradation pathways of the polymer matrix components. Poly(vinyl alcohol) (PVA) undergoes thermodegradation initiated by hydroxyl groups, which promotes depolymerization accompanied by the formation of acetic acid and carbonyl-containing products [
23,
24]. In other words, the presence of hydroxyl groups renders PVA thermally sensitive. Poly(ethylene glycol) diacrylate (PEGDA), in contrast, decomposes primarily through the hydrolysis of ester linkages and subsequent β-scission upon heating, yielding oligomeric fragments and volatile products [
25]. Thus, PEGDA behaves as a typical polyester, with hydrolysis preceding chain cleavage during degradation. An increase in the PEGDA fraction enhances the crosslink density and decreases the water-binding capacity of the hydrogel (the network becomes stiffer and retains less moisture), whereas higher PVA content promotes water uptake and facilitates hydrolytic processes (the network becomes more open and “absorptive”). These structural and compositional factors explain the observed shifts in decomposition temperatures and the characteristic shapes of the DTG peaks (
Figure 3). Broad DTG peaks correspond to gradual and distributed degradation, while narrow peaks indicate rapid and localized decomposition events. Similar degradation patterns have been reported for other interpenetrating polymer networks based on PVA and polyacrylates, where hydrophilicity and crosslink density are key factors determining thermal stability. Hence, the obtained data are consistent with previously described degradation mechanisms of analogous polymeric systems.
It should be emphasized that the parameters Tmax and DTG peak half-width (FWHM) have practical significance for predicting the operational performance of the studied materials. A shift in Tmax toward higher temperatures reflects improved thermal stability of the polymer network, which is crucial for technological processes such as hydrogel drying or sterilization and for long-term storage. The FWHM value, in turn, characterizes the nature of the degradation process: a broader peak indicates gradual and uniform decomposition, whereas a narrower peak corresponds to abrupt, localized breakdown—potentially associated with localized heat accumulation. From a practical perspective, these differences provide insight into the stability and degradation kinetics of the materials, which is particularly relevant for their use as sorbents or carriers of biologically active compounds, where controlled decomposition or “clean” burnout without carbon residue may be desirable.
In addition to thermogravimetric results, supplementary rheological, structural, and morphological data are provided in
Supplementary Materials S3 (Figures S3–S7). These studies confirm the sufficient mechanical strength, stable microstructure, and ordered phase morphology of the IPN hydrogels—demonstrating that the materials are not only thermally stable but also structurally robust under mechanical loading, thereby highlighting their potential for biomedical applications.
For samples (b) and (c), the variations in T
max and water-binding capacity are consistent with the results summarized in
Table 1, confirming the critical role of PVA content and crosslink density in determining water retention. In contrast, an increase in PEGDA concentration to 11 wt.% at a fixed 3 wt.% PVA (sample d) leads to the formation of a denser network, which limits the amount of bound water and shifts the main decomposition stage to a lower temperature range (300–400 °C). This behavior suggests that overly rigid networks may degrade earlier due to the accumulation of internal stresses and restricted diffusion processes.
Thermal degradation of polymer materials is directly linked to their operational reliability and technological performance [
22]. For hydrogels [
26]—used as sorbents, drug carriers, and functional biomaterials—water retention capacity and resistance to sterilization treatments are particularly important, as these properties determine whether the material can withstand drying, sterilization, or long-term storage. These characteristics are governed by thermal stability, which defines the synthesis parameters, storage conditions, and practical usability of the material [
17].
Activation energy (Ea) is one of the key parameters characterizing the thermal stability of polymers. Analyzing its variation as a function of the degree of conversion (α) makes it possible to identify the sequence of decomposition stages—i.e., which bonds break first and which remain the most stable—to determine the most vulnerable structural elements, and to evaluate the influence of polymer architecture and crosslink density on the durability of the matrix.
Isoconversional methods, including the Friedman [
27] and Ozawa–Flynn–Wall (OFW) [
28,
29] approaches, enable the calculation of
Ea without prior assumptions about the reaction mechanism. In other words, they allow one to analyze the kinetics without presuming a specific reaction pathway in advance. This makes such methods particularly effective for studying complex, multicomponent systems such as hydrogels.
It should be emphasized that similar studies on PVA/PEGDA–PEGMA hydrogels (PEGMA 11 wt.% in all samples, H2O 75–81 wt.%) have not been conducted previously. For the first time, samples with different degrees of crosslinking were compared by calculating the distribution of activation energy as a function of α, thus revealing how the energetic barrier changes step-by-step during the degradation process. This approach made it possible to identify the critical stages of thermal degradation, determine the influence of PVA content and PEGDA concentration on thermal stability, and clarify the degradation mechanism. The combined application of the Friedman and OFW methods provided a comprehensive description of thermolysis kinetics, significantly enhancing the understanding of hydrogel stability in this system and offering new possibilities for targeted optimization of their properties.
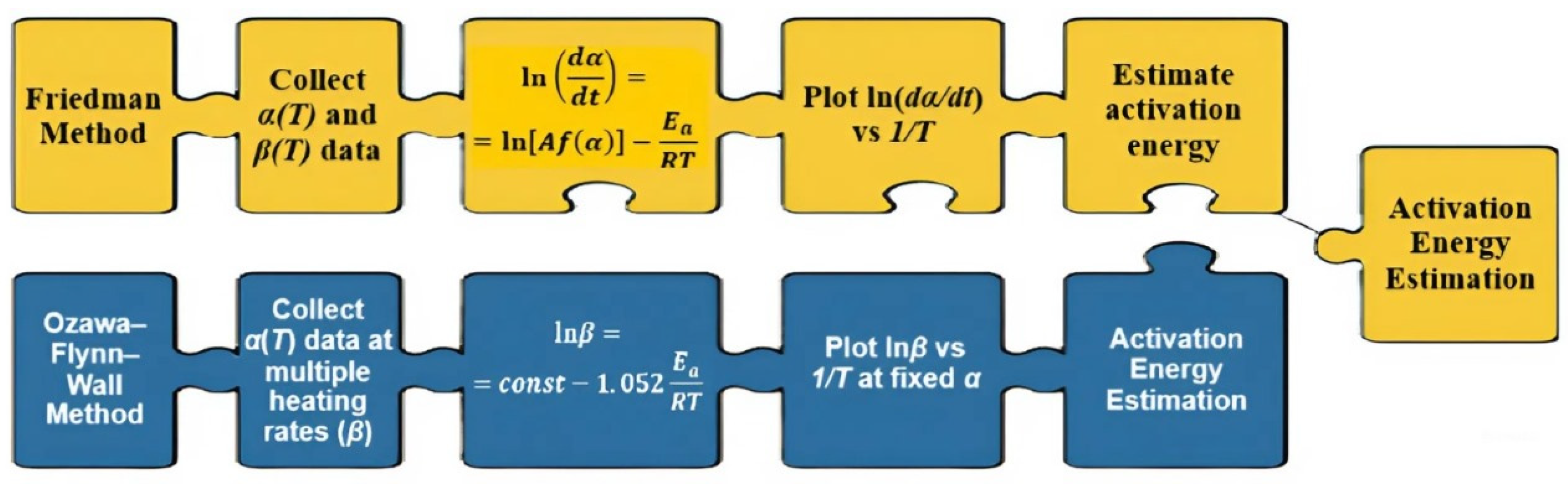
The kinetic analysis was performed using the Friedman and Ozawa–Flynn–Wall (OFW) isoconversional methods.
Figure 4 schematically illustrates the difference between these approaches: the Friedman method is based on the differential form of the Arrhenius equation and analyzes the dependence of ln(dα/dt) on 1/T at fixed conversion degrees (i.e., focusing on the reaction rate at specific stages), whereas the OFW method applies an integral form and examines the dependence of lnβ on 1/T at different heating rates (i.e., considering the overall reaction process). The combined use of these complementary methods ensures the consistency of results and provides a more complete and reliable description of the thermal degradation mechanism of hydrogels.
Following the schematic diagram (
Figure 4), which illustrates the distinctions between the differential (Friedman) and integral (Ozawa–Flynn–Wall, OFW) approaches, the results of their application to the experimental data are presented below.
