Development and Characterization of Biodegradable Films on Native and Esterified Peruvian Purple Yam (Dioscorea trifida) Starches and Tara Gum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Analysis of Peruvian Purple Yam Tubers
2.3. Starch Extraction
2.4. Starch Modification (Esterification with OSA)
2.5. Characterization of Native and Esterified Starches
2.5.1. Proximate Analysis and Apparent Amylose Content
2.5.2. Degree of Substitution (DS)
2.5.3. Particle Size Distribution
2.5.4. Thermal Properties
2.5.5. Scanning Electron Microscopy (SEM)
2.5.6. Infrared Spectroscopy (FTIR)
2.5.7. X-Ray Diffraction (XRD) Pattern and Crystallinity
2.5.8. Rheological Characteristics
2.6. Film Preparation
2.7. Film Characterization
2.7.1. Film Appearance and Thickness
2.7.2. Moisture Content (MC) and Solubility in Water (SW)
2.7.3. Water Vapor Permeability (WVP)
2.7.4. Optical Properties
2.7.5. Mechanical Properties
2.7.6. Disintegrability Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis of Peruvian Purple Yam Tubers (PYTs) and Their Starch (PYS)
3.2. Starch Characterization
3.2.1. Apparent Amylose Content and Degree of Substitution (DS)
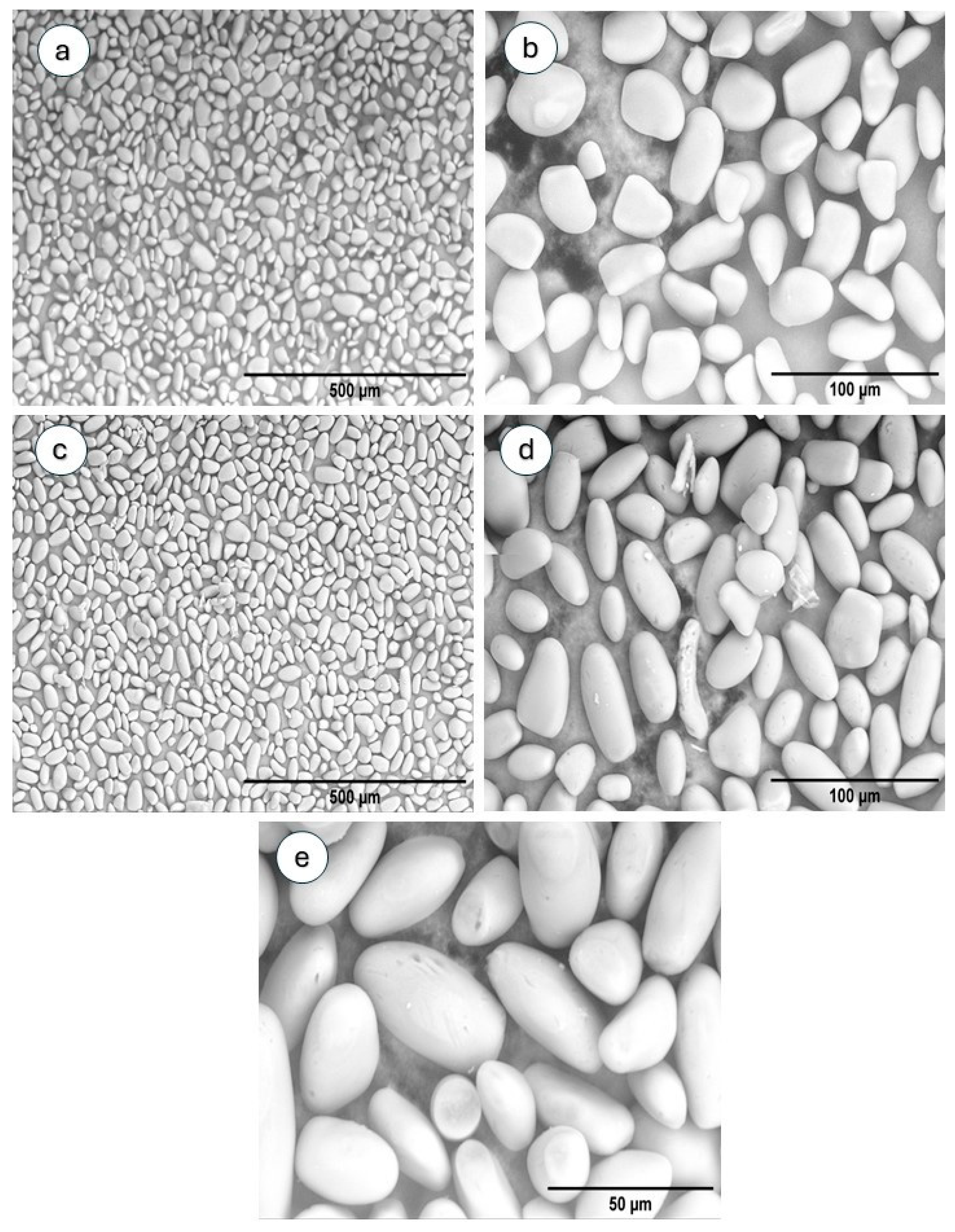
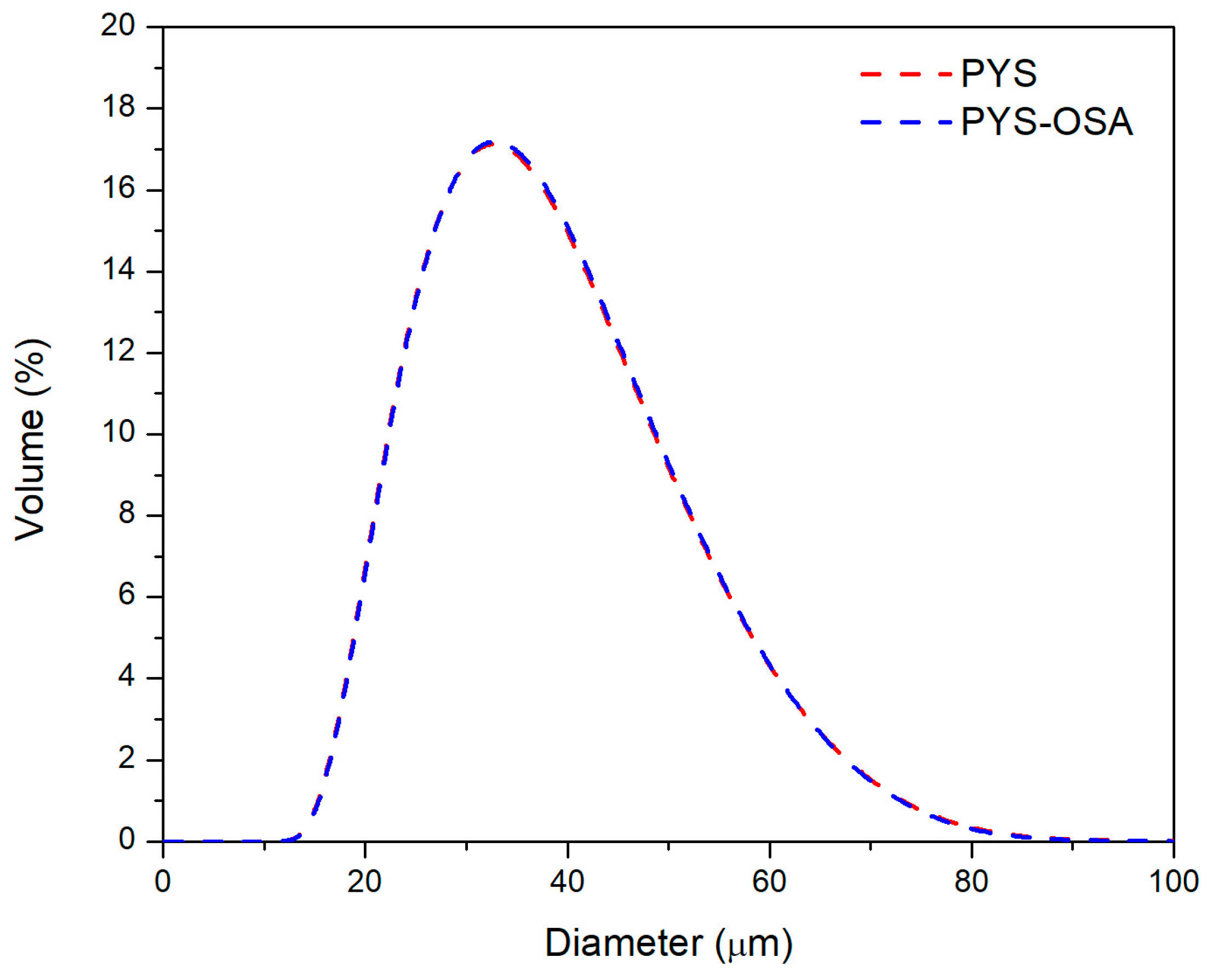
