3.1. Physical and Morphological Analysis of the Fibers
The physical characteristics of fibers play a crucial role in determining their suitability for various applications in the paper and cardboard industries.
Table 1 presents the morphological properties of fibers from the tegument of mango, which are essential for evaluating their potential applications. The fiber length was 1.08 mm, while the fiber diameter was 19.1 µm, with a wall thickness of 6.0 µm. These values are comparable to other short fibers derived from various agro-industrial waste sources, such as rapeseed straw, maize stalk, and sunflower stalk [
23]. However, the observed high fiber wall thickness can negatively impact multiple properties, such as folding endurance, since the paper sheet may not be well-formed. According to the coefficients obtained, the Runkel ratio indicates that the drying process of the paper will be affected by the thickness of the cell wall. Regarding the flexibility coefficient, the sheets will present low flexibility due to the reduced size of the lumen relative to the diameter of the fiber. In this case, the fibers will collapse somewhat due to the lack of contact surface [
24]. The ratios suggest that the sheet formation with the fibers of the tegument might exhibit unfavorable properties. Nonetheless, the development of the plate does not need a high flexibility coefficient or extensive folding endurance because the material is for single-use applications and does not require repeated opening and reclosure [
25]. The quality of the plates could be improved by mixing the mango fibers with a fiber lumen width higher than the fiber wall thickness.
3.2. Chemical Composition and Structural Analysis of the Fibers
The chemical composition of the untreated fibers and alkali cellulosic pulp is shown in
Table 2. After the alkali treatment, there was a significant decrease (
p < 0.05) in both organic and water extractives. These changes can be attributed to the effective removal of impurities and surface cleaning due to the NaOH reactions with lipophilic substances (resins, waxes, sterols, fats, or fatty acids) and polar fractions (tannins, gums, or sugars) [
15]. The alkali cellulosic pulp exhibited a significantly higher (
p < 0.05) holocellulose content of 84.7% compared to the untreated fibers, which had 67.2%. This difference can be attributed to the rise in cellulose content and the removal of amorphous constituents such as hemicelluloses and extractives [
26]. The increased holocellulose content offers several advantages in the development of the material, including enhanced strength and biocompatibility [
27]. No significant differences (
p > 0.05) in lignin content were observed following the alkali treatment because hemicelluloses were removed more than lignin, primarily due to their more readily solubilized structure [
11]. However, it is noteworthy that the lignin content in the studied material is lower than that of other residues, such as garlic and onion [
28]. According to findings from Lorenzo-Santiago and Rendón-Villalobos [
29], the lignin content in the alkali-treated mango tegument was reported to be 0.64%, which is much lower than our findings of 14.65%. This difference may be attributed to the additional treatments not included in our investigation. These treatments include bleaching and acid hydrolysis. Henrique et al. [
30] found that the lignin content in untreated mango fibers was 23.85% higher than our results of 15.41%. The alkali treatment resulted in a cellulosic pulp characterized by reduced extractives and elevated holocellulose content.
Regarding the high volume of the NaOH solution compared with the tegument weight (hydromodule 1:9, w:v), these are conditions for batch-type digesters, like the one used in the present work for obtaining enough impregnation of the mango’s tegument and the fibers separation. However, at the industrial level, the use of continuous-type digesters is more efficient and complies with the same purpose at a lower pulp to alkali solution hydromodule (1:4, w:v). On the other hand, it is well known that the processes for producing cellulosic pulp generate undesirable contaminants. However, nowadays, most of the industrial processes have the regeneration and recirculation of the produced liquors already considered. Therefore, their application to the non-conventional agro-industrial wastes as starting materials diminishes the environmental impact while attempting to reduce the impact of these wastes in the area surrounding the mango fruit industry location.
FTIR spectroscopy is a valuable technique for examining the structure of constituents and detecting chemical changes in lignocellulosic materials during treatments.
