Activation of Inflammatory and Apoptosis Pathways on Human Gingival Fibroblasts Exposed to Dental Resin Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation for Eluates Deriving from GR, BF, and FU
2.2. Cell Culture Establishment
2.3. Cell Metabolic Activity
2.4. Experimental Design
- -
- hGFs cultured with complete medium for 24 h (Control Groups, CTRL);
- -
- hGFs cultured with eluate derived from GR for 24 h;
- -
- hGFs cultured with eluate derived from BF for 24 h;
- -
- hGFs cultured with eluate derived from FU for 24 h;
- -
- hGFs cultured with complete medium for 1 week;
- -
- hGFs cultured with eluate derived from GR for 1 week;
- -
- hGFs cultured with eluate derived from BF for 1 week;
- -
- hGFs cultured with eluate derived from FU for 1 week.
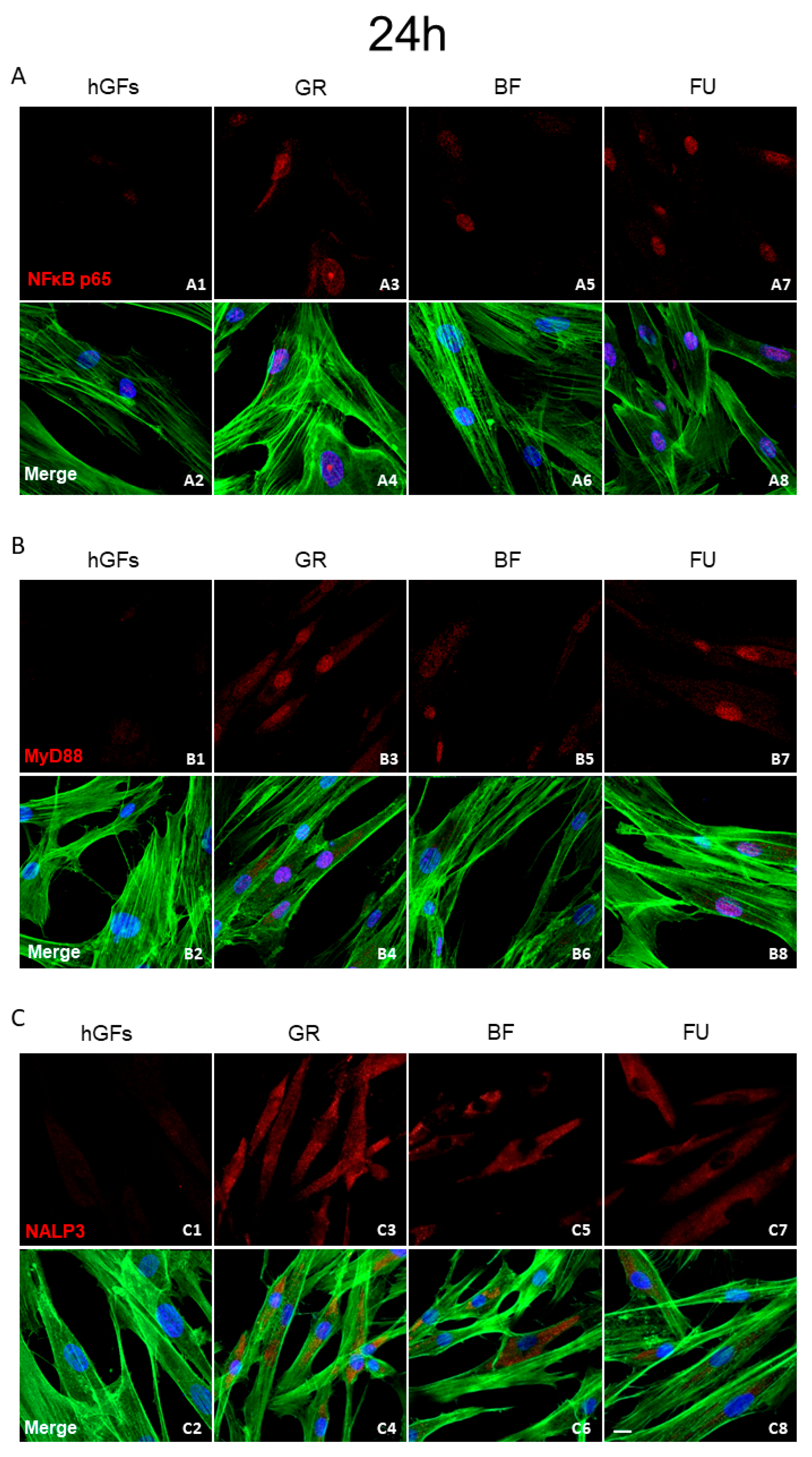
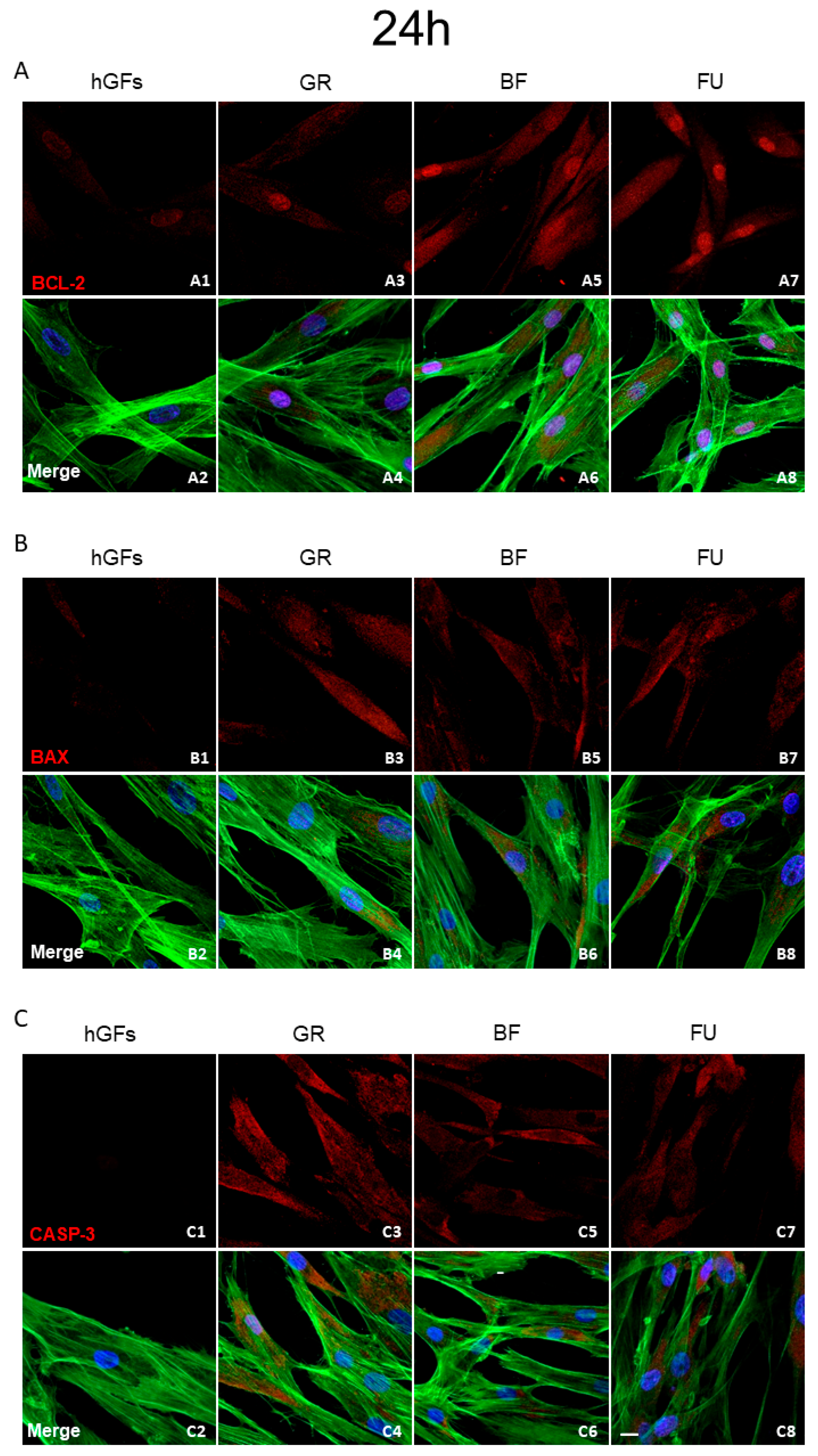
2.5. Immunofluorescence Staining for Confocal Laser Scanning Microscope (CLSM) Detection
2.6. RNA Isolation and Real-Time RT-PCR
2.7. Statistical Analysis
3. Results
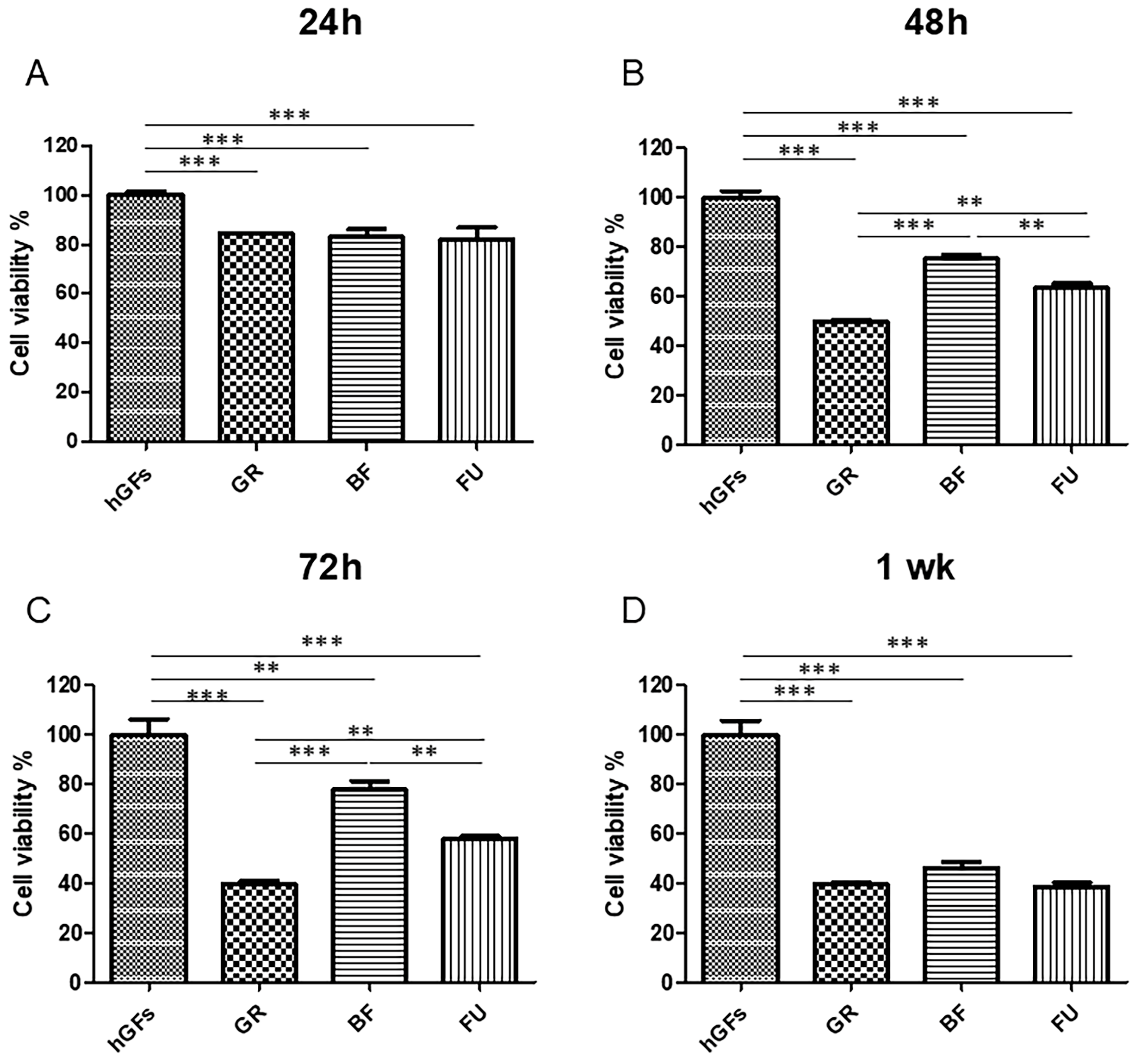
3.1. Effects of Dental Composites on hGFs
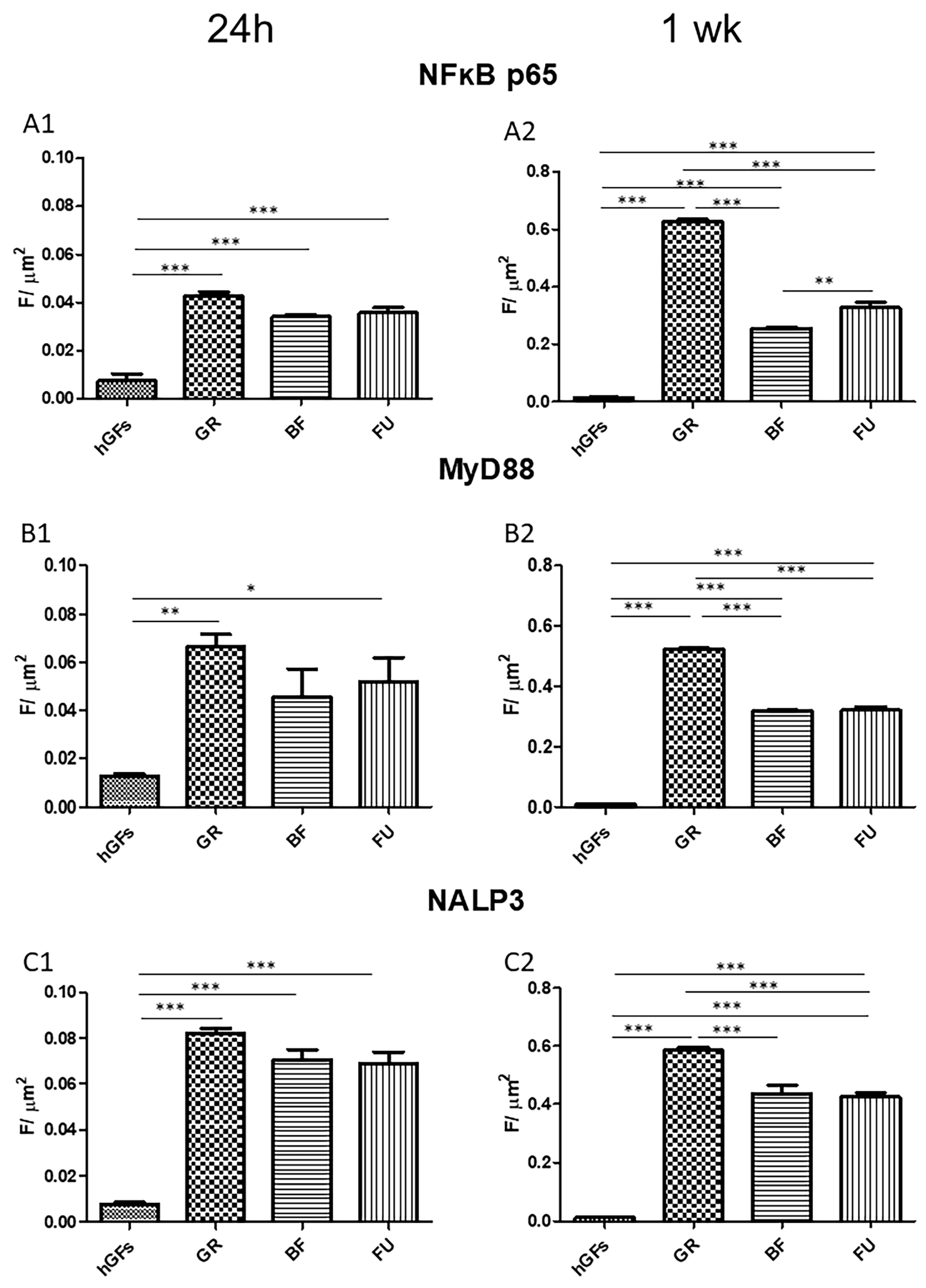
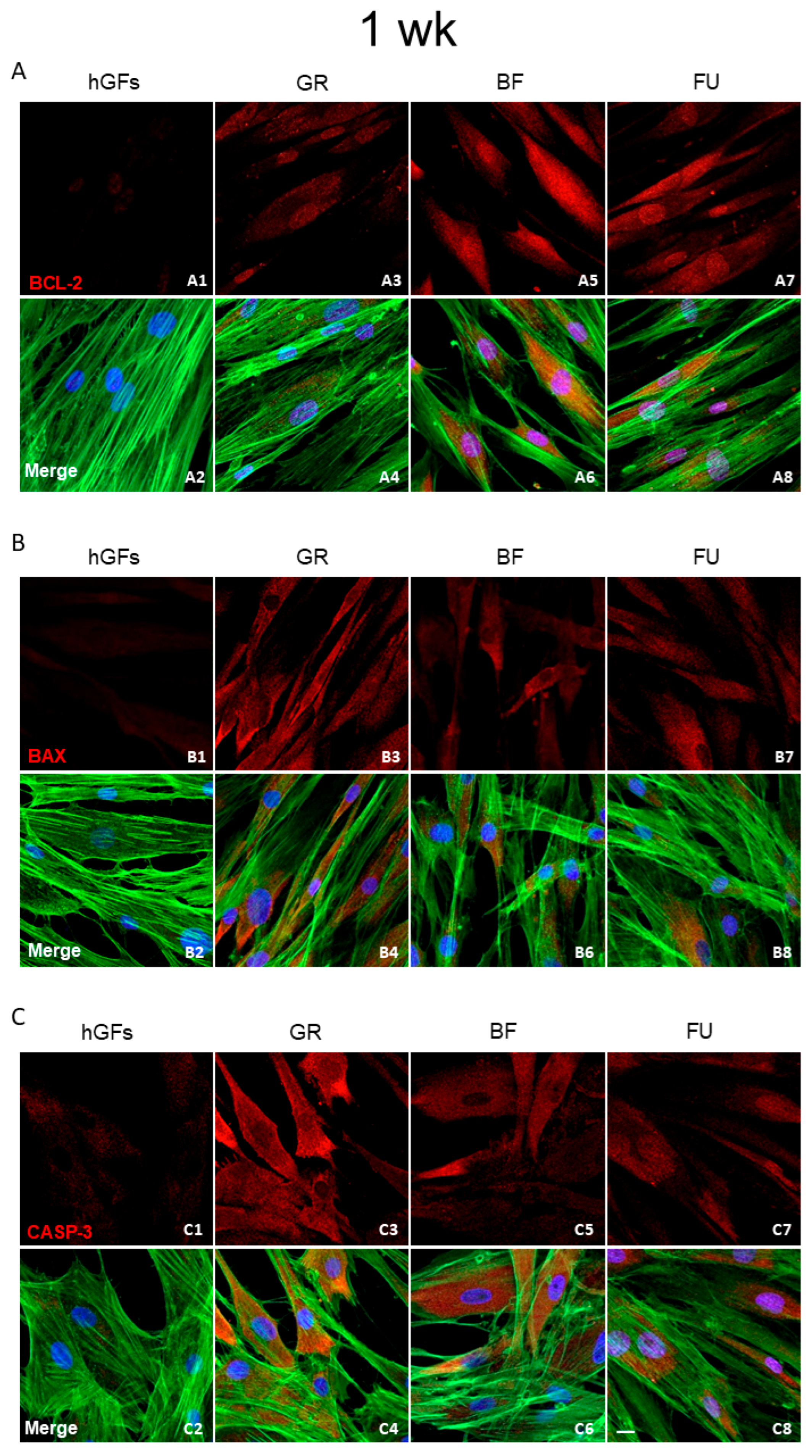
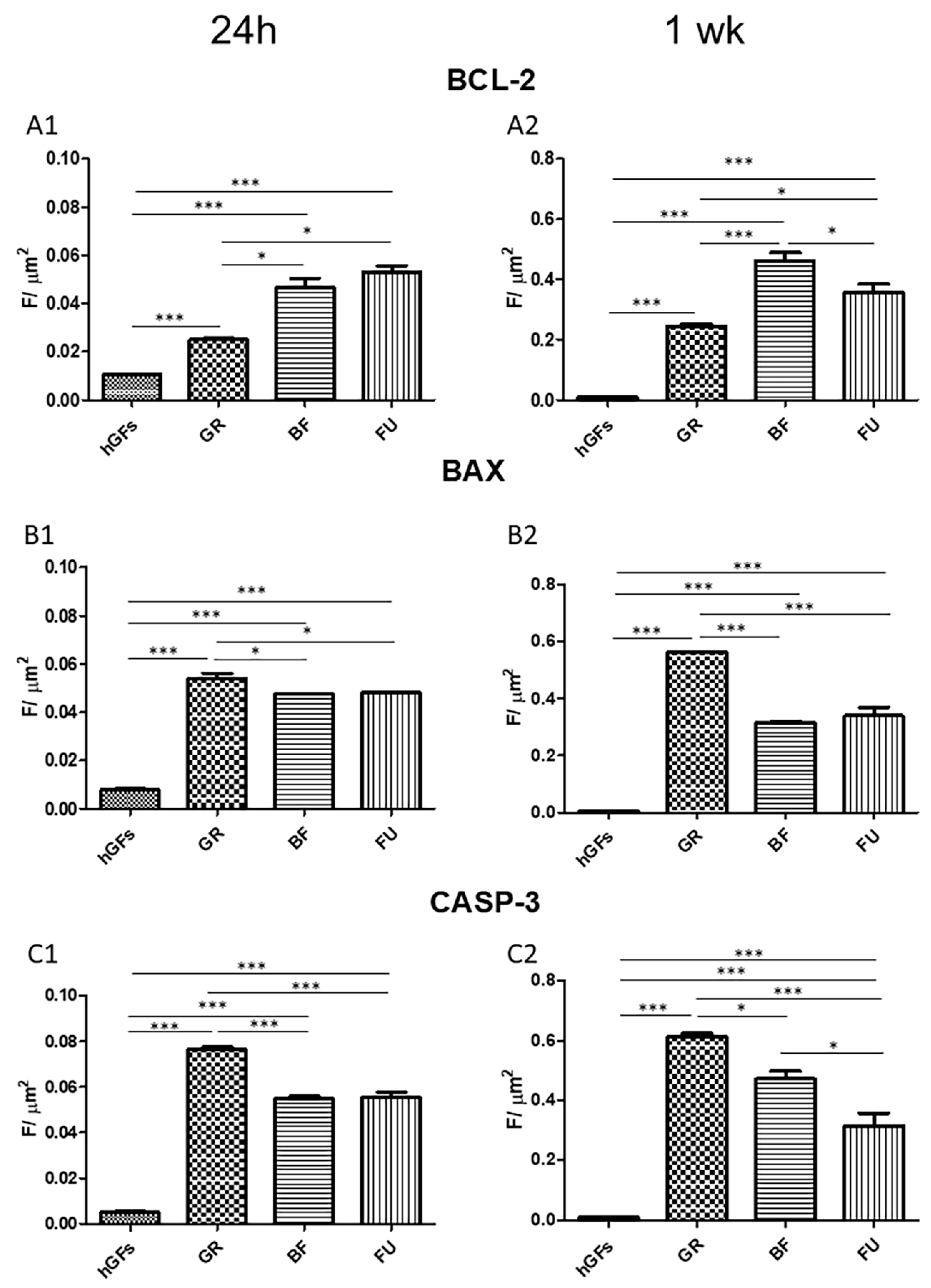
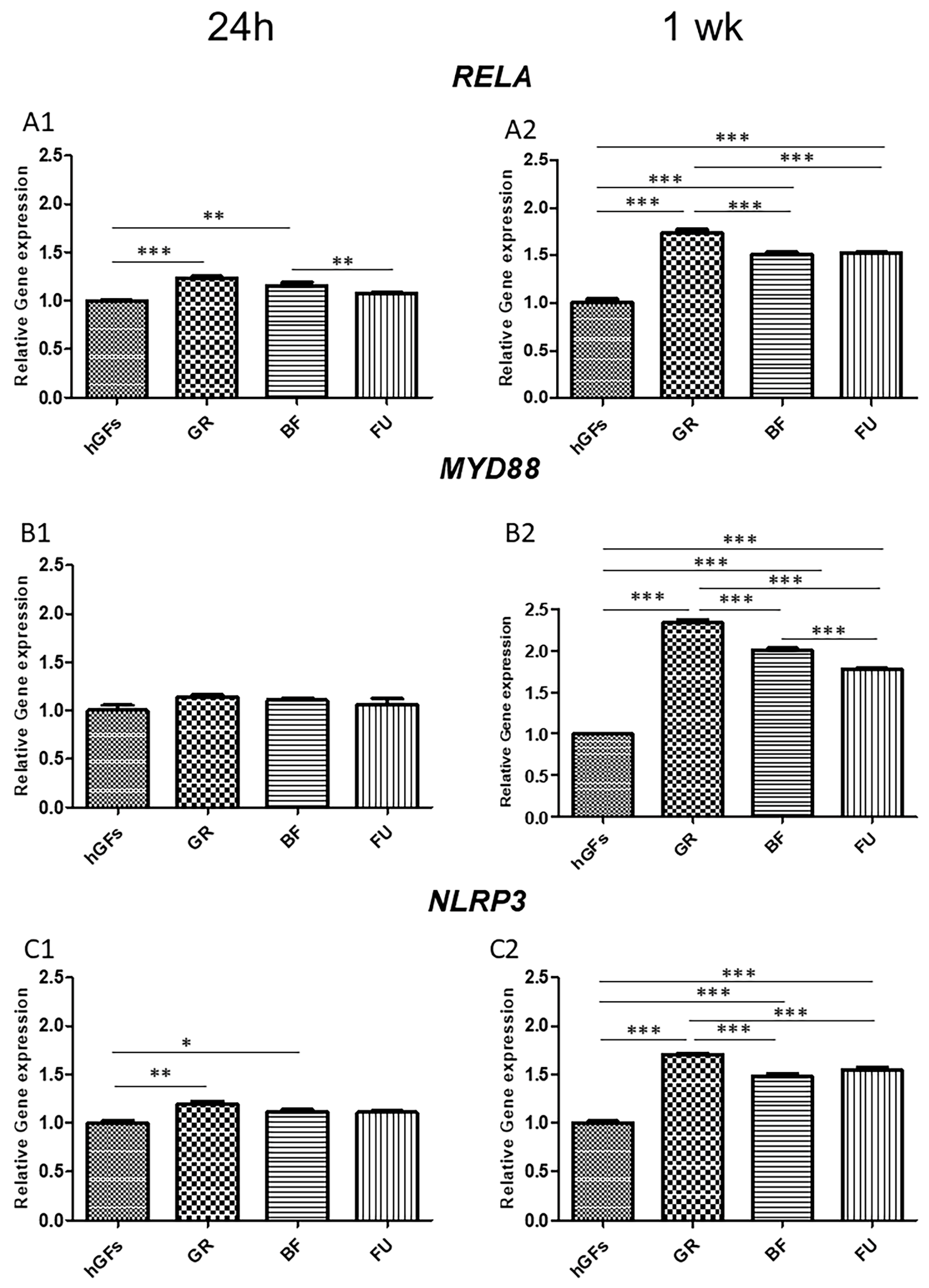
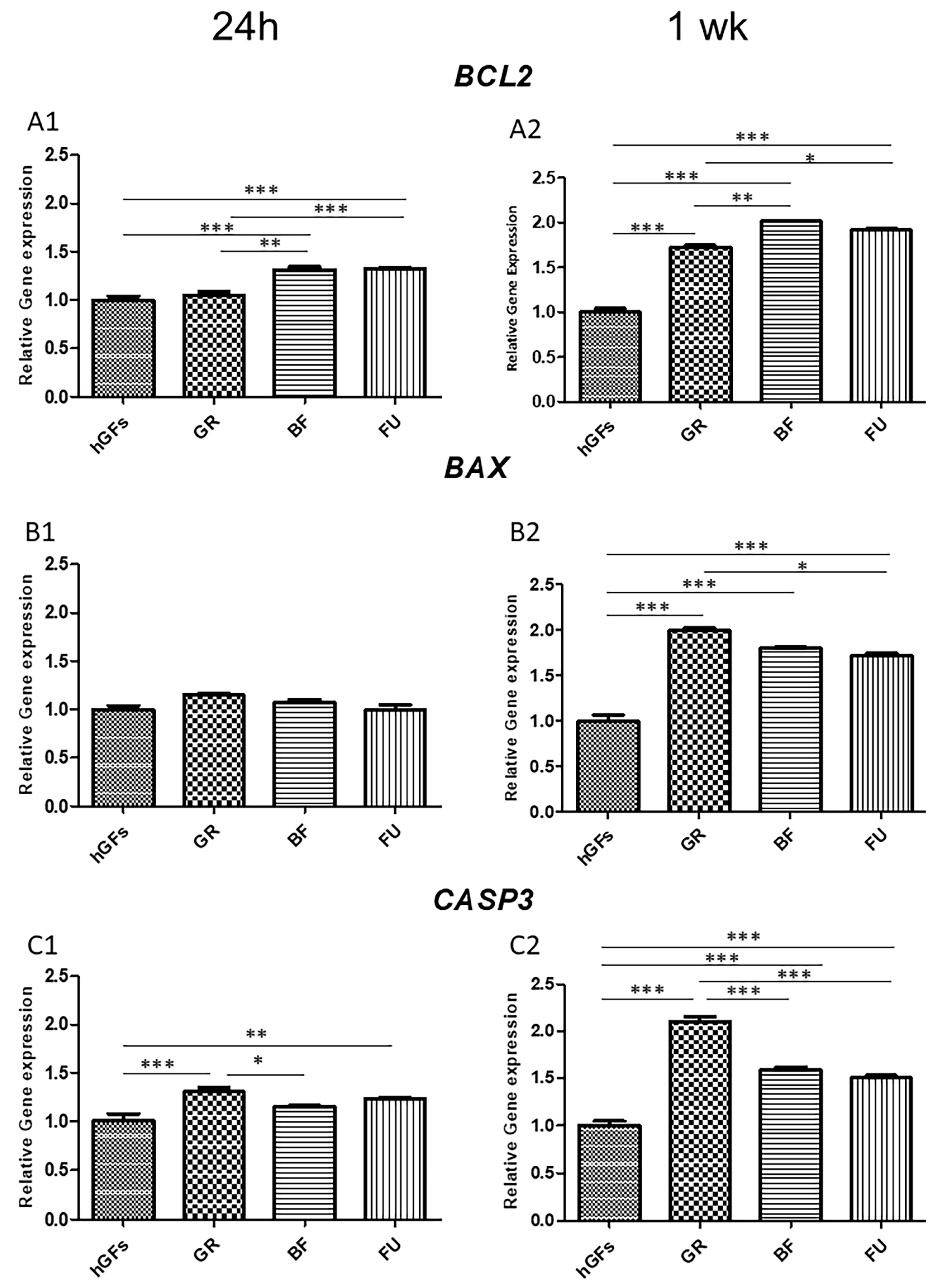
3.2. Dental Composite Treatment Modulated the Pro-Inflammatory and Apoptotic Markers in hGFs Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Murdoch-Kinch, C.A.; McLean, M.E. Minimally invasive dentistry. J. Am. Dent. Assoc. 2003, 134, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Schrickel, K.; Watts, D.C.; Becker, M.; Draenert, M.E. Direct and indirect monomer elution from an RBC product family. Dent. Mater. 2021, 37, 1601–1614. [Google Scholar] [CrossRef]
- Durner, J.; Wellner, P.; Hickel, R.; Reichl, F.X. Synergistic interaction caused to human gingival fibroblasts from dental monomers. Dent. Mater. 2012, 28, 818–823. [Google Scholar] [CrossRef]
- Völkel, W.; Colnot, T.; Csanády, G.A.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin. Oral Investig. 2008, 12, 1–8. [Google Scholar] [CrossRef]
- Fong, H.; Dickens, S.H.; Flaim, G.M. Evaluation of dental restorative composites containing polyhedral oligomeric silsesquioxane methacrylate. Dent. Mater. 2005, 21, 520–529. [Google Scholar] [CrossRef]