3.2.2. SEM and Particle Size Distribution
3.2.3. FT-IR Analysis
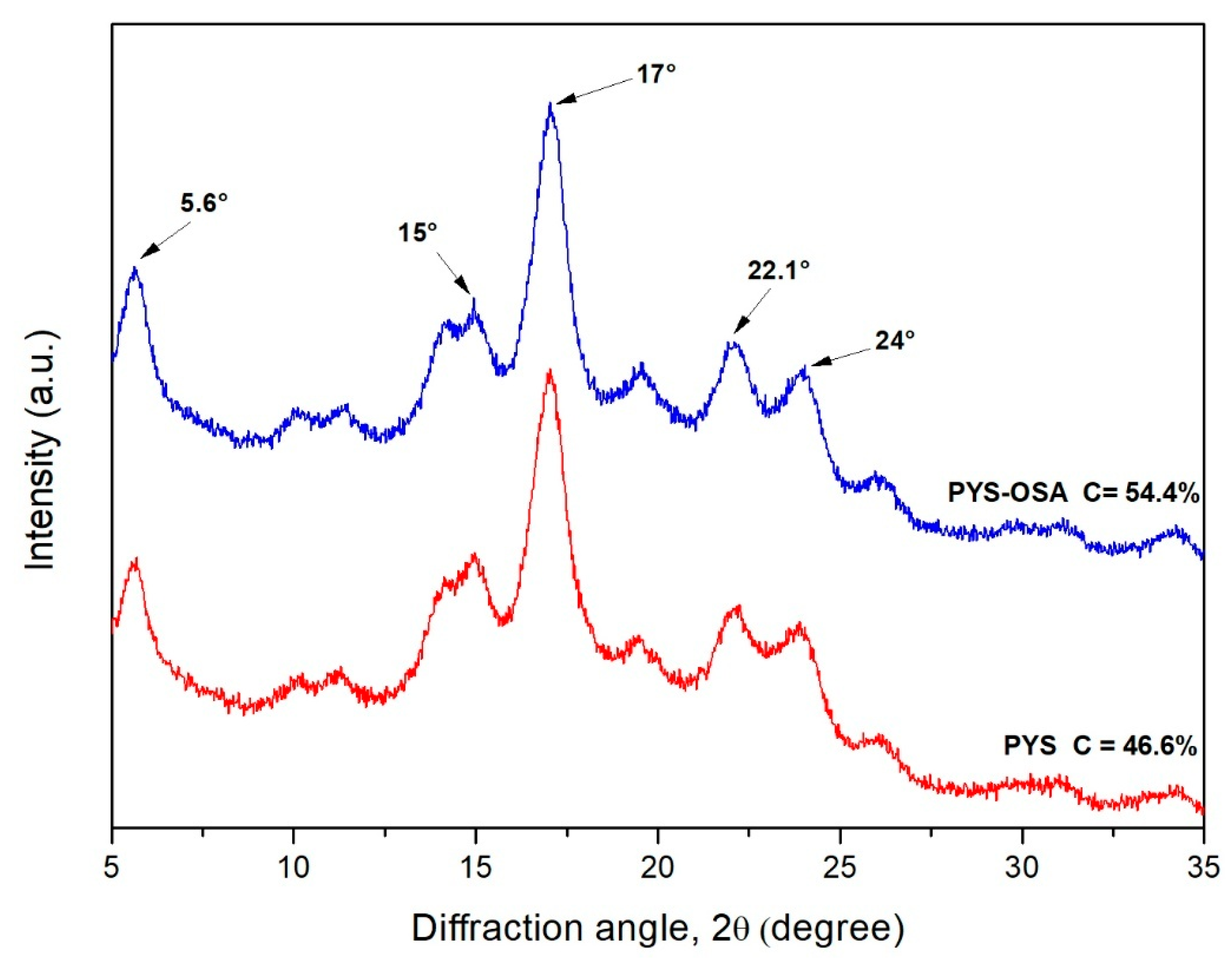
3.2.4. X-Ray Diffraction and Crystallinity
3.2.5. Thermal Properties
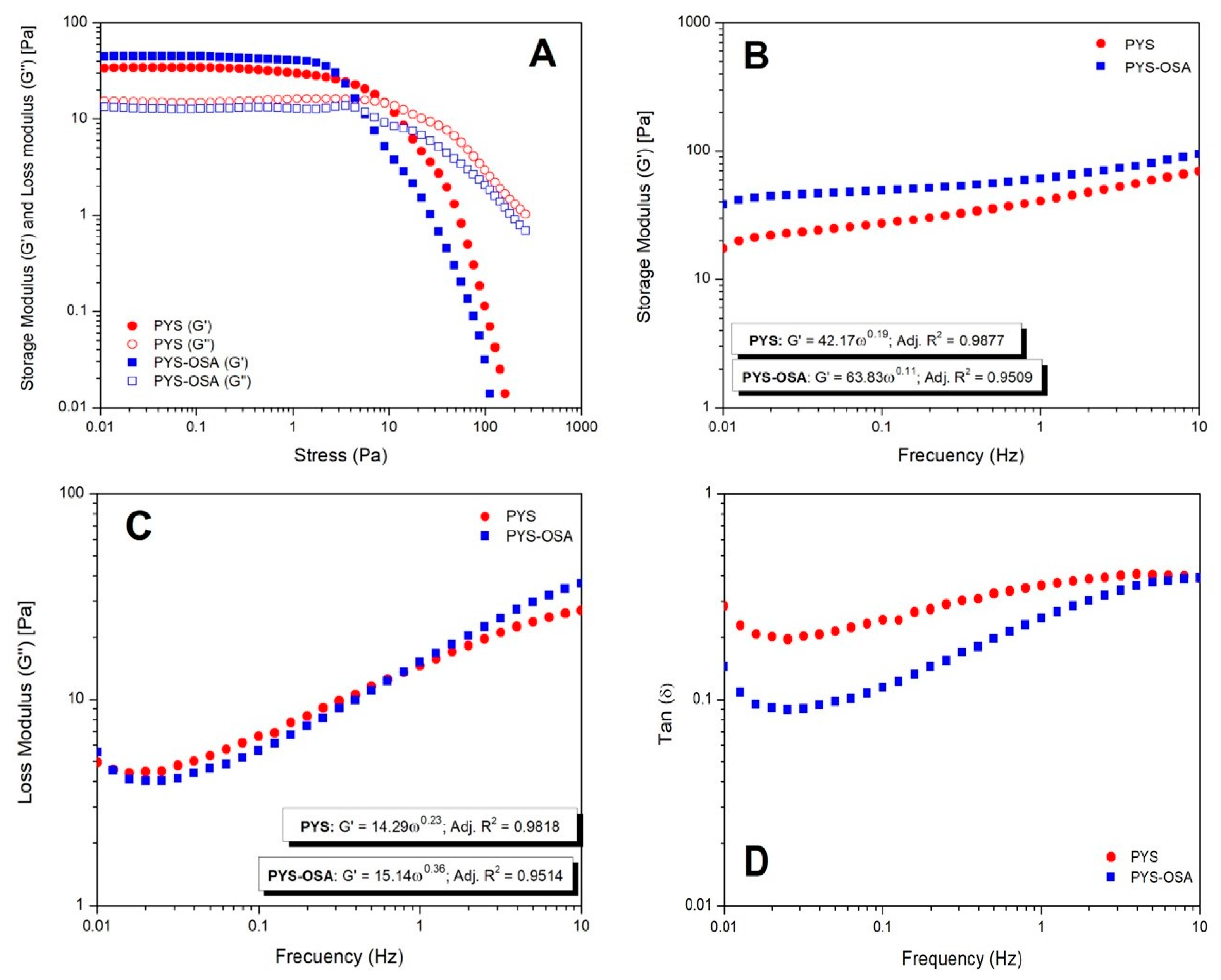
3.2.6. Rheological Characteristics
- Rotational Tests
- Oscillatory Tests
3.3. Film Characterization
3.3.1. Film Appearance and Thickness
3.3.2. Moisture Content (MC), Solubility in Water (SW), and Water Vapor Permeability (WVP)
3.3.3. Optical Properties
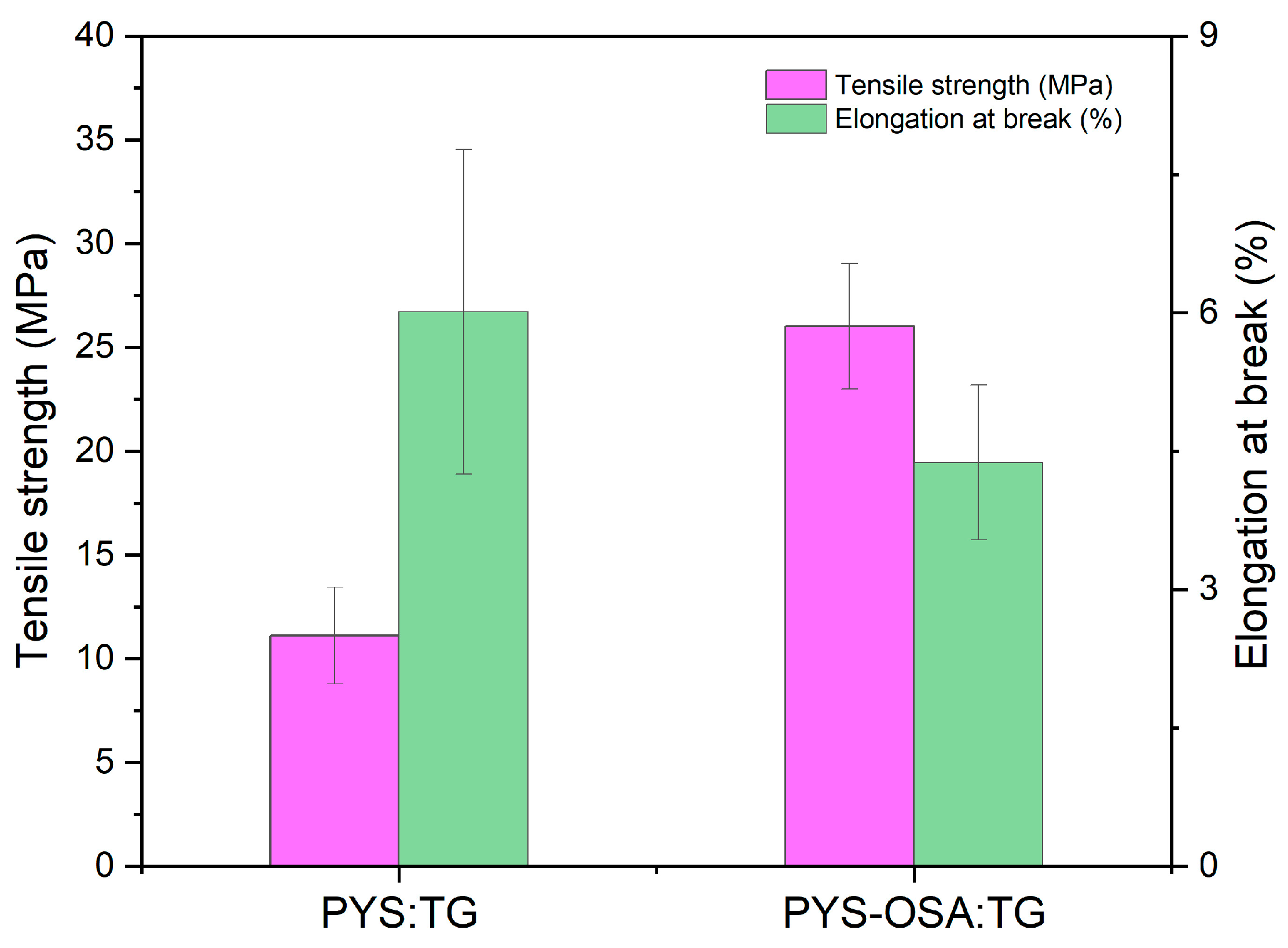
3.3.4. Mechanical Properties
3.3.5. FT-IR Analysis of Films
3.3.6. Disintegrability Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Córdoba, L.J.; Galecio-Rojas, M.; Peña-Carrasco, F.; Ibarz, A.; Velezmoro-Sánchez, C.; Martínez-Tapia, P. Effect of Ultraviolet-Irradiation on the Physicochemical and Disintegrability Properties of Nanocomposite Tunta Starch:Tara Gum Films Reinforced with Starch Nanocrystals. Polym. Plast. Technol. Mater. 2024, 63, 299–311. [Google Scholar] [CrossRef]
- Pérez-Córdoba, L.J.; Sánchez-Pizarro, A.; Vélez-Erazo, E.M.; Peña-Carrasco, E.F.; Pasquel-Reátegui, J.L.; Martínez-Tapia, P.; Velezmoro-Sánchez, C. Bitter Potato Starch-based Film Enriched with Copaiba Leaf Extract Obtained Using Supercritical Carbon Dioxide: Physical–Mechanical, Antioxidant, and Disintegrability Properties. J. Appl. Polym. Sci. 2024, 141, e55243. [Google Scholar] [CrossRef]