Figure 2 shows the FTIR spectra of untreated fibers and alkali-treated cellulosic pulp. The broad band observed in the range of 3000 to 3600 cm
−1 in untreated fibers was attributed to the stretching vibrations of hydroxyl groups in cellulose. However, after the alkali treatment, a remarkable decrease in the intensity of this band was observed. This reduction indicated a significant decrease in the hydroxyl group content, which can be attributed to the removal of amorphous cellulose and hemicelluloses. As a result, the surface of the material becomes more hydrophobic [
31]. In addition, the peak observed at 1730 cm
−1, which is attributed to the carbonyl group (C=O) of the acetyl group in hemicellulose, disappeared after the removal of hemicelluloses following alkali treatment [
32]. The signal at 1237 cm
−1 indicates that the stretching of C-O bonds in the acetyl groups of hemicellulose diminished after obtaining alkali cellulosic pulp. Similarly, the peak at 1030 cm
−1, indicative of the stretching of C-O bonds in hemicellulose, showed a significant reduction in intensity [
33]. The small signal at 1506 cm
−1, attributed to the lignin’s presence [
32], did not show variation after the alkali treatment. This behavior is consistent with the similar level of lignin found in the untreated fibers and alkali cellulosic pulp.
3.3. Scanning Electronic Microscopy (SEM)
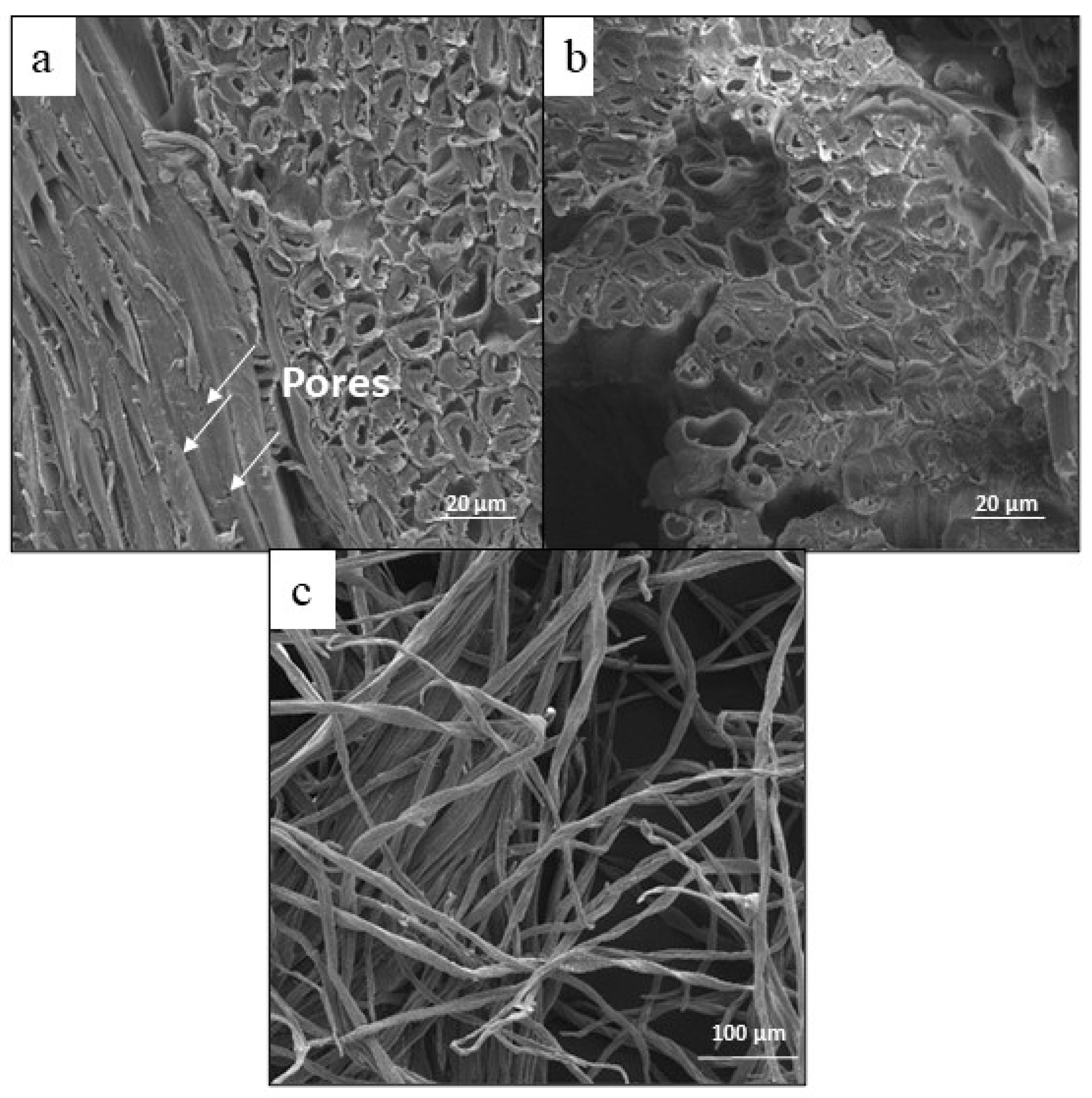
SEM micrographs of the untreated tegument fibers are presented in
Figure 3. The tegument of the mango exhibits a distinctive compact fibrous structure, which shows a level of structural complexity. Additionally, it features tiny pores that are distributed throughout its surface. The structure comprises an epidermis, a cellular inner region, and the lumen, similar to wheat straw fibers [
34].