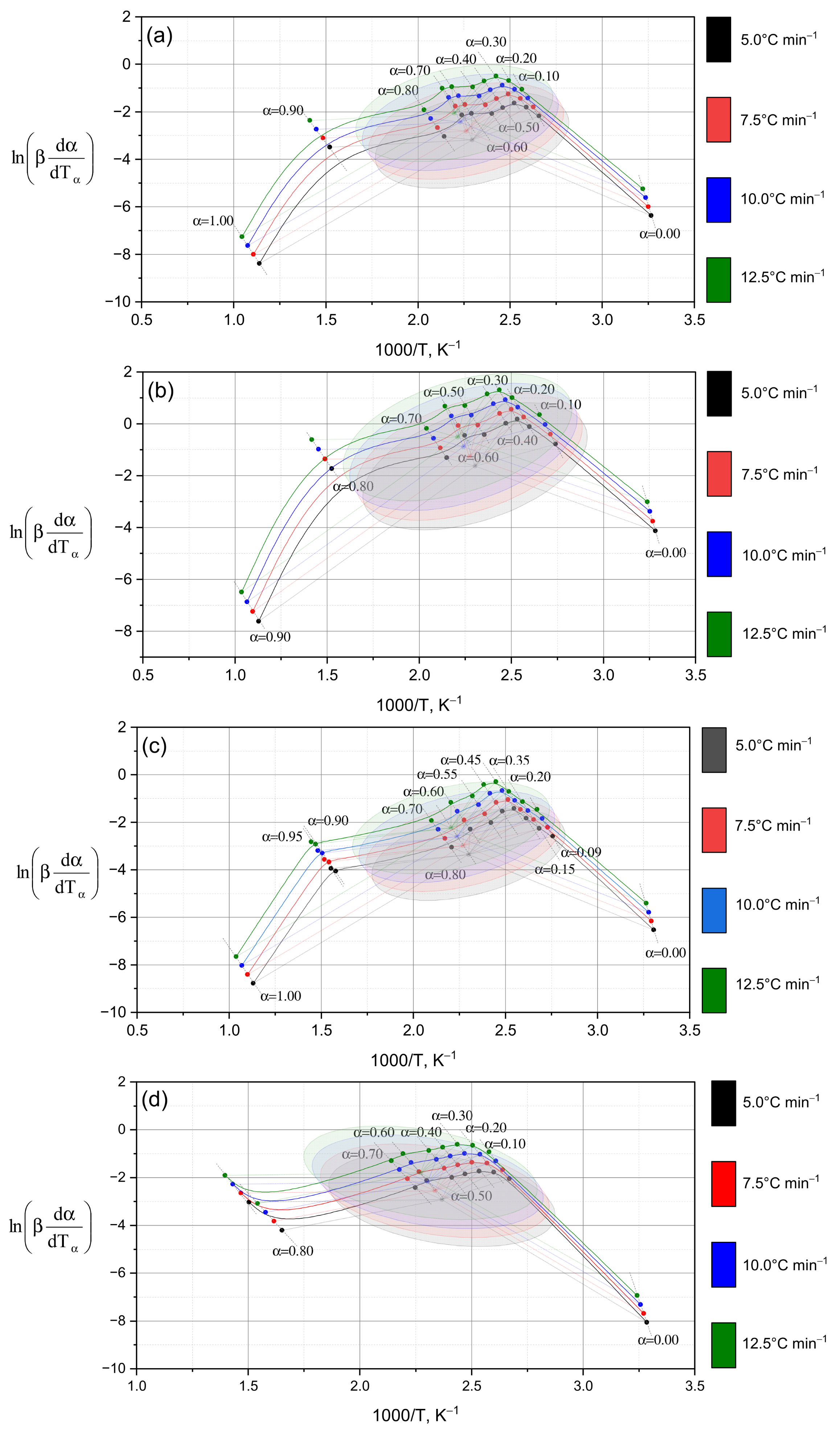
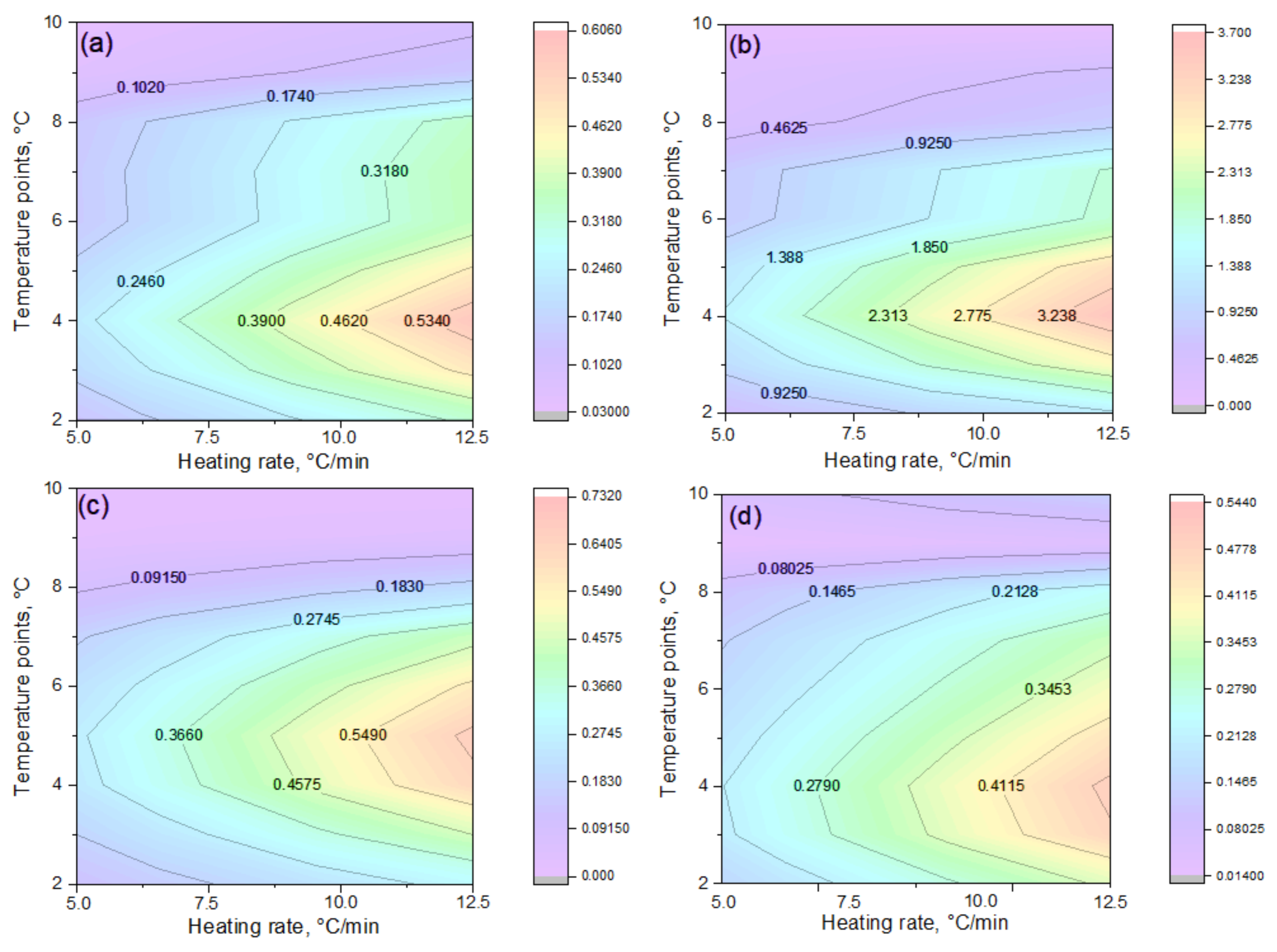
Figure 5 shows the kinetic dependencies constructed for different heating rates (from 5 °C to 12.5 °C), which made it possible to calculate the distribution of activation energy across the conversion degrees.
The dependencies of ln(βdα/dt) on 1/T for various conversion degrees (α = 0.0–1.0) are shown in
Figure 5. As α increases, the curves shift and reach their maximum values within the central ellipsoidal region (α = 0.1–0.8), followed by a subsequent decrease. This behavior reflects a general kinetic trend: the activation energy decreases with increasing conversion degree [
30]—in other words, as the reaction progresses, less energy is required to sustain it.
This trend is quantitatively confirmed by the data in
Table 2, where a consistent decrease in activation energy from α = 0.1 to α = 0.9 is observed for all samples studied (a–e). For sample (a) (
Figure 5a), the Friedman method yields values decreasing from 101.87 to 86.86 kJ·mol
−1 (101.87 → 95.09 → 92.66 → 87.73 → 86.86), fully consistent with the results obtained using the OFW method. In the initial stages (α = 0.1–0.3), the maximum activation energy values (~100–107 kJ·mol
−1) are recorded in all cases, reflecting the destruction of the most stable chemical bonds within the polymer structure [
31] (these correspond to the “strongest building blocks” of the material). As the process proceeds (α = 0.5–0.8), the activation energy decreases to ~84–87 kJ·mol
−1, indicating the involvement of less stable bonds (that is, “weaker links” begin to decompose).