- Yang, Y.; Reichl, F.X.; Shi, J.; He, X.; Hickel, R.; Högg, C. Cytotoxicity and DNA double-strand breaks in human gingival fibroblasts exposed to eluates of dental composites. Dent. Mater. 2018, 34, 201–208. [Google Scholar] [CrossRef]
- Wataha, J.C. Predicting clinical biological responses to dental materials. Dent. Mater. 2012, 28, 23–40. [Google Scholar] [CrossRef]
- Styllou, M.; Reichl, F.X.; Styllou, P.; Urcan, E.; Rothmund, L.; Hickel, R.; Högg, C.; Scherthan, H. Dental composite components induce DNA-damage and altered nuclear morphology in gingiva fibroblasts. Dent. Mater. 2015, 31, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Kopperud, H.M. Preparation and characterization of Bis-GMA-free dental composites with dimethacrylate monomer derived from 9,9-Bis [4-(2-hydroxyethoxy)phenyl]fluorene. Dent. Mater. 2018, 34, 1003–1013. [Google Scholar] [CrossRef]
- Krifka, S.; Petzel, C.; Hiller, K.A.; Frank, E.M.; Bosl, C.; Spagnuolo, G.; Reichl, F.X.; Schmalz, G.; Schweikl, H. Resin monomer-induced differential activation of MAP kinases and apoptosis in mouse macrophages and human pulp cells. Biomaterials 2010, 31, 2964–2975. [Google Scholar] [CrossRef]
- Huang, F.M.; Kuan, Y.H.; Lee, S.S.; Chang, Y.C. Cytotoxicity and genotoxicity of triethyleneglycol-dimethacrylate in macrophages involved in DNA damage and caspases activation. Environ. Toxicol. 2015, 30, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.H.; Huang, F.M.; Lee, S.S.; Li, Y.C.; Chang, Y.C. Bisgma stimulates prostaglandin E2 production in macrophages via cyclooxygenase-2, cytosolic phospholipase A2, and mitogen-activated protein kinases family. PLoS ONE 2013, 8, e82942. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.H.; Huang, F.M.; Li, Y.C.; Chang, Y.C. Proinflammatory activation of macrophages by bisphenol A-glycidyl-methacrylate involved NFκB activation via PI3K/Akt pathway. Food Chem. Toxicol. 2012, 50, 4003–4009. [Google Scholar] [CrossRef]
- Huang, F.M.; Chang, Y.C.; Lee, S.S.; Yeh, C.H.; Lee, K.G.; Huang, Y.C.; Chen, C.J.; Chen, W.Y.; Pan, P.H.; Kuan, Y.H. BisGMA-induced cytotoxicity and genotoxicity in macrophages are attenuated by wogonin via reduction of intrinsic caspase pathway activation. Environ. Toxicol. 2016, 31, 176–184. [Google Scholar] [CrossRef]