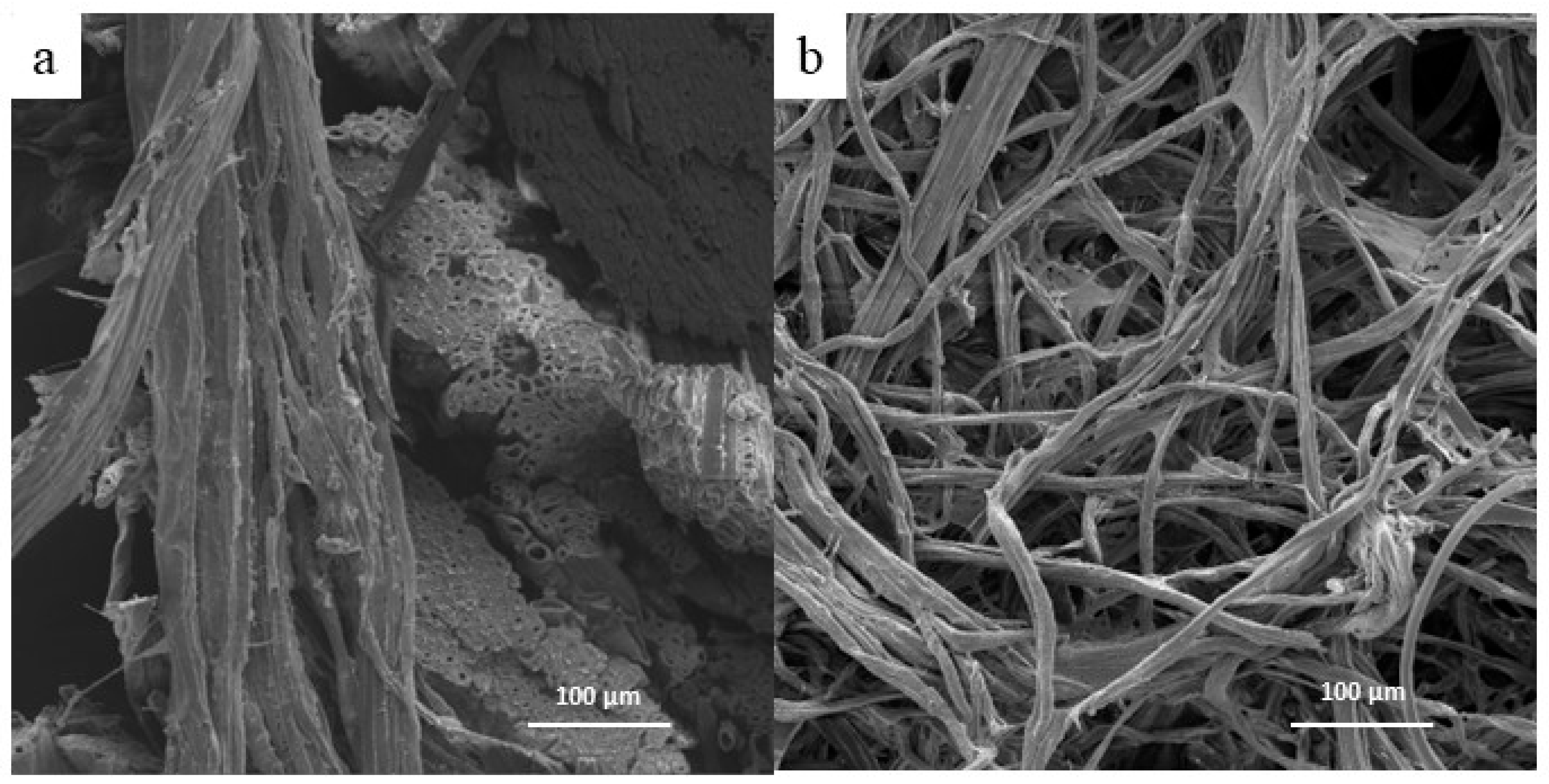
Figure 4 shows the fibers before (a) and after the alkali treatment (b). This treatment removed hemicellulose and other impurities present on the fiber surface. It is essential to note that the treatment caused significant modifications to the complex structure within the fibers of the tegument, resulting in their separation. Cordeiro et al. [
9] also observed the removal of impurities from the fiber surface of the tegument of mango through alkali treatment and hydrogen peroxide, leading to the production of bleached cellulosic pulp. In the present work, the fiber arrangement on the tegument of mango changed as indicated by the FTIR results, leading to the release of fibers.
3.4. Physical and Mechanical Properties of Paper Sheets
Refining is the mechanical process of separating, fibrillating, cutting, and hydrating fibers to improve strength and sheet formation and promote bonding with other fibers. The fibers are modified due to internal fibrillation, external fibrillation, or fiber shortening [
35].
Table 3 presents the effect of refining time on beating level, grammage, thickness, density, folding endurance, tear index, and breaking length. The sheets’ best physical and mechanical properties were achieved in a refining of 10 min without excessive energy consumption. The cellular morphology and fiber length affect the refining time. In the case of mango cellulosic fibers, which are relatively short, a lower refining time is required compared to longer fibers such as pine wood (30 min) [
36]. Refining cellulosic pulp for 10 min reduced the thickness of the sheets and increased their density. These results can be attributed to enhanced fiber-fiber interaction, resulting in higher-density sheets. In addition, the tear index and breaking length exhibited significant enhancements corresponding to the increase in refining times, similar to the findings observed in Tunisian Alfa stem fibers [
37]. The tear index increased at different refining times due to the interaction between fibers through hydrogen bonds [
6].