- Iffath, R.; Ara, R.; Ahmed, T.; Biswas, A. Fabrication and Characterization of Waste Eggshell Microparticles Reinforced Biodegradable Composite Packaging Films Enriched with Pectin and Orange Peel Essential Oil. Appl. Food Res. 2025, 5, 100735. [Google Scholar] [CrossRef]
- Da Costa, J.C.; Lima Miki, K.S.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of Biodegradable Films Based on Purple Yam Starch/Chitosan for Food Application. Heliyon 2020, 6, e03718. [Google Scholar] [CrossRef]
- Martínez, P.; Vilcarromero, D.; Pozo, D.; Peña, F.; Manuel Cervantes-Uc, J.; Uribe-Calderon, J.; Velezmoro, C. Characterization of Starches Obtained from Several Native Potato Varieties Grown in Cusco (Peru). J. Food Sci. 2021, 86, 907–914. [Google Scholar] [CrossRef]
- Do Nascimento, W.F.; Siqueira, M.V.B.M.; Raz, L.; Veasey, E.A. Dioscorea trifida L.f.: A Little Known South American Species. In Varieties and Landraces; Elsevier: Amsterdam, The Netherlands, 2023; pp. 55–68. [Google Scholar] [CrossRef]
- Siqueira, M.V.B.M.; Do Nascimento, W.F.; Raz, L.; Costa, F.M.; Veasey, E.A. Yam (Dioscorea spp.) Cultivation and Landraces with Market Potential in South America. In Varieties and Landraces; Elsevier: Amsterdam, The Netherlands, 2023; pp. 35–53. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Santos-Buelga, C.; Pérez-Alonso, J.J.; Yáñez, J.A.; Dueñas, M. HPLC-DAD-ESI/MS Identification of Anthocyanins in Dioscorea trifida L. Yam Tubers (Purple Sachapapa). Eur. Food Res. Technol. 2010, 230, 745–752. [Google Scholar] [CrossRef]
- Alva, W.; Obregón, R.; Ruiz, S. Banco de Germoplasma En Sachapapa (Dioscórea Trífida) En Tingo María. RevIA 2017, 7, 1–3. [Google Scholar]
- Odicio, J.E.; Tuisima, L.L.; Guillén, W.; Bonzano del Águila, E.H. Manual de Manejo Agronómico de Raíces y Tubérculos Tropicales; Instituto Nacional de Innovación Agraria—INIA: Lima, Peru, 2023.
- Santos, S.D.J.L.; Pires, M.B.; Amante, E.R.; Da Cruz Rodrigues, A.M.; Da Silva, L.H.M. Isolation and Characterization of Starch from Purple Yam (Dioscorea trifida). J. Food Sci. Technol. 2022, 59, 715–723. [Google Scholar] [CrossRef]
- Pérez, E.; Gibert, O.; Rolland-Sabaté, A.; Jiménez, Y.; Sánchez, T.; Giraldo, A.; Pontoire, B.; Guilois, S.; Lahon, M.-C.; Reynes, M.; et al. Physicochemical, Functional, and Macromolecular Properties of Waxy Yam Starches Discovered from “Mapuey” (Dioscorea trifida) Genotypes in the Venezuelan Amazon. J. Agric. Food Chem. 2011, 59, 263–273. [Google Scholar] [CrossRef]
- Jiang, Q.; Gao, W.; Li, X.; Xia, Y.; Wang, H.; Wu, S.; Huang, L.; Liu, C.; Xiao, P. Characterizations of Starches Isolated from Five Different Dioscorea L. Species. Food Hydrocoll. 2012, 29, 35–41. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Morales, N.J.; Pérez, E.; Tapia, M.S.; Famá, L. Physico-Chemical Properties of Edible Films Derived from Native and Phosphated Cush-Cush Yam and Cassava Starches. Food Packag. Shelf Life 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Mukherjee, K.; Dutta, P.; Badwaik, H.R.; Saha, A.; Das, A.; Giri, T.K. Food Industry Applications of Tara Gum and Its Modified Forms. Food Hydrocoll. Health 2023, 3, 100107. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, D.; Wang, L. Preparation and Physical Properties of Tara Gum Film Reinforced with Cellulose Nanocrystals. Int. J. Biol. Macromol. 2016, 86, 606–612. [Google Scholar] [CrossRef] [PubMed]
- NTP 206.011:2018; Bizcochos, Galletas y Pastas o Fideos. Determinación de Humedad. Dirección de Normalización—INACAL: Lima, Peru, 2018; pp. 2–4.