- Trubiani, O.; Ballerini, P.; Murmura, G.; Pizzicannella, J.; Giuliani, P.; Buccella, S.; Caputi, S. Toll-like receptor 4 expression, interleukin-6,-8 and Ccl-20 release, and NF-KB translocation in human periodontal ligament mesenchymal stem cells stimulated with LPS-P. gingivalis. Eur. J. Inflamm. 2012, 10, 81–89. [Google Scholar] [CrossRef]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1beta (IL-1beta) processing pathway. Sci. Signal. 2010, 3, cm2. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Yang, L.C.; Chang, Y.C.; Yeh, K.L.; Huang, F.M.; Su, N.Y.; Kuan, Y.H. Protective Effect of Rutin on Triethylene Glycol Dimethacrylate-Induced Toxicity through the Inhibition of Caspase Activation and Reactive Oxygen Species Generation in Macrophages. Int. J. Mol. Sci. 2022, 23, 11773. [Google Scholar] [CrossRef]
- Norbury, C.J.; Hickson, I.D. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 367–401. [Google Scholar] [CrossRef] [PubMed]
- Dolka, I.; Król, M.; Sapierzyński, R. Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved caspase-3 and p53) expression in canine mammary tumors: An immunohistochemical and prognostic study. Res. Vet. Sci. 2016, 105, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, J.; Kraft, A.S. Bax-induced apoptotic cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 529–531. [Google Scholar] [CrossRef]
- Ouyang, Y.B.; Giffard, R.G. MicroRNAs affect BCL-2 family proteins in the setting of cerebral ischemia. Neurochem. Int. 2014, 77, 2–8. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Festjens, N.; Declercq, W.; Vanden Berghe, T.; Vandenabeele, P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007, 14, 44–55. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Y.; He, S.; Cheng, J.; Gong, Y.; Zhang, Z.; Yang, X.; Xu, B.; Liu, X.; Li, C.Y.; et al. Dying glioma cells establish a proangiogenic microenvironment through a caspase 3 dependent mechanism. Cancer Lett. 2017, 385, 12–20. [Google Scholar] [CrossRef]
- Mukai, M.; Kusama, T.; Hamanaka, Y.; Koga, T.; Endo, H.; Tatsuta, M.; Inoue, M. Cross talk between apoptosis and invasion signaling in cancer cells through caspase-3 activation. Cancer Res. 2005, 65, 9121–9125. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Zhao, Z.; Xiao, Y.; Sengupta, S.; Xiao, Y.; Zhang, R.; Lauber, K.; Wesselborg, S.; Feng, L.; et al. Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. J. Biol. Chem. 2006, 281, 29357–29368. [Google Scholar] [CrossRef]