On the other hand, the lower folding endurance values remained consistent across various refining times, ranging from 1.00 to 1.17. A thick cell wall affects the folding endurance of the sheet, resulting in a bulky material with a coarse surface and a high void volume [
38]. The cell wall thickness of the fiber of tegument was 6.03 µm, which is relatively thick compared with the fiber cell walls of
Acacia (2.51 µm) that can form sheets with high folding endurance [
39]. Although a round and flat plate does not require high folding endurance, a potential strategy for enhancing this property in mango sheets is incorporating a proportion of long fibers during the beating step. Long fibers possess a robust network structure that imparts high resistance to folding. Combining such fibers into the sheet can result in a higher-density sheet due to the strong network formed.
Table 4 presents the three formulations of mango and pine cellulosic pulps (up to 50% concentration) to enhance the mechanical properties. The 70:30 formulation demonstrated superior mechanical properties in terms of tear index, folding endurance, and breaking length compared with the formulation 100:0. These features can be attributed to the synergistic interactions between the long and short fibers present on the surface [
7]. Incorporating cellulosic pulp pine improved the mechanical properties due to individual strength, and the inter-bonding of long fibers was higher than that of short fibers. The resulting network exhibited a notable presence of hydrogen bonds that efficiently bonded with the short fibers, filling the void spaces [
7]. Measuring the porosity of mango sheets (100:0) was not feasible due to the air flux freely passing from one side to the other without resistance. The addition of pine fibers significantly improved the air resistance of the samples, with the 50:50 formulation demonstrating the highest level of air resistance. This performance can be attributed to the interaction between the long and short fibers, which generated denser networks and reduced the pore size, consequently decreasing air transport [
40]. We selected formulation 70:30 for the development of plates due to similar values in mechanical properties and a high proportion of mango cellulosic pulp.