- COVENIM.1195.80:1980; Alimentos, Determinación de Nitrógeno, Método de Kjeldahl. Fondonorma: Caracas, Venezuela, 1980; pp. 1–10.
- NMX-F-427-NORMEX-2019; Alimentos, Determinación de Grasa (Método Gravimétrico por Hidrólisis Ácida), Método Mojonnier. Sociedad Mexicana de Normalizaciòn y Certificación S.C.: Mexico City, Mexico, 2019.
- NMX-F-607-NORMEX-2020; Alimentos, Determinación de Cenizas en Alimentos, Método de Prueba. Sociedad Mexicana de Normalizaciòn y Certificación S.C.: Mexico City, Mexico, 2020.
- AOCS. Official Method of American Oil Chemists Society, 7th ed.; AOCS: Urbana, IL, USA, 2017. [Google Scholar]
- AOAC. Official Method of Analysis, 22nd ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2023.
- Martínez, P.; Peña, F.; Bello-Pérez, L.A.; Núñez-Santiago, C.; Yee-Madeira, H.; Velezmoro, C. Physicochemical, Functional and Morphological Characterization of Starches Isolated from Three Native Potatoes of the Andean Region. Food Chem. X 2019, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Peña, F.; Bello-Pérez, L.A.; Yee-Madeira, H.; Ibarz, A.; Velezmoro, C. Ultrasound-Assisted Esterification of Andean Native Potato Starches Increases the Degree of Substitution and Reaction Efficiency. Potato Res. 2024, 67, 711–732. [Google Scholar] [CrossRef]
- Hoover, R.; Ratnayake, W.S. Determination of total amylose content of starch. Curr. Protoc. Food Anal. Chem. 2001, 1, E2.3. [Google Scholar] [CrossRef]
- Timgren, A.; Rayner, M.; Dejmek, P.; Marku, D.; Sjöö, M. Emulsion Stabilizing Capacity of Intact Starch Granules Modified by Heat Treatment or Octenyl Succinic Anhydride. Food Sci. Nutr. 2013, 1, 157–171. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; Nijsse, J.; Blonk, H.C.G.; Bialek, L.; Schumm, S.; Langton, M. Effect of Mechanical and Thermal Treatments on the Microstructure and Rheological Properties of Carrot, Broccoli and Tomato Dispersions. J. Sci. Food Agric. 2011, 91, 207–217. [Google Scholar] [CrossRef]
- Martínez, P.; Betalleluz-Pallardel, I.; Cuba, A.; Peña, F.; Cervantes-Uc, J.M.; Uribe-Calderón, J.A.; Velezmoro, C. Effects of Natural Freeze-Thaw Treatment on Structural, Functional, and Rheological Characteristics of Starches Isolated from Three Bitter Potato Cultivars from the Andean Region. Food Hydrocoll. 2022, 132, 107860. [Google Scholar] [CrossRef]
- Steffolani, M.E.; León, A.E.; Pérez, G.T. Study of the Physicochemical and Functional Characterization of Quinoa and Kañiwa Starches. Starch Stärke 2013, 65, 976–983. [Google Scholar] [CrossRef]
- Condés, M.C.; Añón, M.C.; Mauri, A.N.; Dufresne, A. Amaranth Protein Films Reinforced with Maize Starch Nanocrystals. Food Hydrocoll. 2015, 47, 146–157. [Google Scholar] [CrossRef]
- Qian, S.-Y.; Tang, M.-Q.; Gao, Q.; Wang, X.-W.; Zhang, J.-W.; Tanokura, M.; Xue, Y.-L. Effects of Different Modification Methods on the Physicochemical and Rheological Properties of Chinese Yam (Dioscorea opposita Thunb.) Starch. LWT 2019, 116, 108513. [Google Scholar] [CrossRef]
- Goswaim, T.H.; Maiti, M.M. Biodegradability of Gelatin—PF Resin Blends by Soil Burial Method. Polym. Degrad. Stab. 1998, 61, 355–359. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Chaves Ribeiro, A.E.; Gondim, Í.C.; Alves Dos Santos, E.; Resende De Oliveira, É.; Mendes Coutinho, G.S.; Soares Júnior, M.S.; Caliari, M. Isolation and Characterization of Yam (Dioscorea alata L.) Starch from Brazil. LWT 2021, 149, 111843. [Google Scholar] [CrossRef]
- Pérez, E.; Rolland-Sabaté, A.; Dufour, D.; Guzmán, R.; Tapia, M.; Raymundez, M.; Ricci, J.; Guilois, S.; Pontoire, B.; Reynes, M.; et al. Isolated Starches from Yams (Dioscorea Sp) Grown at the Venezuelan Amazons: Structure and Functional Properties. Carbohydr. Polym. 2013, 98, 650–658. [Google Scholar] [CrossRef]
- Rached, L.B.; de Vizcarrondo, C.A.; Rincón, A.M.; Padilla, F. Evaluación de harinas y almidones de mapuey (Dioscorea trifida), variedades blanco y morado. Arch. Latinoam. Nutr. 2006, 56, 375–383. [Google Scholar]
- Elmi Sharlina, M.S.; Yaacob, W.A.; Lazim, A.M.; Fazry, S.; Lim, S.J.; Abdullah, S.; Noordin, A.; Kumaran, M. Physicochemical Properties of Starch from Dioscorea Pyrifolia Tubers. Food Chem. 2017, 220, 225–232. [Google Scholar] [CrossRef]