It should be noted that the rate of activation energy decrease varies between samples. In sample (b) (
Figure 5b), a sharp decline occurs between α = 0.1 (107.47 kJ·mol
−1) and α = 0.3 (95.54 kJ·mol
−1), while in sample (d) (
Figure 5d), the decrease is more gradual. Such heterogeneity confirms the multistage nature of the decomposition process: each stage proceeds via its own mechanism. A more detailed comparison of samples (c) and (d) (
Figure 5c,d) shows that at the initial stage, both exhibit nearly identical activation energy values (~103 kJ·mol
−1 by Friedman and ~100 kJ·mol
−1 by OFW). However, at intermediate stages (α = 0.3–0.5), sample (d) shows higher values (95.78 kJ·mol
−1 by OFW vs. 90.44 kJ·mol
−1 for sample (c)), suggesting the participation of stronger bonds (its network “resists” decomposition longer). At later stages (α = 0.7–0.9), activation energy decreases more sharply in sample (c), while in sample (d) the values remain comparatively high—indicating that this material still requires greater energy input for degradation. Thus, the differences in activation energy evolution point to distinct decomposition mechanisms: sample (c) transitions to less energy-intensive stages, whereas sample (d) maintains a high energy barrier.
To verify the consistency between the Friedman and Ozawa–Flynn–Wall methods, statistical analysis was performed (
Table 3). According to the ANOVA results, no significant differences between the two methods were detected (
p > 0.05 for all samples), which can be attributed to the relatively high data dispersion and averaging over the entire sample set (in other words, the “spread” of data masks local variations). However, a paired
t-test, which is more sensitive to local differences [
32], revealed statistically significant discrepancies (
p < 0.05) for samples (a–c), whereas for sample (d) no significant difference was found (
p = 0.20). Hence, the distinction between the two statistical outcomes reflects the differing sensitivities of the methods: ANOVA captures general trends (“a coarse filter”), while the
t-test detects subtle sample-level variations (“fine adjustment”). The convergence of results for sample (d) indicates a stable and uniform decomposition mechanism, whereas the discrepancies observed for samples (a–c) confirm the kinetic complexity and multistage character of their thermal degradation.
Thus, the data presented in
Figure 5 and
Table 2 and
Table 3 demonstrate that the reactions under study do not follow a single kinetic model. The variation in activation energy as a function of the conversion degree (α) indicates a change in the reaction mechanism as the process progresses (i.e., the decomposition pathway evolves over time), which justifies the application of model-independent isoconversional methods (Friedman and Ozawa–Flynn–Wall) for an accurate description of the reaction kinetics.
The differences observed in the thermal parameters of the TGA curves (
Figure 3) are reflected in the distribution of activation energy (
Ea) calculated using isoconversional methods. As shown in the subsequent analysis, an increase in PEGDA content leads to a higher activation energy barrier for thermal degradation, which correlates with the enhanced crosslink density and the restricted diffusion of moisture within the polymer matrix. In contrast, samples with higher PVA content exhibit lower activation energy values, attributed to their higher hydrophilicity and the presence of hydrolyzable fragments that facilitate the initiation of decomposition.
However, the use of isoconversional methods alone does not always provide an accurate description of the behavior of multicomponent or modified systems, where several decomposition stages often overlap, meaning that multiple reactions occur simultaneously and influence each other. In such cases, it becomes particularly important to apply approaches that do not require an a priori selection of a kinetic model and are capable of distinguishing overlapping processes.
To study the thermal degradation of complex systems, model-free kinetic methods are considered highly effective, as they do not rely on predefined reaction mechanisms—allowing the data to “speak for themselves” without being constrained by prior model assumptions. One of the most powerful tools for such analysis is the nonparametric kinetics (NPK) method [
33,
34], which is based on mathematical processing of thermogravimetric data obtained at different heating rates, i.e., by comparing mass-loss curves recorded under varying thermal conditions. Unlike traditional isoconversional approaches (e.g., Friedman or Ozawa–Flynn–Wall), the NPK method enables the effective separation of overlapping stages and thus provides a more detailed interpretation of multicomponent processes. In other words, it can “deconvolute” multiple parallel reactions and reveal their individual contributions.
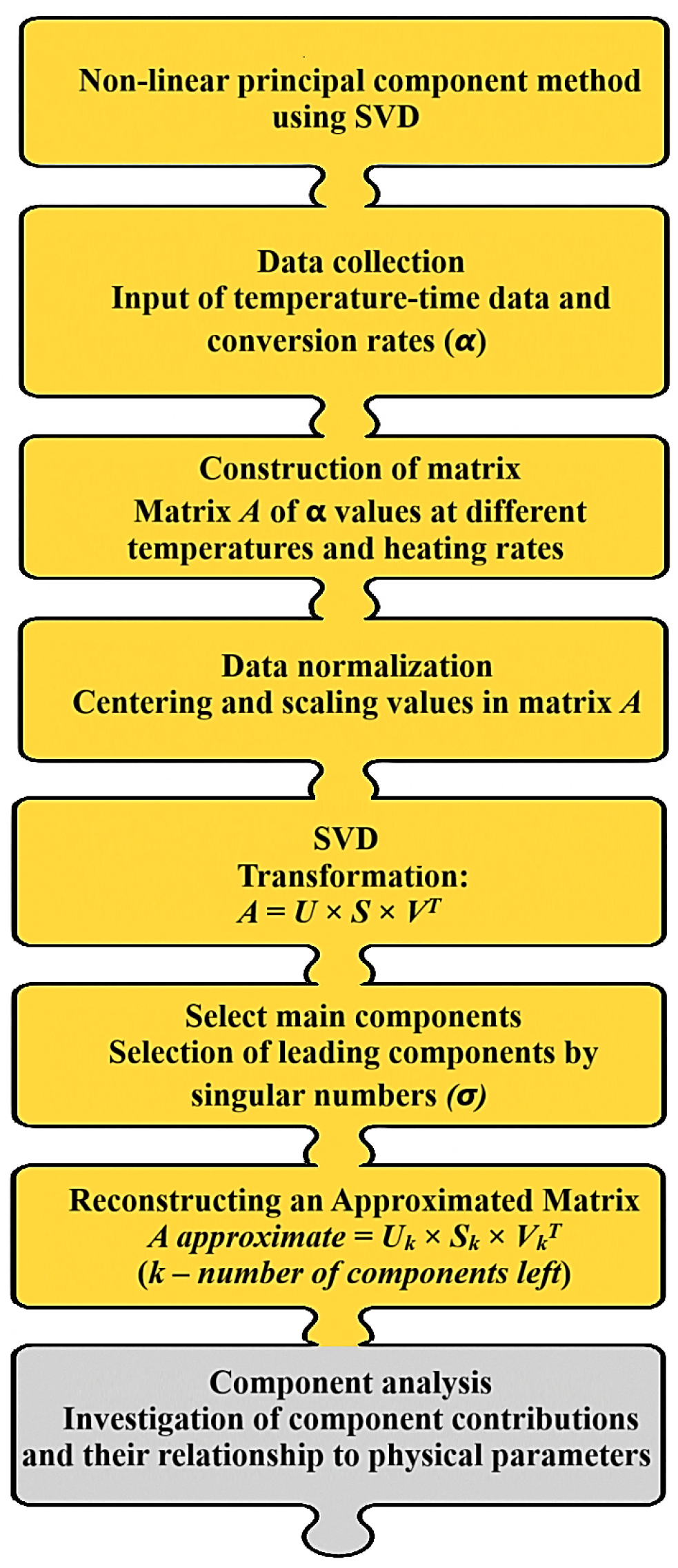
The NPK algorithm represents experimental data as a matrix containing the degree of conversion and the corresponding reaction rates—essentially a data array that reflects how fast the reaction proceeds at different temperatures. Applying singular value decomposition (SVD) to this matrix allows it to be decomposed into principal components, each corresponding to an individual kinetic process, analogous to identifying dominant patterns in complex datasets. This approach facilitates the separation of overlapping decomposition stages, which is particularly valuable when studying modified systems, and ensures more accurate data approximation with reduced errors compared to model-based methods, as it eliminates distortions caused by incorrect model assumptions.