Water resistance is an important parameter when developing tableware using natural fibers. The hydrophilicity of natural fibers can be effectively modified by incorporating chemical substances like AKD. This additive alters the hydrophilic nature of natural fibers into a hydrophobic surface by reacting with cellulose hydroxyl groups to give a β-keto ester bond, which increases hydrophobicity.
Table 5 shows the physical and mechanical properties of formulated sheets added with 1.5% (
w/
w) AKD. The contact angle drop test for the two formulations was >120°, indicating that the materials were water-resistant and could be used with intermediate-moisture foods [
5]. The mechanical properties of folding endurance and breaking length exhibited improvement through the interaction with long pine fibers in formulation 70:30 [
6].
The inclusion of AKD transformed the sheets’ chemical surface from hydrophilic to hydrophobic by introducing hydrophobic functional groups to the cellulose. Structurally, the lactone rings in AKD react with the hydroxyl groups, forming β-keto esters and creating a hydrophobic film [
41]. Our results are similar to those of tableware (cups) designed with sugarcane bagasse: bamboo (70:30) [
6]. As the mix showed low water resistance, (0.5–1.0) % of AKD was added to give a cup with a water contact angle of 127°, slightly higher than the results of our plates. In another work, plates made with rice straws showed a water contact angle as low as 10.2°, and after coating with AKD at 12.2–30.3 g/m
2, the contact angle increased to (94.9–119.2)° [
7]. Slightly lower than our results with mango seed plates. These cases confirm the low water resistance of cellulosic pulp obtained from non-wood sources, a drawback that is controlled by the addition of AKD.
3.5. Barrier Properties of the Tableware Plate
Several properties should be tested in single-use tableware made from natural fibers and designed to be used in contact with food. It is essential to understand the behavior of the plates when they come into contact with fats/oils and water (in liquid and vapor form) since their principal function is to contain foods with diverse compositions.
Table 6 presents the barrier properties of the plates: water resistance, grease resistance, and WVTR. The results were compared with those of a commercial tableware plate made of wheat straw.
As for grease resistance, the kit scale ranges from 0 to 12, with 12 denoting the highest level of resistance and 0 indicating no resistance. All the formulated tableware plates showed no grease resistance, as shown by the kit rating value of 0. In contrast, the commercial plate demonstrated moderate resistance with a kit rating of 6. The lack of grease resistance in our containers can be attributed to the high porosity of the material and the high AKD capacity to interact with oil [
42]. Most commercial plates contain kaolin as a filler, which blocks the pores and delays grease penetration. As the mango/pine samples (100:0 and 70:30) formulations did not include this filler, its addition must be studied to improve their properties. However, as reported in this work, our plates still have the application to contain dry food like dry snacks or dried fruits, which are intended to be in contact with the plate for short times.
The Cobb
60 value was used to determine the water absorption capacity of the samples, representing the mass of water absorbed in a specific area for 60 s. It was observed that the mango/pine samples (100:0 and 70:30) displayed lower water resistance than the commercial ones because they absorbed a relatively higher amount of water. The addition of AKD significantly increased surface hydrophobicity, attributed to the interaction between alkyl and cellulose hydroxyl groups. While the Cobb
60 value in the mango/pine samples was higher than that of the commercial one, when compared with other materials coated with chitosan or cellulose nanofibers, these still demonstrate superior resistance to water absorption [
40,
43].
The WVTR values for the samples were from130 to 158 g/(m
2 day) under typical testing conditions and from 792 to 1093 g/(m
2 day) under extreme conditions. Under extreme testing conditions, the WVTR increased nearly seven times due to high water vapor flow. Nevertheless, the samples exhibited no surface cracks under both typical and extreme conditions, demonstrating that they could be stable even under high temperature and humidity conditions. Paper or paperboard’s water vapor transport mechanism involves diffusion through empty voids in the fiber structure. However, in samples treated with AKD, their alkyl groups and hydrophobic chains disrupted the water vapor absorption and, consequently, its diffusion [
40,
43].