- Abgeunde, O.K.; Mu, T.H.; Chen, J.W.; Deng, F.M. Physicochemical characterization of sweet potato starches popularly used in Chinese starch industry. Food Hydrocoll. 2013, 33, 169–177. [Google Scholar] [CrossRef]
- Lopez-Silva, M.; Bello-Perez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Effect of Amylose Content in Morphological, Functional and Emulsification Properties of OSA Modified Corn Starch. Food Hydrocoll. 2019, 97, 105212. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Hou, H.; Li, X.; Dong, H.; Wang, W.; Zhang, H. Ultrasound-Assisted Preparation of Octenyl Succinic Anhydride Modified Starch and Its Influence Mechanism on the Quality. Food Chem. X 2020, 5, 100077. [Google Scholar] [CrossRef]
- Emmambux, M.N.; Taylor, J.R. Morphology, physical, chemical, and functional properties of starches from cereals, legumes, and tubers cultivated in Africa: A review. Starch Staerke. 2013, 65, 715–729. [Google Scholar] [CrossRef]
- Mao, X.; Lu, J.; Huang, H.; Gao, X.; Zheng, H.; Chen, Y.; Li, X.; Gao, W. Four Types of Winged Yam (Dioscorea alata L.) Resistant Starches and Their Effects on Ethanol-Induced Gastric Injury in Vivo. Food Hydrocoll. 2018, 85, 21–29. [Google Scholar] [CrossRef]
- Zainal Abiddin, N.F.; Yusoff, A.; Ahmad, N. Effect of Octenylsuccinylation on Physicochemical, Thermal, Morphological and Stability of Octenyl Succinic Anhydride (OSA) Modified Sago Starch. Food Hydrocoll. 2018, 75, 138–146. [Google Scholar] [CrossRef]
- Chakraborty, G.; Kumar, Y.; Sharanagat, V.S. Effect of Ultrasonication on OSA Esterified Surface Modification of Sorghum (Sorghum bicolor (L.) Moench) Starch. Int. J. Biol. Macromol. 2025, 288, 138634. [Google Scholar] [CrossRef] [PubMed]
- Leon-Bejarano, M.; Durmus, Y.; Ovando-Martínez, M.; Simsek, S. Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut By-Products Extracts. Foods 2020, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Pająk, P.; Gałkowska, D.; Juszczak, L.; Khachatryan, G. Octenyl Succinylated Potato Starch-Based Film Reinforced by Honey-Bee Products: Structural and Functional Properties. Food Packag. Shelf Life 2022, 34, 100995. [Google Scholar] [CrossRef]
- Martens, B.M.J.; Gerrits, W.J.J.; Bruininx, E.M.A.M.; Schols, H.A. Amylopectin Structure and Crystallinity Explains Variation in Digestion Kinetics of Starches across Botanic Sources in an in Vitro Pig Model. J. Anim. Sci. Biotechnol. 2018, 9, 91. [Google Scholar] [CrossRef]
- He, X.; Li, M.; Gong, X.; Niu, B.; Li, W. Biodegradable and Antimicrobial CSC Films Containing Cinnamon Essential Oil for Preservation Applications. Food Packag. Shelf Life 2021, 29, 100697. [Google Scholar] [CrossRef]
- Niu, X.; Ma, Q.; Li, S.; Wang, W.; Ma, Y.; Zhao, H.; Sun, J.; Wang, J. Preparation and Characterization of Biodegradable Composited Films Based on Potato Starch/Glycerol/Gelatin. J. Food Qual. 2021, 2021, 6633711. [Google Scholar] [CrossRef]
- Saberi, B.; Thakur, R.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Optimization of Physical and Optical Properties of Biodegradable Edible Films Based on Pea Starch and Guar Gum. Ind. Crops Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- López, O.V.; García, M.A.; Zaritzky, N.E. Film Forming Capacity of Chemically Modified Corn Starches. Carbohydr. Polym. 2008, 73, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, C.; Shenoy, R.; Shetty, P.; Srinivasulu, M.; Nayak, R. Formulation and Characterization of Starch-Based Novel Biodegradable Edible Films for Food Packaging. J. Food Sci. Technol. 2023, 60, 2858–2867. [Google Scholar] [CrossRef] [PubMed]
- Pająk, P.; Socha, R.; Królikowska, K.; Grzyb, J.; Hetmańczyk, J.; Zachariasz, P. Characterization of Octenyl Succinylated Potato-Starch Based Films Enriched with Extracts from Various Honey-Bee Products. Int. J. Biol. Macromol. 2025, 285, 138293. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Guha, P. Modelling the Effect of Guar Gum on Physical, Optical, Barrier and Mechanical Properties of Potato Starch Based Composite Film. Carbohydr. Polym. 2018, 200, 498–507. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Barrier, Mechanical and Optical Properties of Plasticized Yam Starch Films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- López-Padilla, A.; Cortés-Rodríguez, M.; Ortega-Toro, R. Development and Comparative Analysis of Hard and Soft Wheat Flour Films Enriched with Yellow and White Chlorella Vulgaris Algae. Polymers 2025, 17, 785. [Google Scholar] [CrossRef]