- Lauber, K.; Bohn, E.; Kröber, S.M.; Xiao, Y.J.; Blumenthal, S.G.; Lindemann, R.K.; Marini, P.; Wiedig, C.; Zobywalski, A.; Baksh, S.; et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 2003, 113, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Ho, Y.C.; Lee, S.S.; Li, Y.C.; Lai, M.Y.; Kuan, Y.H. Cytotoxicity and Apoptotic Mechanism of 2-Hydroxyethyl Methacrylate via Genotoxicity and the Mitochondrial-Dependent Intrinsic Caspase Pathway and Intracellular Reactive Oxygen Species Accumulation in Macrophages. Polymers 2022, 14, 3378. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chiang, C.Y.; Chiang, Y.W.; Lee, M.W.; Lee, C.Y.; Chen, H.Y.; Lin, H.W.; Kuan, Y.H. Toxic Effects of Urethane Dimethacrylate on Macrophages Through Caspase Activation, Mitochondrial Dysfunction, and Reactive Oxygen Species Generation. Polymers 2020, 12, 1398. [Google Scholar] [CrossRef] [PubMed]
- Ditrichova, D.; Kapralova, S.; Tichy, M.; Ticha, V.; Dobesova, J.; Justova, E.; Eber, M.; Pirek, P. Oral lichenoid lesions and allergy to dental materials. Biomed. Pap. 2007, 151, 333–339. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Trachtenberg, F.; Barregard, L.; Tavares, M.; Cernichiari, E.; Daniel, D.; McKinlay, S. Neuropsychological and renal effects of dental amalgam in children: A randomized clinical trial. JAMA 2006, 295, 1775–1783. [Google Scholar] [CrossRef]
- Hensten-Pettersen, A. Skin and mucosal reactions associated with dental materials. Eur. J. Oral Sci. 1998, 106, 707–712. [Google Scholar] [CrossRef]
- Schätzle, M.; Land, N.P.; Anerud, A.; Boysen, H.; Bürgin, W.; Löe, H. The influence of margins of restorations of the periodontal tissues over 26 years. J. Clin. Periodontol. 2001, 28, 57–64. [Google Scholar] [CrossRef]
- Stanislawski, L.; Lefeuvre, M.; Bourd, K.; Soheili-Majd, E.; Goldberg, M.; Périanin, A. TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species. J. Biomed. Mater. Res. Part A 2003, 66, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Juloski, J.; Köken, S.; Ferrari, M. Cervical margin relocation in indirect adhesive restorations: A literature review. J. Prosthodont. Res. 2018, 62, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Demarco, F.F.; Correa, M.B.; Horta, B.; Barros, A.J.; Peres, K.G.; Peres, M.A. Multilevel analysis of the association between posterior restorations and gingival health in young adults: A population-based birth cohort. J. Clin. Periodontol. 