For clarity,
Figure 6 presents a schematic diagram of the PCA-based analysis, illustrating the formation of the initial data matrix, SVD, selection of principal components, and evaluation of their contribution to the overall reaction process—that is, how the method extracts individual reaction mechanisms from the experimental data.
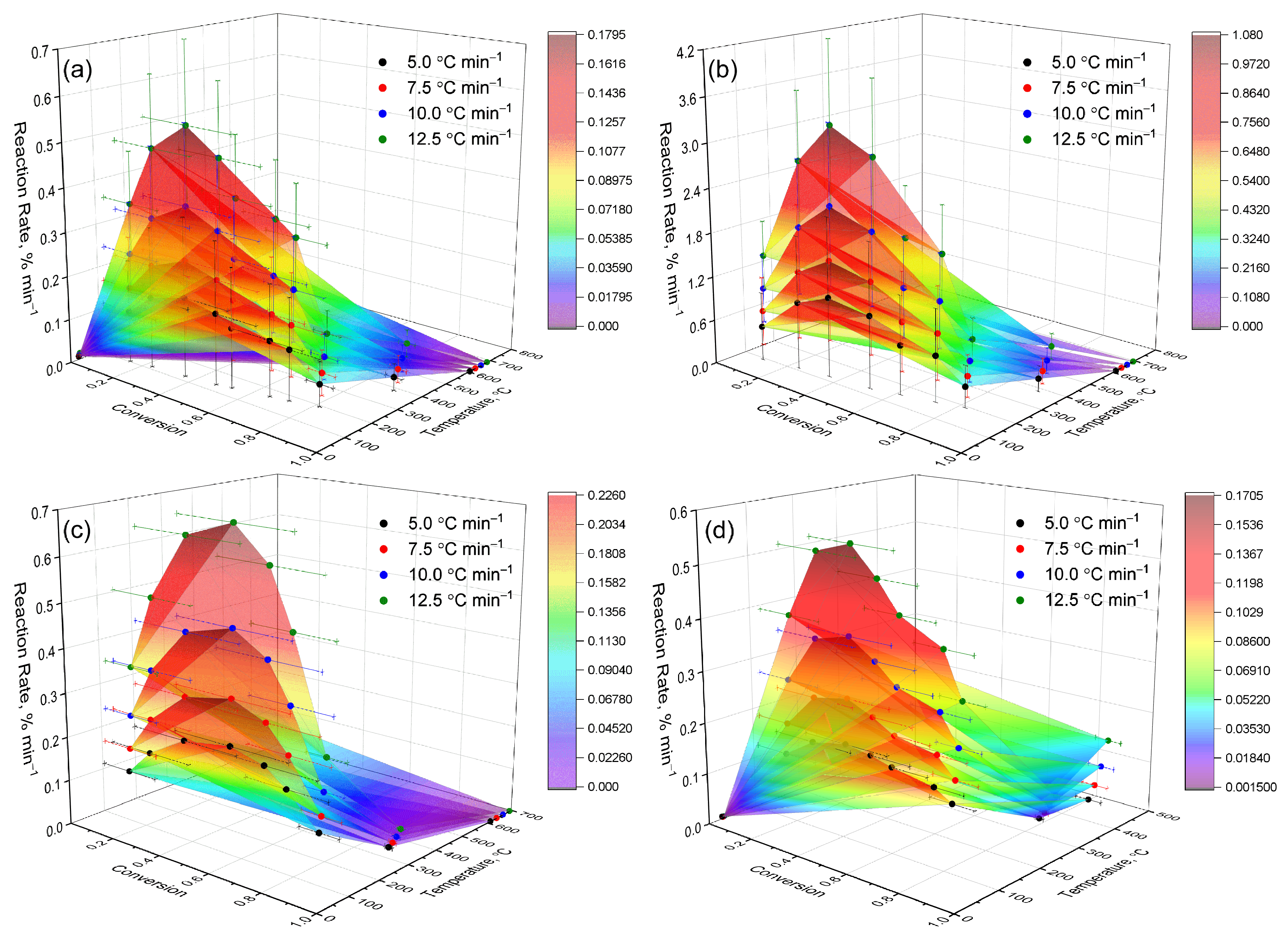
For a more detailed analysis of the thermokinetic characteristics of the studied systems, the dependence of the decomposition rate (dα/dT) on temperature (T) and the degree of conversion (α) was visualized in the form of three-dimensional plots (
Figure 7), illustrating how rapidly the material decomposes at different temperatures and stages of the process. The resulting surfaces represent visual “kinetic landscapes” that allow us to track the dynamics of thermal decomposition across the entire range of temperatures and conversions—in other words, they reveal where the reaction is most active and how it evolves during heating. This approach makes it possible to identify the characteristic features of each sample’s behavior, including shifts in the temperature ranges of active decomposition and the differences associated with structural modifications and complexation effects [
35], such as the influence of polymer composition or intermolecular interactions on network stability.
The subsequent analysis provides a step-by-step description of the kinetic characteristics identified for each of the samples.
Figure 7a–d show three-dimensional dependencies of the reaction rate (dα/dT) on temperature (T) and conversion degree (α) at heating rates of 5.0, 7.5, 10.0, and 12.5 °C·min
−1. The construction of 3D surfaces enabled the identification of distinct features of the thermal decomposition of hydrogels, clearly illustrating the main zones of activity and their shifts depending on the heating rate. All numerical values of the maximum decomposition rates were obtained from the experimental 3D dependencies (
Figure 7).
The three-dimensional plots revealed fundamental differences among the samples. Sample (a) exhibited moderate activity and a simplified kinetic pattern, with decomposition occurring predominantly in a single stage without significant process overlap. Sample (b) demonstrated the highest reactivity and a multistage mechanism, attributed to the increased mobility of the polymer network—its less dense structure facilitates chain movement and accelerates the reaction. Sample (c) showed a narrow zone of activity and rapid attenuation of the process, indicating diffusion limitations and high thermal stability, as heat and volatile products are released more slowly while the structure remains stable. Sample (d) occupied an intermediate position: the reaction proceeded more gradually and over a wider range of temperatures, reflecting a less compact structure and the participation of secondary processes, such as side reactions or the gradual decomposition of residual fragments.
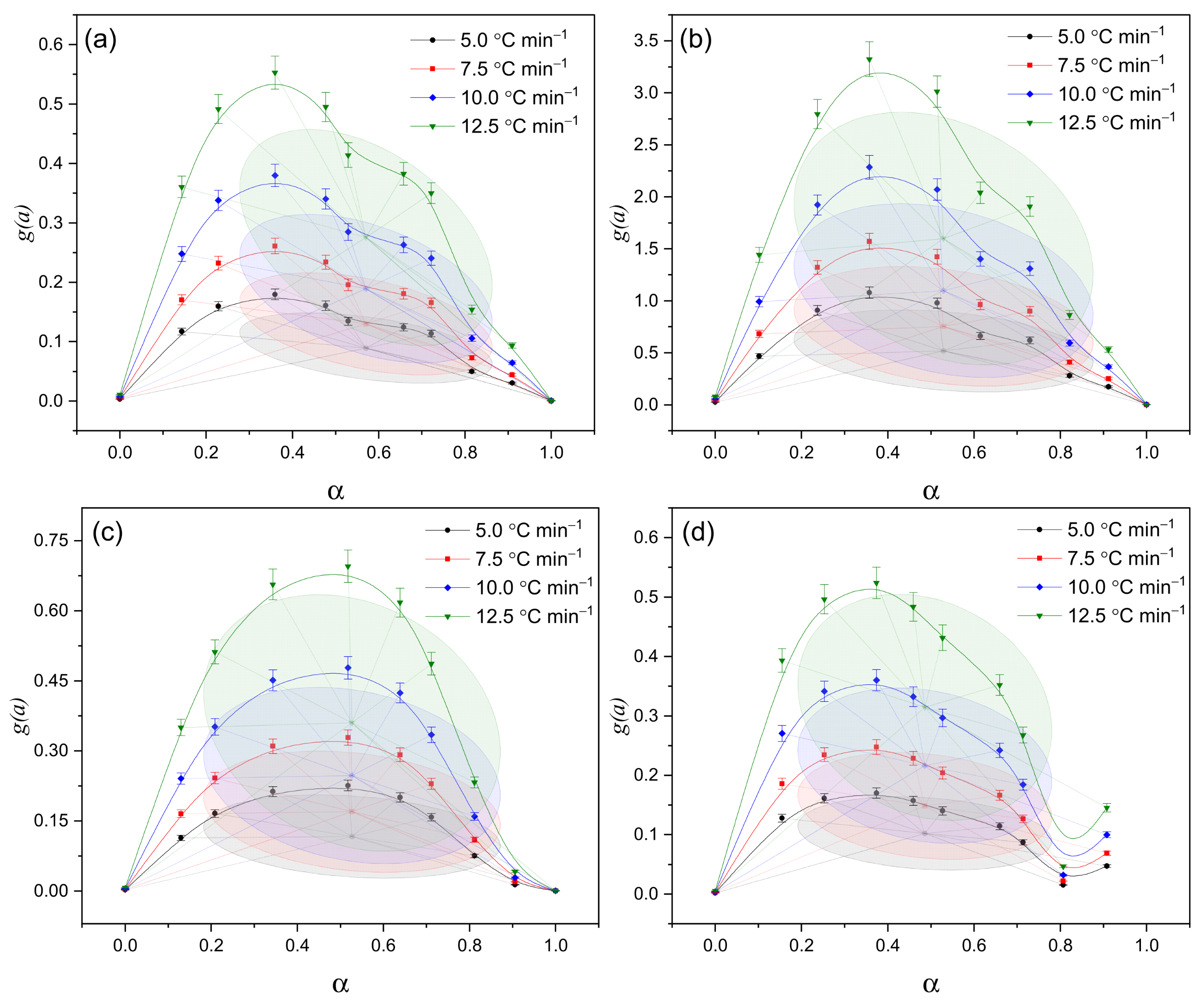
The g(α)–α approximation data (
Figure 8) confirm the previously identified distinctions between the samples. In sample (c), the process is diffusion-limited, meaning that the movement of volatile products slows down the overall reaction rate. In sample (d), the reaction proceeds more gradually and remains active over an extended range of conversion, indicating a prolonged and continuous decomposition process. In contrast, sample (a) exhibits a simplified kinetic behavior with moderate reactivity, corresponding to an almost single-stage process with minimal complexity.