3.6. Overall Migration (OM)
Table 7 shows the OM results for formulated materials and commercial samples in aqueous, acidic, and lipophilic food simulants. In the case of the aqueous food simulant (10% ethanol), a 30 min exposure at 40 °C yielded negative OM values ranging from −13.36 to −4.98 mg/dm
2 attributed to the absorption of material of the simulant due to the content of ethanol. The formulation 100:0 demonstrated the highest absorption value, which can be explained by its porous structure that allows the simulant to permeate the plate more readily. Concerning the lipophilic food simulant (50% ethanol), the materials also absorbed liquid, resulting in negative values ranging from −14.14 to −1.96 mg/dm
2. Similarly to the aqueous food simulant findings, the 100:0 formulation showed the highest absorption value, attributed to the higher ethanol content in the simulant that facilitated the transfer through the porous structure of the plate [
44]. On the contrary, the OM in acidic food simulant was higher than the established limit by the European Commission (≤10 mg/dm
2) for both the commercial and the developed materials. The contact of the samples with 3% acetic acid resulted in the random scission of the cellulose chains, forming carbohydrate molecules that underwent oxidation and promoted hydrolysis [
45]. These products were released into the simulant, contributing to the OM value. According to the established limit for the European Commission, the evaluated materials exhibited a higher migration in acidic food simulant, failing to comply with the regulation. However, this regulation was established to test materials mainly made of plastics and does not represent contact with paper or paperboard. It appears that the testing conditions exaggerated the actual contact between solid food and the paper plates. Moreover, the presence of AKD was insufficient to seal the porous surface or the fibers and prevent the diffusion of ethanol or the hydrolysis of the cellulose chains. Tanpichai et al. [
46] applied several layers of chitosan coating on cellulose-based paper, decreasing the absorption of liquids and reducing OM. Future work in our research is to find a bio-based and biodegradable coating material that effectively fills the pores and cavities of the plates and prevents the release of products of the hydrolysis of the fibers.
3.7. Biodegradation
Figure 5 presents the biodegradation curves of the studied materials in a controlled soil aerobic biodegradation test, comparing them with positive and negative controls. The biodegradation percentage of positive control (pine cellulose pulp) was 75.03 ± 0.70% at 182 days. According to ASTM D5988, the biodegradation run was considered valid because the positive control achieved a biodegradation higher than 70% after six months. Concerning the samples, the biodegradation percentage during 182 days of incubation for mango paper was 62.14 ± 0.87%, whereas for mango paper + AKD, it was 67.65 ± 0.92%. Biodegradation occurred in the samples at different rates since this process can be influenced by various factors such as cellulose molecular weight, crystallinity, and hydrophilicity [
47]. The variations in biodegradation percentage between pine cellulosic pulp and mango paper can be attributed to differences in their lignin content. The industrial bleaching process applied to pine cellulosic pulp removed lignin, facilitating enhanced microbial access to cellulose during composting. In contrast, in mango paper, the presence of lignin hindered biodegradation, resulting in a reduced breakdown of cellulose fibers [
48,
49]. At the end of the assay, the biodegradation percentage of mango paper + AKD was higher than that of mango paper; it could be attributed to the high humidity (50%) and alkali pH (8.65), which influenced AKD hydrolysis [
50]. The void spaces (free volume) could improve the enzymatic biodegradation rate. Togo and Hagihara [
51] found a direct correlation between free volume and biodegradation rate with films developed with amorphous poly lactic acid. In the present work, the biodegradation curve showed two distinct stages: the lag phase (first 21 days of incubation), in which microorganisms adapt to the substrate and are characterized by low production of CO
2, and the log phase (from 22 to 182 days) corresponds to biodegradation, in which the microorganisms obtain energy for their metabolic reactions with a significant increase in CO
2 production. The plateau stage was not observed in the curve before 182 days of incubation.