2013, 40, 1126–1131. [Google Scholar] [CrossRef]
- Noor, E.; Al-Bayaty, F.H. A review on predisposing and modifying factors of periodontal disease. J. Adv. Med. Res. 2015, 5, 5–23. [Google Scholar]
- Larato, D.C. Influence of a composite resin restoration on the gingiva. J. Prosthet. Dent. 1972, 28, 402–404. [Google Scholar] [CrossRef]
- Della Rocca, Y.; Traini, E.M.; Trubiani, O.; Traini, T.; Mazzone, A.; Marconi, G.D.; Pizzicannella, J.; Diomede, F. Biological Effects of PMMA and Composite Resins on Human Gingival Fibroblasts: An In Vitro Comparative Study. Int. J. Mol. Sci. 2024, 25, 4880. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Lanuti, P.; Della Rocca, Y.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Transforming Growth Factor-Beta1 and Human Gingival Fibroblast-to-Myofibroblast Differentiation: Molecular and Morphological Modifications. Front. Physiol. 2021, 12, 676512. [Google Scholar] [CrossRef] [PubMed]
- Caputi, S.; Trubiani, O.; Sinjari, B.; Trofimova, S.; Diomede, F.; Linkova, N.; Diatlova, A.; Khavinson, V. Effect of short peptides on neuronal differentiation of stem cells. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419828613. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Della Rocca, Y.; Oliva, S.; Rajan, T.S.; Trubiani, O.; Murmura, G.; Diomede, F.; Pizzicannella, J. Enhanced Extracellular Matrix Deposition on Titanium Implant Surfaces: Cellular and Molecular Evidences. Biomedicines 2021, 9, 1710. [Google Scholar] [CrossRef]
- Mahaney, B.L.; Meek, K.; Lees-Miller, S.P. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem. J. 2009, 417, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Lottner, S.; Shehata, M.; Hickel, R.; Reichl, F.X.; Durner, J. Effects of antioxidants on DNA-double strand breaks in human gingival fibroblasts exposed to methacrylate based monomers. Dent. Mater. 2013, 29, 991–998. [Google Scholar] [CrossRef]