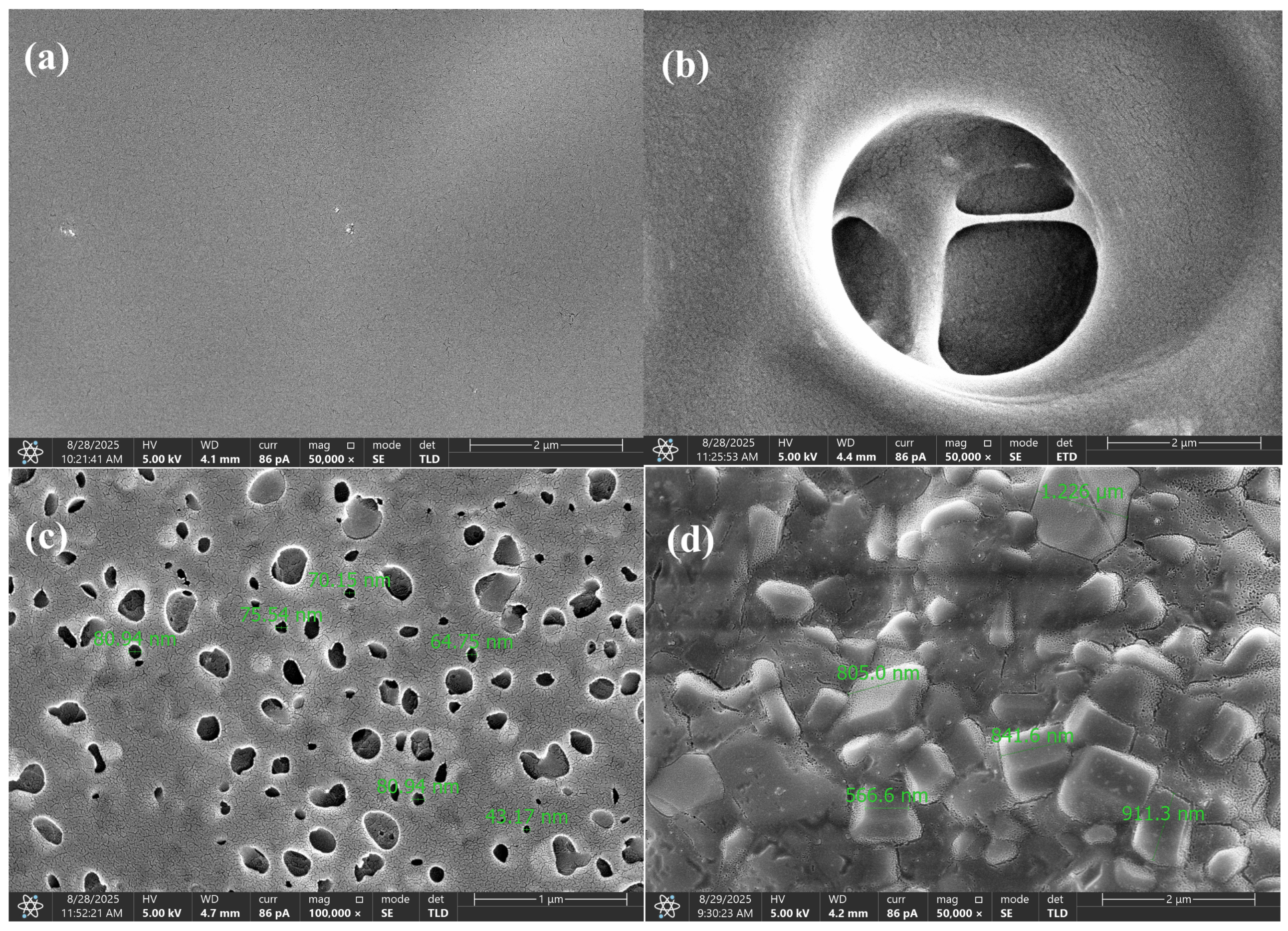
Morphological transformations (
Figure 9) have a direct impact on the reaction kinetics and activation energy. At the early stage (150 °C), the dense structure of the composition inhibits the diffusion of volatile products, thereby requiring higher activation energy to initiate destructive reactions—i.e., the material must absorb more heat before decomposition begins. In the range of 250–450 °C, the formation of gas bubbles and the development of nanoporous domains significantly enhance heat transfer and gas diffusion, lowering the energy barrier and accelerating the decomposition of the polymer matrix. As the sample becomes more porous, it also becomes more reactive.
At approximately 600 °C, a carbonized, crystallite-like structure with high thermal stability is formed; its further transformation again demands increased activation energy, indicating that the residual material becomes more resistant to degradation. Thus, the evolution of morphology exhibits a nonlinear behavior: from increased energy consumption during the initial dehydration phase (associated with a compact structure), to a decrease in the active zone of gas release and pore formation (corresponding to structural loosening), followed by another increase during the formation of a rigid carbon matrix.
The
Supplementary Materials S4 and S5 (Figures S8–S12) include IR spectra and SEM micrographs of the composition at different temperatures, which confirm these structural transitions by clearly demonstrating the emergence of porosity and the gradual carbonization of the material.
These observations are consistent with the results of the parametric analysis (
Table 4), particularly regarding the activation energy and pre-exponential factor, which describe how easily the reaction is initiated. For sample (a), the activation energy was determined to be 102.70 ± 0.38 kJ·mol
−1 with an average pre-exponential factor of
= (29.30 ± 0.20) × 10
13 s
−1, indicating a pronounced kinetic barrier—i.e., the reaction requires a significant amount of energy to initiate, and the material remains relatively stable at moderate temperatures.
For sample (b), which exhibited the highest decomposition rate, the lower average activation energy (93.67 ± 0.45 kJ·mol−1) combined with a smaller pre-exponential factor ( = (1.45 ± 0.05) × 1013 s−1) suggests a more energetically accessible but less stable process, meaning that the material decomposes more readily upon heating.
In contrast, sample (c), characterized by a low decomposition rate and sharp decay, shows the minimum pre-exponential factor ( = (0.05 ± 0.01) × 1013 s−1), which corresponds to limited kinetic activity and a narrow temperature range of decomposition—i.e., the reaction proceeds rapidly within a short interval and then ceases.
Sample (d), on the other hand, demonstrates an activation energy of 103.59 ± 0.26 kJ·mol−1 and maintains reactivity over a broader range of conversion degrees, consistent with its smooth profile on the 3D kinetic maps. This indicates a gradual, uniform degradation process and structural stability under heating conditions.