- Karnwal, A.; Rauf, A.; Jassim, A.Y.; Selvaraj, M.; Al-Tawaha, A.R.M.S.; Kashyap, P.; Kumar, D.; Malik, T. Advanced Starch-Based Films for Food Packaging: Innovations in Sustainability and Functional Properties. Food Chem. X 2025, 29, 102662. [Google Scholar] [CrossRef]
- Gujral, H.; Sinhmar, A.; Nehra, M.; Nain, V.; Thory, R.; Pathera, A.K.; Chavan, P. Synthesis, Characterization, and Utilization of Potato Starch Nanoparticles as a Filler in Nanocomposite Films. Int. J. Biol. Macromol. 2021, 186, 155–162. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of Bioplastics in Natural Environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]




| Component | PYTs | PYS |
|---|---|---|
| Moisture content (%) | 76.42 ± 0.16 | 13.53 ± 0.02 |
| Total protein (%, db) | 6.87 ± 0.29 | 0.31 ± 0.10 |
| Fat (%, db) | 1.02 ± 0.23 | 1.47 ± 0.03 |
| Fiber (%, db) | 2.71 ± 0.14 | 0.13 ± 0.02 |
| Ash (%, db) | 3.27 ± 0.08 | 0.06 ± 0.00 |
| Phosphorus (mg/100 g, db) | 94.72 ± 0.72 | 13.38 ± 0.04 |
| Component | PYS | PYS-OSA |
|---|---|---|
| Apparent amylose content (%) | 30.69 ± 0.72 b | 19.97 ± 0.16 a |
| Degree of substitution | -- | 0.023 |
| Color parameters | ||
| L* | 93.59 ± 0.05 a | 95.96 ± 0.39 b |
| a* | 2.29 ± 0.16 b | 0.08 ± 0.02 a |
| b* | 1.08 ± 0.06 a | 1.32 ± 0.14 a |
| W (%) | 93.11 ± 0.02 a | 95.61 ± 0.25 b |
| Particle size distribution | ||
| D [4,3] (μm) | 34.5 | 34.9 |
| Crystallinity (%) | 46.6 | 54.4 |
| Differential scanning calorimetry | ||
| To (°C) | 71.75 ± 0.09 b | 68.30 ± 0.59 a |
| Tp (°C) | 75.83 ± 0.08 b | 73.27 ± 0.06 a |
| Tc (°C) | 81.57 ± 0.16 b | 78.81 ± 0.18 a |
| ΔH (J/g) | 14.27 ± 0.11 a | 13.18 ± 1.43 a |
| Characteristic | PYS:TG | PYS-OSA:TG |
|---|---|---|
| Moisture content (%) | 11.37 ± 1.67 a | 17.14 ± 0.87 b |
| Thickness (mm) | 0.076 ± 0.004 a | 0.074 ± 0.002 a |
| Solubility in water (%) | 0.25 ± 0.06 b | 0.42 ± 0.09 a |
| WVP × 10−10 (g·m−1 s−1 Pa−1) | 1.10 ± 0.26 a | 1.33 ± 0.22 b |
| Density (g/cm3) | 2.02 ± 0.02 a | 1.93 ± 0.11 b |
| Optical properties | ||
| L* | 96.65 ± 0.26 a | 97.09 ± 0.05 b |
| ΔE* | 2.74 ± 0.24 b | 2.17 ± 0.05 a |
| YI | 0.29 ± 0.03 b | −0.05 ± 0.00 a |
| WI | 96.50 ± 0.26 a | 97.06 ± 0.00 b |
| Opacity (mm−1) | 2.42 ± 0.26 a | 2.76 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornejo, P.; Chalco, N.; Gutiérrez, S.; Junco, K.; Lopinta, R.; Peña-Carrasco, F.; Velezmoro-Sánchez, C.; Martínez-Tapia, P. Development and Characterization of Biodegradable Films on Native and Esterified Peruvian Purple Yam (Dioscorea trifida) Starches and Tara Gum. Polymers 2025, 17, 2754. https://doi.org/10.3390/polym17202754
Cornejo P, Chalco N, Gutiérrez S, Junco K, Lopinta R, Peña-Carrasco F, Velezmoro-Sánchez C, Martínez-Tapia P. Development and Characterization of Biodegradable Films on Native and Esterified Peruvian Purple Yam (Dioscorea trifida) Starches and Tara Gum. Polymers. 2025; 17(20):2754. https://doi.org/10.3390/polym17202754
Chicago/Turabian StyleCornejo, Paola, Naomi Chalco, Sebastian Gutiérrez, Katherine Junco, Ronal Lopinta, Fiorela Peña-Carrasco, Carmen Velezmoro-Sánchez, and Patricia Martínez-Tapia. 2025. "Development and Characterization of Biodegradable Films on Native and Esterified Peruvian Purple Yam (Dioscorea trifida) Starches and Tara Gum" Polymers 17, no. 20: 2754. https://doi.org/10.3390/polym17202754
APA StyleCornejo, P., Chalco, N., Gutiérrez, S., Junco, K., Lopinta, R., Peña-Carrasco, F., Velezmoro-Sánchez, C., & Martínez-Tapia, P. (2025). Development and Characterization of Biodegradable Films on Native and Esterified Peruvian Purple Yam (Dioscorea trifida) Starches and Tara Gum. Polymers, 17(20), 2754. https://doi.org/10.3390/polym17202754