- Tsitrou, E.; Kelogrigoris, S.; Koulaouzidou, E.; Antoniades-Halvatjoglou, M.; Koliniotou-Koumpia, E.; van Noort, R. Effect of extraction media and storage time on the elution of monomers from four contemporary resin composite materials. Toxicol. Int. 2014, 21, 89–95. [Google Scholar] [CrossRef]
- De Angelis, F.; Mandatori, D.; Schiavone, V.; Melito, F.P.; Valentinuzzi, S.; Vadini, M.; Di Tomo, P.; Vanini, L.; Pelusi, L.; Pipino, C.; et al. Cytotoxic and Genotoxic Effects of Composite Resins on Cultured Human Gingival Fibroblasts. Materials 2021, 14, 5225. [Google Scholar] [CrossRef]
- Yamashita, U.; Sugiura, T.; Yoshida, Y.; Kuroda, E. Effect of endocrine disrupters on macrophage functions in vitro. J. UOEH 2005, 27, 1–10. [Google Scholar] [CrossRef]
- Della Rocca, Y.; Traini, E.M.; Diomede, F.; Fonticoli, L.; Trubiani, O.; Paganelli, A.; Pizzicannella, J.; Marconi, G.D. Current Evidence on Bisphenol A Exposure and the Molecular Mechanism Involved in Related Pathological Conditions. Pharmaceutics 2023, 15, 908. [Google Scholar] [CrossRef]
- Bertoldi, C.; Monari, E.; Cortellini, P.; Generali, L.; Lucchi, A.; Spinato, S.; Zaffe, D. Clinical and histological reaction of periodontal tissues to subgingival resin composite restorations. Clin. Oral Investig. 2020, 24, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.; Ungvári, K.; Györgyey, Á.; Kukovecz, Á.; Turzó, K.; Nagy, K. Human epithelial tissue culture study on restorative materials. J. Dent. 2014, 42, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Nakata, R.; Takahashi, S.; Inoue, H. Recent advances in the study on resveratrol. Biol. Pharm. Bull. 2012, 35, 273–279. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Fan, T.; Liu, J.; Su, X.; Fan, S.; Liu, Q.; et al. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Int. J. Mol. Sci. 2013, 14, 14105–14118. [Google Scholar] [CrossRef]
- Yao, H.; Rahman, I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem. Pharmacol. 2012, 84, 1332–1339. [Google Scholar] [CrossRef]
- Finkel, T.; Deng, C.X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Y.; Sun, Y.; Gao, Q.; Li, H.; Yang, Z.; Wang, Y.; Jiang, X.; Yu, B. Target of MCC950 in Inhibition of NLRP3 Inflammasome Activation: A Literature Review. Inflammation 2020, 43, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Busch, K.; Kny, M.; Huang, N.; Klassert, T.E.; Stock, M.; Hahn, A.; Graeger, S.; Todiras, M.; Schmidt, S.; Chamling, B.; et al. Inhibition of the NLRP3/IL-1β axis protects against sepsis-induced cardiomyopathy. J. Cachexia Sarcopenia Muscle 2021, 12, 1653–1668. [Google Scholar] [CrossRef]