Additionally, the parameters of the Šesták–Berggren model support the identified distinctions: for sample (c), the values m = 0.48 and n = 0.45 indicate strong diffusion limitations and a localized nature of the reaction (i.e., the process occurs in confined regions and is restricted by molecular mobility). In contrast, for sample (d) (m = 0.62; n = 1.05), the kinetics correspond to a process taking place over a broader range of conversion degrees and temperatures, suggesting a smoother and more prolonged decomposition.
In the present study, a comprehensive approach was applied to the kinetic analysis of the thermal decomposition of IPN hydrogels, combining both model-free and model-based methods (i.e., approaches that either assume or do not assume a predefined reaction mechanism). Each selected method served a specific analytical purpose aimed at constructing an integrated understanding of the degradation mechanisms:
The Friedman and Ozawa–Flynn–Wall (OFW) models were used to evaluate the variation in activation energy (Ea) as a function of the conversion degree (α), allowing us to trace how the energetic barrier changes throughout the decomposition process and to identify its multistage and kinetically complex character.
The NPK method was applied to determine the functional dependence f(α) without the need to predefine the mechanism—a crucial advantage in systems with overlapping reactions where individual processes cannot be easily distinguished.
The Šesták–Berggren model was employed to describe autocatalytic effects (where the reaction products promote further decomposition), which are characteristic of highly crosslinked structures and are not revealed by other kinetic models.
Thus, the combined use of these approaches provided a multilevel interpretation of the thermal degradation mechanisms—from the identification of individual stages and activation parameters to the formal dependencies of the reaction rate.
Statistical analysis (
Table 4) confirmed these conclusions. The analysis of variance (ANOVA) revealed no significant differences between the Friedman and Šesták–Berggren methods (
p > 0.05 for all samples). However, the paired
t-test indicated that for samples (a–c), the differences are statistically significant (
p < 0.05), whereas for sample (d) no significant difference was observed (
p = 0.20). The convergence of results for sample (d) demonstrates a high degree of agreement between the two approaches and confirms a stable, uniform decomposition mechanism (i.e., the reaction proceeds evenly without abrupt transitions). In contrast, the discrepancies observed for samples (a–c) reflect the complexity and multistage nature of their kinetics, where different reactions become activated within distinct temperature ranges. These findings are consistent with the data obtained from the three-dimensional plots (
Figure 8) and the
g(α)–α approximations (
Figure 6).
Thus, qualitative analysis based on 3D dependencies and quantitative evaluations using the NPK and Šesták–Berggren methods provide a consistent picture of the kinetic behavior of the studied hydrogels. Sample (a) follows a relatively simple kinetic model with moderate activity, indicating that decomposition occurs in one or two stages without significant overlap of processes. Sample (b) exhibits high reactivity but limited thermal stability—it decomposes rapidly but is less resistant to further heating. In contrast, samples (c) and (d) demonstrate higher stability, differing in their kinetic mechanisms and structural factors: sample (c) shows a narrow kinetic window and diffusion-controlled decomposition (slow degradation limited by molecular mobility), while sample (d) displays a more extended process with a broader temperature range of activity and prolonged degradation.
The practical significance of these differences lies in the ability to tailor hydrogel composition to specific functional requirements. For instance, sample (b), characterized by high reactivity, is suitable for applications that demand rapid or programmable degradation, such as drug delivery systems, temporary implants, or materials with tunable degradation times. Sample (a), which exhibits moderate activity and relatively high activation energy, is advantageous for applications requiring a balance between degradation rate and thermal stability, such as protective coatings or thermoresistant polymer matrices. The enhanced stability of samples (c) and (d) makes them promising candidates for long-term use in heat-resistant hydrogels, barrier coatings, and biomedical implants, where high mechanical and thermal endurance are essential.
Figure 10 presents thermal maps of the reaction rate distribution (dα/dt) plotted in “temperature–heating rate” coordinates for hydrogels of different compositions. For sample (a), the zone of maximum activity is concentrated within a narrow temperature range at high heating rates, while at lower rates, the process proceeds more gradually and is more prolonged, indicating kinetics of moderate intensity with a limited number of stages. The heat maps confirm the previously observed trends: sample (b) exhibits maximum reactivity and a multistage decomposition behavior, suggesting the superposition of several concurrent reactions. In sample (c), the activity zone is confined and decays rapidly, consistent with its dense network structure and reduced chain mobility that hinder degradation. Sample (d) demonstrates a broader range of thermal activity with a gradual decline in reaction rate, indicative of a prolonged decomposition mechanism. Sample (a) occupies an intermediate position, combining moderate activity with a simple kinetic profile, and can thus be regarded as a transitional case between fast- and slow-degrading systems.
A comparative analysis of thermal maps (
Figure 10) confirms the conclusions obtained from the results of 3D dependencies,
g(α)–α approximation, and parametric analysis (
Table 3 and
Table 4). The heat maps (
Figure 10) confirmed that sample (b) has the maximum reactivity and multistage decomposition (see description above), while sample (c) demonstrates a narrow activity window and rapid decay (see
Figure 7 and
Figure 8), which is consistent with its dense lattice. Sample (d) is characterized by a more prolonged destruction process and preservation of activity in the later stages, which is consistent with its high activation energy and Šesták–Berggren model parameters. Sample (a) occupies an intermediate position, combining a moderate decomposition rate with a relatively simple kinetic picture.
Thus, the thermal map data not only illustrate the temperature-dependent reaction activity of the hydrogels but also provide additional confirmation of the structurally determined differences in their kinetic behavior, namely the relationship between network density, chain mobility, and degradation rate.
Thermokinetic analysis (
Figure 7,
Figure 8,
Figure 9 and
Figure 10,
Table 3 and
Table 4) revealed significant differences in the stability and decomposition mechanism of hydrogels. To compare these results with the behavior in a humid environment, the dehydration kinetics characterizing the moisture retention capacity and structure of the polymer network were additionally studied. Quantitative analysis was performed using linear approximation of experimental data in time coordinates based on the analytical solution of Fick’s equation. The slope of the approximated straight-line k was used to calculate the effective diffusion coefficient D using the expression:
where
sample thickness in meters. All calculated parameters—slope
k, coefficient of determination
R2, and corresponding
D values—are summarized in
Table 5.
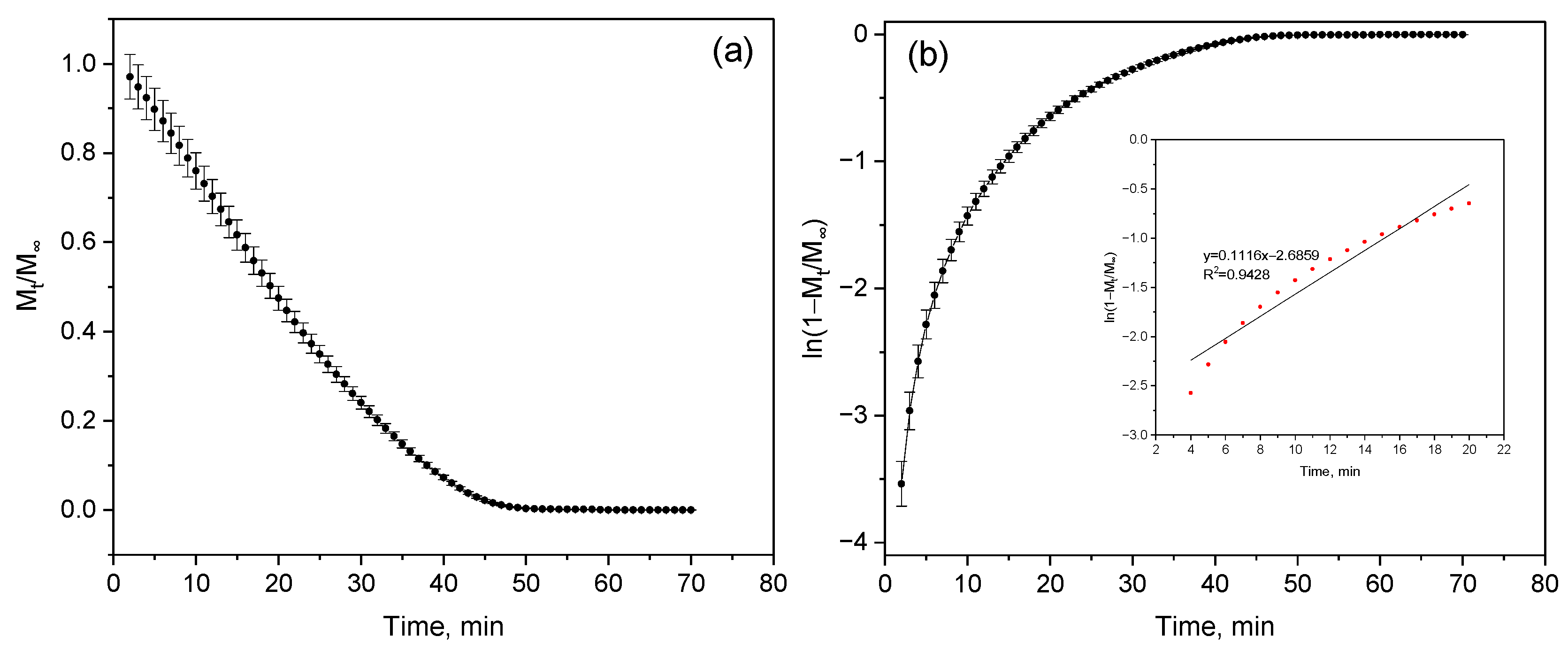
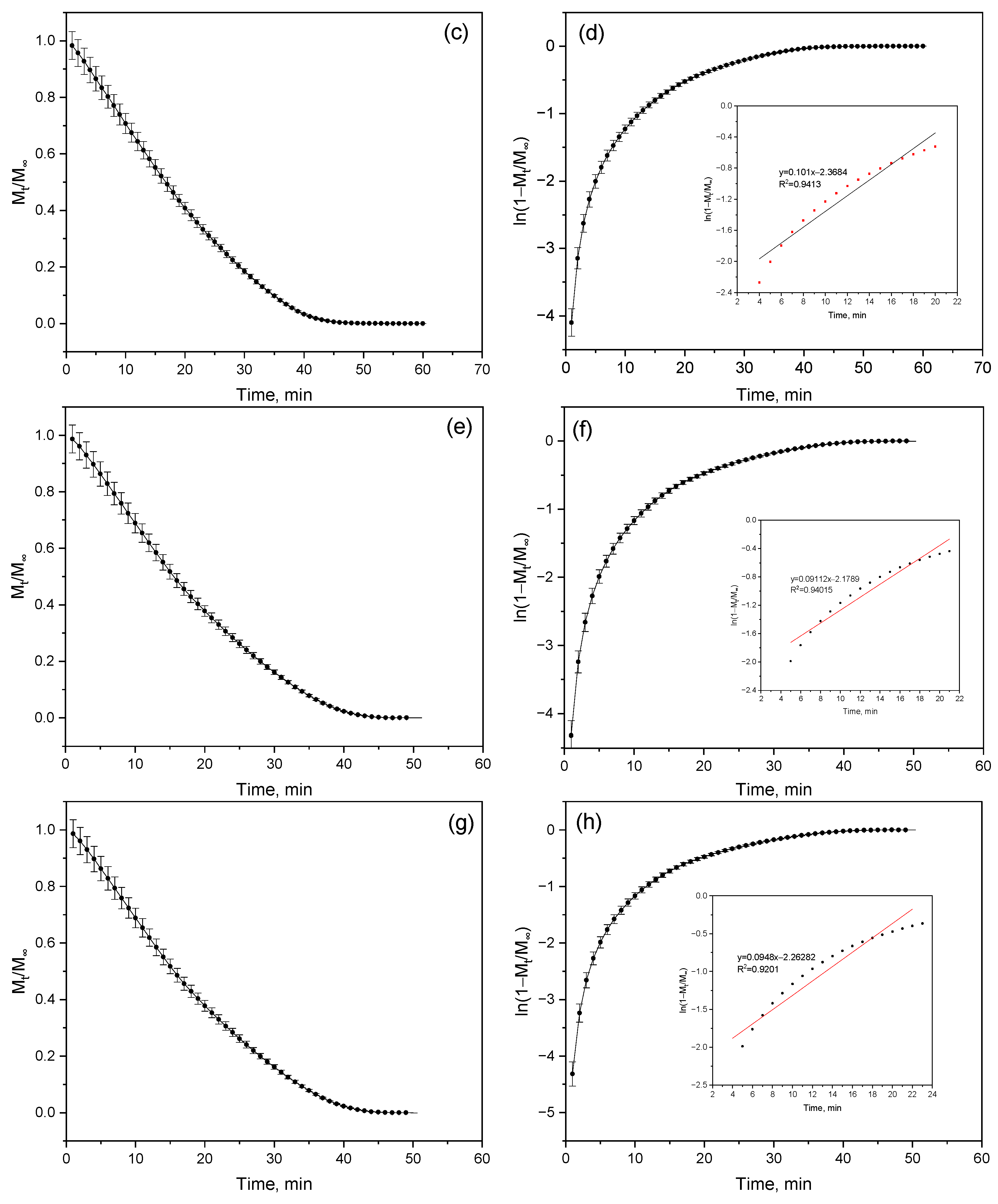
The highest slope value (k = 0.12 ± 0.001 min
−1) was recorded for sample (a) (
Figure 11a,b), corresponding to the maximum diffusion coefficient D = (2.74 ± 0.02) × 10
−8 m
2/min—that is, in this sample, moisture migrates within the structure at the highest rate (
Table 6). A decrease in the slope for samples (b) (k = 0.10 ± 0.002 min
−1) and (c) (k = 0.09 ± 0.002 min
−1) leads to the expected reduction in D to (2.28 ± 0.05) × 10
−8 m
2/min (
Figure 11c,d) and (2.05 ± 0.05) × 10
−8 m
2/min (
Figure 11e,f), respectively. Interestingly, samples (c) and (d) exhibit identical slope values; however, their diffusion coefficients differ slightly—(2.05 ± 0.05) × 10
−8 and (2.16 ± 0.23) × 10
−8 m
2/min, respectively. Although the difference is small, it indicates the influence of microstructural factors—specifically, the distribution of pores and capillaries within the polymer. Such variations affect the initial moisture content of the samples, as in more porous materials, moisture is retained differently, altering the efficiency of its transport.
A decrease in the coefficient of determination (R
2 = 0.92) for sample (d) suggests a deviation from a purely diffusion-controlled mechanism (
Figure 11g,h), indicating that moisture transfer in this case does not fully obey Fick’s law and may involve additional processes such as sorption or structural relaxation.
Thus, a clear trend is observed: as the dehydration rate (slope k) decreases, the diffusion coefficient also decreases. The slower the water evaporates, the more difficult it becomes for it to diffuse through the polymer matrix. This reflects increased resistance to moisture transfer due to structural densification, which reduces water permeability and enhances the material’s stability under humid conditions.
Analysis of thermogravimetric data and dehydration kinetics showed that the high dehydration rate of sample (a) is consistent with its simplified kinetics and moderate stability, as determined from 3D graphs (
Figure 7 and
Figure 8). For sample (b), lower diffusion coefficient values reflect an energetic but less stable process, which is confirmed by its high reactivity on thermal maps (
Figure 10) and reduced
values (
Table 3). Sample (c) is characterized by minimal diffusion coefficients and a narrow dehydration window, which coincides with the parameters of the Šesták–Berggren model (
Table 3), indicating strong diffusion limitations. Sample (d), similar to the thermolysis results, demonstrates intermediate behavior: moderate diffusion coefficient values (
Table 5) and an extended temperature range of activity on 3D graphs.
Thus, the combination of thermogravimetric analysis, approximation, and calculations based on the Fick model proves that changes in composition control both the destruction mechanism and moisture transfer.