- Oguchi, T.; Ono, R.; Tsuji, M.; Shozawa, H.; Somei, M.; Inagaki, M.; Mori, Y.; Yasumoto, T.; Ono, K.; Kiuchi, Y. Cilostazol Suppresses Aβ-induced Neurotoxicity in SH-SY5Y Cells through Inhibition of Oxidative Stress and MAPK Signaling Pathway. Front. Aging Neurosci. 2017, 9, 337. [Google Scholar] [CrossRef]
- Lu, C.C.; Chiang, J.H.; Tsai, F.J.; Hsu, Y.M.; Juan, Y.N.; Yang, J.S.; Chiu, H.Y. Metformin triggers the intrinsic apoptotic response in human AGS gastric adenocarcinoma cells by activating AMPK and suppressing mTOR/AKT signaling. Int. J. Oncol. 2019, 54, 1271–1281. [Google Scholar] [CrossRef]
- Taghizadeh, F.; Hosseinimehr, S.J.; Zargari, M.; Karimpour Malekshah, A.; Talebpour Amiri, F.B. Gliclazide attenuates cisplatin-induced nephrotoxicity through inhibiting NF-κB and caspase-3 activity. IUBMB Life 2020, 72, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Pan, Y.; Wang, M.; Wang, Y.; Lin, H.; Jiang, L.; Lin, D.; Cheng, H. Comparative analysis of leaching residual monomer and biological effects of four types of conventional and CAD/CAM dental polymers: An in vitro study. Clin. Oral Investig. 2022, 26, 2887–2898. [Google Scholar] [CrossRef] [PubMed]
- Alamoush, R.A.; Kushnerev, E.; Yates, J.M.; Satterthwaite, J.D.; Silikas, N. Response of two gingival cell lines to CAD/CAM composite blocks. Dent. Mater. 2020, 36, 1214–1225. [Google Scholar] [CrossRef] [PubMed]

| Experimental Group | Material | Manufacturer | Batch | Composition |
|---|---|---|---|---|
| GR | GrandioSO -Shade A2- (Nanohybrid) | Voco GmbH (Anton-Flettner-Straße 1-3, 27472, Cuxhaven, Germany) | 2213772 | 89% (w/w) fillers (1 μm glass ceramic filler, 20 nm–40 nm silicon dioxide fillers), Bis-GMA, Bis-EMA, TEGDMA. |
| BF | Enamel Plus HRi Biofunction -Shade BF2- (Nanohybrid) | Micerium S.p.A (Via Guglielmo Marconi, 83, 16036, Avegno, Genova, Italy) | 2024006140 | 74% in weight (60% in volume) fillers (0.005 μm–0.05 μm silicon dioxide fillers), (0.2–3.0 μm glass fillers), Urethane dimethacrylate, Tricyclodecane dimethanol dimethacrylate. |