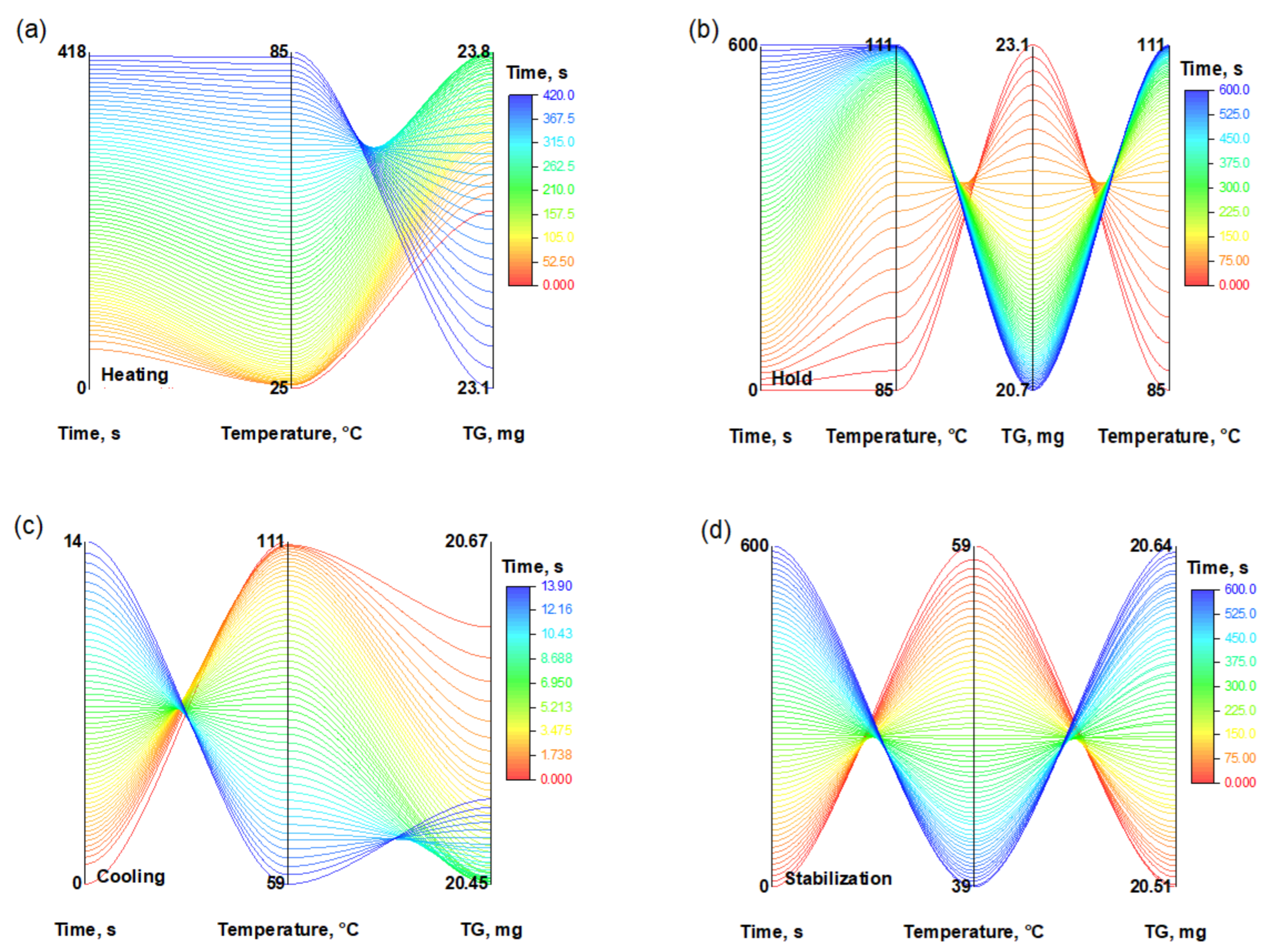
In addition to kinetic and diffusion analysis, the thermal stability of the PVA/PEGDA (3/11) hydrogel under repeated thermal exposure was evaluated. Thermogravimetric analysis (TG) was used to monitor mass changes during 20 consecutive thermal cycles, each consisting of heating (up to 100 °C), isothermal holding, cooling, and subsequent stabilization at 30 °C. As shown in
Figure 12, each stage is characterized by a specific temperature profile and corresponding mass variations.
The most pronounced mass losses (
Figure 12) were recorded during the isothermal holding stage, which can be attributed to thermal decomposition and desorption processes. In contrast, during the cooling and stabilization stages, slower TG changes or slight mass recovery were observed, likely due to moisture reabsorption.
The color scale represents the duration of the experiment. The graphs illustrate the decrease in sample mass at each stage, reflecting the degradation and evaporation processes occurring during the thermal cycle.
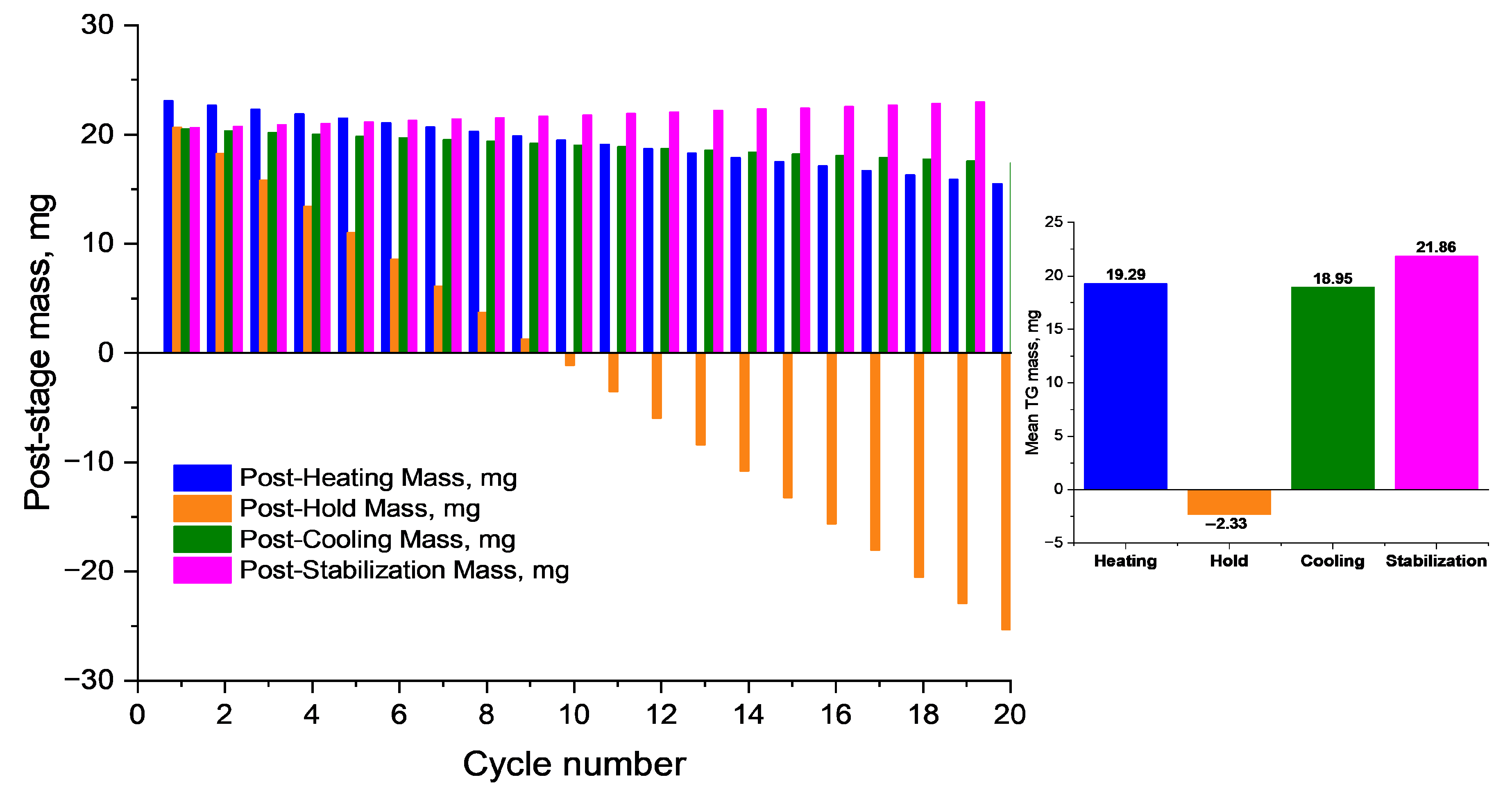
The summarized data (
Figure 13) show that after 20 cycles, the sample retains more than 65% of its initial mass, confirming its thermal stability and reversible moisture exchange capability—key characteristics for use in biomedical devices, drug delivery systems, and smart materials operating under cyclic thermal conditions.
The results obtained in the present study expand and complement previously published data on the thermal stability of PVA- and PEG-based hydrogels [
9,
10,
17,
18]. In contrast to the aforementioned studies, which focused either on physically or chemically crosslinked systems separately, or employed limited kinetic analysis methods, our work is based on the investigation of interpenetrating polymer networks (IPNs) incorporating both PEGDA and PEGMA. It also implements a comprehensive approach that combines several modeling and statistical methods (Friedman, OFW, NPK, Šesták–Berggren, ANOVA, and
t-tests). This approach enabled a more detailed characterization of the stages of thermal degradation, their kinetic parameters, and their dependence on composition, thereby expanding the possibilities for controlling the thermal stability of hydrogels in applied contexts, including biomedical and engineering applications.