| FU | 3M™ Filtek™ Universal Restorative—Shade A2 | 3M ESPE (2510 Conway Avenue St. Paul, MN 55144-1000 USA) | 10544071 | Silane treated ceramic (40–70 by wt%) *, silica (1–5 wt%) *, and zirconia (1–5 wt%) *, Ytterbium Fluoride (1–10 wt%) *, Aromatic Urethane Dimethacrylate, Diurethane Dimethacrylate, 1,12-Dodecane Dimethycrylate |
| Gene | Primer 1 | Primer 2 |
|---|---|---|
| Sequence (5′-3′) | Sequence (5′-3′) | |
| RELA | 5′-CGAGCTTGTAGGAAAGGACTG-3′ | 5′-TGACTGATAGC-CTGCTCCAG-3′ |
| MYD88 | 5′-CGGTCTCCTCCACATCCT-3′ | 5′-GCCGGATCTCCAAGTACTCA’-3′ |
| NLRP3 | 5′-GAATGCCTTGG GAGACTCAG-3′ | 5′-AGATTCTGATT AGTGCTGAGTACC-3′ |
| BCL2 | 5′-GATGACTGAGTACCTGAACCG 3′ | 5′-ACCAATCTTGT AGGACTGACC-3′ |
| BAX | 5′-TCTGAGCAGATCATGAAGACAG-3′ | 5′-AGCCAGGAGAAATCAAACAGAG-3′ |
| CASP3 | 5′-CTCTGGAATATCCCTGGACAAC-3′ | 5′GTTTGCTGCATCGACATCTG-3′ |
| B2M | 5′-GGACTGGTCTT-TCTATCTCTTGT-3′ | 5′-ACCTCCATGATGCTGCTTAC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Angelis, F.; Sorrentino, E.; Mazzone, A.; Della Rocca, Y.; Pizzicannella, J.; Trubiani, O.; Iezzi, G.; D’Arcangelo, C.; Marconi, G.D.; Diomede, F. Activation of Inflammatory and Apoptosis Pathways on Human Gingival Fibroblasts Exposed to Dental Resin Composites. Polymers 2025, 17, 2779. https://doi.org/10.3390/polym17202779
De Angelis F, Sorrentino E, Mazzone A, Della Rocca Y, Pizzicannella J, Trubiani O, Iezzi G, D’Arcangelo C, Marconi GD, Diomede F. Activation of Inflammatory and Apoptosis Pathways on Human Gingival Fibroblasts Exposed to Dental Resin Composites. Polymers. 2025; 17(20):2779. https://doi.org/10.3390/polym17202779
Chicago/Turabian StyleDe Angelis, Francesco, Edoardo Sorrentino, Antonella Mazzone, Ylenia Della Rocca, Jacopo Pizzicannella, Oriana Trubiani, Giovanna Iezzi, Camillo D’Arcangelo, Guya Diletta Marconi, and Francesca Diomede. 2025. "Activation of Inflammatory and Apoptosis Pathways on Human Gingival Fibroblasts Exposed to Dental Resin Composites" Polymers 17, no. 20: 2779. https://doi.org/10.3390/polym17202779
APA StyleDe Angelis, F., Sorrentino, E., Mazzone, A., Della Rocca, Y., Pizzicannella, J., Trubiani, O., Iezzi, G., D’Arcangelo, C., Marconi, G. D., & Diomede, F. (2025). Activation of Inflammatory and Apoptosis Pathways on Human Gingival Fibroblasts Exposed to Dental Resin Composites. Polymers, 17(20), 2779. https://doi.org/10.3390/polym17202779








